Abstract
Context
Klinefelter Syndrome (KS) is the most frequent sex chromosome disorder in males. Due to hypergonadotropic hypogonadism, treatment with testosterone replacement therapy (TRT) is commonly indicated. There are no international guidelines for the most appropriate TRT in KS.
Objective
We aimed to evaluate how different routes of testosterone administration impact testosterone-responsive variables, as well as the development of later metabolic diseases and other complications.
Methods
We conducted a retrospective study covering 5 years from 2015 to 2020. Data on TRT, biochemical parameters, bone mineral density (BMD), medications, comorbidity, and karyotyping were derived from electronic patient records and The Danish Cytogenetic Register.
Results
A total of 147 KS males were included: 81 received injection TRT, 61 received transdermal TRT, and 5 did not receive TRT. Testosterone levels were similar in the 2 TRT groups (P = 0.9), while luteinizing hormone and follicle-stimulating hormone levels were higher in the group receiving transdermal TRT (P = 0.002). Levels of cholesterol, blood glucose, hemoglobin A1c, hemoglobin, hematocrit, liver parameters, prostate-specific antigen, and spine and hip BMD were similar in the 2 treatment groups (Ps > 0.05).
Conclusion
TRT, irrespective of route of administration, affects androgen-responsive variables similarly in males with KS. Neither long-acting injection nor transdermal gel seem to reduce the risk of metabolic diseases significantly. These results should encourage clinicians in seeking the route of administration resulting in the highest degree of adhesion to treatment based on individual patient preferences. Implementation of shared decision-making with patients may be important when choosing TRT.
Keywords: Klinefelter syndrome, testosterone supplementation, route of administration, retrospective study
Klinefelter syndrome (KS) is caused by 1 extra X chromosome [1, 2]. KS occurs approximately in 150 per 100 000 live born male births [1]. The diagnosis is often delayed due to the absence of overt clinical signs and unawareness of KS among clinicians [3], with a mean age at diagnosis of 27 years [4]. In addition, only a minority (25%-40%) of all males with KS are ever diagnosed [5]. KS males present a broad phenotypic spectrum from mild to severe symptoms and the pattern of morbidity is complex [6]. One cardinal sign is small and firm testes with decreased function, leading to azoospermia and reduced testosterone production [6]. Hypergonadotropic hypogonadism is a hallmark of KS, starting in adolescence and continuing in adulthood, when serum testosterone levels inadvertently fall to low normal range or below and gonadotropins become elevated [7]. Hypogonadism is associated with development of later metabolic diseases [8-10]. The metabolic profile in male individuals with KS includes obesity, dyslipidemia, insulin resistance, and a tendency toward thrombosis, adding to an overall increased risk of metabolic and cardiovascular diseases [11, 12]. Obesity is frequent in KS males, with increased weight, hip and waist circumference, increased total and abdominal fat mass, and an average body mass index (BMI) above the normal range [13]. A consequence hereof is an unfavorable lipid profile, with decreased HDL-cholesterol and increased total cholesterol, LDL-cholesterol, and triglycerides [9, 10]. The prevalence of type 2 diabetes is also increased in males with KS [14, 15], as is the risk of venous thromboembolism [8, 14, 16-18]. Furthermore, KS is associated with low bone mineral density (BMD), resulting in osteopenia or osteoporosis and leading to a higher risk of fractures [19, 20]. An affected micro-architecture of bone is present, much similar to what is observed among postmenopausal women [21]. The impaired metabolic profile in KS males increases morbidity and mortality [14].
Due to hypergonadotropic hypogonadism, testosterone replacement therapy (TRT) is described as being at the core of care of KS males, although there are no international guidelines for handling details on treatment. However, the European Academy of Andrology created guidelines on KS providing recommendations and suggestions for diagnostic tests, monitoring, and overall aspects of treatment [22]. The purpose of TRT in KS is restoration of normal serum testosterone levels and prevention of an unfavorable metabolic profile and development of later metabolic diseases [23]. A recent meta-analysis of the effect of TRT in KS concluded that while TRT could induce improvements in BMD, body composition, and hematocrit, it seems insufficient in normalizing the metabolic profile [20]. Another study suggested that testosterone treatment is protective for the development of deep vein thrombosis (DVT) [8, 18]. TRT is most frequently administered either by intramuscular injection of long-acting testosterone or transdermally via patch or gel preparations, and infrequently via oral administration. The advantages of injectable TRT are its less-frequent dosing and relatively stable testosterone levels. The disadvantages are anaphylaxis and discomfort at injection site, as well as infrequent lipid embolism in the lungs [24, 25]. Transdermal gel is applied once daily. The most important advantage is its easy application and a steadier testosterone level, whereas disadvantages include risk of transfer of testosterone to others and skin irritation [26-29]. Today the choice of route of administration mostly depends on patients’ preference at our institution, and the choice is made in a “shared decision-making” fashion after thorough discussion of pros and cons.
The aim of this study was to clarify if route of administration of TRT in individuals with KS has differential effects on hormone levels and development of metabolic diseases. We also assessed the frequency of unwarranted effects and safety parameters measured during treatment, such as prostate-specific antigen (PSA), hematocrit, and hemoglobin.
Methods
This is a retrospective study covering 5 years. We identified 179 males with KS who consistently visited our outpatient clinic from 2015 to 2020 at the Department of Endocrinology at Aarhus University Hospital. The inclusion criteria were an ICD-10 diagnosis of KS (DQ984, DQ980, DQ981). We excluded 24 KS males due to wrong diagnosis (n = 4), death during follow-up period (n = 3), age below 18 years of age (n = 1) and lack of measurements of the variables studied since 2015 in spite of receiving TRT (n = 16). Furthermore, we excluded KS males with supernumerary constellations of sex chromosomes, which resulted in exclusion of 7 treated KS males with either 48,XXYY, 48,XXXY, or 49,XXXXY karyotypes and 1 untreated KS male with 48,XXXY. A total of 147 males with KS were thus included in the study, including 142 treated KS males and 5 untreated KS males. All treated KS males presented symptoms of androgen deficiency prior to starting TRT. One untreated KS male had never been treated due to lack of symptoms of androgen deficiency. Two untreated KS males had received testosterone therapy in the past for a shorter period, but they had not since 2015 due to choosing not to take any medication in spite of symptoms of androgen deficiency (N = 2). Two untreated KS males did not receive TRT because of fertility treatment with testicular sperm extraction (TESE). Date of karyotyping was assigned as the date of diagnosis and were gathered from The Danish Cytogenetic Register (DCCR). Among the treated individuals, 133 had a 47,XXY karyotype and 4 had mosaicism (46,XY/47,XXY). Five treated KS males did not have karyotyping data recorded in the DCCR due to being diagnosed abroad (n = 3), karyotyping of bone marrow (n = 1), and due to unknown reason (n = 1). Untreated KS males had either 47,XXY (n = 4) or 46,XY/47,XXY (n = 1).
Data were extracted from electronic patient records. The data included details on the applied TRT, including route of administration, dosage, date of initiation of TRT, and changes in administered TRT formulations. Changes in route of administration were determined after thorough discussion with a shared decision-making approach. We exclusively use injection TRT in the form of Nebido (1000 mg given on average every twelfth week) and transdermal TRT in the form of Testogel or tostran (administered daily). Medications were noted, including the use of statins, antihypertension drugs, antidiabetic, antidepressant, and antipsychotic medications. Comorbidities were noted, including asthma/chronic obstructive pulmonary disease, diabetes, hypertension, depression, anxiety, attention-deficit/hyperactivity disorder (ADHD), psychosis, DVT, and arterial thrombosis. The latest available height and weight was noted, and BMI calculated as body weight in kilograms divided by height in meters squared (kg/m2). The latest available systolic and diastolic blood pressure was noted. If a dual-energy x-ray absorptiometry (DXA) scan was performed, the latest values of spine and hip BMD were noted. DXA scans were not conducted at any prespecified time point. Smoking habits were noted as well.
Comparisons of continuous variables for the injection and transdermal group were performed with the latest recorded values. It was noted whether the specific parameter had been measured for all measures of weight, height, BMI, biochemical parameters, and BMD (Tables 1 and 2). The graphical presentations were performed with the longitudinal data extracted during the treatment period.
Table 1.
Characteristics of KS males: untreated, treated (47,XXY and 46,XY/47,XXY), and treated (unknown karyotypes)
| Variable | Untreated KS N = 5 | Treated (47,XXY & 46,XY/47,XXY) N = 137 | Treated (unknown karyotypes) N = 5 |
|---|---|---|---|
| Age (years) | 43.0 ± 12.6 | 41.8 ± 11.5 | 43.8 ± 15.9 |
| Age at diagnosis of Klinefelter (years)a | 25.4 ± 21.9 | 26.0 ± 12.7 | N/A |
| Weight (kg)b | 90.5 ± 9.5 | 96.8 ± 19.4 | 88.2 ± 20.2 |
| Height (cm)c | 184 ± 4 | 186 ± 8 | 182 ± 8 |
| BMId | 24.4 ± 2.2 | 27.7 ± 5.4 | 26.6 ± 5.9 |
| Age at initiation of TRT (years)e | |||
| 18-49 years | N/A | 121 (89%) | 4 (80%) |
| 50-64 years | N/A | 14 (10%) | 1 (20%) |
| 65 + years | N/A | 1 (1%) | 0 (0%) |
| Medication | |||
| Statins | 0 (0%) | 15 (11%) | 0 (0%) |
| Antidiabetics | 0 (0%) | 8 (6%) | 0 (0%) |
| Antihypertensives | 0 (0%) | 19 (14%) | 2 (40%) |
| Antidepressants | 0 (0%) | 14 (10%) | 1 (20%) |
| Antipsychotics | 0 (0%) | 6 (4%) | 1 (20%) |
| Comorbidity | |||
| Asthma/COPD | 1 (20%) | 22 (16%) | 0 (0%) |
| Diabetes | 0 (0%) | 8 (6%) | 0 (0%) |
| Hypertension | 0 (0%) | 18 (13%) | 2 (40%) |
| Depression | 0 (0%) | 17 (12%) | 1 (20%) |
| Anxiety | 0 (0%) | 9 (7%) | 0 (0%) |
| ADHD | 0 (0%) | 15 (11%) | 0 (0%) |
| Psychosis | 0 (0%) | 5 (4%) | 1 (20%) |
| DVT | 0 (0%) | 3 (2%) | 0 (0%) |
| Arterial thrombosis | 0 (0%) | 1 (1%) | 0 (0%) |
| Osteopeniaf | 0 (0%) | 29 (49%) | 0 (0%) |
| Osteoporosisg | 0 (0%) | 0 (0%) | 0 (0%) |
| Karyotypesh | |||
| Klinefelter XXY | 4 (80%) | 133 (97%) | N/A |
| Klinefelter Mosaic | 1 (20%) | 4 (3%) | N/A |
| Swapping in treatment | |||
| No | 4 (80%) | 86 (63%) | 3 (60%) |
| Yes | 1 (20%) | 51 (37%) | 2 (40%) |
| Smoker | 2 (40%) | 19 (14%) | 0 (0%) |
Data are expressed as mean ± SD or number of patients and percentage.
Swapping in treatment = No to “Swapping in treatment”: KS males had never shifted in between formulations of TRT. Yes to “Swapping in treatment”: KS males did shift in between TRT formulations 1 or more times and were included by the latest route of administration.
Abbreviations: ADHD, attention-deficit/hyperreactive disorder; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; N/A, not available; TRT, testosterone replacement therapy.
a Age at diagnosis: N = 5/136/N/A.
b Weight: N = 4/116/5.
c Height: N = 2/105/5.
d BMI: N = 2/104/5.
e Age at Initiation of TRT: N = N/A/136/5.
f Osteopenia/ g Osteoporosis: N = 1/59/1.
h Karyotypes: N = 5/137/N/A.
Table 2.
Demographic, anthropometric, hormonal, and densitometric characteristics of treated KS males
| Variable | N = Injection/Gel | Injection | Gel | P value |
|---|---|---|---|---|
| Age (years) | 81/61 | 40.6 ± 11.5 | 43.5 ± 11.6 | 0.1 |
| Age at diagnosis of Klinefelter (years) | 76/60 | 23.8 ± 12.3 | 28.7 ± 12.8 | 0.03* |
| Age at initiation of TRT (years) | 80/61 | 33.0 ± 11.3 | 36.8 ± 11.5 | 0.05 |
| Cumulated treatment period (years) | 80/61 | 7.1 ± 3.0 | 6.2 ± 2.8 | 0.08 |
| Weight (kg) | 68/53 | 96.8 ± 21.4 | 95.9 ± 16.7 | 0.8 |
| Height (cm) | 63/47 | 186 ± 8 | 185 ± 7 | 0.3 |
| BMI | 62/47 | 27.5 ± 5.4 | 27.8 ± 5.6 | 0.8 |
| Daily dosage (mg) | 84/65 | 15.3 | 41.0 | ** |
| Serum testosterone (8.4-34.0) (nmol/L) | 78/58 | 15.9 (2.5-90.0) | 15.2 (0.6-55.0) | 0.9 |
| Serum FSH (1.2-15.8) (IU/L) | 63/57 | 5.3 (0.1-62.0) | 26.0 (0.2-69.0) | 0.002* |
| Serum LH (1.7-8.6) (IU/L) | 71/59 | 2.9 (0.1-50.0) | 14.0 (0.1-68.0) | 0.002* |
| HDL-cholesterol (>1) (mmol/L) | 40/34 | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.3 |
| LDL-cholesterol (<3) (mmol/L) | 42/32 | 2.9 ± 0.9 | 3.1 ± 1.0 | 0.3 |
| Triglycerides (<2) (mmol/L) | 40/34 | 1.6 (0.5-7.3) | 1.9 (0.7-7.9) | 0.2 |
| Total cholesterol (<5) (mmol/L) | 41/34 | 4.9 ± 1.0 | 5.2 ± 1.0 | 0.1 |
| Blood glucose (mmol/L) | 58/45 | 6.1 (4.5-14.5) | 6.2 (4.5-11.9) | 0.4 |
| HbA1c (<48) (mmol/mol) | 44/40 | 37 (30-94) | 37 (25-67) | 0.9 |
| Hemoglobin (8.3-10.5) (mmol/L) | 72/54 | 9.7 ± 0.8 | 9.7 ± 0.9 | 0.6 |
| Hematocrit (0.4-0.5) (EVF) | 44/32 | 0.46 ± 0.04 | 0.46 ± 0.04 | 0.9 |
| PSA (<0.5) (µg/L) | 47/41 | 0.7 (0.2-5.7) | 0.6 (0.1-10.7) | 0.3 |
| Alanine transaminase (10-70) (IU/L) | 60/49 | 27 (14-92) | 25 (11-126) | 0.8 |
| Lactate dehydrogenase (105-205) (U/L) | 37/31 | 170 ± 32 | 171 ± 19 | 0.9 |
| Alkaline phosphatase (35-105) (U/L) | 45/34 | 68.9 ± 21.1 | 74.1 ± 21.7 | 0.3 |
| Bilirubin total (5-25) (µmol/L) | 42/33 | 10 (5-35) | 10 (5-29) | 0.8 |
| Systolic blood pressure (mmHg) | 78/56 | 134 ± 16 | 135 ± 14 | 0.7 |
| Diastolic blood pressure (mmHg) | 78/56 | 80.8 ± 11.2 | 84.4 ± 9.4 | 0.05 |
| Spine BMD (≤ −2.5) | 35/25 | −0.54 ± 1.32 | −0.72 ± 0.94 | 0.5 |
| Hip BMD (≤ −2.5) | 35/25 | −0.23 ± 0.94 | −0.50 ± 0.55 | 0.2 |
Data are expressed as N = total patients included in test, and results in mean ± SD or median (range). Injection TRT (Nebido). Transdermal/gel TRT (Tostran, Testogel).
Abbreviations: BMD, bone mineral density; FSH, follicle-stimulating hormone; HbA1c, glycated hemoglobin A1c; LH, luteinizing hormone; PSA, prostate-specific antigen; TRT, testosterone replacement therapy.
* Statistical significance P < 0.05. ** Not comparable.
Assays
Testosterone was measured by liquid chromatography–tandem mass spectrometry using Perkin Elmer’s CHS Steroid MS kit. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were analyzed on the Architect i2000 platform (Abbott) by the chemiluminescent microparticle immunoassay method using the corresponding kits. The working ranges were 0.05 to 150 IU/L and 0.07 to 250 IU/L, respectively. Plasma lipids and triglycerides were measured using an automated commercially available system (Aeroset, Abbott Diagnostics, Abbott Park Laboratories). The coefficient of variation was <5%. Glycated hemoglobin A1c (HbA1c) was measured by commercially available high-performance liquid chromatography. Hepatic enzymes were determined on a Cobas INTEGRA (Roche). Prostate-specific antigen (PSA) was measured on ADVIA Centaur/XPT. Normative values for these assays are presented in Figure legends.
Dual-Energy X-Ray Absorptiometry
We measured total and regional fat mass (g) and lean body mass (g) by DXA using a Hologic 2000/w osteodensitometer (Hologic, Waltham, MA, USA). The system software provided the mass of lean body, fat, and bone mineral for the whole body and specific regions. Appendicular, trunk, and visceral trunk fat mass and trunk and appendicular lean body mass were extracted. The coefficient of variation for the DXA scans was <2%, as estimated by repeated measurements.
Ethics
The study was approved by the local review board at Aarhus University Hospital and since ethical approval was not required, as the analyses of patient records did not involve renewed contact with patients, the study was registered with and approved by the Danish Data Protection Agency (j. nr. 1-16-02-622-20).
Statistics
Stata/IC version 15.1 (StataCorp LLC, College Station, TX, USA) was used for statistical data analyses. Normality was assessed by quantile-quantile (QQ)-plots of absolute values. Comparisons of continuous variables were performed using Student’s independent t test (mean ± SD) for normally distributed variables, or Mann Whitney U-test (median with range) for nonparametric data. For all variables, we compared KS males receiving TRT as injections and transdermal. A P < 0.05 was considered statistically significant. Due to the explanatory nature of the current study, we did not correct for multiple inferences.
Results
Characteristics of KS Males
In total, 142 KS males received TRT, while 5 KS males did not receive TRT at the time of observation (Table 1). The frequency of somatic and psychiatric diseases did not differ between KS males with the karyotypes 47,XXY and 46,XY/47,XXY (N = 137) and with unknown karyotype (N = 5) (Table 1), and these groups were combined in the ensuing analyses. During the treatment period, a number of patients chose to change TRT from injection to transdermal application or vice-versa; 63% of treated KS males had no change in route of administration, while 37% changed route of administration and are here included by the latest route of administration (Table 1). The main reason for change in route of administration was that patients were not satisfied with the kind of TRT they were presently receiving. Two patients changed to transdermal TRT because they had suspected side effects from injection therapy in the form of transient lipid embolism in the lungs.
Injection TRT Versus Transdermal TRT
Of the included KS males, 81 received intramuscular injection of long-acting testosterone (injection TRT group), and 61 received transdermal applied gel preparations (transdermal TRT group) at the beginning of the treatment period (Table 2). Age at participation, age at initiation of TRT, and cumulated period of TRT treatment did not differ between the injection TRT group and transdermal TRT group. Age at diagnosis was significantly higher among those receiving transdermal TRT. There were no differences in any of the anthropometric values between the 2 TRT groups (Table 2). However, BMI increased with age in both groups (Fig. 1). Serum testosterone was similar in the 2 groups, while serum FSH and serum LH were significantly higher among those receiving transdermal TRT. Hemoglobin, hematocrit, PSA, cholesterol, blood glucose, HbA1c, liver parameters, and spine and hip BMD were similar in the 2 groups (Table 2). During the observation period, DVT and arterial thrombosis were recorded in 2% and 1% of participants, respectively (Table 1).
Figure 1.
The association between age and BMI of KS males.
Effectiveness of TRT During 5 Years of Observation
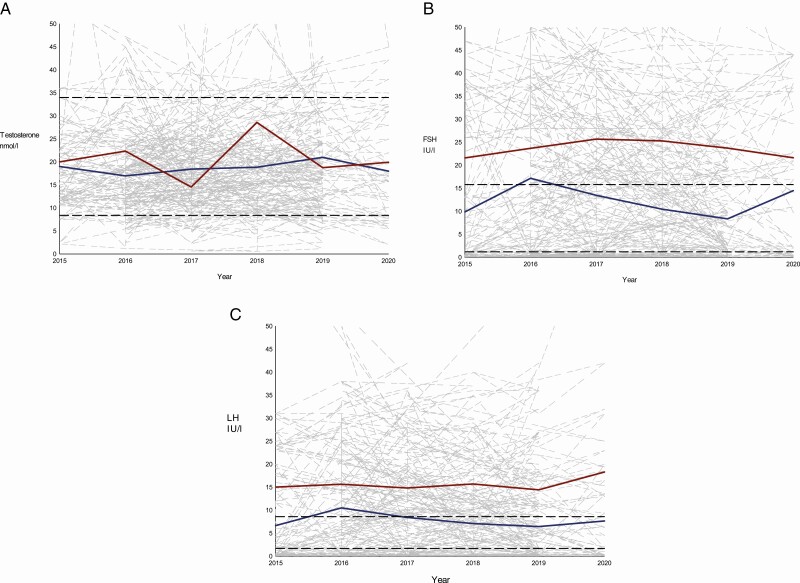
During the observation period it was evident that individuals had point estimates of the biochemical measurements that deviated from normative ranges (Figs. 2 and 3). Low levels of testosterone were at some point seen in 10% of KS males treated with injection TRT and in 20% of KS males treated with transdermal TRT. Testosterone was within normative limits in 85% of KS males treated with injection TRT and in 65% of KS males treated with transdermal TRT (Fig. 2A). Among KS males treated with injection TRT, the levels of FSH and LH were within normative limits in 29% and 16%, respectively. In KS males treated with transdermal TRT, the levels of FSH and LH were within normative limits in 22% and 17%, respectively. In KS males treated with injection TRT, elevation of FSH and LH were seen in 30% and 33%, respectively. In KS males treated with transdermal TRT, elevation of FSH and LH were seen in 64% and 65%, respectively, at some point during a treatment period of 5 years, (Fig. 2B and 2C). In KS males treated with injection TRT, levels of FSH and LH below normative levels were found in 41% and 51%, respectively. In KS males treated with transdermal TRT, levels of FSH and LH below normative levels were found in 14% and 18%, respectively (Fig. 2B and 2C). In KS males treated with injection TRT the testosterone-responsive variables, hemoglobin and hematocrit, were within normative limits in 77% and 78%. In KS males treated with transdermal TRT the testosterone-responsive variables, hemoglobin and hematocrit, were within normative limits in 82% and 74%, respectively. In KS males treated with injection TRT, increased levels of hemoglobin and hematocrit were seen in 21% and 20%, respectively. In KS males treated with transdermal TRT, increased levels of hemoglobin and hematocrit were seen in 15% and 24%, respectively (Fig. 3A and 3B). PSA was normal in 99% (injection TRT) and 95% (transdermal TRT) of KS males. Only 1% of the injection TRT group and 5% of the transdermal TRT group, had point estimates above the normative limit of PSA (Fig. 3C).
Figure 2.
Sex hormones in treated KS males from 2015 to 2020. The underlying gray lines represent the individual patients and their continuous values of A: Testosterone, B: FSH and C: LH over time. The dashed black lines represent the normative limits: A: 8-34 nmol/L, B: 1.2 - 15.8 IU/L, and C: 1.7 - 8.6 IU/L. The blue line represents the yearly average of A: Testosterone, B: FSH and C: LH for the injection group. The red line represents the yearly average of A: Testosterone, B: FSH and C: LH for the transdermal group. For graph A, B and C values > 50 are not presented to optimize the graphical expression.
Figure 3.
Hemoglobin, Hematocrit and PSA (testosterone-responsive variables) in treated KS males from 2015-2020. The underlying gray lines represent the individual patients and their continuous values of A: Hemoglobin, B: Hematocrit and C: PSA over time. The dashed black lines represent the normative limits: A: 8.3-10.5 mmol/L, B: 0.4-0.5 mmol/L and C: <5 µg/L. The blue line represents the yearly average of A: Hemoglobin, B: Hematocrit and C: PSA for the injection group. The red line represents the yearly average of A: Hemoglobin, B: Hematocrit and C: PSA for the transdermal group. For graph A and B values below 7 and 0.35, respectively, and for graph A, B and C values above 13.0, 0.60 and 6.0 respectively, are not presented to optimize the graphical expression.
In KS males treated with injection TRT or transdermal TRT, 31% had levels of HDL-cholesterol below normative limits. In KS males treated with injection or transdermal TRT, elevated levels of total cholesterol, LDL, and triglycerides were seen in 48%, 48%, and 41%, and in 56%, 49%, and 45%, respectively (Supplementary Figure 1) [30]. Elevated levels of HbA1c above 48 mmol/mol indicative of diabetes were seen in 9% (injection TRT) and in 8% (transdermal TRT) of KS males (Supplementary Figure 2) [30]. Liver parameters of KS males during the treatment period were mostly within normative limits (Supplementary Figure 3) [30]. There were no correlations between dosage of TRT, administered via the injection or transdermal route, and serum testosterone (Supplementary Figure 4), or serum LH and FSH (Supplementary Figure 5 & 6) [30]. There were no correlations between lipid fractions and testosterone levels (Supplementary Figure 7) [30].
Discussion
To our knowledge this is the first study presenting real-world clinical data concerning TRT in males with KS and how different routes of administration of TRT impact testosterone-responsive measures, such as serum testosterone, FSH, LH, hemoglobin, hematocrit, BMD, PSA, and metabolic measures.
Among KS males we demonstrated similar levels of testosterone during treatment, showing that injection TRT and transdermal TRT had comparable efficacy in terms of normalizing serum testosterone. The differences in serum FSH and LH, on the other hand, were significant and large. Gonadotropins were significantly elevated in the transdermal TRT group compared with the injection TRT group, likely because transdermally-administered testosterone gel has a short half-life period [28, 29, 31]. The transdermal group receive a dose once daily, which leads to an initial increase in testosterone, followed by a subsequent decrease in testosterone until the next application of testosterone, which may only transiently normalize FSH and LH [28, 29, 31]. The difference in the level of the gonadotropins could as well indicate insufficient TRT in males treated with transdermal TRT. However, all other androgen-responsive parameters, such as hemoglobin, lipids, HbA1c, DXA scans, PSA, etc., were similar in the 2 groups, indicating that it is more likely that differences in pharmacodynamics between injection and transdermal TRT are the cause for this difference in the level of the gonadotropins. However, neither serum testosterone nor LH and FSH were correlated with TRT dose, indicating that other mechanisms, such as sex hormone binding globulin and CAG repeats in the androgen receptor [2, 32-34], as well as age and adiposity [35], may influence circulating testosterone. Increased values of hemoglobin and hematocrit are known side effects of testosterone treatment, and a predictor for DVT and arterial thrombosis, which occurred in 2% and 1%, respectively, of KS males included in this study. Here, hemoglobin, hematocrit, and PSA were not different when comparing injection and transdermal TRT, but increased values were observed frequently at differing timepoints during treatment in both groups. PSA is monitored during testosterone treatment since increased dosage of TRT can lead to increased values of PSA, and because of a hypothetical fear of inducing prostate cancer; however, PSA was within normative limits for the vast majority in both the injection TRT and transdermal TRT group. Age at diagnosis was significantly higher among those receiving transdermal TRT, which indicates that age at diagnosis could influence the choice of treatment. About a third of all treated KS males swapped treatment during the observation period. Overall, the androgen-responsive parameters were similar in the patients that swapped treatment compared with the ones that remained on the same treatment during the entire period.
Hypogonadism in KS males is part of the explanation for development of metabolic diseases in KS, although it is also plausible that genetic factors play a role [2, 36]. KS males present with obesity, dyslipidemia, insulin resistance, and a tendency toward thrombosis, resulting in an increase in risk of cardiovascular diseases [11, 12]. Many of the KS males included in this study were overweight (BMI > 25 kg/m2) or obese (BMI > 30 kg/m2) [37, 38], and we found a significant association between age and increase in BMI, indicating a concurrent age-dependent increase in BMI, which is also seen in healthy eugonadal men [39]. In a recent meta-analysis, it was shown that body fat was significantly higher in untreated KS males compared with healthy controls, and that TRT resulted in a reduction in fat mass, but with no modification in BMI and waist circumference [20], a finding in line with a recent randomized controlled study [40]. Tentatively, results from this study do not suggest that different routes of administration affect the prevalence of obesity.
In this study, dyslipidemia with decreased HDL-cholesterol and increased LDL-cholesterol, triglycerides, and total cholesterol was seen in many KS males during a treatment period of 5 years, but neither route of administration seemed to be directly associated with a reduced risk of dyslipidemia. Pizzocaro et al found that TRT was ineffective in ameliorating the skewed lipid profile in KS males [20]. Those data are also in agreement with our results showing no correlation between any lipid fractions and testosterone levels. The mechanisms causing dyslipidemia in KS are largely unknown [2].
In this study, 6% of KS males with 47,XXY and 46,XY/47,XXY had diabetes, and were treated with antidiabetic medication, with no effect of route of administration on the diabetes profile. Conflicting results concerning the effect of testosterone on glucose homeostasis have been presented in previous studies, in which TRT seemed to improve insulin sensitivity in KS males in some studies [41] but not in other studies [20, 42]. The reasons for reduced insulin sensitivity in KS males have not been fully elucidated, although obesity is a strong predictor of type 2 diabetes. Likewise, known risk factors are health habits, including smoking [43]. In this study, 14% of the KS males were smokers.
Osteopenia was seen in 49% of KS males with 47,XXY and 46,XY/47,XXY, with no difference due to route of administration. Borst et al found that older hypogonadal men receiving TRT at 125 mg/week IM injection for 1 year had an increase in lumbar spine BMD at 4% and in hip BMD at 2%, whereas no increase in BMD was seen for patients treated with transdermal TRT [44]. Pizzocaro et al found in their recent meta-analysis that BMD were better in testosterone treated than untreated hypogonadal KS males [20], while other studies indicated that TRT did not fully reverse bone abnormalities in KS men [14].
Conclusively, during real-life circumstances, long-acting injection TRT and transdermal gel TRT are not significantly associated with a reduced risk of any of the metabolic diseases. Further investigation of route of administration is necessary to clarify the associations between TRT formulations and development of later metabolic diseases.
Many KS males present in the outpatient clinic with a range of diseases, indicating significant comorbidity. Visits at outpatient clinics at a specialized center are recommended after 3 to 6 months of therapy, and thereafter annually to follow the response to TRT, to assess adverse effects, quality of life, and to investigate the adherence to therapies, which is a primary determinant of treatment success [2]. The choice of route of administration depends on patient preference at our outpatient clinic and neither injection nor transdermal TRT is reserved for those with most severe hypogonadism. Some of the side effects of TRT include acne, snoring suggestive of sleep apnea, male pattern baldness, gynecomastia, elevation of PSA, benign prostatic hyperplasia, polycythemia, elevated hematocrit levels, elevated hemoglobin levels, and suppression of residual spermatogenesis [23, 27], although the latter is usually not relevant in KS. The findings reported here may support clinicians in shared decision-making when considering the appropriate treatment strategies for KS males, taking both treatment efficacy side effects and treatment adherence into account.
Limitations
Given the retrospective observational nature of the current study and the fact that about a third of all treated KS males swapped treatment, it is important to state that future prospective studies with proper randomization are necessary. We made no formal corrections for multiple testing due to the explorative nature of the study. Thus, the results should be interpreted with caution. Measurements of biochemical parameters including testosterone were not conducted on exact time points for many different practical reasons in clinical practice, but we recommended that those receiving transdermal TRT should have testosterone and other analytes measured after 23 to 24 hours, while those receiving injection TRT should have a nadir measurement performed, just before receiving yet another injection. We did not adjust for swaps between TRT formulations. Furthermore, adherence to therapy is a confounder when assessing the effect of treatment in general. Adherence to treatment is very difficult to monitor and measure. We have no information of how adherent the KS males in this study were. It is possible that patients treated with daily gel may forget it or skip it occasionally, which will lead to underestimation of the efficacy of transdermal treatment. At the same time, KS males treated with injection TRT can forget or skip the injection, underestimating the treatment efficacy of injection treatment.
Conclusion
TRT, irrespective of route of administration, leads to similar effects based on androgen-responsive variables. We found no difference in side effects, such as elevated hematocrit, hemoglobin, PSA, or BMD, when comparing injection TRT and transdermal TRT. We conclude that neither long-acting injection nor transdermal gel seem to reduce the risk of the metabolic diseases significantly, thereby leading to similar effects on the metabolic profile. The results suggest that clinicians should discuss the most desirable route of administration for every individual male with KS, which can result in the highest degree of adhesion to treatment based on individual patient preferences. Shared decision-making is a key component of patient-centered health care, allowing the patient to contribute to the medical decision-making process, in this way arriving at the most optimal route of administration for each male with KS.
Acknowledgments
Claus H. Gravholt and Anne Skakkebæk are members of the European Reference Network on Rare Endocrine Conditions (ENDO-ERN), Project ID number 739543.
Financial Support: The study was financially supported by research grants from Health Research Foundation of Central Denmark Region, The Novo Nordisk Foundation (NNF13OC0003234, NNF15OC0016474, NNF20OC0060610) and the Familien Hede Nielsen foundation.
Author Contributions: C.H.G. and A.K. conceived the study, A.K. gathered and analyzed data and wrote the manuscript, with significant input from C.H.G., S.C., and A.S.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- DVT
deep venous thrombosis
- DXA
dual-energy x-ray absorptiometry
- FSH
follicle-stimulating hormone
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- KS
Klinefelter syndrome
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- PSA
prostate-specific antigen
- TRT
testosterone replacement therapy
Additional Information
Disclosures: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this paper. The authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to legal restrictions eg, their containing information that could compromise the privacy of research participants.
References
- 1. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622-626. [DOI] [PubMed] [Google Scholar]
- 2. Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr Rev. 2018;39(4):389-423. [DOI] [PubMed] [Google Scholar]
- 3. Nieschlag E, Ferlin A, Gravholt CH, et al. The Klinefelter syndrome: current management and research challenges. Andrology. 2016;4(3):545-549. [DOI] [PubMed] [Google Scholar]
- 4. Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194(1):24-28. [DOI] [PubMed] [Google Scholar]
- 5. Berglund A, Viuff MH, Skakkebæk A, Chang S, Stochholm K, Gravholt CH. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet J Rare Dis. 2019;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364(9430):273-283. [DOI] [PubMed] [Google Scholar]
- 7. Arver S, Luong B, Fraschke A, et al. Is testosterone replacement therapy in males with hypogonadism cost-effective? An analysis in Sweden. J Sex Med. 2014;11(1):262-272. [DOI] [PubMed] [Google Scholar]
- 8. Chang S, Skakkebaek A, Davis SM, Gravholt CH. Morbidity in Klinefelter syndrome and the effect of testosterone treatment. Am J Med Genet C Semin Med Genet. 2020;184(2):344-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis SM, DeKlotz S, Nadeau KJ, Kelsey MM, Zeitler PS, Tartaglia NR. High prevalence of cardiometabolic risk features in adolescents with 47,XXY/Klinefelter syndrome. Am J Med Genet C Semin Med Genet. 2020;184(2):327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bojesen A, Kristensen K, Birkebaek NH, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29(7):1591-1598. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA; United Kingdom Clinical Cytogenetics Group . Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90(12):6516-6522. [DOI] [PubMed] [Google Scholar]
- 12. Chang S, Christiansen CF, Bojesen A, Juul S, Münster AB, Gravholt CH. Klinefelter syndrome and testosterone treatment: a national cohort study on thrombosis risk. Endocr Connect. 2020;9(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang S, Skakkebæk A, Trolle C, et al. Anthropometry in Klinefelter syndrome-multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J Clin Endocrinol Metab. 2015;100(3): E508-E517. [DOI] [PubMed] [Google Scholar]
- 14. Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91(4):1254-1260. [DOI] [PubMed] [Google Scholar]
- 15. Bojesen A, Høst C, Gravholt CH. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16(6):396-401. [DOI] [PubMed] [Google Scholar]
- 16. Zöller B, Ji J, Sundquist J, Sundquist K. High risk of venous thromboembolism in Klinefelter syndrome. J Am Heart Assoc. Published online May 20, 2016. 2016;5(5)e003567. doi:10.1161/JAHA.116.003567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salzano A, Arcopinto M, Marra AM, et al. Klinefelter syndrome, cardiovascular system, and thromboembolic disease: review of literature and clinical perspectives. Eur J Endocrinol. 2016;175(1):R27-R40. [DOI] [PubMed] [Google Scholar]
- 18. Chang S, Biltoft D, Skakkebæk A, et al. Testosterone treatment and association with thrombin generation and coagulation inhibition in Klinefelter syndrome: a cross-sectional study. Thromb Res. 2019;182:175-181. [DOI] [PubMed] [Google Scholar]
- 19. Stagi S, Di Tommaso M, Manoni C, et al. Bone mineral status in children and adolescents with Klinefelter syndrome. Int J Endocrinol. 2016;2016:3032759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pizzocaro A, Vena W, Condorelli R, et al. ; King, Klinefelter ItaliaN Group . Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020;43(12):1675-1687. [DOI] [PubMed] [Google Scholar]
- 21. Shanbhogue VV, Hansen S, Jørgensen NR, Brixen K, Gravholt CH. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. J Bone Miner Res. 2014;29(11):2474-2482. [DOI] [PubMed] [Google Scholar]
- 22. Zitzmann M, Aksglaede L, Corona G, et al. European academy of andrology guidelines on Klinefelter syndrome endorsing organization: European Society of Endocrinology. Andrology. 2021;9(1):145-167. [DOI] [PubMed] [Google Scholar]
- 23. Chang S, Skakkebæk A, Gravholt CH. Klinefelter syndrome and medical treatment: hypogonadism and beyond. Hormones (Athens). 2015;14(4):531-548. [DOI] [PubMed] [Google Scholar]
- 24. Zhang GY, Gu YQ, Wang XH, Cui YG, Bremner WJ. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl. 1998;19(6):761-768. [PubMed] [Google Scholar]
- 25. Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long-acting testosterone undecanoate injections in hypogonadal men: an 84-week phase III clinical trial. J Androl. 2010;31(5):457-465. [DOI] [PubMed] [Google Scholar]
- 26. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85(12):4500-4510. [DOI] [PubMed] [Google Scholar]
- 27. Tsametis CP, Isidori AM. Testosterone replacement therapy: for whom, when and how? Metabolism. 2018;86:69-78. [DOI] [PubMed] [Google Scholar]
- 28. Sansone A, Sansone M, Selleri R, et al. Monitoring testosterone replacement therapy with transdermal gel: when and how? J Endocrinol Invest. 2019;42(12):1491-1496. [DOI] [PubMed] [Google Scholar]
- 29. Arver S, Stief C, de la Rosette J, Jones TH, Neijber A, Carrara D. A new 2% testosterone gel formulation: a comparison with currently available topical preparations. Andrology. 2018;6(3):396-407. [DOI] [PubMed] [Google Scholar]
- 30. Kabilan A, Skakkebæk A, Chang S, Gravholt CH. Supplementary material. figshare. Posted March 25, 2021. https://doi.org/10.6084/m9.figshare.14308733.v1
- 31. Olsson H, Sandström R, Neijber A, Carrara D, Grundemar L. Pharmacokinetics and bioavailability of a new testosterone gel formulation in comparison to Testogel® in healthy men. Clin Pharmacol Drug Dev. 2014;3(5):358-364. [DOI] [PubMed] [Google Scholar]
- 32. Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J Clin Endocrinol Metab. 2004;89(12):6208-6217. [DOI] [PubMed] [Google Scholar]
- 33. Plymate SR, Leonard JM, Paulsen CA, Fariss BL, Karpas AE. Sex hormone-binding globulin changes with androgen replacement. J Clin Endocrinol Metab. 1983;57(3):645-648. [DOI] [PubMed] [Google Scholar]
- 34. Bojesen A, Hertz JM, Gravholt CH. Genotype and phenotype in Klinefelter syndrome - impact of androgen receptor polymorphism and skewed X inactivation. Int J Androl. 2011;34(6 Pt 2):e642-e648. [DOI] [PubMed] [Google Scholar]
- 35. Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6(3):208-215. [DOI] [PubMed] [Google Scholar]
- 36. Skakkebæk A, Nielsen MM, Trolle C, et al. DNA hypermethylation and differential gene expression associated with Klinefelter syndrome. Sci Rep. 2018;8(1):13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176-s185. [PubMed] [Google Scholar]
- 38. Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eat Weight Disord. 2018;23(3):293-302. [DOI] [PubMed] [Google Scholar]
- 39. Rolf C, von Eckardstein S, Koken U, Nieschlag E. Testosterone substitution of hypogonadal men prevents the age-dependent increases in body mass index, body fat and leptin seen in healthy ageing men: results of a cross-sectional study. Eur J Endocrinol. 2002;146(4):505-511. [DOI] [PubMed] [Google Scholar]
- 40. Høst C, Bojesen A, Erlandsen M, et al. A placebo-controlled randomized study with testosterone in Klinefelter syndrome: beneficial effects on body composition. Endocr Connect. 2019;8(9):1250-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fink J, Matsumoto M, Tamura Y. Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids. 2018;138:161-166. [DOI] [PubMed] [Google Scholar]
- 42. Jiang-Feng M, Hong-Li X, Xue-Yan W, et al. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98(5):1331-1335. [DOI] [PubMed] [Google Scholar]
- 43. O’Connor MJ, Snyder EA, Hayes FJ. Klinefelter syndrome and diabetes. Curr Diab Rep. 2019;19(9):71. [DOI] [PubMed] [Google Scholar]
- 44. Borst SE, Shuster JJ, Zou B, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to legal restrictions eg, their containing information that could compromise the privacy of research participants.