Supplemental Digital Content is available in the text
Keywords: Breast cancer, lncHSAT164, Single-nucleotide polymorphisms
Abstract
Background:
Single-nucleotide polymorphisms (SNPs)-associated genes and long non-coding RNAs (lncRNAs) can contribute to human disease. To comprehensively investigate the contribution of lncRNAs to breast cancer, we performed the first genome-wide lncRNA association study on Han Chinese women.
Methods:
We designed an lncRNA array containing >800,000 SNPs, which was incorporated into a 96-array plate by Affymetrix (CapitalBio Technology, China). Subsequently, we performed a two-stage genome-wide lncRNA association study on Han Chinese women covering 11,942 individuals (5634 breast cancer patients and 6308 healthy controls). Additionally, in vitro gain or loss of function strategies were performed to clarify the function of a novel SNP-associated gene.
Results:
We identified a novel breast cancer-associated susceptibility SNP, rs11066150 (Pmeta = 2.34 × 10−8), and a previously reported SNP, rs9397435 (Pmeta = 4.32 × 10−38), in Han Chinese women. rs11066150 is located in NONHSAT164009.1 (lncHSAT164), which is highly expressed in breast cancer tissues and cell lines. lncHSAT164 overexpression promoted colony formation, whereas lncHSAT164 knockdown promoted cell apoptosis and reduced colony formation by regulating the cell cycle.
Conclusions:
Based on our lncRNA array, we identified a novel breast cancer-associated lncRNA and found that lncHSAT164 may contribute to breast cancer by regulating the cell cycle. These findings suggest a potential therapeutic target in breast cancer.
Introduction
Breast cancer is a leading cause of cancer-related deaths in women worldwide.[1] The 5-year prevalence statistics indicate that approximately 11% of breast cancers worldwide occur in China, and the incidence has increased rapidly in recent decades.[2] Genome-wide association studies and exome sequencing have been widely used to identify disease-associated susceptibility single-nucleotide polymorphisms (SNPs)/genes/loci,[3–10] and >180 breast cancer-associated SNPs have been identified in over 100 susceptibility genes/loci. The majority of identified SNPs fall outside of the protein-coding regions, which comprise only 2% of the whole genome.[11,12]
In the whole genome, the vast majority of transcribed sequences does not encode proteins and are thus called non-coding RNAs. Among these non-coding transcripts, transcripts of long non-coding RNAs (lncRNAs) can work together with other proteins and participate in various biological processes,[13–16] including carcinogenesis, tumor growth, and patient prognosis. Recent technological advances in high-throughput sequencing have created an opportunity to identify breast cancer-associated lncRNAs and generated compelling evidence that lncRNAs contribute to the metastasis of breast cancer.[17–20] In addition, the SNPs in lncRNA regions can also promote disease development.[21,22] However, investigations about lncRNAs are still limited due to small sample sizes and weak statistical power, rendering it imperative to thoroughly study the correlation between lncRNA SNPs and disease development.
To comprehensively investigate the contribution of lncRNAs to breast cancer, we designed a genome-wide lncRNA array containing >800,000 SNPs and performed a genome-wide lncRNA association study on Han Chinese women. Furthermore, we investigated the function of SNP-associated genes in breast cancer.
Methods
Ethical approval
This study was approved by the Ethics Committee of Anhui Medical University (No. 20131301). All participants provided written informed consent. The current study was conducted according to the principles of the Declaration of Helsinki.
lncRNA array
Based on the NONCODE database, the Run-Sheng Chen team independently designed an lncRNA array,[12,23] which included >800,000 SNPs in total. Among these SNPs, 425,000 were located in the whole-genome non-coding region, 8187 were located in the non-coding gene regulatory region (promoter and enhancer regions), and 11,466 were located in the miRNA binding region. A total of 130,831 of these SNPs were recruited from RegulomeDB (https://www.regulomedb.org/regulome-search/), and 11,764 were associated with tumors, immune diseases, and cardiovascular diseases. This lncRNA array covers 150,000 SNPs in the Illumina Human OmniZhongHua array (https://www.illumina.com/products/by-type/microarray-kits/infinium-omni-zhonghua.html),[24] and >60% of SNPs in this lncRNA array were distributed in the Illumina Human OmniZhongHua array SNP genome linkage disequilibrium (LD) region. All SNP data were collected from the 1000 Genomes Project (r2 = 0.8).
Study subjects
A total of 5634 cases and 6308 healthy controls were enrolled in our study through a collaborative consortium in China. All cases were diagnosed with breast cancer by at least two pathologists, and their clinical information was collected by professional investigators with a comprehensive clinical check-up. All of the healthy controls were clinically determined to be breast cancer free and to have no family history of breast cancer (including first- and second-degree relatives). Peripheral blood was collected from all participants with an anticoagulation tube and stored in a −80°C freezer for sequencing analysis. Specimens of cancer tissues and para-cancerous tissues were obtained with informed consent from patients (all Han Chinese women) at the No. 2 Hospital, Anhui Medical University (Hefei, China), between July 2011 and September 2016. For specimens of para-carcinoma tissues or healthy controls, we collected a small amount of adipose/skin tissue from breast cancer patients who underwent radical mastectomy. All tissue samples were stored in liquid nitrogen immediately after surgical resection. All participants provided written informed consent, and all investigators were blinded to the group allocation during the experiment when assessing outcomes.
Quality control (QC) and statistical analysis
PLINK 1.07 software (developed by Christopher Chang with support from NIH-NIDDK's Laboratory of Biological Modeling, the Purcell Lab and others; USA)[25] was used to examine potential genetic relatedness based on pairwise identity by state for all the successfully genotyped samples. A principal component analysis (PCA)-based approach was used to assess population outliers and stratification.[26] All human leukocyte antigen SNPs on chromosome 6 between 25 and 34 Mb and SNPs on non-autosomes were removed. We excluded SNPs with a call rate <99%, a minor allele frequency <0.0001, and/or a significant deviation from Hardy-Weinberg equilibrium in the controls (P < 10−4) during each stage. LD pruning was carried out by using the PLINK option “-indep-pairwise 50 5 0.20.”
Cell culture
MCF10A, MC7F, T47D, and HEK293T cell lines were purchased from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences. MCF10A cells were grown in Dulbecco's modified eagle medium/nutrient mixture F-12 (DMEM/F12; 1:1, Gibco, Life, USA) medium supplemented with 10% fetal bovine serum (FBS) (Gibco, Australia), 10 μg/mL insulin (Macklin, China), 20 ng/mL epidermal growth factor (EGF) (Peprotech, China), 0.5 μg/mL hydrocortisone Macklin, China), and 100 U/mL penicillin-streptomycin. MCF7 cells were maintained in Dulbecco's modified eagle medium (DMEM; Gibco, Life, USA) supplemented with 10% FBS (Gibco, Australia) and 100 U/mL penicillin-streptomycin. T47D cells were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Gibco, Life, USA) supplemented with 10% FBS (Gibco, Australia) and 100 U/mL penicillin-streptomycin. All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and confirmed to be mycoplasma free.
RNA extraction and quantitative real-time PCR (RT-qPCR) analysis
The total RNA from the cell lines, human breast cancer tissues, and para-cancerous tissues used in this study, was extracted with TRIzol reagent and incubated with DNase I (Thermo Fisher, USA) to remove genomic DNA. First-strand complementary DNA (cDNA) was synthesized by using the SuperScript III Reverse Transcriptase Kit (Thermo Fisher, USA). Relative RNA levels determined by RT-qPCR were measured on a Rotor-Gene Q real-time PCR machine (Qiagen, Germany). Glyceraldehyde-3-phosphate dehydrogenase was employed as an internal control. The relative expression of RNAs was calculated using the 2−ΔΔCt method.
Subcellular fractionation
The separation of nuclear and cytosolic fractions was performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher, USA) according to the manufacturer's instructions. RNA was extracted and RT-qPCR was performed to assess lncHSAT164 expression in the nuclear and cytosolic fractions.
5′ and 3′ rapid amplification of cDNA ends (RACE) and northern blot analysis
5′ and 3′ RACE was performed with a SMARTer® RACE 5′/3′ Kit (Takara, China). PCR products were obtained by using SeqAmpTM DNA Polymerase (Takara, China) and separated on a 2% agarose gel. Gel bands were purified, cloned into the pEASY-T5 Zero vector (Transgen, China), and sequenced at Beijing Genomics Institute. Northern blot analysis was carried out with a NorthernMax-Gly Kit (Ambion, USA) according to the manufacturer's instructions. For this procedure, 30 μg of the total RNA was mixed with the glyoxal loading dye, denatured, and loaded onto a 1% agarose gel to detect lncHSAT164.
Plasmid construction, transfection, and lentivirus infection
Full-length lncHSAT164 was amplified according to RACE results and cloned into the pcDNA3.1 vector (Invitrogen, Waltham, MA, USA) according to the manufacturer's instructions. In total, 5 × 105 cells were seeded into each well of a six-well plate and transfected with plasmids upon reaching 80% to 90% confluence. The recombinant plasmid pcDNA3.1 (normal control [NC])-lncHSAT164+ (164+) and the control plasmid pcDNA3.1 (normal control [NC]) were constructed and transfected separately into cells using Lipofectamine 3000 (Invitrogen, USA) according to the instruction manual. Cells were harvested 48 h after transfection for further experiments.
Short hairpin RNAs against lncHSAT164 were designed and cloned into the enhanced green fluorescent protein-puromycin-pll3.7 plasmid. Based on the psPAX2-pMD2G lentiviral system (Addgene, Beijing, China), a lentivirus was constructed according to the manufacturer's instructions. After lentivirus infection, 1 μg/mL puromycin (Invitrogen, USA) was added for selection, and 48 to 72 h later, the cells were harvested for further experiments.
Colony formation assay
Cells (1 × 103 cells/well) were seeded in a six-well plate and incubated for 10 to 15 days at 37°C. Then, the cells were washed twice in phosphate buffered solution (PBS), fixed with 90% ethanol for 15 min, and stained with 0.1% crystal violet for 20 min. Images of colonies were taken with a digital camera, and the number of colonies was analyzed by ImageJ v1.8.0 software.
Flow cytometry analyses of apoptosis and the cell cycle
For apoptosis analysis, target cells were transferred to a 15 mL centrifuge tube, and annexin V binding buffer was added. After being centrifugated at 1000 rpm for 5 min at 4°C, the cells were washed three times in PBS. Then, 100 μL of binding buffer was added, and 5 μL of Annexin V-APC and 1 μL of 100 μg/mL propidium iodide (PI) stain (Thermo Fisher, USA) were added and incubated in the dark for 25 min. For cell cycle analysis, target cells were fixed with 75% ice-cold ethanol at 4°C overnight. Then, the cells were suspended in PBS supplemented with 100 mg/mL RNAse A for 30 min at 37°C and then stained with 50 μg/mL PI (Thermo Fisher, USA) in the dark at room temperature for 15 min. Finally, a total of 20,000 cells were analyzed on a FACSCalibur flow cytometer equipped with Cell Quest software (BD Biosciences, USA).
Results
Identification of breast cancer-associated SNPs
Based on our lncRNA array, 1496 cases and 1257 controls of Han Chinese women were genotyped in the first stage. After QC filtering and PCA, 1675 SNPs with P values < 10−4 were identified [Supplementary Table 1, http://links.lww.com/CM9/A495]. Based on the previously reported SNP selection criteria,[27,28] 70 SNPs with P values < 10−5 were selected for replication through the Sequenom MassARRAY system. An independent cohort including 4138 cases and 5051 controls was involved in the second replication stage, and after QC, 23 SNPs were replicated (P value < 0.05). A meta-analysis of the 70 SNPs in this lncRNA array discovery and replication stage study identified two variant SNPs and two suggestive SNPs [Figure 1 and Table 1]. All four SNPs were subjected to PCA [Supplementary Figure 1A–H, http://links.lww.com/CM9/A492]. The novel breast cancer-associated SNP verified in this work, rs11066150 (Pmeta = 2.34 × 10−8, odds ratio [OR] = 1.13), is located at 12q24.13. According to the NONCODE database, rs11066150 was located in the NONHSAT164009.1 transcript intron (lncHSAT164). A previously reported breast cancer-associated variant rs9397435 (CCDC170, Pmeta = 4.32 × 10−38, OR = 1.41) was also confirmed by our data,[3] which suggests that the genotype data in this lncRNA array are highly reliable for future studies.
Figure 1.
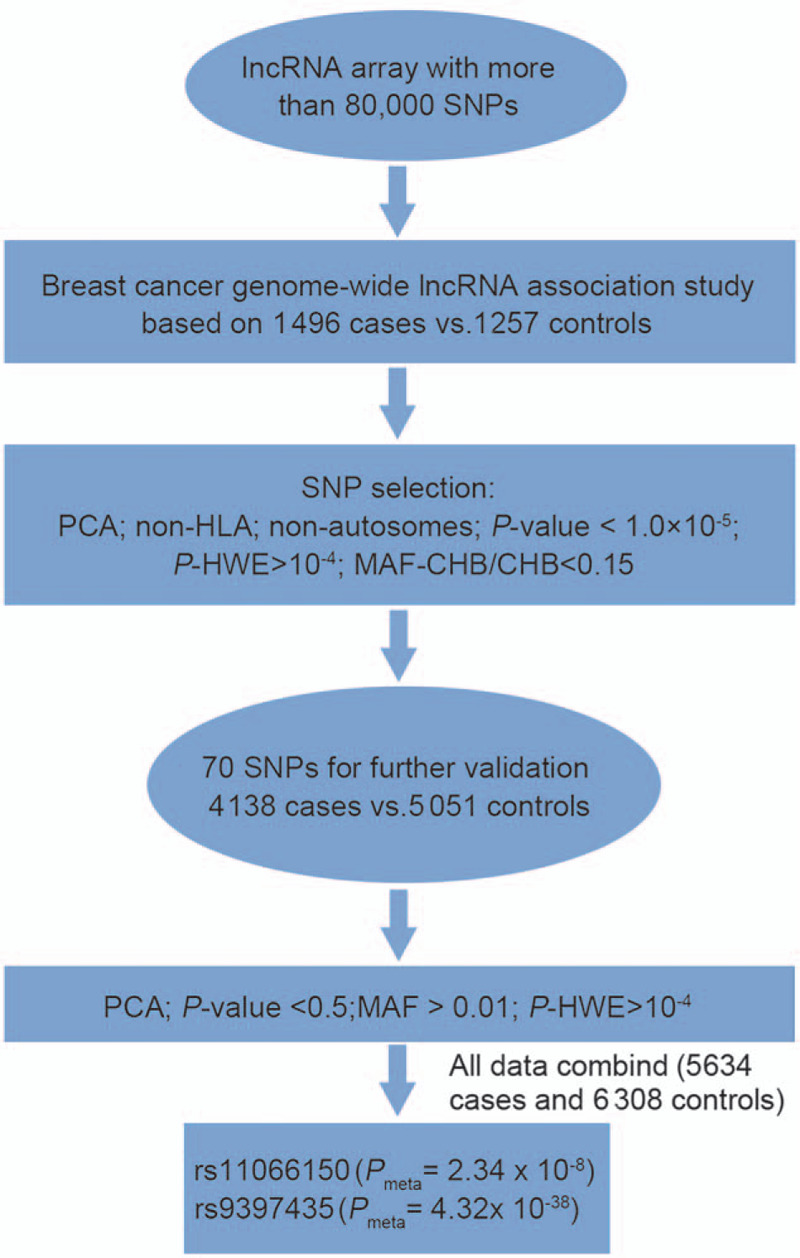
Flow chart of the genome-wide lncRNA analysis of breast cancer in Chinese Han women. CHB: Chinese Han Beijing; HWE: Hardy-Weinberg equilibrium; lncRNA: Long non-coding RNA; MAF: Minor allele frequency; PCA: Principal component analysis; SNPs: Single-nucleotide polymorphisms.
Table 1.
Association of the two stages and meta-analyses based on logistic regression.
| lncRNA array | Genotyping validation | Meta | ||||||||||||||
| SNP | BP (hg19) | Non-coding RNA transcript annotation | Allele | F_A | F_U | P value | OR (95% CI) | P-HWE | F_A | F_U | P value | OR (95% CI) | P-HWE | P value | HetISq | HetPVal |
| rs9397435 | chr6_151951220 | ∗N/A | G/A | 0.3759 | 0.2825 | 4.34 × 10−13 | 1.53 (1.36–1.72) | 0.4452 | 0.3669 | 0.2914 | 4.05 × 10−27 | 1.41 (1.32–1.50) | 0.1397 | 4.32 × 10−38 | 33.1 | 0.2216 |
| rs11066150 | chr12_112518803 | NONHSAT164009.1: intron_1 | A/G | 0.4276 | 0.3721 | 3.50 × 10−5 | 1.26 (1.13–1.41) | 0.8564 | 0.4235 | 0.3933 | 3.91 × 10−5 | 1.13 (1.07–1.20) | 0.2234 | 2.34 × 10−8 | 64.6 | 0.09297 |
| rs12537 | chr22_30423460 | NONHSAT192799.1: exon_1 | T/C | 0.1922 | 0.2387 | 3.44 × 10−5 | 0.76 (0.67–0.86) | 0.3143 | 0.1772 | 0.1964 | 9.97 × 10−4 | 0.88 (0.82–0.95) | 0.3676 | 8.84 × 10−7 | 73.5 | 0.05202 |
| rs62112521 | chr19_51162756 | ∗N/A | A/G | 0.4278 | 0.4865 | 1.72 × 10−5 | 0.79 (0.71–0.88) | 0.778 | 0.4305 | 0.4507 | 6.61 × 10−3 | 0.92 (0.87–0.98) | 0.8408 | 8.80 × 10−6 | 83.6 | 0.01366 |
N/A: No comment information. BP: Base position; CI: Confidence interval; HWE: Hardy-Weinberg equilibrium; lncRNA: Long non-coding RNA; OR: Odds ratio; SNP: Single-nucleotide polymorphism.
Characterization of lncHSAT164 in breast cancer
According to the NONCODE database, rs11066150 is located in the lncHSAT164 transcript, which is >867 bp. It is highly expressed in HepG2 (hepatocellular carcinoma cell line exosomes), invasive non-functional pituitary adenomas exosomes (NFPAs), non-invasive NFPAs, and tuberculosis patients serum (active tuberculosis patients serum exosomes), but did not express in A431 cell line (squamous cell carcinoma cell line exosomes) and MCF7 cell line (human breast cancer cell line exosomes) [Supplementary Figure 2, http://links.lww.com/CM9/A493]. We performed the qPCR analysis with four paired breast cancer tissues and para-cancerous tissues and found that lncHSAT164 was more highly expressed in the cancer tissues than in the para-cancerous tissues [Figure 2A]. Furthermore, the qPCR analysis was performed among the normal breast cell line MCF10A and the breast cancer cell lines MCF7 and T47D. The results demonstrated that lncHSAT164 was highly expressed in T47D cells [Figure 2B]. To determine the full length of lncHSAT164, 5′ and 3′ RACE [Supplementary Figure 3A, http://links.lww.com/CM9/A494] and Northern blotting were performed [Figure 2C]. Our results revealed that lncHSAT164 is an ∼1700-nucleotide-long transcript with a polyadenylated tail.
Figure 2.
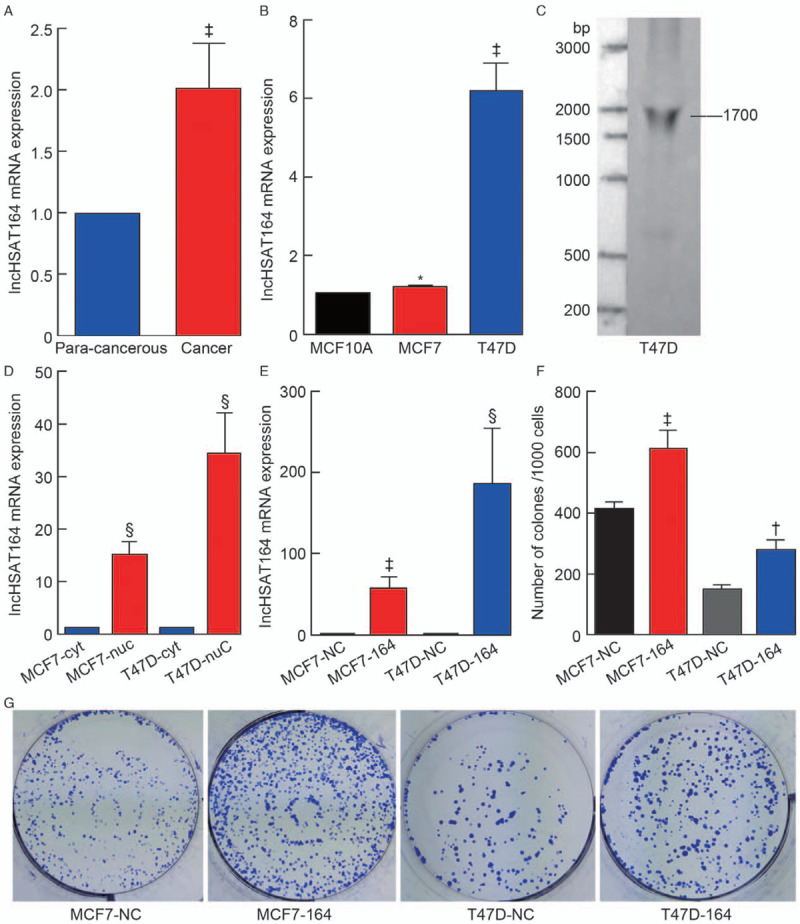
Characterization of lncHSAT164 in breast cancer. (A) lncHSAT164 mRNA expression in four paired human breast cancer tissues and para-cancerous tissues. (B) Relative lncHSAT164 expression levels in breast cancer cells. (C) Northern blot analysis of lncHSAT164 in T47D cells. (D) Subcellular localization of lncHSAT164 in breast cancer cells. Blue indicates cytoplasmic extracts. Red indicates nuclear extracts. (E) lncHSAT164 was overexpressed in MCF7 and T47D cells. (F–G) Overexpressed lncHSAT164 promotes the clonogenic potential of MCF7 and T47D cells. Data are presented as the mean ± standarad error (SE), and P values were computed by unpaired Student's t test. ∗P > 0.05; †P < 0.05; ‡P < 0.01; §P < 0.001.
Next, we examined the subcellular localization of lncHSAT164 in the MCF7 and T47D cell lines and discovered that lncHSAT164 predominately resided in the nucleus [Figure 2D]. Colony formation assays illustrated that clonogenic survival remarkably increased following lncHSAT164 overexpression [Figure 2F–G]. We reasoned that lncHSAT164 might play an important role in the nucleus and could promote the growth of breast cancer cells.
lncHSAT164 knockdown regulates cell proliferation and the cell cycle
To further investigate the function of lncHSAT164 in breast cancer, a lentivirus was constructed to knockdown lncHSAT164 in T47D cells [Figure 3A]. In addition, face shorting was used to examine the impact of lncHSAT164 knockdown on cell apoptosis and the cell cycle, and the results showed that the number of apoptotic cells increased after lncHSAT164 knockdown (P < 0.01) [Figures 3B and 3D]. Fewer cells remained in the G0/G1 phase, but more cells were arrested in the G2/M phase (P < 0.05) [Figures 3C and 3E]. Colony formation assays revealed that clonogenic survival greatly decreased after lncHSAT164 knockdown [Figures 3F and 3G], and this result was also found for MCF7 cells [Supplementary Figure 3B–D, http://links.lww.com/CM9/A494]. Subsequently, a pull-down analysis was applied to identify lncHSAT164-associated proteins, but unfortunately, we failed to identify any related proteins (data not shown). Based on the above analysis, it is reasonable to suggest that lncHSAT164 could promote tumor proliferation by regulating cell proliferation and the cell state.
Figure 3.
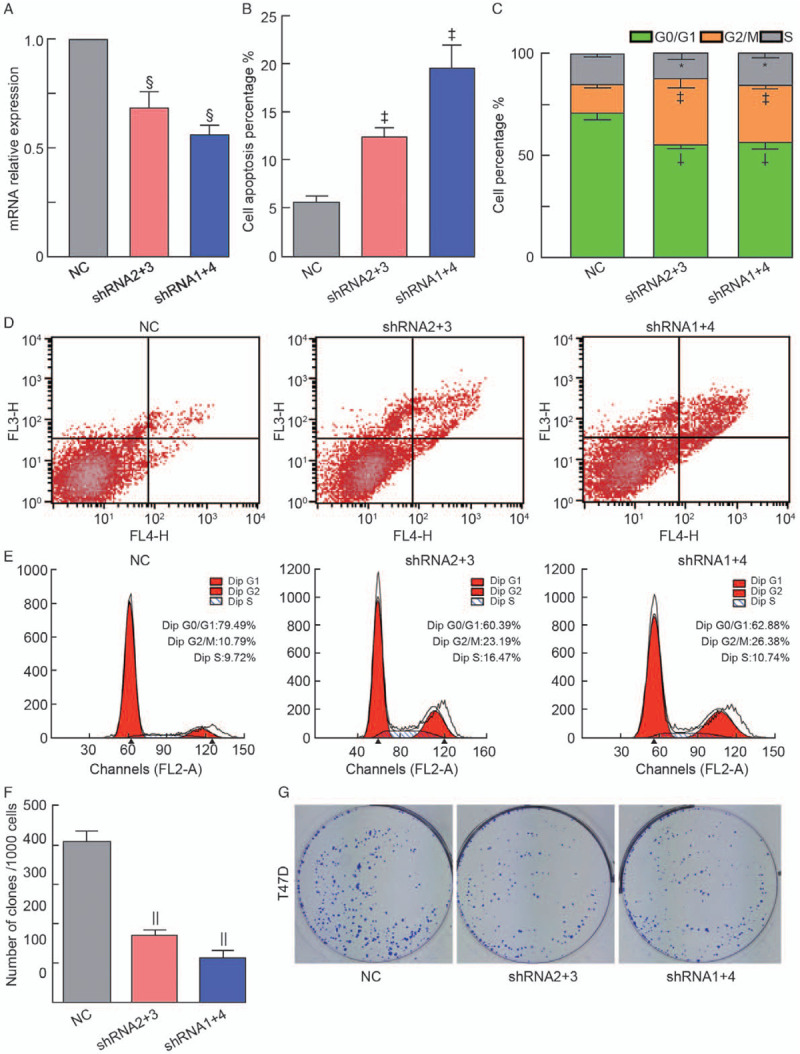
lncHSAT164 knockdown increases cell apoptosis and disturbs the cell cycle in breast cancer. (A) mRNA expression of lncHSAT164 after its knockdown in T47D cells. (B) Apoptosis analysis of the lncHSAT164-deficient T47D cell line. (C) Cell cycle analysis of the lncHSAT164-deficient T47D cell line. (D) and (E) Cell apoptosis and cell cycle analyses were assessed by flow cytometry assays after lncHSAT164 knockdown. (F) and (G) Downregulated lncHSAT164 reduced the clonogenic potential of T47D cells. Data are presented as the means ± standard error (SE), and P values were computed by an unpaired Student's t test. ∗P > 0.05; †P < 0.05; ‡P < 0.01; §P < 0.001; ||P < 0.0001. NC: Normal control; shRNA: Short hairpin RNA.
Discussion
To our knowledge, this study was among the first attempts to perform a genome-wide lncRNA analysis based on an lncRNA array. In addition to the target sites in non-coding areas, this lncRNA array also includes thousands of SNPs in the Illumina Human OmniZhongHua array and its genome LD region. Despite the fact that only 1675 SNPs in our array had P values < 10−4, resulting in lower array recovery than in the Illumina Human OmniZhongHua array, the current study still supports the existence of the breast cancer-associated SNP rs9397435 reported previously,[3,29] which also demonstrates that the data presented herein are reliable. The aforementioned results also suggest that this lncRNA array can be widely used to identify more disease-associated SNPs in future studies.
In this study, we identified a novel breast cancer-associated SNP, rs11066150 on Han Chinese. This SNP is an intron variant located on chr12q24.13. Based on the NONCODE database, rs11066150 was annotated to transcript lncHSAT164. It was first identified by Sharon et al[30] in 2013 based on the single-molecule long-read sequencing technology. However, they did not go further and conduct functional studies on lncHAST164. Due to the limitation of samples, we did not study the relationship between rs11066150 variant and lncHSAT164 expression, and further studies are needed to clarify their correlations.
Currently, lncRNAs have been verified to be involved in breast cancer progression by regulating cell proliferation and metastasis.[31] lncHSAT164, as a newly identified lncRNA, is highly expressed in breast cancer and other cancers. Downregulated lncHSAT164 can regulate the cell cycle and promote cell apoptosis in breast cancer cell lines. These findings suggest a potential therapeutic target in breast cancer. As previously reported, pull-down analysis or chromatin immunoprecipitation sequencing (ChiP-seq) analysis can be performed to identify lncRNA binding proteins or miRNAs,[19,32–34] but we did not obtain any positive data associated with lncHSAT164 in the current study. Therefore, the mechanisms by which lncHSAT164 contributes to breast cancer are still unclear, and further studies are required to fully understand how lncHSAT164 predisposes patients to breast cancer and other cancers.
Acknowledgements
The authors would like to thank all the participating patients and healthy controls, and they would also like to thank all the doctors and nurses in the Department of Oncology, No. 2 Hospital, Anhui Medical University.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81320108016) and the Natural Science Foundation of Higher Education of Anhui Province of China (No. KJ2017A181).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Xu JK, Li GZ, Li Z, Li WJ, Chen RS, Zhang B, Zhang XJ. Genome-wide long non-coding RNA association study on Han Chinese women identifies lncHSAT164 as a novel susceptibility gene for breast cancer. Chin Med J 2021;134:1138–1145. doi: 10.1097/CM9.0000000000001429
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.cmj.org).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016; 159:395–406. doi: 10.1007/s10549-016-3947-0. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou AC, Kartsonaki C, Sinilnikova OM, Soucy P, McGuffog L, Healey S, et al. Common alleles at 6q25.1 and 1p11.2 are associated with breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet 2011; 20:3304–3321. doi: 10.1093/hmg/ddr226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet 2008; 40:17–22. doi: 10.1038/ng.2007.53. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Chen MY, Shen YJ, Zhuo XB, Gao P, Zhou FS, et al. A large-scale, exome-wide association study of Han Chinese women identifies three novel loci predisposing to breast cancer. Cancer Res 2018; 78:3087–3097. doi: 10.1158/0008-5472.CAN-17-1721. [DOI] [PubMed] [Google Scholar]
- 6.Milne RL, Kuchenbaecker KB, Michailidou K, Beesley J, Kar S, Lindström S, et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet 2017; 49:1767–1778. doi: 10.1038/ng.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft P, Haiman CA. GWAS identifies a common breast cancer risk allele among BRCA1 carriers. Nat Genet 2010; 42:819–820. doi: 10.1038/ng1010-819. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman J, Fejerman L, Hu D, Huntsman S, Li M, John EM, et al. Identification of novel common breast cancer risk variants at the 6q25 locus among Latinas. Breast Cancer Res 2019; 21:3.doi: 10.1186/s13058-018-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 2010; 42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoussaini M, Fletcher O, Michailidou K, Turnbull C, Schmidt MK, Dicks E, et al. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet 2012; 44:312–318. doi: 10.1038/ng.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature 2012; 489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang SS, Zhang LL, Guo JC, Niu YW, Wu Y, Li H, et al. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res 2018; 46:D308–D314. doi: 10.1093/nar/gkx1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. Cell 2010; 142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 2013; 154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet 2013; 45:1392–1398. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet 2018; 50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niknafs YS, Han S, Ma T, Speers C, Zhang C, Wilder-Romans K, et al. The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1 in breast cancer progression. Nat Commun 2016; 7:12791.doi: 10.1038/ncomms12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betts JA, Moradi Marjaneh M, Al-Ejeh F, Lim YC, Shi W, Sivakumaran H, et al. Long noncoding RNAs CUPID1 and CUPID2 mediate breast cancer risk at 11q13 by modulating the response to DNA damage. Am J Hum Genet 2017; 101:255–266. doi: 10.1016/j.ajhg.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin A, Li C, Xing Z, Hu Q, Liang K, Han L, et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol 2016; 18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu WL, Jin L, Xu A, Wang YF, Thorne RF, Zhang XD, et al. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. Nat Cell Biol 2018; 20:492–502. doi: 10.1038/s41556-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 21.Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet 2007; 39:1181–1186. doi: 10.1038/ng1007-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Z, Chen X, Liu L, Zhu C, Xu J, Yin X, et al. The polymorphism rs13259960 in SLEAR predisposes to systemic lupus erythematosus. Arthritis Rheumatol 2020; 72:985–996. doi: 10.1002/art.41200. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Li H, Fang S, Kang Y, Wu W, Hao Y, et al. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res 2016; 44:D203–D208. doi: 10.1093/nar/gkv1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Wu WJ, Yang JK, Cheng H, Zuo XB, Lai W, et al. Two new susceptibility loci 1q24.2 and 11p11.2 confer risk to severe acne. Nat Commun 2014; 5:2870.doi: 10.1038/ncomms3870. [DOI] [PubMed] [Google Scholar]
- 25.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 27.Wen L, Zhu C, Zhu Z, Yang C, Zheng X, Liu L, et al. Exome-wide association study identifies four novel loci for systemic lupus erythematosus in Han Chinese population. Ann Rheum Dis 2018; 77:417.doi: 10.1136/annrheumdis-2017-211823. [DOI] [PubMed] [Google Scholar]
- 28.Zuo X, Sun L, Yin X, Gao J, Sheng Y, Xu J, et al. Whole-exome SNP array identifies 15 new susceptibility loci for psoriasis. Nat Commun 2015; 6:6793.doi: 10.1038/ncomms7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacey SN, Sulem P, Zanon C, Gudjonsson SA, Thorleifsson G, Helgason A, et al. Ancestry-shift refinement mapping of the C6orf97-ESR1 breast cancer susceptibility locus. PLoS Genet 2010; 6:e1001029.doi: 10.1371/journal.pgen.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharon D, Tilgner H, Grubert F, Snyder M. A single-molecule long-read survey of the human transcriptome. Nat Biotechnol 2013; 31:1009–1014. doi: 10.1038/nbt.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 2015; 27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Xiao Z-D, Han L, Zhang J, Lee S-W, Wang W, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol 2016; 18:431–442. doi: 10.1038/ncb3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing Z, Lin A, Li C, Liang K, Wang S, Liu Y, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell 2014; 159:1110–1125. doi: 10.1016/j.cell.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grelet S, Link LA, Howley B, Obellianne C, Palanisamy V, Gangaraju VK, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol 2017; 19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]


