Supplemental Digital Content is available in the text.
Introduction:
Critical illness results in physical impairments which may be mitigated by intensive care unit (ICU)-based early mobility. This initiative aimed to develop and implement ICU-based mobility guidelines for critically ill children.
Methods:
A multidisciplinary team developed and implemented ICU-based mobility guidelines. Guideline implementation success was determined by comparing utilization of physical (PT) and occupational therapies (OT) and changes in functional status scale scores in preimplementation and postimplementation cohorts. The team also assessed barriers and adverse events.
Results:
Thirty-four patients were identified preimplementation and 55 patients postimplementation. PT/OT consultation by 72 hours occurred in 44 (81.5%) of patients postimplementation compared to 6 (17%) preimplementation (P < 0.001). Implementation did not result in more ICU-based therapy sessions or shorter time to active therapies. High deferral rates for PT/OT sessions [PT: n = 72 (46.2%) preimplementation versus 112 (39.4%) postimplementation; OT: n = 71 (46.1%) preimplementation versus 134 (41.5%) postimplementation] occurred. No difference in new morbidity between cohorts was identified. Barriers to treatment included the patient’s sedation status, severity of illness, and patient availability.
Conclusions:
Implementation of ICU-based mobility guidelines resulted in a 4-fold increase in PT/OT consultation. They did not result in increased treatment sessions due to frequent deferrals. Future guidelines should focus on interventions to address identified barriers to treatment in a critically ill pediatric population.
INTRODUCTION
Children with critical illness are at risk of developing worsening functional status during their hospitalization that persists after discharge, known as postintensive care syndrome (PICS).1–4 In addition to comorbidities and critical illness, iatrogenic complications of common intensive care unit (ICU) medications and immobility contribute to physical impairments during critical illness.5,6
ICU-based mobility programs focus on increasing mobility for critically ill patients to reduce the impact of PICS and promote a return to prior physical baseline.7,8 In most studies, “early” mobility occurs within 72 hours of admission as muscle wasting begins within this time frame.9 Many centers have successfully implemented guidelines for mobility in adult and pediatric ICUs.10–14 In adults, these initiatives lead to many benefits including decreased delirium, shortened length of stay, and improved emotional health without compromising safety.10–13,15 Although studies suggest feasibility and safety of early mobility programs in the pediatric ICU (PICU), data showing improved outcomes for children are lacking and further research is needed.8,16
With increasing evidence of the benefit of early mobility in critically ill patients, our PICU team performed an assessment of their current physical therapy (PT) and occupational therapy (OT) practices. Low utilization of PT and OT with high therapy session deferral rates was identified, showing a need for improvement.17 Also, families reported daily living activities and physical mobility as highly valued patient outcomes.18 We therefore, aimed to develop and implement ICU-based mobility guidelines through a quality improvement (QI) initiative. This initiative’s primary aim was to safely increase ICU mobility activities by implementing a unit-based guideline and assessing barriers to such implementation.
METHODS
Context
ICU-based mobility guideline implementation occurred in the 36-bed medical-surgical PICU at the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, a tertiary care center. The ICU team includes residents, fellows, nurse practitioners, attending physicians, bedside nurses, and respiratory therapists. Physical therapists and occupational therapists are shared among all inpatient hospital units in the 315-bed hospital. Therapists utilize a team-based approach Monday-Saturday, performing evaluations together and developing an individualized plan of care. For patients who cannot participate actively, therapists provide treatment 1–2 times per week per the American Physical Therapy Association’s recommendations for the acute care environment.19 This QI initiative was approved by the University of Pittsburgh Medical Center Quality Improvement Review Committee.
Intervention
ICU-based Functional Mobility Guidelines.
To develop an evidence-based guideline focused on ICU-based PT and OT, we convened a multidisciplinary team to review evidence, evaluate current practices, and consider barriers in our ICU. The multidisciplinary team included stakeholder representatives from the PICU physician staff, nursing staff, physical therapists, occupational therapists, respiratory therapists, and rehabilitation medicine physicians.
The team developed a consensus opinion on factors necessary for ICU-based mobility guidelines for our unit. The team focused on early and appropriate therapies for all patients in the PICU, with specific consideration for illness severity. The team delineated appropriate patient eligibility for passive and active therapies based on illness and current ICU organ support through a novel clinical classification tool. This tool defined 4 clinical classifications categories based on organ dysfunction and ICU therapies. A clinical classification category of 1 indicates the lowest severity of illness and a clinical classification category of 4 indicates the highest severity of illness. The clinical classifications of patient status were derived from a previous randomized controlled trial of early rehabilitation for neurocritical care patients.8 The goals of using this clinical classification tool were to enhance communication between providers, encourage consistent decision-making among various disciplines, and provide appropriate therapies to facilitate safe yet effective therapy for patients. Examples of clinical classification by patient organ dysfunction and the appropriate associated therapy are found in Figure 1. Full details are found in eFigure 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A257.
Fig. 1.
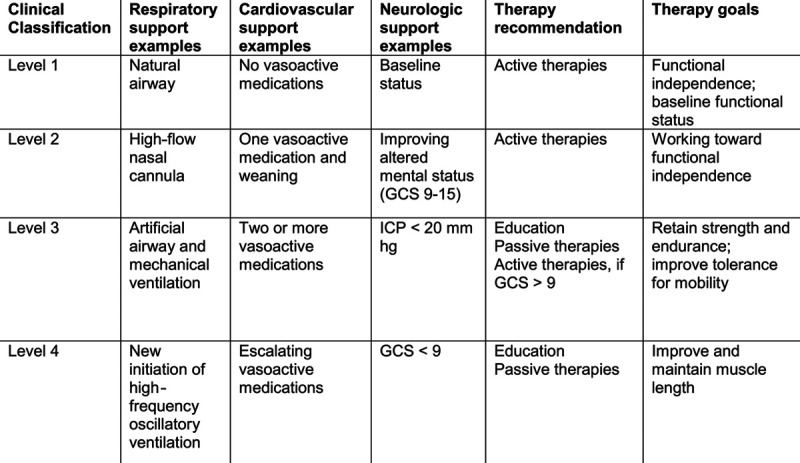
Clinical classification categories based on patient severity of illness with examples of qualifying organ dysfunction and intensive care therapy utilization and recommended PTs and OTs. GCS, Glasgow Coma Scale; ICP, intracranial pressure.
Our final guidelines focused on ease and timeliness of consultations. Specifically, our guidelines suggested that all patients in the ICU have a PT and OT consultation and evaluation within 72 hours of admission. To facilitate this, we developed an automatic order embedded within the PICU admission order set in the electronic medical record (EMR). Clinicians could also place a PT or OT consultation order on any patient sooner than 72 hours if clinically indicated. Implementation of the guidelines and the automatic order set occurred on March 1, 2018.
Unit Education
The multidisciplinary team identified education for staff including physicians, trainees, nurses and therapists as a crucial to successful implementation. Therefore, a robust month-long educational effort occurred during February 2018 intending to educate staff on the benefits and safety of ICU mobility to promote buy-in and culture change of all staff.20 Physician education occurred during QI meetings involving detailed presentations and through email communications with guideline updates throughout the implementation phase. Nursing education occurred in person through PowerPoint presentations during morning debriefings and journal club presentations of recent publications focused on ICU mobility by study personnel and a nurse manager. Nursing educational credits were applied for attendance. All educational sessions occurred in a closed conference room and attendance was recorded and reviewed by study personnel. Nurses unable to attend group sessions received one-on-one bedside education. OTs and PTs received training during departmental meetings. Formal guidelines were distributed to all staff and were available online through an internal site.
Data Collection and Interventions
Assessment of this initiative involved preimplementation data collection from October to November 2017 and postimplementation data collection from April to May 2018. The team collected data prospectively on all patients admitted to the pediatric ICU for greater than 72 hours. Exclusion criteria included ICU length of stay of less than 72 hours. Data collected on patients included demographic information of age in years, race, and gender. Characteristics of the hospitalization collected from the EMR included: primary reason for admission defined by organ system as respiratory disease, cardiovascular disease, gastrointestinal disease, neurologic disease, oncologic disease, or sepsis/septic shock; source of admission classified as emergency department, outside hospital transfer, inpatient transfer, operating room or other; length of ICU stay in days; hospital length of stay in days and discharge disposition classified as home, long-term care facility, skilled nursing facility, hospice care, inpatient rehabilitation, or death. Severity of illness was captured at 72 hours of admission and classified based on the guideline clinical classification tool (Fig. 1). The team collected mechanical ventilation utilization at 72 hours, defined as intubation or tracheostomy on ventilator support.
Assessment of preillness baseline, admission, and discharge functional status was performed using the functional status scale (FSS). The FSS is a measure of functional outcomes and specifically assesses respiratory status, feeding function, motor function, communication, sensory function, and mental status.21 This assessment was performed using a parental report of patient baseline functional status and EMR review for admission and discharge functional status.
Evaluation of PT and OT utilization included the date of PT and OT consultations, the number of PT and OT sessions per patient during the hospitalization, and the number of reasons for PT and OT deferrals in the ICU. A deferral indicates a physical therapist or occupational therapist approached the patient for a therapy session, but the session was not initiated. The team collected time to the first active therapy, including active range of motion, in-bed mobility, side-of-bed sitting, out-of-bed sitting, transfers, righting and balance skills, and pregait skills and ambulation (eTable 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A257).
Adverse safety events (ASEs) during therapy sessions were defined as loss of vascular access, dislodgement of endotracheal tube, falls, or any significant change in vital signs requiring cessation of therapy session. The development of deep thromboembolism and pressure ulcers were also noted. A timeline of our study events is available in eFigure 2, Supplemental Digital Content 1, http://links.lww.com/PQ9/A257.
Measures
The primary outcome was PT and OT consultation measured as the percentage of patients with PT or OT consultation by 72 hours of admission. Secondary outcome measures included the number of PT and OT sessions per patient, PT and OT session deferral rate (measured as a ratio determined by the number of PT and OT sessions completed during their hospitalization divided by the number of PT and OT sessions attempted during hospitalization), PT and OT outpatient referrals, and discharge to an inpatient rehabilitation unit. In addition, time to active therapy in days was assessed for patients with a clinical classification of 3 and 4. These patients include mechanically ventilated patients who historically have not received active therapy in our ICU.
The team evaluated the development of new morbidity as defined by an increase of three or more in FSS at hospital discharge from pre-morbid baseline based on parental report.2,22 Any increase in FSS, ICU and hospital length of stay were also evaluated.
Barrier Assessment
As a part our QI process, barriers to adherence were assessed. Therapists documented the number of and reasons for deferrals in the EMR to inform future guidelines. Source of deferral was classified as either caregiver/parent, nursing staff, therapist, or patient availability if deferral was related to the patient not receiving therapy due to a procedure, testing, or otherwise physically outside of the ICU. We utilized this information to develop a key driver diagram to inform the next cycle of our initiative.
Statistical Analysis
Patient demographics and hospitalization characteristics were compared using chi square analysis or Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. A regression analysis was performed to determine the impact of implementing change in functional status adjusting for severity of illness based on the clinical classification score, baseline functional status, and patient age. All statistical tests were 2-sided, and significance was set at P < 0.05. All analyses were performed using STATA version 15.0 (StataCorp, College Station, Tex.).
Ethical Considerations
During the development of the guidelines, the implications of increased PT and OT consultations on hospital resource utilization were considered, as therapists are shared between all units. After review of current evidence, hospital leadership determined that the benefit of therapy justified the increased utilization of therapists in the ICU.
RESULTS
Overall, 34 patients during the preimplementation phase and 55 patients during the postimplementation phase met criteria for inclusion. Table 1 outlines demographic comparisons between preimplementation and postimplementation cohorts. Notably, patients in the preimplementation cohort were older, but did not differ in gender or race (10 versus 5 years, P < 0.001). The most common reason for admission was a respiratory disorder, and approximately half of all patients required mechanical ventilation at 72 hours, regardless of cohort. Sixteen patients in the preimplementation cohort (47.1%) and 31 patients (56.4%) in the postimplementation cohort had a clinical classification of 3 or 4 at 72 hours of admission indicating moderate–high severity of illness.
Table 1.
Patient Characteristics by Preimplementation and Postimplementation of ICU-based Mobility Guidelines
| Characteristic | Preimplementation (n = 34) | Postimplementation (n = 55) | P |
|---|---|---|---|
| Age in years, mean ±SD | 9.9 ± 9.0 | 5.0 ± 5.6 | <0.001 |
| Female | 15 (44.1) | 27 (49.1) | 0.65 |
| Race | 0.45 | ||
| White | 25 (73.5) | 41 (77.4) | |
| Black | 5 (14.7) | 6 (11.3) | |
| Hispanic | 1 (2.9) | 4 (7.6) | |
| Other | 3 (8.8) | 8 (14.5) | |
| Admission source | 0.12 | ||
| Emergency department | 17 (50) | 21 (38.2) | |
| Inpatient transfer | 10 (29.4) | 11 (20.0) | |
| Outside hospital | 6 (17.7) | 10 (18.2) | |
| Operating room | 1 (2.9) | 13 (23.6) | |
| Primary admission diagnosis | 0.10 | ||
| Respiratory disorder | 19 (55.9) | 29 (53.7) | |
| Neurologic disorder | 8 (23.5) | 3 (5.6) | |
| Sepsis | 4 (11.8) | 8 (14.8) | |
| Gastrointestinal disorder | 2 (5.9) | 11 (20.4) | |
| Cardiac disorder | 1 (2.9) | 2 (3.7) | |
| Oncologic disorder | 0 (0.0) | 1 (1.9) | |
| Mechanical ventilation at 72 h | 17 (50.0) | 25 (46.3) | 0.93 |
| Clinical classification score at 72 h | 0.66 | ||
| 1 | 8 (23.5) | 9 (16.4) | |
| 2 | 10 (29.4) | 15 (27.3) | |
| 3 | 14 (41.2) | 24 (43.6) | |
| 4 | 2 (5.9) | 7 (12.7) |
All data represented as N (%) unless indicated.
Consultation of PT and OT by 72 hours occurred in 44 (81.5%) of patients in the postimplementation cohort compared to 6 (17%) in the preimplementation cohort (P < 0.001). However, early consultation to PT did not increase the number of ICU-based PT sessions (2.5 ± 3.3 versus 3.2 ±2.8, P = 0.44) or OT sessions (2.4 ± 3.3 versus 2.5± 3.2, P = 0.53) (Table 2). Deferral rates were similar between groups. A summary of reported indications for PT or OT deferrals in the postimplementation phase are displayed in Table 3. The majority of deferrals were related to patients sleeping (n = 13), being temporarily unavailable (n = 12), or high severity of illness (n = 11). A second attempt at therapy occurred 21.3% of the time after a deferral during the preimplementation phase and 29.4% postimplementation (P = 0.20).
Table 2.
PT and OT Utilization during Hospitalization and Outcomes Preimplementation and Postimplementation of ICU Functional Mobility Guidelines
| Preimplementation | Postimplementation | P | |
|---|---|---|---|
| PT and OT consult order at 72 h | 6 (17.7) | 44 (81.5) | <0.001 |
| Clinical Classification Score at initial PT and OT evaluation | |||
| No evaluation | 16 (48.5) | 3 (5.9) | <0.001 |
| 1 | 7 (21.2) | 9 (17.7) | |
| 2 | 2 (6.1)) | 16 (31.4) | |
| 3 | 7 (21.2) | 18 (35.3) | |
| 4 | 1 (3.0) | 5 (9.8) | |
| Deferred PT sessions | 72 (46.2) | 112 (39.4) | 0.23 |
| Deferred OT sessions | 71 (46.1) | 134 (41.5) | 0.62 |
| Days till active PT from time of consultation, median (IQR) | 3.5 (2, 23.5) | 1 (1, 5) | 0.33 |
| Days till active OT from time of consultation, median (IQR) | 2.5 (1, 4) | 2 (1, 5.5) | 0.81 |
| PT and/or OT recommended at discharge by therapist | 14 (41.2) | 32 (61.8) | 0.06 |
| Discharge to inpatient rehabilitation center | 1 (2.9) | 4 (7.4) | 0.38 |
All data represented as n (%) unless indicated.
IQR, interquartile range.
Table 3.
Documented Reasons for PT or OT Deferral Postimplementation of ICU Functional Mobility Guidelines
| Source | No. Deferrals | Summary of Reasons | Examples |
|---|---|---|---|
| Nursing | 28 | Patient sleeping (n = 13) Patient agitated (n = 8) Severity of illness too high (n = 5) | “Nursing deferred as patient not medically appropriate” “Nursing deferred as she was turning off the patient’s paralytic at this time” “Patient desatting…and bradycardic with care per nursing” |
| Unavailable | 15 | Patient temporarily out of ICU (n = 7) Beside procedure (n = 5) Patient discharged from PICU (n = 3) | “Off floor for barium swallow” |
| Therapist | 10 | Severity of illness too high (n = 3) PT or OT not indicated (n = 6) | “Unable to ambulate secondary to high ventilator settings” “No PT or OT concerns” |
| Parental | 8 | Parent declined (n = 4) Therapy not needed (n = 1) Parent providing therapy (n = 3) | “Parent states therapy not needed” “Parent reports no issues with dependent transfer or recliner at this time…” |
ASEs were similar between groups and are reported in eTable 2, Supplemental Digital Content 1, http://links.lww.com/PQ9/A257. The most common ASE was change in vital signs requiring discontinuation of therapy. Notably, no loss in vascular access or endotracheal tube displacement occurred.
Implementation of an ICU-based mobility program did not reduce the development of new morbidity at hospital discharge [4 patients (12.9%) in the preimplementation group versus 4 patients (7.8%) in the postimplementation group; P = 0.42]. In addition, there was no significant difference in PICU or hospital length of stay, or duration of mechanical ventilation (Table 4). After risk-adjustment for patient age, baseline functional status, and clinical classification score, there was still no association between implementation cohort and new morbidity [odds ratio (OR) 0.22, confidence interval (CI): 0.035–1.38, P = 0.12].
Table 4.
Patient Outcomes Preimplementation and Postimplementation of ICU Functional Mobility Guidelines
| Patient Outcome | Preimplementation (n = 34) | Postimplementation (n = 55) | P |
|---|---|---|---|
| PICU length of stay in days, median (IQR) | 8 (6, 17) | 9 (6, 20) | 0.71 |
| Hospital length of stay in days, median (IQR) | 12 (8, 23) | 17 (9, 28) | 0.42 |
| Mechanical ventilation days, median (IQR) | 7 (5, 15) | 7 (5, 10) | 0.73 |
| Mortality, n (%) | 3 (8.8) | 1 (1.8) | 0.15 |
| Preillness functional status scale, median (IQR) | 7.5 (6, 14) | 10 (6, 16) | 0.37 |
| Hospital admission functional status scale, median (IQR) | 18 (14, 23) | 17 (13, 21) | 0.24 |
| Hospital discharge functional status scale, median (IQR) | 11 (6, 14) | 11 (6, 17) | 0.37 |
| Any increase in functional status scale*, n (%) | 7 (22.6) | 8 (15.7) | 0.34 |
| New morbidity*, n (%) | 4 (12.9) | 4 (7.8) | 0.30 |
*n = 31 in preimplementation and 54 in postimplementation due to mortality exclusion.
IQR, interquartile range.
Finally, increased PT and OT consultation during ICU admission did not significantly increase referrals to outpatient PT and OT with 41% receiving referrals before implementation and 62% receiving referrals after implementation (P = 0.06). There was also no difference in discharge to inpatient rehabilitation between the preimplementation (n = 1) and postimplementation (n = 4) groups (P = 0.38).
DISCUSSION
Implementation of ICU-based mobility guidelines resulted in PT and OT consultation for four out of every 5 patients by 72 hours of admission in our ICU. Despite earlier consultation, guideline implementation did not increase the number of PT and OT sessions per ICU day due to high deferral rates. In our cohort, early consultation did not decrease time to active therapies, which are most likely to improve functional limitations.
Our study has several important findings. First, although implementation of guidelines increased early access to PT and OT through earlier consultations, patients did not receive more therapy sessions in the setting of frequent deferrals. The deferral rate was similar to a prior study of PT and OT in our ICU.17 A common reason for deferral was high severity of illness. Our clinical guidelines addressed the severity of illness and provided appropriate therapies for patients with high severity of illness. Given this disparity, further education is necessary to ensure patients receive treatment. Another common reason for deferral was the sedation status of the patient. Sedation is a barrier to early mobilization and likely impacted adherence to our guidelines.23 Sedation in a critically ill pediatric population differs from adult populations because of developmental status, safety concerns, and ability to assess pain and anxiety in young or neurologically impaired children.24 Improved sedation practices to safely provide comfort while allowing for interaction are crucial to mobility facilitation. Specifically, developing a protocol for a reduction in sedation before therapy may be beneficial in appropriate scenarios.
Another barrier to increased therapy was deferrals for patients unable to participate when therapists arrived, due to ongoing procedures or imaging. In the majority of cases, therapists were unable to return to the bedside later in the day to complete the therapy session. During our initiative, we maintained the same number of therapists (a ratio of 1 therapist per 60 hospital beds) despite an increase in consultations. Therapists are often a shared resource throughout children’s hospitals which may lead to inadequate staff availability to mobilize patients safely.10 Other programs have recognized this barrier and addressed it differently.12 First, increased staffing of ICU-dedicated therapists has been utilized in some institutions leading to increased availability.25,26 The cost-saving effect of early mobility may provide an opportunity to increase financing for needed staff.27 Second, the development of nursing-driven protocols and mobility activities have facilitated early mobility in ICUs constrained by limited therapists.28 Nursing-driven protocols require increased training but benefit patients through increased accessibility and enhance cultural change through nursing empowerment.14 In addition to nursing-driven protocols, family-focused interventions may increase acceptance of early therapy and satisfaction in patient care. Family and patient stakeholders were not included in our initial process and future development of our guidelines will benefit significantly from their input.
In our small cohort, there was no reduction in the development of new morbidity following implementation of our guidelines. Despite no improvement in functional status, earlier and protocolized consultation of OT and PT did result in a trend toward increased outpatient referral for OT and PT services. The increase in referral may indicate an earlier recognition of functional deficits and more long-term follow-up may be necessary to see the full benefit of therapy. Future cycles of our initiatives will focus on improving our guidelines through factors identified and displayed in a key driver diagram (Fig. 2).
Fig. 2.
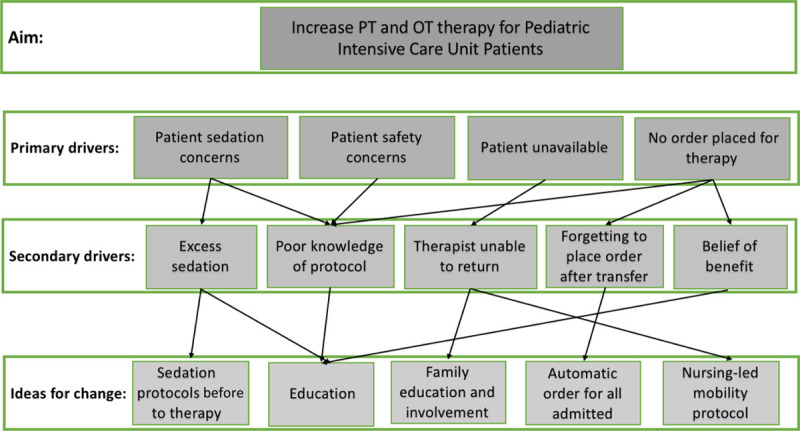
Key driver diagram describing targeted changes for the next cycle of ICU-based mobility initiative.
There are several limitations to our study. First, although our initiative led to increased consultation of PT and OT in the ICU, it did not lead to increased therapy sessions, decreased time to active therapies, or decreased morbidity. This is likely because the high deferral rates of therapists at the bedside led to inadequate therapy negating any potential benefit of early consultation. Other limitations include age differences between the cohorts. Our postimplementation cohort was significantly younger, and this may increase the need for sedation and reduce participation in mobilization. Also, the postimplementation phase occurred during the winter months when PICUs have higher census, further straining resources. Future evaluation should account for this seasonality issue. Our study was also a single-center study limiting results generalizability to other hospital systems. Furthermore, the study’s sample size limits the conclusions that may be drawn regarding the relationship between mobility and functional outcomes. Last, the FSS may not be sufficiently sensitive to assess functional decline modifiable by early mobility. Evaluation of patient outcomes that are directly linked to mobility will be key to enhance future studies.
CONCLUSIONS
Implementation of a mobility program through a QI initiative in our tertiary PICU increased early consultations of PT and OT for patients but did not increase PT or OT session frequency. PT and OT deferrals remained high despite a unit-wide educational effort. Future cycles of this initiative will address identified barriers including patient sedation status and therapist availability. Future programs will aim to incorporate nurse and family-driven delivery of treatment.
ACKNOWLEDGMENTS
The authors would like to thank Nicholas J. Jackson, PhD, MPH, for his statistical consultation. Ann and Michael Popper for support of the Critical Illness Recovery for ChiLdrEn (CIRCLE) program at Children’s Hospital of Pittsburgh.
Supplementary Material
Footnotes
Presented at the Society of Critical Care Medicine Congress, February 2019.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Ames SG, Alessi LJ, Chrisman M, Stanger M, Corboy D, Sinha A, Fink EL. Development and Implementation of Pediatric ICU-based Mobility Guidelines: A Quality Improvement Initiative. Pediatr Qual Saf 2020;00:e414.
Published online May 19, 2021
Disclosure The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med. 2000; 28:1173–1179. [DOI] [PubMed] [Google Scholar]
- 2.Pollack MM, Holubkov R, Funai T, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014; 15:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013; 14:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson RS, Asaro LA, Hutchins L, et al. Risk factors for functional decline and impaired quality of life after pediatric respiratory failure. Am J Respir Crit Care Med. 2019; 200:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos Santos C, Hussain SN, Mathur S, et al. ; MEND ICU Group; RECOVER Program Investigators; Canadian Critical Care Translational Biology Group. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016; 194:821–830. [DOI] [PubMed] [Google Scholar]
- 6.Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011; 183:364–371. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009; 373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink EL, Beers SR, Houtrow AJ, et al. ; PICU-Rehabilitation Study Group. Early protocolized versus usual care rehabilitation for pediatric neurocritical care patients: a randomized controlled trial. Pediatr Crit Care Med. 2019; 20:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013; 310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 10.Betters KA, Hebbar KB, Farthing D, et al. Development and implementation of an early mobility program for mechanically ventilated pediatric patients. J Crit Care. 2017; 41:303–308. [DOI] [PubMed] [Google Scholar]
- 11.Tsuboi N, Nozaki H, Ishida Y, et al. Early mobilization after pediatric liver transplantation. J Pediatr Intensive Care. 2017; 6:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel HJ, Needham DM, Morris PE, et al. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013; 41(9 suppl 1):S69–S80. [DOI] [PubMed] [Google Scholar]
- 13.Fraser D, Spiva L, Forman W, et al. Original research: implementation of an early mobility program in an ICU. Am J Nurs. 2015; 115:49–58. [DOI] [PubMed] [Google Scholar]
- 14.King BJ, Steege LM, Winsor K, et al. Getting patients walking: a pilot study of mobilizing older adult patients via a nurse-driven intervention. J Am Geriatr Soc. 2016; 64:2088–2094. [DOI] [PubMed] [Google Scholar]
- 15.Klein K, Mulkey M, Bena JF, et al. Clinical and psychological effects of early mobilization in patients treated in a neurologic ICU: a comparative study. Crit Care Med. 2015; 43:865–873. [DOI] [PubMed] [Google Scholar]
- 16.Wieczorek B, Ascenzi J, Kim Y, et al. PICU up!: impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care Med. 2016; 17:e559–e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui LR, LaPorte M, Civitello M, et al. Physical and occupational therapy utilization in a pediatric intensive care unit. J Crit Care. 2017; 40:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasek TA, Burns C, Treble-Barna A, et al. Important outcomes for parents of critically ill children. Crit Care Nurse. 2019; 39:74–79. [DOI] [PubMed] [Google Scholar]
- 19.Practice Committee of the Section on Pediatrics APTA. Section on Pediatrics Fact Sheet. 2013. Available at https://pediatricapta.org/includes/fact-sheets/pdfs/PEDS_Factsheet_FrequencyAndDuration.pdf. Accessed May 6, 2020.
- 20.Hopkins RO, Choong K, Zebuhr CA, et al. Transforming PICU culture to facilitate early rehabilitation. J Pediatr Intensive Care. 2015; 4:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack MM, Holubkov R, Glass P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Functional status scale: new pediatric outcome measure. Pediatrics. 2009; 124:e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagiela LM, Barbaro RP, Quasney MW, et al. Outcomes and patterns of health care utilization after hospitalization for pediatric critical illness due to respiratory failure. Pediatr Crit Care Med. 2019; 20:120–127. [DOI] [PubMed] [Google Scholar]
- 23.Saliski M, Kudchadkar SR. Optimizing sedation management to promote early mobilization for critically ill children. J Pediatr Intensive Care. 2015; 4:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best KM, Asaro LA, Curley MAQ; Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) Study Investigators. Sedation management for critically ill children with pre-existing cognitive impairment. J Pediatr. 2019; 206:204–211.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi N, Hiratsuka M, Kaneko S, et al. Benefits of early mobilization after pediatric liver transplantation. Pediatr Crit Care Med. 2019; 20:e91–e97. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JK, Lohse B, Bento HA, et al. Improving outcomes for critically ill cardiovascular patients through increased physical therapy staffing. Arch Phys Med Rehabil. 2019; 100:270–277.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013; 41:717–724. [DOI] [PubMed] [Google Scholar]
- 28.Hunter OO, George EL, Ren D, et al. Overcoming nursing barriers to intensive care unit early mobilisation: a quality improvement project. Intensive Crit Care Nurs. 2017; 40:44–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


