Abstract
Acute pulmonary embolism (APE) is a common and prognostically significant complication of COVID-19 infection. We investigated the clinical characteristics and chest CT findings of COVID-19 positive patients complicated with APE. A retrospective, record-based, case-series study was performed examining 483 patients admitted to King Saud Medical City during the pandemic, from April 2020 to June 2020. Of these, 92 patients who underwent chest CT scans were included in the final analysis. The incidence of APE, clinical presentations, radiological patterns, and patient outcomes were assessed and compared against those for patients without PE. The incidence of APE was 22% [95% confidence interval (95% CI): 19%–39%], detected by chest CT. Men constituted 85.0% of patients, with a mean age of 48.9 ± 16.7 years. For most patients with APE, risk factors for thromboembolism were established but did not differ significantly from those without PE. The mean D-dimer level of 9.1 (range 7.0–10.2) was significantly higher among patients diagnosed with APE (OR: 1.021; 95% CI: 1.012–1.028; P = 0.001) compared with that in patients without PE. Moreover, the mean levels of lactate dehydrogenase (LDH, 628.5; range: 494.0–928.3; OR: 1.002; 95% CI: 1.000–1.003; P = 0.02), C-reactive protein (CRP; 158.5; range: 105.3–204.5; OR: 1.025; 95% CI: 1.015–1.035; P = 0.001), and cardiac troponin (3.5; range; 2.6–3.8; OR: 1.016; 95% CI: 0.971–1.067; P = 0.01) were also significantly higher in patients with APE than those in patients with PE. The chest CT presentations of APE included massive, segmental, and sub-segmental APE. The need for Intensive Care Unit admission was higher among patients diagnosed with APE, who presented a fatality rate of 10%.. Our study pointed to the incidence and predictors of APE in COVID-19 patients. High levels of D-dimer, CRP, cardiac troponin, and LDH should alert the clinician to the possibility of APE in COVID-19 patients..
Keywords: Pulmonary embolism, COVID_19, Coagulopathy, Saudi Arabia
1. Introduction
The highly communicable disease, known as coronavirus disease 2019 (COVID-19), associated with the infection of the lower respiratory tract and caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has reached pandemic proportions worldwide since December 2019 [1].
The first case of COVID-19 was announced in the Kingdom of Saudi Arabia on March 2, 2020, with the bulk of confirmed cases diagnosed in returning travelers or their immediate contacts. The total number of confirmed COVID-19 cases reached 313,911 by August 30, 2020, including 3840 associated fatalities [2,3].
COVID-19 presents with a broad range of clinical manifestations, and the typical onset is associated with the presentation of non-specific symptoms, such as fever, dry cough, and fatigue. Multiple systems, including the respiratory, cardiovascular, neurological, musculoskeletal, and gastrointestinal systems, may be affected. The condition may rapidly deteriorate to acute respiratory distress syndrome (ARDS) in high-risk patients such as elderly and those with other medical comorbidities, such as heart, lung, kidney, or liver disorders [4].
COVID-19 infections are frequently correlated with the activation of the coagulation system, defined as COVID-19 coagulopathy [5]. Thrombotic symptoms result in the development of important sequelae among patients infected with COVID-19, leading to significant morbidity and mortality [6]. Recent studies have indicated that most serious COVID-19 patients who require Intensive Care Unit (ICU) admission present with a hypercoagulable state, which may result in the development of thromboembolic events [7]. The infection has been reported to cause endothelial dysfunction and an inflammatory condition, resulting in increased thrombin production and decreased fibrinolysis [8]. Combined with hypoxia due to severe pneumonia, these conditions can encourage thrombosis, leading to the increased viscosity of the blood [9].
Computed tomography (CT) has played a major role in the diagnosis of COVID-19 since the initial discovery of the disease and has helped to identify those patients with severe complications, including pulmonary embolism (PE) [10,11]. This study aimed to assess the incidence of acute PE (APE) among COVID-19 patients and to compare their clinical presentations, laboratory test results, and outcomes against those for patients without PE.
2. Patients and methods
A retrospective descriptive, record-based, case-series, single-center study was performed, which included adult COVID-19-confirmed patients who were hospitalized at King Saud Medical City between April 2020 and June 2020. We examined all 483 medical files for consecutively admitted COVID-19 positive patients. We included all 92 of these patients who underwent a chest CT scan during their hospital admission in this study. The COVID-19 suspicion-to-confirmation process was driven by the recommendations of the Saudi Centers for Disease Control and Prevention (updated in June 2020), based on the best available data and evidence [12]. A confirmed COVID-19 infection was defined as a positive polymerase chain reaction (PCR) test. The necessity of a CT scan during hospital admission of COVID-19 positive patients is to assess the severity of COVID-19 pneumonia and rule out PE. The medical criteria used to indicate chest CT scan included the deterioration of respiration that was not associated with other factors, acute respiratory distress without improvement, despite specific treatment, and increasing D-dimer levels, in discordance with other inflammatory parameters. All CT images were reviewed independently by two experts, and the final decisions reported here were reached by consensus. All demographic data, clinical presentation data (including clinical symptoms and signs), comorbidities, laboratory findings, and outcomes were assessed and compared between COVID-19 patients with and without PE. Laboratory investigations included the PCR results for SARS-CoV-2 infection, complete blood counts, cardiac enzymes [troponin I, creatinine kinase (CK), CK-MB], D-dimer, C-reactive protein (CRP), prothrombin time, lactate dehydrogenase (LDH), glycated hemoglobin (Hb A1C), and liver and renal function tests.
This study was approved by the King Saud Medical City ethics committee. This study was performed in accordance with the Helsinki declaration and was approved by the Local Ethics and Research Committee.
2.1. Statistical analysis
Microsoft Excel 2016 and the Social Science Statistical Package (SPSS, version 26.0) for Windows (SPSS IBM., Chicago, IL) were used to analyze the results. Continuous variables that were normally distributed are presented as the mean ± standard deviation (SD), with a 95% confidence interval (95% CI), whereas non-normal variables are presented as the median and interquartile range (IQR). Categorical variables are presented as frequencies and percentages. A p-value of less than 0.05 was considered significant. The Student’s t-test was performed to compare the means of normally distributed variables between groups, whereas the Mann–Whitney U test was used to compare the means of non-normal variables. To compare the distributions of categorical variables between groups, the χ2 test or Fisher’s exact test was used. Effect modifications were evaluated by stratification, and statistical interactions were evaluated using multivariate logistic regression analysis, using main predictor variables and their product terms in a binary logistic regression analysis.
3. Results
Of 483 COVID-19 positive patients who were admitted to the hospital during the study period, 92 (19.04%) patients underwent chest CT scans. Patients were categorized according to their chest CT scan results into two groups: one with APE and one without PE (Non-PE).
The demographic characteristics of both groups are presented in Table 1. In both the APE and non-PE groups, men represented the majority of patients, at 17 (85.0%) and 55 (76.4%), respectively. The mean ages of patients underwent chest CT scans was 48.9 ± 16.7 in the APE group and 47.7 ± 14.8 years in non-PE group. Most patients in both groups were non-Saudi nationals, including 17 (85.0%) in the APE group and 55 (76.4%) in non-PE group. The diagnosis of PE, based on results of chest CT, occurred later in the APE group than in the non-APE group, by an average of 4 days (range 1.0–12.8 days). However, this difference was not significant compared with the non-PE group (average 1 day, range 1.0–12.0 days).
Table 1.
Demographics and clinical characteristics of the studied groups.
| APE N = 20 |
Non-PE N = 72 |
P. value | OR (95%C·I) | P. value | ||
|---|---|---|---|---|---|---|
| Demographic data, n %: | ||||||
| Age (years) (mean ± SD) | 48.9 ± 16.7 | 47.7 ± 14.8 | 0.7 | 1.005 (0.973–1.039) | 0.7 | |
| Sex | Female | 3 (15.0%) | 1 (23.6%) | 0.4 | 1.752 (0.457–6.706) | 0.4 |
| Male | 17 (85.0%) | 55 (76.4%) | ||||
| Nationality | Non-Saudi | 17 (85.0%) | 50 (69.4%) | 0.2 | 0.401 (0.107–1.51) | 0.2 |
| Saudi | 3 (15.0%) | 22 (30.6%) | ||||
| Delay between admission and CT diagnosis (Days) | 4.0 (1.0–12.8) | 1.0 (1.0–12.0) | 0.5 | 1.032 (0.970–1.098) | 0.3 | |
| Incidence (Total No. = 92) | 20 (21.7%) | 72 (78.3%) | 0.001** | 22.0 (19.0–39.0) | 0.01* | |
| History of risk factors, n %: | ||||||
| Diabetes Mellites | 7 (35.0%) | 28 (38.9%) | 0.7 | 0.846 (0.301–2.379) | 0.7 | |
| Hypertension | 4 (20.0%) | 24 (33.3%) | 0.2 | 0.5 (0.151–1.66) | 0.3 | |
| Chronic Kidney Disease | 0 (0.0%) | 21 (29.2%) | 0.01* | 0.128 (0.016–1.017) | 0.05* | |
| Smoker | 1 (5.0%) | 15 (20.8%) | 0.09 | 0.2 (0.025–1.617) | 0.1 | |
| Obesity (body mass index over 30) | 0 (0.0%) | 4 (5.6%) | 0.3 | 0.673 (0.045–1.031) | 0.3 | |
| Cerebro Vascular Accidents | 3 (15.0%) | 7 (9.7%) | 0.5 | 1.639 (0.383–7.014) | 0.5 | |
| Ischemic Heart Disease | 2 (10.0%) | 4 (5.6%) | 0.5 | 1.889 (0.320–11.146) | 0.5 | |
| Lung disease. | 2 (10.0%) | 5 (6.9%) | 0.6 | 1.489 (0.267–8.318) | 0.6 | |
| Clinical presentation, n %: | ||||||
| Fever | 16 (80.0%) | 56 (77.8%) | 0.8 | 1.143 (0.335–3.904) | 0.8 | |
| Shortness of breath | 16 (80.0%) | 59 (81.9%) | 0.8 | 0.881 (0.253–3.074) | 0.8 | |
| Cough | 15 (75.0%) | 62 (86.1%) | 0.2 | 0.484 (0.144–1.627) | 0.2 | |
| Pneumonia | 6 (30.0%) | 8 (11.1%) | 0.03* | 3.429 (1.026–11.454) | 0.04* | |
| Laboratory investigations, n %: | ||||||
| Hemoglobin, (g/dl) | 11.9 ± 2.3 | 11.7 ± 2.7 | 0.6 | 1.043 (0.860–1.266) | 0.6 | |
| White Blood Cells, (10 ^9/L) | 9.9 (4.7–15.8) | 7.0 (4.2–12.0) | 0.3 | 0.997 (0.978–1.017) | 0.7 | |
| Leucopenia, n (%) | 13 (65.0%) | 45 (62.5%) | 0.8 | 1.114 (0.396–3.138) | 0.8 | |
| Platelets, (10 ^9/L) | 254.0 (202.0–335.3) | 272.0 (173.3–363.0) | 0.9 | 1.000 (0.997–1.003) | 0.8 | |
| INR | 1.1 (1.0–1.2) | 1.1 (1.1–1.2) | 0.2 | 0.018 (0.001–1.679) | 0.08 | |
| Creatine kinase (U/L) | 154.0 (104.0–306.0) | 135.5 (56.3–361.0) | 0.3 | 1.000 (1.000–1.001) | 0.3 | |
| Lactate Dehydrogenase (U/L) | 628.5 (494.0–928.3) | 534.0 (219.5–749.8) | 0.04* | 1.002 (1.000–1.003) | 0.02* | |
| Creatine kinase-MB (ng/ml) | 2.3 (0.5–14.5) | 1.0 (0.4–3.8) | 0.1 | 1.032 (0.990–1.076) | 0.1 | |
| Cardiac troponin I (ng/mL) | 3.5 (2.6–3.8) | 0.04 (0.01–0.2) | 0.001** | 1.016 (0.971–1.067) | 0.01* | |
| D-dimer (mg/L) | 9.1 (7.0–10.2) | 1.0 (0.6–1.3) | 0.001** | 1.021 (1.012–1.028) | 0.001** | |
| C-reactive protein (mg/L) | 158.5 (105.3–204.5) | 24.0 (3.5–36.0) | 0.001** | 1.025 (1.015–1.035) | 0.001** | |
| Creatinine (mmol/L) | 86.0 (56.6–100.3) | 79.0 (61.8–94.0) | 0.7 | 0.998 (0.989–1.007) | 0.6 | |
| Urea (mmol/L) | 5.3 (4.4–13.0) | 6.0 (4.7–9.8) | 0.7 | 0.972 (0.918–1.029) | 0.3 | |
| Blood sugar (mmol/L) | 8.0 (7.7–10.6) | 10.4 (5.0–16.0) | 0.6 | 0.962 (0.889–1.041) | 0.3 | |
| Aspartate aminotransferase (AST) (U/L) | 49.0 (33.3–87.0) | 44.5 (28.3–80.3) | 0.8 | 1 (0.991–1.009) | 0.9 | |
| Alanine aminotransferase (ALT) (U/L) | 29.5 (26.3–55.0) | 33.5 (20.0–71.0) | 0.9 | 0.993 (0.973–1.014) | 0.5 | |
| Total bilirubin (umol/L) | 18.0 (8.6–23.8) | 9.3 (7.8–14.0) | 0.01* | 1.036 (0.999–1.074) | 0.05* | |
| Sodium (mmol/L) | 140.4 ± 2.9 | 140.4 ± 2.7 | 0.9 | 0.997 (0.831–1.195) | 0.9 | |
| Complications, n %: | ||||||
| ARDS (Acute Respiratory Distress Syndrome) | 0 (0.0%) | 2 (2.8%) | 0.4 | 0.792 (0.118–1.027) | 0.7 | |
| Septic shock | 2 (10.0%) | 3 (4.2%) | 0.3 | 2.556 (0.397–16.463) | 0.3 | |
| RF (Respiratory failure) | 3 (15.0%) | 7 (9.7%) | 0.5 | 1.639 (0.383–7.014) | 0.5 | |
| ICU admission | 19 (95.0%) | 39 (54.2%) | 0.001** | 16.077 (2.042–126.597) | 0.01* | |
| Death Rate | 2 (10.0%) | 3 (4.2%) | 0.3 | 2.556 (0.397–16.463) | 0.3 | |
Age, HB, and Na are represented as Mean ± SD; the data were analyzed by student t test. While Sex, Nationality, Delay, Smoker, Obesity, HTN, CKD, DM, CVA, IHD, Lung dis., Leucopenia, SOB, Fever, Cough, Pneumonia, ARDS, Septic shock, RF, ICU admission and Death Rate are represented as frequency and percent; the data were analyzed by X2 test. But Delay, WBC, PLT, INR, CK, LDH, CKMB, cTn I, D.dimer, CRP, Cr, Urea, B. sugar, AST, ALT, T. bilirubin and Na are represented as Median with Interquartile range (25%–75%), the data were analyzed by Mann-whitney U test.
OR; Odd Ratio, C·I; Confidence Interval, p-value calculated depend on logistic regression analysis.
Septic shock is defined by persisting hypotension requiring vasopressors to maintain a mean arterial pressure of 65 mm Hg or higher and a serum lactate level greater than 2 mmol/L (18 mg/dL) despite adequate volume resuscitation.
p. value < 0.05 is significant,
p. value < 0.01 is highly significant.
Diabetes, identified in 35.0% (7/20) patients, was the most prevalent comorbidity identified in the APE group, followed by hypertension, which was identified in 20.0% (4/20) patients. For most patients with APE, risk factors for thromboembolism were established, but these did not differ significantly from those in patients without PE, except for a history of chronic kidney disease, which was absent from the APE group.
Fever and shortness of breath were the most commonly identified clinical presentations for both groups, with no significant difference in incidence. However, pneumonia incidence was significantly higher among the APE group, identified in 30.0% (6/20) of patients in the APE group compared with only 11.1% (8/20) patients in the non-PE group (P = 0.03). Patients with APE were more frequently admitted to the ICU than those without PE [19 (95.0%) vs 39 (54.2%) patients, P = 0.001]. Moreover, the in-hospital fatality rate was 2 (10.0%) in the APE group versus 3 (4.2%) in the non-PE group.
The laboratory tests for patients with APE showed significantly elevated levels compared with those for patients in the non-PE group. The mean D-dimer [9.1 (range: 7.0–10.2) vs. 1.0 (range: 0.6–1.3); OR: 1.021; 95% CI: 1.012–1.028; P = 0.001], LDH [628.5 (range: 494.0–928.3) vs. 534.0 (range: 219.5–749.8); OR: 1.002; 95% CI: 1.000–1.003; P = 0.02], CRP [158.5 (range: 105.3–204.5) vs. 24.0 (range: 3.5–36.0); OR: 1.025; 95% CI: 1.015–1.035; P = 0.001], cardiac troponin [3.5 (range: 2.6–3.8) vs. 0.04 (range: 0.01–0.2); OR: 1.016; 95% CI: 0.971–1.067; P = 0.01], and total bilirubin [18.0 (range: 8.6–23.8) vs. 9.3 (range: 7.8–14.0); OR: 1.036; 95% CI: 0.999–1.074; P = 0.05] levels were all significantly elevated in the APE group vs. the non-PE group.
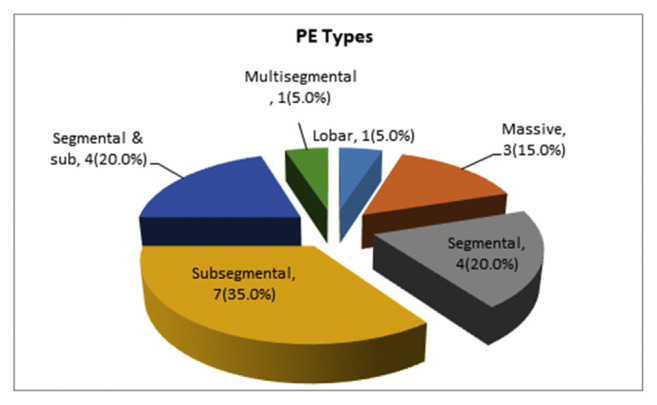
According to Saudi MOH Protocol for Patients of Confirmed with COVID-19, which KSMC follows, thromboprophylaxis with low molecular weight heparin (LMWH) should be considered in all patients (including non-critically ill) who require hospital admission for COVID-19 infection, in the absence of any contraindications (active bleeding and platelet count less than 25 × 109/L; monitoring is advised in severe renal impairment; abnormal PT or APTT is not a contraindication). Dose of LMWH depend on level of D-dimer; is it less or more than 1 mcg/mL. The decision to conduct chest CT scan was based on acute hemodynamic or respiratory status deterioration and suspicion of PE. The CT findings for patients with APE displayed sub-segmental PE in 7 patients (35.0%), segmental PE in 4 patients (20.0%), both segmental and sub-segmental PE in 4 patients (20.0%), multi-segmented PE in 1 patient (5.0%), lobar PE in 1 patient (5.0%), and massive PE in 3 patients (15.0%, Fig. 1).
Fig. 1.
Different CT findings in COVID-19 patients with acute pulmonary embolism.
4. Discussion
The incidence of APE among our COVID-19 patients was 22% (95% CI: 19%–39%), based on chest CT results. The incidence of APE among COVID-19 patients based on pulmonary CT angiography was reported between 23% and 30% in recent studies [13,14], which is nearly similar to our results (22%). This high prevalence of PE among COVID-19-positive patients supports an increasing awareness of the relationship between COVID-19 and hypercoagulable conditions [15].
Our patients presented with classic COVID-19 manifestations, including fever, cough, and shortness of breath. Similarly, several publications have reported the diagnosis of PE among patients with COVID-19 who presented with common COVID-19 manifestations [16,17]. Unlike Grillet et al., who reported the delayed diagnosis of APE among COVID-19 patients, after a mean of 12 days from symptom onset [13], our patients were diagnosed with APE nearly four days earlier.
Significant differences between the CRP and D-dimer levels between the APE and non-PE groups have been observed, which may suggest that COVID-19-positive patients with higher inflammation rates are more likely to experience thrombotic events. Elevated levels of D-dimer in patients with COVID-19-associated pneumonia have also been reported by several studies [18,19]. Several studies have shown a strong association between elevated D-dimer levels and poor COVID-19 prognosis [20–23]. However, a positive D-dimer test is not an absolute diagnostic criterion for PE because other clinical conditions (for example, cancer, infection, pregnancy, or postoperative status) have also been associated with high D-dimer levels [24,25].
The laboratory findings for the PE group in our study also showed significantly higher levels of Troponin, LDH, and total bilirubin compared with the non-PE group. Similarly, Guo et al. reported that the levels of plasma troponin had a positive, direct, linear correlation with the levels of high-sensitivity plasma CRP, which indicated that myocardial injury might be closely linked to inflammatory pathogenesis during COVID-19 progression [26]. In addition, elevated troponin T concentrations in patients with PE have been associated with higher in-hospital mortality rates [27]. Moreover, increased CRP levels were associated with adverse aspects of COVID-19, such as ARDS, and increased LDH levels have also have been associated with an increased risk of ARDS [28], higher ICU admission rates [29], and increased mortality [20,25].
A previous study, which described a series of 8 patients complicated with PE following COVID-19 infection, also reported high levels of D-dimer, total bilirubin, liver enzymes, and LDH [30].
Our APE patients showed a high frequency (95.0%) of ICU admission, which agrees with the study by Klok et al., who reported a high incidence of PE with thrombotic complications among positive COVID-19 patients admitted to the ICU (65/75; 87%) [31]. In addition, Grillet et al. reported that COVID-19 Patients with pulmonary embolus were more likely than those without to be admitted to the critical care unit, (17/23; 74%) vs (22/77; 29%) patients, respectively; P, 0.001 [18].
Our study identified that specific clinical data might serve as potential predictors for PE among COVID-19-positive patients. Multivariate analysis revealed that higher levels of D-dimer, CRP, LDH, cardiac troponin, and total bilirubin levels were crucial predictors of APE complicating COVID-19 infection. Our observations meet with Helms J et al. who stated that Despite anticoagulation, a high number of COVID-19 patients experienced life-threatening thrombotic complications, the most common of which were pulmonary embolisms (16.7%), during their ICU admission [32].
Our study reaffirmed the link between increased D-dimer levels and the development of ARDS in COVID-19 patients, which is considered to be a risk factor for micro-PE. Moreover, previous studies [20,33,34] have reported that D-dimer levels tend to be a critical parameter for the assessment of COVID-19 severity. D-dimer also adds value to the prediction of APE risk among COVID-19-positive patients. Our study showed that APE risk factors in COVID-19 patients do not include the typical thrombo-embolic risk factors; instead, independent clinical and laboratory predictors can be used to assess risk, which reflects the significant contribution of inflammation.
A recent study reported higher significant mortality in COVID-19 patients with PE as compared with non-PE patients (16/32; 50%) vs (52/192; 27%), respectively; P, 0.010 [35]. In our study, the fatality rate was insignificantly higher in APE group than in those in non-APE group. This insignificance may be related to quite small sample size.
PE, in our study, was most frequently observed in the subsegmental and segmental pulmonary arteries, or both (75%), whereas the remaining cases were distributed between multisegmented, lobar, and massive PE. This finding agrees with previous studies that reported that PE in COVID-19 is more typically identified in the peripheral parts of the lungs [34–39], which is compatible with the observation of microthrombi formation [38,39]. These sites also tend to conform to the most typical COVID-19 opacity distribution [38]; however, other studies have also reported massive PE among COVID-19 patients [27,40].
Our research limitations include the retrospective study design and the constraint to a single health system. However, our results may help to alert the medical community regarding the increased risk of PE among COVID-19 patients and provided evidence of some potentially predictive factors that can be used to identify COVID-19 patients at high risk for PE.
We conclude that due to symptom overlap, the differentiation between COVID-19 and PE presents a diagnostic challenge for doctors in emergency care. More than one-fifth of patients hospitalized for COVID-19 pneumonia presented with APE on chest CT. The early diagnosis and timely treatment of COVID-19 patients complicated with APE are crucial for reducing mortality. These results suggest that chest CT evaluations should be more widely used in patients with COVID-19 pneumonia, especially those with marked D-dimer elevation.
Footnotes
Author contribution
Conception and design of Study: Samah I Abohamr, Mubarak A Aldossari, Eman Elsheikh. Literature review: Samah I Abohamr, Sara W Abulhamid, Eman Elsheikh. Acquisition of data: Hala A Amer, Hiba M Saadeddin. Analysis and interpretation of data: Samah I Abohamr, Fayaz A Bhat, Eman Elsheikh. Research investigation and analysis: Hiba M Saadeddin, Sara W Abulhamid, Fayaz A Bhat. Data collection: Hala A Amer, Hiba M Saadeddin, Sara W Abulhamid, Fayaz A Bhat. Drafting of manuscript: Sara W Abulhamid, Eman Elsheikh. Revising and editing the manuscript critically for important intellectual contents: Samah I Abohamr, Mubarak A Aldossari, Eman Elsheikh. Data preparation and presentation: Samah I Abohamr, Hala A Amer, Eman Elsheikh. Supervision of the research: Samah I Abohamr, Mubarak A Aldossari. Research coordination and management: Samah I Abohamr, Eman Elsheikh.
Disclosure
Author has nothing to disclose with regard to commercial support.
References
- 1.Rotzinger D, Beigelman-Aubry C, von Garnier C, Qanadli S. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020 Jun;190:58–9. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MOH news - MOH reports first case of coronavirus infection. 2020 https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2020-03-02-002.aspx.
- 3.Ministry of Health Moh. Command and Control center, national public health events, 2020. Ministry of Health; 2020. Available from: https://www.moh.gov.sa/en/CCC/Pages/default.aspx. [Google Scholar]
- 4.Abohamr S, Abazid R, Aldossari M, Amer H, Badhawi O, Aljunaidi O, et al. Clinical characteristics and in-hospital mortality of COVID-19 adult patients in Saudi Arabia. SMJ. 2020 Nov 1;41(11):1217–26. doi: 10.15537/smj.2020.11.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020 Jul;18(7):1559–61. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020 Jun;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017 Jan;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019 Sep;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009 Dec;193(6):1488–93. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 11.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 Aug;296(2):E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saudi Center for Disease prevention and Control. Novel corona virus (2019-nCoV) infection guidelines V1.0. Saudi Center for Disease Prevention and Control Ministry of Health; Kingdom of Saudi Arabia: 2020. [Google Scholar]
- 13.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020 Sep;296(3):E186–8. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020 Sep;296(3):E189–91. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakr Y, Giovini M, Leone M, Pizzilli G, Kortgen A, Bauer M, et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0.eCollection.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiology: Cardiothoracic Imaging. 2020 Apr 1;2(2):e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danzi GB, Loffi M, Galeazzi G, Gherbesi E.Acute pulmonary embolism, and COVID-19 pneumonia: a random association Eur Heart J 2020May1441191858 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020 Jun;55(6):327–31. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course, and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 May;18(5):1094–9. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Wang X, Zhang S, et al. Findings of acute pulmonary embolism in COVID-19 patients (3/1/2020) SSRN. 2020 [Google Scholar]
- 23.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr;18(4):844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Gal G, Righini M, Wells PS. D-dimer for pulmonary embolism. JAMA. 2015 Apr 28;313(16):1668–9. doi: 10.1001/jama.2015.3703. [DOI] [PubMed] [Google Scholar]
- 25.Ehmann MR, Hinson JS.Diagnosis of pulmonary embolism with d-dimer testing N Engl J Med 2020March12382111074–5. 10.1056/NEJMc1917227. [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol 2020July157811–8. 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sang CJ, Heindl B, Von Mering G, Rajapreyar I. Massive pulmonary embolism in a COVID-19 patient: a case report. Eur Heart J Case Rep. 2020 Oct;4(FI1):1–5. doi: 10.1093/ehjcr/ytaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and Death in patients with coronavirus disease 2019 pneumonia in wuhan, China JAMA Intern Med 2020July11807934–43. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 Jun;95(6):E131–4. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 30.Hékimian G, Lebreton G, Bréchot N, Luyt CE, Schmidt M, Combes A.Severe pulmonary embolism in COVID-19 patients: a call for increased awareness Crit Care 2020June2241274. 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020 Jul;191:148–50. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 Jun;46(6):1089–98. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey K, Iteen A, Nicolini R, Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.011. S0735-6757(20)30239-30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 Jun;18(6):1421–4. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scudiero F, Silverio A, Di Maio M, Russo V, Citro R, Personeni D, et al. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. 2021 Feb;198:34–9. doi: 10.1016/j.thromres.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease. Thromb Res. 2020 Sep;193:86–9. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of over-diagnosis. Arch Intern Med. 2011 May 9;171(9):831–7. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li T, Kicska G, Kinahan PE, Zhu C, Oztek MA, Wu W. Clinical and imaging findings in COVID-19 patients complicated by pulmonary embolism. MedRxiv. 2020 doi: 10.1101/2020.04.20.20064105. [DOI] [Google Scholar]
- 39.Seung-Ick Cha, Kyung-Min Shin, Jung-Woo Lee, Jongmin Lee, Shin-Yup Lee, Chang-Ho Kim, et al. Clinical characteristics of patients with peripheral pulmonary. Embolism Jung Resp. 2010;80:500–8. doi: 10.1159/000277929. [DOI] [PubMed] [Google Scholar]
- 40.Pishgahi M, Ansari Aval Z, Hajimoradi B, Bozorgmehr R, Safari S, Yousefifard M. Massive pulmonary thromboembolism in patients with COVID-19; report of three cases. Arch Acad Emerg Med. 2020;8(1):e58. [PMC free article] [PubMed] [Google Scholar]