Supplemental Digital Content is available in the text.
Introduction:
Peripheral blood culture contamination (BCC) can lead to an initiation of unnecessary antimicrobial treatment, further laboratory tests, increased length of stay, and increased costs. This study describes a 12-month quality improvement (QI) program to reduce the BCC rate in a neonatal unit by 50%.
Methods:
The QI team focused on standardizing processes to align with best practices using process mapping and cause and effect diagrams. Plan-Do-Study-Act (PDSA) 1: inoculation of blood culture bottles with the introduction of transfer device; PDSA 2: preparation of the skin for peripheral intravenous cannula insertion; PDSA 3: aseptic technique education package; and PDSA 4: optimizing blood volume of blood collected for culture. The team used statistical process control methodology to detect special cause variation.
Results:
Compliance with the standard processes as part of PSDA 1 improved from a mean level of 50% to 100% and for PDSA 2 improved from a mean level of 50% to 95%. After implementation of PDSA 3, scores on a relevant knowledge test increased from a mean of 39% (pretraining test; n = 10) to 92% (posttraining test; n = 10) (P < 0.001). Postimplementation of the processes for PDSA 4, a minimum of 1 mL was collected in 94% of blood culture collection events (n = 450) (mean 1.1 mL; range 0.5–3.5 mL). Special cause variation occurred after the implementation of the PDSA cycles. During the baseline period, the BCC rate was 2.0% and decreased to 1.0% postinterventions implementation.
Conclusions:
Interventions focused on standardizing practices around collection of blood cultures in neonates were associated with fewer contaminants.
This study is reported according to the SQUIRE 2.0 guidelines.
INTRODUCTION
Background
Sepsis is a significant cause of both mortality and morbidity in neonates.1 Blood cultures are the gold standard test for identifying bacteremia in patients in whom sepsis is suspected, making it a crucial and commonly used diagnostic tool within neonatal units.2 However, the growth of contaminants in blood cultures presents a diagnostic challenge for clinicians and weakens the reliability of blood culture results. Multiple studies conducted since 1990 show the costs associated with each contaminated blood culture as between USD $2,844 and $10,078.3–6 Gander et al7 described an average added cost of USD $8,720 per contamination event. Meanwhile, Alahmadi et al3 showed that contaminated blood cultures increase the length of stay by up to 5.4 days.
The standard benchmark for blood culture contamination (BCC) rates is 3% as set out by the Clinical and Laboratory Standards Institute.2 BCC rates in adults are between 0.6% and 6%.8 Several studies in neonatal populations demonstrate that BCC occurs with rates of between 2.6% and 18%.9–12 The higher rates of contamination in neonates is attributed to difficult blood sampling.9,13 There are multiple methods identified to reduce contamination including skin preparation, culture bottle preparation, single versus double needle for bottle inoculation, source of culture, use of dedicated phlebotomy teams, use of sterile blood culture collection kits, use of initial specimen diversion devices, and the use of sterile gloves.7,8,10,14–20 Multiple studies show that the introduction of standardized protocols to support blood culture collection methods and staff education on best practices significantly reduces BCC rates.5,14,18,21
Local Problem Description
In October 2018, there was a concern raised by medical and nursing staff regarding the rate of central line-associated blood stream infections in the neonatal unit. A review of the organisms grown in blood cultures for the preceding 12 months suggested a larger issue was likely causing BCC events. A subsequent audit of all positive blood cultures demonstrated that, although the overall BCC rate was below the standard benchmark of 3%,2 there was variation in the monthly contamination rates (0.0%–5.7%). For the baseline period (October 2017 to April 2019), 1,944 blood cultures were collected, of which 39 were contaminants (2.0%). An observational audit of blood culture collection practices revealed significant variation among staff with inconsistent culture collection methods. There were frequent and significant breaches in the aseptic technique during the collection process itself. There was a concern raised that if this was not adequately addressed, the BCC rate could increase and the breaches in aseptic technique during other procedures could lead to significant adverse events such as line sepsis. Consequently, the team decided it was feasible and desirable to decrease the baseline BCC rate from 2.0%.
Project Aim.
Reduce the overall contamination rate from 2.0% to 1.0% (50% reduction) by March 2020.
METHODS
Context
This quality improvement (QI) initiative was conducted in South Australia in a Level 6 (Level IV as per the American Academy of Pediatrics) unit with 14 neonatal intensive care beds and 34 special care beds. It is located within a children’s hospital and is responsible for providing care for inborn babies and outborn babies requiring transfer for specialist care. The unit averages 1,400 admissions per year which include premature infants born from 23 weeks as well as infants with congenital surgical and cardiac conditions.
Interventions
QI Team.
Following identification of BCC rates as a concern as a concern, a multi-disciplinary QI team was established to address the issue. This working group included neonatologists, advanced neonatal trainees (fellows), pediatric residents, neonatal nurse practitioners, nurses, midwives, nurse educators, infection control staff, and an infectious diseases specialist.
Audit and Process Mapping.
An audit of blood cultures, review of current practice, and literature review were conducted between December 2018 and February 2019. A process map outlining how blood cultures were collected within the unit was developed, and at each step, observed variations in practice were discussed (Supplemental Digital Content 1, http://links.lww.com/PQ9/A255). Aspects of blood culture collection potentially contributing to BCC were reviewed and used to develop a cause and effect diagram (Supplemental Digital Content 1, http://links.lww.com/PQ9/A255). A multivoting and weighted voting process was used to narrow the possible factors contributing to BCC, to a total of 6. These included: not cleaning blood culture bottles, not changing needles before inoculating blood culture bottles, inadequate skin antisepsis, repalpating the skin, poor understanding of aseptic technique by the residents, and insufficient blood volumes. The 6 factors became the basis of the 4 interventions [Plan-Do-Study-Act (PDSA)cycles 1–4].
PDSA Cycle 1: Standardizing Processes for Inoculation of Blood Culture Bottles
The unit did not have a protocol for handling and inoculating blood cultures bottles, and as a result, practices between staff members varied. The rubber stoppers of blood culture bottles are not sterile.8,20 The CAP Q-Probes study showed a significantly lower rate of contamination in hospitals where the antiseptic was applied to the tops of blood culture bottles before innoculation.22 A meta-analysis concluded that the double-needle technique decreased contamination rates from 3.7% to 2.0%.15 In the past, this practice has been discouraged due to the risk of needle stick injuries. In April 2019, a practice change guideline to standardize how blood cultures were handled and inoculated was introduced (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256). A pilot was conducted with 10 staff members to ensure that the new protocol was clear and easy to follow before rolling out to the neonatal unit.
PDSA Cycle 2: Standardizing Preparation of Skin for Peripheral IV Cannula Insertion
The neonatal unit uses 1% Chlorhexidine and 70% isopropyl alcohol swabs as part of the standard aseptic technique. On many occasions, the duration of cleaning and drying time were insufficient. In discussion with the local infection control team, and following a literature review, it was agreed that 1% Chlorhexidine and 70% isopropyl alcohol swabs were appropriate, and its use continued. This conclusion was supported by a meta-analysis of 6 randomized controlled trials that concluded alcohol-containing products were associated with low rates of contamination.23 Traditionally, skin antiseptics have been applied in concentric circles; however, Stonecypher24 suggested that cleaning in a back and forth motion creates friction that cleans more effectively and reduces bacteria load of the dermal layer. In June 2019, a practice change guideline to standardize the method by which skin is prepared for peripheral IV cannulation (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256) was introduced.
PDSA Cycle 3: Aseptic Technique Education Package
During initial observational audit and surveys, limited understanding of aseptic technique amongst the medical staff was identified. The team developed an education package including a presentation, pamphlet (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256), and poster. The presentation is now a regular component of orientation for all new and returning resident medical staff. Each new staff member is now required to demonstrate understanding and performance of aseptic technique. This same education package is included in orientation for all new nursing and midwifery staff members. This education material was developed through a series of small pilots with medical staff members during June and July 2019. Staff were asked to answer a series of questions about aseptic technique before and after the education package to ensure that it had its desired effect (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256).
PDSA Cycle 4: Optimizing Volume of Blood Collected for Culture
There was significant variation in what was considered adequate volume of blood to collect for a culture. During the observational audit period, volumes of 0.2–1.5 mL were collected. It has been clearly demonstrated that the volume of blood collected is a key variable in detecting true bacteremia.2 The recommendation is for a minimum of 1 mL of blood to be obtained from infants with suspected sepsis before initiating antimicrobial therapy.25,26 Blood culture sensitivity decreases by 10%–40% when 0.5 mL is inoculated compared to 1 mL.27 Gonsalves et al28 demonstrated in a retrospective study of infants and children with at least 1 blood culture collected, that higher rates of contamination occurred at lower blood collection volumes. A change of practice was introduced with the aim to collect 1 mL from all neonatal patients. Although the goal was for 1 mL from all babies weighing less than 1,000 g, the minimum collection volume could be lowered to 0.5 ml (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256).
The team developed a neonatal blood culture sticker for staff to record the patient’s details, weight, and blood culture volume obtained (Supplemental Digital Content 2, http://links.lww.com/PQ9/A256). Additionally, a blood culture record book was created to allow monitoring of total aggregate volumes collected.
Studies of the Interventions
BCC rates were reviewed monthly, which allowed for assessing the impact of the interventions on the primary outcome measure. Observational audits were conducted regularly as process measures for each intervention and PDSA cycle. This provided opportunities to address staff concerns or questions regarding the practice changes. Circumstances surrounding each BCC event were reviewed.
Measures
Outcome Measures.
The primary outcome measure was the rate, reported as a percentage, of contaminated blood cultures. This was calculated for each month. Overall rates for the baseline period (October 2017 to April 11, 2019) were calculated. The study period was from April 12, 2019 to February 29, 2020. Study period BCC inclusion points were determined by P chart SPC rules and BCC rates determined, based on those data points.
A blood culture was classified as contaminated if it grew a microorganism commonly accepted as normal skin flora and appears on the Center for Disease Control list of known contaminants.29 Data for the period of October 2017 to March 2019 were identified through a list of all the blood cultures obtained from the local clinical information system. This list was manually reviewed to identify all positive blood cultures and cross-checked with the data recorded by infection control regarding positive blood cultures. For the study period, individual patient records were reviewed daily to record the number of blood cultures taken and identify positive results.
Balancing Measures.
Number of skin burns, unsuccessful blood culture sampling events, or any other event related to the process changes reported through our local electronic safety learning system.
Process Measures.
Compliance with the standard process for inoculation of blood culture bottles (PDSA cycle 1), and for the preparation of skin for peripheral IV cannula insertion (PDSA cycle 2) were assessed with observational audits of 20 individual blood culture collection events. For PDSA cycle 1, the compliance rate was determined based on compliance with 4 key steps in the process preintervention, and 3 key steps postintervention. For PDSA cycle 2, the compliance rate was determined based on compliance with the 4 key steps in the process. Feedback on the usability and satisfaction with the new protocols were sought. The aseptic technique education package effectiveness (PDSA cycle 3) was used as a process measure. It was assessed through a prepackage and postpackage test that staff completed. These 2 tests were identical and contained questions about the material presented and how aseptic technique is applied in the neonatal unit. Compliance with the recommended volume of blood collected for culture (PDSA cycle 4) was the primary process measure for this cycle.
Missing Data.
Data collection for the baseline period and study period was complete.
Analysis
Statistical process control methods were used for this study.30 Statistical process control (P chart) analyses were performed by using the QI Macros package in Microsoft Excel 2020 (KnowWare International Inc, Denver, Colo.). Center line (mean) and control limits were calculated using statistical process control methods that conform to P chart primary assumptions. Special cause for centerline shift was defined as 8 consecutive points either above or below the centerline.
Ethical Considerations.
Ethics approval was obtained from the Women’s and Children’s Hospital Network Human Research Ethics Committee (WCHN HREC) as part of a QI program (HREC approval number 1030A/6/2021).
RESULTS
Process Measures
Process for Inoculating Blood Culture Bottles (PDSA Cycle 1).
For PDSA cycle 1, audit findings (n = 20) indicated a mean level of compliance with the standardized practices at 50%. After implementing the standardized process, compliance with the standard process, including blood culture bottle cleaning and use of the transfer device was 100%.
Preparation of Skin for Peripheral IV Cannula Insertion (PDSA Cycle 2).
For PDSA cycle 2, audit findings (n = 20) indicated a mean level of compliance with the 4 better practices at 50%. After the implementation of PDSA cycle 2, compliance increased to 95%.
Aseptic Technique Education Package (PDSA Cycle 3).
After implementation of the education package, scores on the test administered increased from a mean of 39% (pretraining test; n = 10) to 92% (posttraining test; n = 10) (P < 0.001).
Blood Volume Collected For Culture (PDSA Cycle 4).
For this PDSA cycle, an audit found a range of 0.2–1.5 mL of blood was obtained per culture (baseline volume). Following this PDSA cycle, a minimum of 1 mL of blood was collected in 94% of blood culture collection events (n = 450; mean 1.1 mL; range 0.5–3.5 mL).
Balancing Measures.
No skin burns associated with blood culture sampling process changes or unsuccessful blood culture sampling events occurred. No other adverse events were reported through our local electronic safety learning system related to this QI initiative.
Outcome Measures.
During the study period (April 12, 2019 to February 29, 2020), 1,110 blood cultures were obtained. Ten cultures were deemed contaminants equating to an overall contamination rate of 0.90%. The baseline (October 2017 to April 11, 2019) contamination rate was 2%. Monthly rates of contamination for the entire project time-period (October 2017 to June 2020) are displayed on a P chart (Fig. 1). Although the study period began on April 12, 2019, SPC chart rules for centerline shift (special cause) were not met until May 2019, approximately 1 month after implementation of all the PDSAs.
Fig. 1.
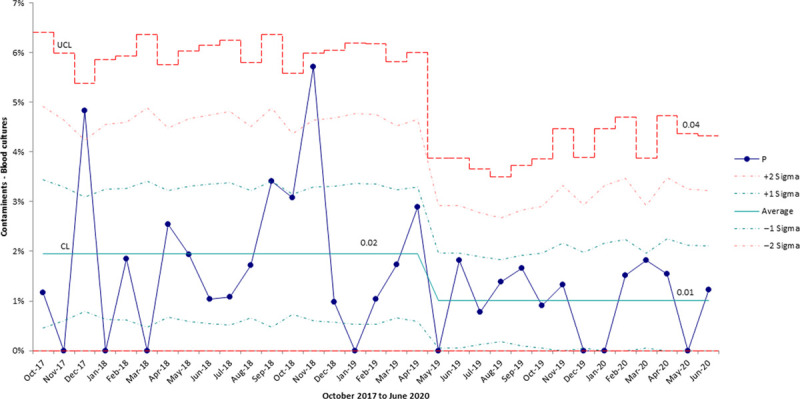
P chart displaying the proportion of contaminated blood cultures in the neonatal unit.
DISCUSSION
This QI study successfully standardized how blood culture bottles were handled including introducing a transfer unit, standardization of preparation of skin for peripheral intravenous cannula insertion, improving knowledge and understanding of aseptic technique, and optimizing blood volume collected for cultures. These interventions led to an overall BCC rate decrease from 2.0% during the baseline period to 0.90% in the study period (>50% reduction). Acknowledging that the baseline BCC rate was already low, we believed there was room for improvement and the success of this QI initiative demonstrated this. It was an invaluable opportunity to introduce QI processes to our neonatal unit.
The change in culture within the unit regarding maintaining asepsis for blood culture collection has had extremely positive effects throughout the unit. There is now a heightened awareness of the importance of asepsis with all interventions, from inserting central lines to accessing intravenous devices for medications.
Interpretation
This QI initiative has demonstrated that standardizing practices and improving staff knowledge and education about blood culture collection is achievable and can have a significant impact on the incidence of BCC events. We demonstrate that it is feasible to achieve extremely low BCC events, even when the baseline BCC rate is low. Our current BCC rate of less that 1% compares favorably to the other studies in neonatal populations, demonstrating that BCC occurs with rates of between 2.6% and 18%.9–12
Limitations
There are several limitations of this study, including being a single-center study as well as the possibility that blood cultures could have been incorrectly identified. Although each positive blood culture was investigated and the case records and charts reviewed by a minimum of 2 clinicians, there is a possibility that either true sepsis was labeled a contaminant or alternatively that a contaminant was labeled as sepsis. The same contamination definition was used throughout the baseline and study periods, minimizing the risk that the definition affected the change in observed contamination rates. All positive blood cultures are now discussed at the weekly joint neonatal and infectious diseases meeting and classified formally as either true or contaminants. There is a risk of association bias within this study. This was identified early and minimized by clearly identifying the definition of a BCC event before commencing classification of specific blood culture results. Due to the need to collect data by manually reviewing patient charts, there is a chance that data could have been missed. This was minimized by comparing the data collected manually with the data obtained by infection control. Although the indications for collecting blood cultures did not change during the study, there is the potential that the demographics differed between the baseline and intervention periods.
Strengths
This study represents the first formal QI project within our neonatal unit that has been successfully completed. It has allowed us to share our experiences with our colleagues in neonatal units in Australia and New Zealand and to demonstrate that very low rates of BCC are possible in neonates.
CONCLUSIONS
This QI study showed that it is possible to improve clinical practices relating to blood culture collection and achieve high compliance rates with practice change.
ACKNOWLEDGMENTS
The authors wish to thank the members of the QI team for their contributions, Dr Chad Andersen as the Head of Unit for his support and Dr Erin Grace for her assistance with data collection.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Allen E, Cavallaro A, Keir AK. A Quality Improvement Initiative to Reduce Blood Culture Contamination in the Neonatal Unit. Pediatr Qual Saf 2021;6:e413.
Published online May 19, 2021
Disclosure A/Professor Keir receives support from a National Health and Medical Research Council (NHMRC) Fellowship (APP1161379). The views expressed in this article are solely the authors’ responsibility and do not reflect the views of the NHMRC. The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017; 390:1770–1780. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. Principles and Procedures for Blood Culture, Approved Guidelines. 1st ed. Clinical and Laboratory Standards Institute; 2007:67. [Google Scholar]
- 3.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011; 77:233–236. [DOI] [PubMed] [Google Scholar]
- 4.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991; 265:365–369. [PubMed] [Google Scholar]
- 5.Alahmadi YM, McElnay JC, Kearney MP, et al. Tackling the problem of blood culture contamination in the intensive care unit using an educational intervention. Epidemiol Infect. 2015; 143:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self WH, Talbot TR, Paul BR, et al. Cost analysis of strategies to reduce blood culture contamination in the emergency department: sterile collection kits and phlebotomy teams. Infect Control Hosp Epidemiol. 2014; 35:1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gander RM, Byrd L, DeCrescenzo M, et al. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009; 47:1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin LM, Inglis GD, Hoellering AB, et al. Relationship between blood culture collection method and proportion of contaminated cultures in neonates. J Paediatr Child Health. 2013; 49:105–108. [DOI] [PubMed] [Google Scholar]
- 10.Silva HL, Strabelli TM, Cunha ER, et al. Nosocomial coagulase negative staphylococci bacteremia: five year prospective data collection. Braz J Infect Dis. 2000; 4:271–274. [PubMed] [Google Scholar]
- 11.Krajčinović SS, Doronjski A, Barišić N, et al. Risk factors for neonatal sepsis and method for reduction of blood culture contamination. Malawi Med J. 2015; 27:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton LF, Gillett HE, Smith-Collins A, et al. A sterile collection bundle intervention reduces the recovery of bacteria from neonatal blood culture. Biomed Hub. 2018; 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modi N, Doré CJ, Saraswatula A, et al. A case definition for national and international neonatal bloodstream infection surveillance. Arch Dis Child Fetal Neonatal Ed. 2009; 94:F8–F12. [DOI] [PubMed] [Google Scholar]
- 14.Feghaly RE, Chatterjee J, Dowdy K, et al. A quality improvement initiative: reducing blood culture contamination in a children’s hospital. Pediatrics. 2018; 142:e20180244. [DOI] [PubMed] [Google Scholar]
- 15.Spitalnic SJ, Woolard RH, Mermel LA. The significance of changing needles when inoculating blood cultures: a meta-analysis. Clin Infect Dis. 1995; 21:1103–1106. [DOI] [PubMed] [Google Scholar]
- 16.Kim NH, Kim M, Lee S, et al. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann Intern Med. 2011; 154:145–151. [DOI] [PubMed] [Google Scholar]
- 17.Rupp ME, Cavalieri RJ, Marolf C, et al. Reduction in blood culture contamination through use of initial specimen diversion device. Clin Infect Dis. 2017; 65:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Self WH, Speroff T, Grijalva CG, et al. Reducing blood culture contamination in the emergency department: an interrupted time series quality improvement study. Acad Emerg Med. 2013; 20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson S. Blood culture contaminants. J Hosp Infect. 2014; 87:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control. 2015; 43:1222–1237. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins K, Huynh S, McNary C, et al. Reducing blood culture contamination rates: a systematic approach to improving quality of care. Am J Infect Control. 2013; 41:1272–1274. [DOI] [PubMed] [Google Scholar]
- 22.Schifman RB, Strand CL, Meier FA, et al. Blood culture contamination: a College of American Pathologists Q-Probes study involving 640 institutions and 497134 specimens from adult patients. Arch Pathol Lab Med. 1998; 122:216–221. [PubMed] [Google Scholar]
- 23.Caldeira D, David C, Sampaio C. Skin antiseptics in venous puncture-site disinfection for prevention of blood culture contamination: systematic review with meta-analysis. J Hosp Infect. 2011; 77:223–232. [DOI] [PubMed] [Google Scholar]
- 24.Stonecypher K. Going around in circles: is this the best practice for preparing the skin? Crit Care Nurs Q. 2009; 32:94–98. [DOI] [PubMed] [Google Scholar]
- 25.Cantey JB, Baird SD. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017; 140:e2017004. [DOI] [PubMed] [Google Scholar]
- 26.Polin RA; Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012; 129:1006–1015. [DOI] [PubMed] [Google Scholar]
- 27.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996; 129:275–278. [DOI] [PubMed] [Google Scholar]
- 28.Gonsalves WI, Cornish N, Moore M, et al. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol. 2009; 47:3482–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN). CDC Master Organisms List. 2019. Available at: www.cdc.gov/nhsn/XLS/master-organism-Com-Commensals-Lists.xlsx. Accessed March 1, 2019.
- 30.Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003; 12:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


