Abstract
Background
This cross-sectional study aimed to track population-based SARS-CoV-2 antibody seropositivity duration across the United States using observational data from a national clinical laboratory registry of patients tested by nucleic acid amplification (NAAT) and serologic assays. Knowledge of antibody seropositivity and its duration may help dictate post-pandemic planning.
Methods
Using assays to detect antibodies to either nucleocapsid (N) or spike (S) proteins performed on specimens from 39,086 individuals with confirmed positive COVID-19 by reverse transcription-polymerase chain reaction (RT-PCR) from March 2020 to January 2021, we analyzed nationwide seropositivity rates of IgG up to 300 days following patients’ initial positive NAAT test. Linear regression identified trends in seropositivity rates and logistic regression tested positive predictability by age, sex, assay type and days post-infection.
Findings
Seropositivity of IgG antibodies to both SARS-CoV-2 S and N-proteins followed a linear trend reaching approximately 90% positivity at 21 days post-index. The rate of N-protein seropositivity declined at a sharper rate, decaying to 68·2% [95% CI: 63·1–70·8%] after 293 days, while S-antibody seropositivity maintained a rate of 87·8% [95% CI: 86·3–89·1%] through 300 days. In addition to antigen type and the number of days post-positive PCR, age and gender were also significant factors in seropositivity prediction, with those under 65 years of age showing a more sustained seropositivity rate.
Interpretation
Observational data from a national clinical laboratory, though limited by an epidemiological view of the U.S. population, offer an encouraging timeline for the development and sustainability of antibodies up to ten months from natural infection and could inform post-pandemic planning.
Keywords: COVID-19, SARS-CoV-2, Antibody seropositivity, Real-world evidence
Research in context.
Evidence before this study
Since antibody testing for SARS-CoV-2 began in the United States, identification of antibody kinetics and seropositivity has provided differing conclusions. With testing for antibodies to both the Nucleocapsid and Spike proteins of SARS-CoV-2 being performed since early 2020, some studies have shown IgG antibody half-life to be at a few weeks, while others have examined IgG levels remaining elevated for 4 or more months. Antibody seropositivity persistence has been shown to last for up to two years in other coronaviruses, such as SARS-CoV, but due to the novelty of SARS-CoV-2, sufficient longitudinal data has not been collected in follow-up to infection to properly view population-level seropositivity rates.
Added value of this study
This study extends the timeframe of available longitudinal data to ten months-worth of follow-up antibody assay results, giving indication that antibody detection is possible for almost a year post-natural infection of COVID-19. Additionally, few studies have been able to track follow-up assays in large samples. While it does not indicate that these antibodies provide protective immunity for that duration, it provides key real-world evidence from a national clinical laboratory of the U.S. population retaining antibodies, for both Spike and Nucleocapsid, at a detectable level.
Implications of all the available evidence
With the world currently directing its efforts to provide vaccines to halt the COVID-19 pandemic, epidemiological evidence of antibody duration can help shape public policy moving forward. Further research is required to quantify antibodies this far from infection and how they may provide or sustain protective immunity, but these results indicate that population-level seropositivity persists for a substantial period of time given the limited timeframe since testing began in the U.S.
Alt-text: Unlabelled box
1. Introduction
Since December 2019, the world has been tasked with rapidly identifying, testing and treating coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to its novelty, one of the difficulties in treatment and planning is the lack of sufficient longitudinal data on humoral immune response to infection [1]. With over 31 million SARS-CoV-2 infections in the United States alone since the first confirmed case in late January 2020, identifying SARS-CoV-2 antibody seropositivity rates and reversion times can help establish a better understanding of how long a population can retain these antibodies. Data from Laboratory Corporation of America® Holdings (Labcorp®), which began providing COVID-19 antibody tests nationwide by late April 2020 [2], provides a near population-level view of natural antibody seropositivity rates. Testing has been performed in every state and territory in the U.S. and these data encompass thousands of unique IgG seropositive results ranging from the time of polymerase chain reaction (PCR) confirmed diagnosis and up to ten months post-COVID-19 diagnosis date.
When exposed to the coronavirus, studies have shown that IgG antibodies can be detected in blood from ten days after onset of symptoms with high sensitivity for specific structures of SARS-CoV-2 [3]. The typical structure of a coronavirus consists of four main proteins: the nucleocapsid (N), envelope (E), membrane (M) and spike (S) proteins. The N-protein is intercellular and pivotal in transcription and replication of viral DNA and therefore highly expressed during infection. The S-protein is highly immunogenic and sits on the surface, responsible for binding to angiotensin converting enzyme 2 (ACE2) to gain entrance into host cells via the receptor binding domain (RBD) [4,5]. While both are immunogenic, effectiveness of antibody response is believed to differentiate between the two: the N-antibody is readily detected early in the serologic response, but the S-antibody, due to its location and function on the viral surface, may be the more important target antigen, as antibodies to the S-antigen have been shown to correlate to viral neutralization in vitro [6,7].
Current understanding of SARS-CoV-2 antibodies is limited by small sample sizes and short-term longitudinal data, with studies so far offering conflicting reports of antibody sustainability [8,9]. Previous research on SARS-CoV, responsible for the SARS outbreak in 2003, demonstrated that IgG antibodies remain detectable for up to two years [10] ,but the novelty of SARS-CoV-2 requires further analysis. One study evaluating 34 participants with mild COVID-19 symptoms for RBD-specific IgG antibodies estimated IgG half-life to be 36 days [11]. Alternatively, some studies have examined IgG levels that remained elevated up to 115 [12] and 120 days [13] after onset of symptoms. Iyer, et al. observed a median time to seroreversion of IgG antibodies to the RBD of S-proteins to be 70·5 days, but found that neutralizing antibody titers did not decrease significantly over 75 days from symptom onset [14]. So far, SARS-CoV-2 antibody studies have mostly examined shorter timeframes, such as a study from Iceland that observed comparable seropositivity rates after 125 days or another from New York that examined similar rates, but in neutralizing capacity through five months [15,16].
The testing performed by Labcorp® from March 2020 to January 2021 provides a large cohort of SARS-CoV-2 PCR-positive individuals with subsequent antibody testing. This provides an opportunity to examine and compare long-term sustainability of both N and S-antibody positivity. Seropositivity rates can thus be observed across the U.S. to help predict sustainability, though this is done by examining rates of the presence of antibodies in the blood after naturally infected patients only, rather than tracking neutralizing titers for individuals over time. Based on current evidence, we hypothesize that antibodies to both S and N-proteins after natural infection may persist for longer than previously thought, thereby providing evidence of sustainability that may influence post-pandemic planning,[17] though longevity may be different between antibodies to the two proteins.
2. Methods
2.1. Assays and data collection
Currently, Labcorp® retains an active, de-identified registry of COVID-19 confirmed patients and their associated laboratory testing. COVID-19 diagnosis was identified by a patient's first nucleic acid amplification test (NAAT) by positive reverse transcription-polymerase chain reaction (RT-PCR) assay between March 1, 2020 and January 31, 2021 via FDA Emergency Use Authorized (EUA) approved method, utilizing samples from nasopharyngeal, oropharyngeal or nasal swabs. Specimens were collected from all 50 U.S. states, classified as Northeast, South, Midwest and West regions according to the U.S. Census Bureau. Date of the first positive RT-PCR test, referred to here as PCR, was considered the index, at t = 0 days. All antibody tests performed for these patients any time after this index PCR test were identified and used for this study, using blood draw date to calculate the number of days since index. The final cohort of patients reflects only those with completed antibody assays. These tests used in our analysis were qualitative assays, identifying presence of IgG to the N-protein or S-protein (specifically, the S1 subunit that contains the RBD) only. Assays can be from multiple manufacturers, but this study limited analysis to the two most frequently used assay platforms at Labcorp® per S (named here as Assays A and B, utilizing ELISA and chemiluminescence immunoassay methodologies, respectively) and N-antibodies (Assays C and D, both chemiluminescence immunoassays), four in total. Specific manufacturers remain anonymous for unbiased comparison. As listed by the FDA EUA, sensitivities 14 days after PCR-positive test of both N-assays are 100% and both S-assays are 97% or higher. Specificity for all four assays are 99% or higher [18].
Analysis of the data is population-based, calculating rates of total positive antibody tests as the numerator and all antibody tests with either positive or negative status as the denominator, per day since first positive PCR. Assay results categorized as “other,” such as inconclusive, equivocal, or contaminated were omitted. Due to variability of test orders over time, the denominator differs each post-index day, with more testing occurring within 21 days of first positive PCR. Seropositivity rates are presented only for the post-index days with at least five blood samples. Patients with more than one follow-up test are counted towards the seropositivity rate for the specific day post-index the blood was drawn from. Quantitative neutralizing antibody capacity was not consistently available for majority of patients.
2.2. Statistical analysis
Statistical analysis and visualizations were performed using Python 3·7 and SciPy package. Locally Weighted Scatterplot Smoothing (LOWESS), a moving average regression, smoothed curves with high variability. To fit a trend to the daily post-index seropositivity rates, regression analysis identified linear trends per protein after reaching height of antibody response at 21 days post-index, with linear equation and p-value reported with significance at p < 0·05. A 95% confidence interval around this trend was achieved through bootstrapping to compensate for lower sample sizes at later days. Intercept values are also reported, representing seropositivity at 21 days post-infection. A sigmoid function, which has been used to predict S-antibody decay [6], was attempted to fit the data, but acceptable convergence could not be achieved, leaving linear regression considered as the best fit until longer post-index data can be accumulated. Logistic regression was used to predict positivity status at the raw individual level and assess predictive variables, with p-value reported for significance. This is separate from the linear regression in that it attempts to identify positivity status for a patient adjusting for age and sex, rather than positivity rate for the population. Continuous variables were normalized on a minimum-maximum scaler. Missing categorical data was imputed as unknown. Both regression analyses grouped specific S and N-assays together, regardless of manufacturer (Assays A and B categorized as S, Assays C and D as N); a sub-analysis used LOWESS to examine differences between all four separately. Comparison of positivity rates between sex and age groups were evaluated using the log-rank test, commonly used in survival analysis, reporting p-value. Due to the conditions of the data de-identification, any patient over 90 years of age had associated dates for blood draw shifted by a substantial margin for additional anonymity, thereby limiting the ability to obtain accurate date differences. These patients were removed from the analysis.
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies [19]. Use of the data within Labcorp®’s COVID-19 registry was approved with waiver of authorization for the use and disclosure of protected health information by Western IRB on March 26, 2020. There was no financial support for this study. All authors are employed by Laboratory Corporation of America® Holdings.
3. Results
Of close to 22 million patients with an index PCR test, 2,547,616 patients tested positive for COVID-19 between March 2020 and January 2021. A total of 39,086 patients had at least one antibody test performed with Labcorp® after their index positive PCR test, also done with Labcorp® (Table 1 for patient characteristics, Supplemental Fig. S1 for flowchart of testing). Of those, 52·4% (n = 20,487) had at least one assay that tested for N and 52·9% (n = 20,694) had at least one assay for S-antibody. Of the whole population, 58·0% (n = 22,657) were female and median age (IQR) was 47 years (27). Majority of patients were located in the South (50·3%, n = 19,645) and Northeast (29·4%, n = 11,494) regions of the U.S., indicative of Labcorp® presence in those regions. Median number of follow-up antibody tests per patient was one test, and median (IQR) time to test for N-assays was 36 (48) days and 52 (109) days for S-assays. Of total antibody test orders (n = 45,061), about 73% came from primary care specialties (Family Medicine, Internal Medicine, Telemedicine, General Practice), reflecting a majority of outpatient settings. Volume of tests per day post-positive PCR index reached a peak at 14 days for both N and S-antibody assays (n = 768 and n = 469, respectively) and followed an exponential decay for the remaining days (Fig. S2). Though N-antibody assays were more abundant at the start of infection, S-antibody assays were more common after about 112 days (refer to Supplemental Table S1 for counts of tests by week), reflecting an increase in data pointing to the importance of S-antibodies in humoral immune response.
Table 1.
Patient counts and demographic information of patients with antibody testing post-first positive PCR test, by antibody protein assay.
| All* | Nucleocapsid | Spike | |
|---|---|---|---|
| N, Unique Patients | 39,086 | 20,487 | 20,694 |
| Sex | |||
| Female | 22,657 (58·0%) | 11,766 (57·4%) | 12,108 (58·5%) |
| Male | 16,375 (42·9%) | 8689 (42·4%) | 8561 (41·4%) |
| Unknown | 54 (0·1%) | 32 (0·2%) | 25 (0·1%) |
| Age, Median (IQR) | 50 (26) | 50 (25) | 49 (29) |
| Age Class, N (years) | |||
| < 18 | 1076 (2·8%) | 550 (2·7%) | 544 (2·6%) |
| 18–30 | 6234 (15·9%) | 2996 (14·6%) | 3497 (16·9%) |
| 31–40 | 6055 (15·5%) | 3216 (15·7%) | 3169 (15·3%) |
| 41–50 | 7047 (18·0%) | 3853 (18·8%) | 3621 (17·5%) |
| 51–60 | 8179 (20·9%) | 4417 (21·6%) | 4242 (20·5%) |
| 61–70 | 6259 (16·0%) | 3289 (16·1%) | 3340 (16·1%) |
| 71–80 | 3186 (8·2%) | 1595 (7·8%) | 1760 (2·6%) |
| 81–90 | 1050 (2·7%) | 571 (2·8%) | 521 (2·5%) |
| N, Patients per US Region | |||
| South | 19,645 (50·3%) | 10,803 (52·7%) | 9851 (47·6%) |
| Northeast | 11,494 (29·4%) | 5805 (28·3%) | 6450 (31·2%) |
| West | 5448 (13·9%) | 2746 (13·4%) | 2943 (14·2%) |
| Midwest | 2446 (6·3%) | 1102 (5·4%) | 1425 (6·9%) |
| Unknown | 53 (0·1%) | 31 (0·2%) | 25 (0·1%) |
| Number of follow-up Antibody tests per patient, median (IQR) | 1 (1) | 1 (0) | 1 (0) |
| Number of days from positive PCR test to antibody assay, median (IQR) | 42 (73) | 36 (48) | 52 (109) |
| N, Total Antibody Tests | 45,061 | 22,180 | 22,881 |
| N, Antibody Tests by Ordering Provider Specialty | |||
| Family Practice | 13,664 (30·3%) | 7010 (31·6%) | 6654 (29·1%) |
| Internal Medicine | 10,226 (22·7%) | 4846 (21·8%) | 5380 (23·5%) |
| Telemedicine | 4733 (10·5%) | 1398 (6·3%) | 3335 (14·6%) |
| General Practice | 4150 (9·2%) | 1986 (9·0%) | 2164 (9·5%) |
| Multispecialty | 2209 (4·9%) | 1115 (5·0%) | 1094 (4·8%) |
| Hospital & Hospital Labor | 2161 (4·8%) | 1437 (6·5%) | 724 (3·2%) |
| Emergency Medicine | 1638 (3·6%) | 970 (4·4%) | 668 (2·9%) |
| Other | 6280 (14·0%) | 3418 (15·4%) | 2862 (12·4%) |
*Some patients had both N and S assays performed, so the All column does not reflect a summation of the Nucleocapsid and Spike totals.
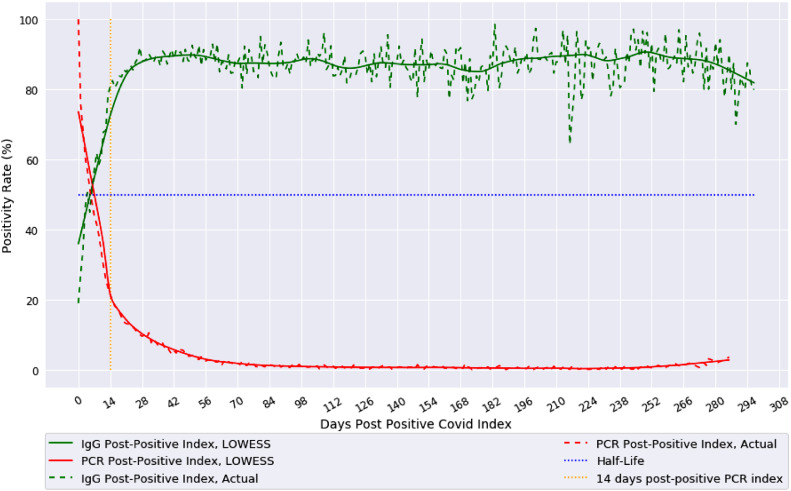
Population IgG seropositivity, when considering all N and S-antibody assays grouped together, reaches 90% at about 21 days post-PCR positive index (Fig. 1). Comparatively, follow-up PCR tests show positive PCR rates decaying to about 20% after 14 days and <1% after 84 days. LOWESS regression provides a smooth curve for both population positivity rates and shows antibody rates remaining around 90%, given the variability, until about 300 days post-index.
Fig. 1.
Population IgG and Follow-up PCR Positivity Rates using LOWESS moving regression.
Actual population-based IgG seropositivity (green) of both nucleocapsid (N) and spike (S) proteins together, and follow-up PCR positivity rate (red) over days from initial positive PCR test for COVID-19 are shown from index of day 0 to 300 days. Alternatively, actual daily index value (dotted line) with LOWESS moving average regression (solid line) are both shown. The 14-day post index (orange) highlights breaks in trends (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
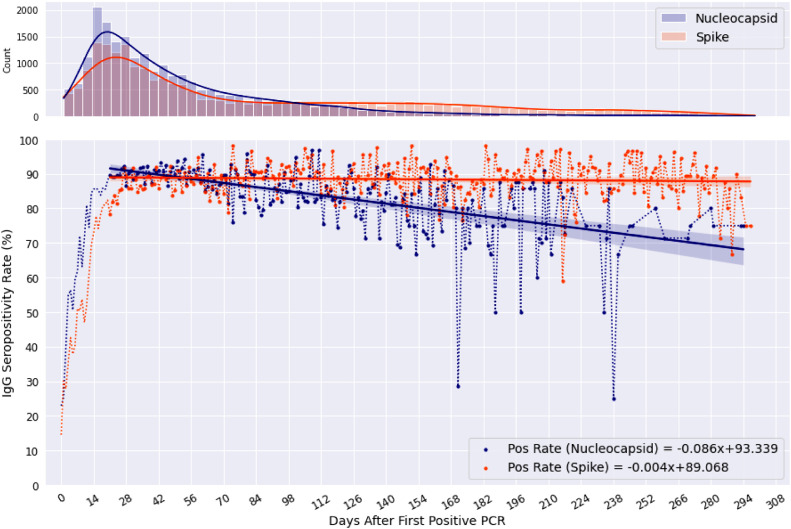
Further investigation of potential differences in population longevity of N-antibody or S-antibody positivity was undergone. Seropositivity of both were sustained above half-life for the duration of the timeframe, with linear trends identifying N-antibody positivity at 68·2% [95% CI: 63·1–70·8%] 293 days post-index and S-antibody remaining at 87·8% [95% CI: 86·3–89·1%] 300 days post-index, the furthest day possible from available data (Fig. 2). Linear regression for both antibodies, starting regression at the height of the antibody response of t = 21 days, yield p < 0·0001 for N-antibodies and p = 0·031 for S-antibodies. Both regressions had negative slopes of –0·086 and −0·004 for N and S, respectively. N-antibodies seroconvert more quickly than S-antibodies, reaching 50% between three to five days versus the seven days it takes for S. Using the resulting linear equations, S-antibodies appear to sustain for longer duration, while the negative trend of N-antibodies suggests a shorter population half-life.
Fig. 2.
Population-based seropositivity rates of Nucleocapsid (N) and Spike (S) protein IgG Assays.
Seropositivity rates of IgG antibodies to Nucleocapsid (blue) and Spike (orange) proteins days following initial positive PCR test, found in the lower plot. Linear regression equation is displayed for both antibodies in the lower right corner, which starts at 21 days post positive PCR. 95% CI is represented by the shaded region above and below each linear line. The upper plot contains a frequency count of tests run (with kernel density plot) per day from index (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
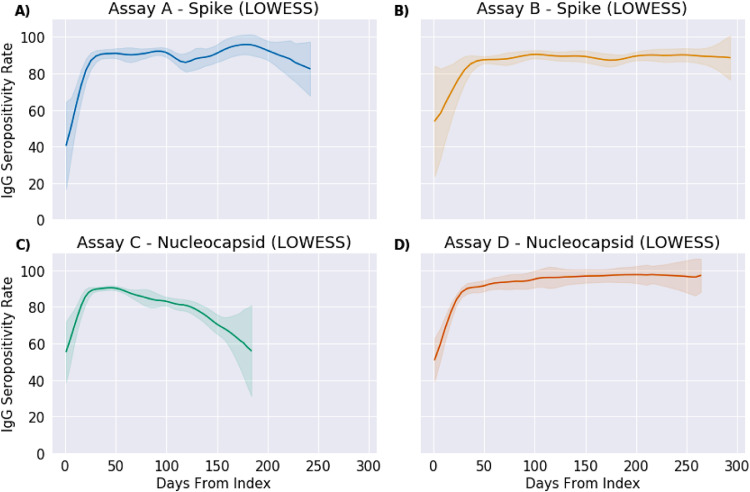
An investigation into the assay platform differences analyzed the four assays, A, B, C and D separately (Fig. 3). For S-assays, a total of 3,989 patients were assessed with Assay A and 17,027 were assessed with Assay B. For N-assays, 16,974 patients had Assay C performed and 3,769 patients had Assay D. This analysis identified comparable smoothed seropositivity profiles via LOWESS between the two S-assay platforms, with Assay B maintaining a relative 89·1% [95% CI: 70·5–100·0%] throughout the 293 days with minimal variation due to daily sample size and Assay A indicating more variability at only 250 days with the beginnings of a downward trend (Fig. 3A,B). The N-assay platforms differed significantly, with Assay C following a sharp decline from peak at 50 days to 57·3% [95% CI: 38·7–75·8%] at 184 days, with limited data available and high variance in later periods compared to Assay D (refer to Supplemental Table S2 for counts by of tests per assay by week), which sustained close to a 96·7% [95% CI: 78·1–100%] seropositivity rate consistently through 260 days and minimal variation (Fig. 3C,D).
Fig. 3.
Comparison of antibody seropositivity over time by protein type and assay platform.
Seropositivity rates of IgG antibodies to Spike (A and B) and Nucleocapsid (C and D) proteins by two separate assay platforms each over days post first positive PCR result for COVID-19. Data is smoothed using LOWESS and fitted with 95% confidence intervals for variance in sample sizes. Comparison between assay platform within protein types show similarities for Spike, but different trends for Nucleocapsid.
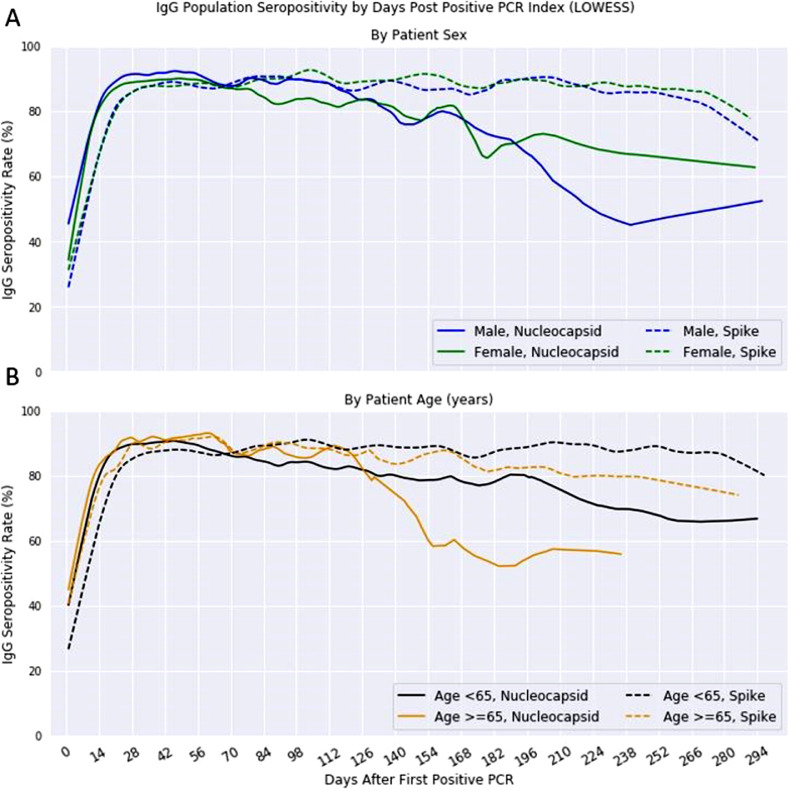
Logistic regression evaluated how IgG seropositivity at an individual patient-level is dependent on assay protein type as well as the number of days from positive PCR index, age and sex of the patient (Table 2). The resulting model had an accuracy of 0·77 and AUC of 0·75. All variables proved to be significant with p < 0·0001 (assay protein, days from index and age) and p = 0·0003 (sex). To visualize the disparities between genders and age, LOWESS smoothed the positivity time series analysis per variable. Differences in gender fluctuated over time, but mostly followed the same trend for males and females in S positivity (log-rank p = 0·65), with males significantly different (log-rank p < 0·001) in N positivity with a sharper decrease post-180 days (Fig. 4A). Age, when classified as either <65 or ≥65 years old, showed a higher seropositivity rate for the younger class in later days for both S and N-antibody assays (Fig. 4B) and both proteins were significantly different by log-rank test (p < 0·001).
Table 2.
IgG Seropositivity Logistic Regression Variable Significance.
| Variable | Coefficient | Standard Error | Z | p | 95% Confidence Interval |
|---|---|---|---|---|---|
| Intercept | −0·2020 | 0·0335 | −6·0363 | <0·0001 | [−0·2675, −0·1364] |
| Days From PCR Index | 4·7443 | 0·0848 | 55·9308 | <0·0001 | [4·5781, 4·9106] |
| Patient Sex (Female) | 0·0771 | 0·0214 | 3·6068 | 0·0003 | [0·0352, 0·1190] |
| Age | 1·4123 | 0·0537 | 26·3085 | <0·0001 | [1·3071, 1·5175] |
| Protein Type (Spike) | −0·3319 | 0·0215 | −15·5347 | <0·0001 | [−0·3738, −0·2901] |
Fig. 4.
Population Nucleocapsid and Spike IgG seropositivity by Demographics.
Nucleocapsid and spike IgG seropositivity rates after first positive PCR test for SARS-CoV-2 using LOWESS moving average for curve smoothing. (A) Rates by gender shown with S IgG following the same trend for males (blue) and females (green), (B) Rates by age, classified as <65 (black) or ≥65 years old (gold) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
4. Discussion
Taken together, both S and N SARS-CoV-2 antibody results offer an encouraging view of how long humans may have protective antibodies against COVID-19, with curve smoothing showing population seropositivity reaching 90% within three weeks, regardless of whether the assay detects N or S-antibodies. Most importantly, this level of seropositivity was sustained with little decay through ten months after initial positive PCR. Many studies have argued to bypass N-antibodies in favor of S, as it has shown to have higher rates of neutralizing capacity [20]. However, others offer conflicting sensitivity analyses when comparing assays detecting both [21]. In support, results of predictive logistic regression indicate that antigen type does affect whether a patient will test positive, leading us to investigate further in separate population-level analyses. Also of note was the significance of both age and sex on seropositivity, two demographic variables that correlate with COVID-19 severity, affecting both males and older patients more [22]. They have not been reported in depth with antibody development and sustainability. A study in China observed higher levels of neutralizing antibody titers in older patients (>60 years) compared to young [23], which is initially seen in these results, but conflictingly, we find a quicker decay in the older class’ rate in later days, significantly different for both protein types by log-rank test. This may indicate the need for more frequent titer screens in an older population. Another study found females to have higher average IgG levels than males during severe COVID-19. While seropositivity rates look similar for males and females in the LOWESS smoothing curve, females do appear to have a higher seropositivity rate longer than males and our results detect these statistically significant factors that warrant further research (of note, the Labcorp® registry identified more females receiving both PCR and antibody testing, which might be attributable to prevalence of testing ordered by OB/GYN providers). Our data does not have clinical indications of COVID-19 severity and therefore cannot evaluate previous research claims.
Our results indicate that seroconversion of N-antibodies reaches 50% by three to five days after first positive PCR and by seven days for S-antibodies. This correlates with current understanding of N-proteins, which are predominantly expressed during early coronaviral infections[24] and induce antibodies the quickest [25] ,which made them the first candidate for early antibody tests. They have previously been detected as early as one day for SARS-CoV [26]. Of note, some patients had antibody testing collected on the same day as their first positive PCR, showing a seropositivity rate of 22% and 15% for N and S, respectively. The authors speculate that some patients may have waited to get tested after the onset of symptoms, were asymptomatic or exposed and got an order for both PCR and antibody tests, or may have had their index PCR performed in an inpatient setting or with a different diagnostic laboratory. However, current research has shown that seroconversion by IgG in cells expressing the S-protein occurred in 50% of hospitalized patients (n = 9) after seven days [27], matching these findings. It is important to highlight again that this analysis does not investigate quantified neutralizing antibody titers, but rather a qualitative assessment of whether there is enough in the blood to be detected.
Interestingly, both antibodies achieve peak of about 90% seropositivity by three weeks post-index. The remaining 10% could reflect immunocompromised individuals with no antibody response, possible false negative antibody testing, false positive PCR testing, or those asymptomatic or with low viral load. Asymptomatic patients have been indicated to exhibit lower antibody levels and functions than those who were symptomatic [28] and neutralizing titers of patient plasma infected with SARS-CoV-2 have shown moderate correlation with both N and S-antibodies [29]. Nevertheless, decay of these rates seems to follow a linear trend after 21 days post-index for the current available data. High variability as time accumulates post-index is observed, but can be attributed to decreased patient follow-up or interest in subsequent testing after initial antibody screening. True SARS-CoV-2 antibody kinetics are still unknown, and require more time from sample collection and monitoring [8].
N-antibody decay occurs more quickly out of the two, but most encouraging is the consistency of S-antibody positivity through 300 days. A similar trend in N-antibody decay compared to S was also found in the sera of patients infected with SARS-CoV, though the cause of the disparity is largely unknown in coronaviruses [30]. Additionally, while it could be argued that the assay threshold differs between the two, Burbelo, et al. found that detection of antibodies against the N-protein is more sensitive than detection of those against the S-protein, especially within 14 days after onset of symptoms, with more comparable sensitivities afterwards [31]. The authors note that the N decline is most likely due to variability in specific assay platform sensitivity decreasing over time, as Assay D showed prolonged seropositivity compared to Assay C (Fig. 3) and would therefore drive the decline in N-antibody as seen in Fig. 2. The decline in the number of available assays over time, especially for N-antibodies after 168 days, is a limitation to the study that may cause some uncertainty, but still informs the general trend of positivity decay. The study also confirmed that N-antibodies generally appear earlier than S, as reflected in our results.
Due to its importance in binding to its host, S-proteins have been the target for most SARS-CoV-2 research. More time and testing will be needed to see if this trend continues linearly, which the authors speculate may change, or decays more sharply in months further out. This study shows that natural antibody presence is detected in the blood for sustained duration in most assays, representing real world evidence of seropositivity, but not necessarily protective immunity from COVID-19; neutralizing titers would be the next step to identify how this reflects the humoral response. Other recent observational studies suggest that antibody seropositivity is associated with protection from infection, but the duration of which is unknown [32]. In addition to neutralizing antibody protection, early studies examining T cell response are encouraging, showing high frequency of S-protein-specific CD4+T cell response in convalescent COVID-19 patients [33,34].
The major limitation of the study is the restrictive nature of the de-identified data, involving no advanced demographic (race, ethnicity, etc.) and diagnostic information (disease severity). Due to the nature of the Labcorp® registry, which may be biased towards specific regions of the country due to market limitations, putative diagnosis of COVID-19 is obtained from positive-PCR results only and not from a healthcare provider's clinical diagnosis. Therefore, disease severity, including whether the patient is symptomatic or not, is unknown. Some studies have shown that severe cases present higher concentration of IgG than mild [35] and speculated that disease severity is an indicator in how quickly antibodies decay [11]. However, given that about one in six people infected remain asymptomatic [36], and S-antibody appears to persist long past 42 weeks in >88% of the population, the authors argue that low viral load could cause insufficient production of antibodies, but this requires further investigation.
As a national diagnostic laboratory, Labcorp® mostly processes samples from outpatient settings, as evidence from the ordering provider specialty breakdown in Table 1. While this may reflect patients who are healthier due to their access to healthcare and diagnostic testing, Labcorp® provides testing to millions of patients across the U.S. with various comorbidities. Additionally, to our knowledge, the index PCR test used here is the first available test to confirm COVID-19 positivity, but it is possible a prior test could have been performed in an inpatient or point-of-care setting. Though this may introduce some uncertainty to our conclusions, the authors note that these instances would lead to under-estimation of antibody longevity, and therefore extend our observation of 300 days of seropositivity.
The use of multiple different qualitative-only SARS-CoV-2 antibody tests inhibits the specificity of how much antibodies remain in the system. Though roughly similar, different manufacturing assays have different sensitivities (sensitivity and specificity of all four assays provided in Methods). Of note, assays detecting S-antibody have lower sensitivities, yet S-antibody seropositivity remains higher in the population. It is also true that qualitative tests may return positive, but true levels at individual patient-level could be low or at the threshold of positivity. Some assays for N-antibody detection have been proven to exhibit decreased sensitivity as days post-positive PCR increase [37], which is supported by the platform analysis in Fig. 3. This difference between Assays C and D is stark, but it indicates a vital aspect of understanding these results. It is important to note that for the purposes of this analysis, more sensitivity is not necessarily better. Our results show how a less sensitive qualitative assay is better able to detect a population signal of declining antibody levels than a more sensitive one, because more of the population falls below the limit of detection at a given point in time. At the individual level the best limit of detection would be the one most closely matched to the clinically relevant immunity threshold, but in the case of SARS-CoV-2, it is not possible to say at this time what is the minimal antibody titer necessary for immunity. Therefore, this does not limit the interpretation of Fig. 2, but allows for an understanding that N-positivity (Assays C and D) reaches an assay threshold level at days further out from index more frequently than S-positivity. Quantification of N and S-antibody titers at such a long period of time, in combination with knowing disease severity, could establish proper models of antibody kinetics and may be part of ongoing clinical trials. Analyzing these observational data at the population-level provides real-world evidence from clinical laboratory data and a large sample size for an epidemiological view of what it may look like once the majority of the country develops antibodies, but a patient-level review will be needed to examine quantification in the blood.
We have demonstrated a sustained positivity rate of antibodies against the SARS-CoV-2 spike protein past ten months post-PCR confirmed COVID-19 infection using data from over 39,000 patients, with linear trends indicating a substantial population half-life. Results from observational and longitudinal population-level data may help guide current and future post-pandemic planning, such as public health restrictions. In addition, these findings show that antibody status is dependent on age, sex, protein type, and the number of days since a patient's positive PCR test. This study is novel in that it provides an epidemiological view from one of the United States’ largest diagnostic laboratories, which has access to some of the most substantial longitudinal data on COVID-19. As more data accumulates, further research may provide insight into long-term presence and kinetics of protective antibodies.
5. Funding
No financial support for this study. All authors are employed by Laboratory Corporation of America® Holdings.
6. Declaration of Competing Interest
The authors report no conflict of interest.
7. Data sharing statement
Data cannot be shared publicly because it is confidential data to Laboratory Corporation of America® Holdings.
8. Author contributions
AS, BP, JW and D. Adcock conceptualized the study. D. Alfego curated the data and performed the analysis with AS. D. Alfego, AS and SL had access to raw data. D. Alfego created the figures, performed literature search and wrote the manuscript. BP, D. Adcock, JW, SL contributed domain knowledge of serological testing and with AS, reviewed and edited the manuscript. SL supervised the study.
Declaration of Competing Interest
None.
Acknowledgments
The authors thank Marcia Eisenberg, Ph.D., Kelly Chun, Ph.D., and Lakshmanan Iyer, Ph.D. (Labcorp®) for their consultation and guidance in writing this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100902.
Appendix. Supplementary materials
References
- 1.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2020;21(2):E26–E35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labcorp. LabCorp-COVID-19-antibody-tests-available-nationwide-no-upfront-out-of-pocket-costs [Internet]. 2020. Available from: https://www.labcorp.com/coronavirus-disease-covid-19/news/LabCorp-COVID-19-Antibody-Tests-Available-Nationwide-No-Upfront-Out-of-Pocket-Costs
- 3.Adams E., Ainsworth M., Anand R., Andersson R., Auckland K., Baillie J.K. Evaluation of antibody testing for SARS-CoV-2 using ELISA and lateral flow immunoassays. medRxiv. 2020 [Google Scholar]
- 4.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehr A.R., Perlman S. Coronaviruses. Vol. 1282. Methods in Molecular Biology; 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandjean L., Saso A., Torres A., Lam T., Hatcher J., Thistlethwayte R. Long-term persistence of spike antibody and predictive modeling of antibody dynamics following infection with SARS-CoV-2. medRxiv. 2020 [Google Scholar]
- 7.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 8.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11(1):49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5(52):eabe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bölke E., Matuschek C., Fischer J.C. Loss of anti–SARS-CoV-2 antibodies in mild Covid-19. N Engl J Med. 2020;383(17):1694–1698. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 14.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;80(370):6521. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18) doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health C for D and R. EUA Authorized serology test performance [Internet]. Vol. 2, Fda. 2020. p. 1–14. Available from: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
- 19.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.McAndrews K.M., Dowlatshahi D.P., Dai J., Becker L.M., Hensel J., Snowden L.M. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight. 2020;5(18):e142386. doi: 10.1172/jci.insight.142386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(6):e00461–20. doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallapaty S. The coronavirus is most deadly if you are older and male - new data reveal the risks. Nature. 2020;585(7823):16–17. doi: 10.1038/d41586-020-02483-2. [DOI] [PubMed] [Google Scholar]
- 23.Wu F., Liu M., Wang A., Lu L., Wang Q., Gu C. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180(10):1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong Y., Ulasli M., Schepers H., Mauthe M., V’kovski P., Kriegenburg F. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J Virol. 2019;94(4):e01925–19. doi: 10.1128/JVI.01925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Che X.Y., Hao W., Wang Y., Di B., Yin K., Xu Y.C. Nucleocapsid protein as early diagnostic marker for SARS. Emerg Infect Dis. 2004;10(11):1947–1949. doi: 10.3201/eid1011.040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 28.Dufloo J., Grzelak L., Staropoli I., Madec Y., Tondeur L., Ois Anna F. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. medRxiv. 2020 doi: 10.1016/j.xcrm.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang L., Sang L., Ye F., Ruan S., Zhong B. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130(10):5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chia W.N., Tan C.W., Foo R., Kang A.E.Z., Peng Y., Sivalingam V. Serological differentiation between COVID-19 and SARS infections. Emerg Microbes Infect. 2020;9(1):1497–1505. doi: 10.1080/22221751.2020.1780951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222:206–213. doi: 10.1093/infdis/jiaa273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey R.A., Rassen J.A., Kabelac C.A., Turenne W., Leonard S., Klesh R. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181(5):672–679. doi: 10.1001/jamainternmed.2021.0366. Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Q., Cui X., Liu X., Peng B., Jiang J., Wang X. The production of antibodies for SARS-CoV-2 and its clinical implication. medRxiv. 2020 2020.04.20.20065953. [Google Scholar]
- 36.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Can. 2020;5(4):223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. medRxiv. 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.