Abstract
Background
For patients with B cell acute lymphocytic leukemia (B-ALL) who underwent allogeneic stem cell transplantation (allo-SCT), many variables have been demonstrated to be associated with leukemia relapse. In this study, we attempted to establish a risk score system to predict transplant outcomes more precisely in patients with B-ALL after allo-SCT.
Methods
A total of 477 patients with B-ALL who underwent allo-SCT at Peking University People's Hospital from December 2010 to December 2015 were enrolled in this retrospective study. We aimed to evaluate the factors associated with transplant outcomes after allo-SCT, and establish a risk score to identify patients with different probabilities of relapse. The univariate and multivariate analyses were performed with the Cox proportional hazards model with time-dependent variables.
Results
All patients achieved neutrophil engraftment, and 95.4% of patients achieved platelet engraftment. The 5-year cumulative incidence of relapse (CIR), overall survival (OS), leukemia-free survival (LFS), and non-relapse mortality were 20.7%, 70.4%, 65.6%, and 13.9%, respectively. Multivariate analysis showed that patients with positive post-transplantation minimal residual disease (MRD), transplanted beyond the first complete remission (≥CR2), and without chronic graft-versus-host disease (cGVHD) had higher CIR (P < 0.001, P = 0.004, and P < 0.001, respectively) and worse LFS (P < 0.001, P = 0.017, and P < 0.001, respectively), and OS (P < 0.001, P = 0.009, and P < 0.001, respectively) than patients without MRD after transplantation, transplanted in CR1, and with cGVHD. A risk score for predicting relapse was formulated with the three above variables. The 5-year relapse rates were 6.3%, 16.6%, 55.9%, and 81.8% for patients with scores of 0, 1, 2, and 3 (P < 0.001), respectively, while the 5-year LFS and OS values decreased with increasing risk score.
Conclusion
This new risk score system might stratify patients with different risks of relapse, which could guide treatment.
Keywords: B cell acute lymphocytic leukemia, Allogeneic stem cell transplantation, Minimal residual disease, Disease status, chronic graft-versus host disease, Patient outcome
Introduction
Outcomes of acute lymphoblastic leukemia (ALL) have improved with the development of treatment measures.[1,2] However, relapse remains the major cause of treatment failure in patients with ALL who either exclusively received chemotherapy or additionally underwent allogeneic stem cell transplantation (allo-SCT).[3,4] For allo-SCT cases, almost all available studies have demonstrated that positive measurable/minimal residual disease (MRD) before[5–12] and after[13–25] transplantation was related to a higher cumulative incidence of relapse (CIR) in both adult and pediatric ALL. A previous study[18] also showed that the positive MRD at the time points, including days +30, +60, +90, +180, and +365 after allo-SCT, were inversely correlated with event-free survival and positively correlated with CIR in pediatric ALL. More recently, we reported the association of both quantitative and qualitative pre-transplantation MRD (pre-MRD)[11] as well as post-transplantation MRD (post-MRD)[25] with increased CIR and inferior survival in patients with ALL who underwent haploidentical allograft transplantation.
For patients with ALL who underwent allo-SCT, other variables in addition to post-MRD have also been demonstrated to be associated with leukemia relapse. For example, patients in the first complete remission (CR1) have better outcomes than those in CR2 or those with advanced disease stage.[26–35] Meanwhile, studies reported by others[26,28,32,36–44] and us[45,46] have shown that the onset of graft-versus-host disease (GVHD) after allo-SCT can reduce CIR. Recent studies found that acute graft-versus-host disease (aGVHD) or chronic graft-versus-host disease (cGVHD) could reduce CIR for patients with positive post-MRD[47] as well as for patients transplanted with advanced disease (≥CR3 or active disease).[39]
However, there are rare data regarding the prognostic significance of the combination of disease status with pre-MRD or post-MRD and GVHD in patients with ALL who are receiving allo-SCT. In addition, long-term survival has improved in patients with ALL who undergo current treatment regimens, but outcomes for T cell ALL are still worse than those for B cell acute lymphocytic leukemia (B-ALL).[48,49] Therefore, we attempted to establish a risk score based on pre-MRD or post-MRD determined by multiparametric flow cytometry (MFC) and disease status as well as cGVHD to explore whether it could predict transplant outcomes more precisely in patients with B-ALL after allo-SCT.
Materials and Methods
Ethical approval
This study met the guidelines of the Helsinki Declaration of 1975 and was approved by the Ethics Committee of Peking University People's Hospital (No. 2014PHB086-01). Informed consent was obtained from all patients or their guardians, and donors.
Patients and study design
This is a retrospective study. A total of 477 patients at the Peking University People's Hospital from December 2010 to December 2015 were enrolled. Donor selection and transplant protocol were performed as previously described.[50] Patients with B-ALL who underwent allo-SCT were included, and patients who lost follow-ups were excluded.
Transplant protocol
Granulocyte colony-stimulating factor (G-CSF; 5 μg per kilogram of body weight per day for 5 days) was used to mobilize granulocytes from the bone marrow (G-BM) and the peripheral blood (G-PB). The target mononuclear cell count was ≥6 × 108 per kilogram of recipient weight. Unmanipulated BM (harvested on day 4 after G-CSF) and PB stem cells (PBSCs, harvested on day 5 after G-CSF) were infused into the recipient on the day of collection. All patients who underwent haploidentical blood and marrow transplantation (HBMT) or HLA-matched sibling donor transplantation (MSDT) received both G-BM and G-PB, while patients who underwent HLA-matched unrelated donor transplantation (MUDT) received G-PB.
The conditioning therapy for the HBMT group was as follows: cytarabine (4 g·m−2·d−1) intravenously on days −10 to −9; busulfan (3.2 mg·kg−1·d−1) intravenously on days −8 to −6; cyclophosphamide (1.8 g·m−2·d−1) intravenously on days −5 to −4; semustine (Me-CCNU, 250 mg·m−2·d−1) orally once on day −3; and anti-T-lymphocyte globulins (ATG, thymoglobulin, 2.5 mg·kg−1·d−1, Genzyme Polyclonals, SAS, France) intravenously on days −5 to −2. MSDT patients received hydroxycarbamide (80 mg·kg−1·d−1) orally on day −10 and a lower dose of cytarabine (2 g·m−2·d−1) on day −9, but otherwise, an identical regimen to that of the HBMT patients without ATG was used. In the MUDT group, patients received the same regimen as the MSDT group but with the addition of ATG, as in the HBMT group. All patients received immunosuppressive agents, including cyclosporine A, mycophenolate mofetil, and short-term methotrexate, to prevent GVHD.[51–53]
MFC detection of MRD
The first day after stem cell infusion was defined as day 1. Multicolor MFC was performed in all patients on bone marrow aspirate samples that were obtained as part of the baseline assessment at diagnosis, across the duration of chemotherapy, before and around days +30, +60, +90, +120, and +180 or more after transplantation.[54–58] In this study, we focused on MRD status pre- and post-transplantation. Different antibody combinations were used as described previously.[17,59] Residual disease ≥0.001% detected by MFC at any time point before and after allo-SCT was considered pre-MRD or post-MRD positive (MRD+), while persistent negative MRD at all time points after transplantation was defined as MRD negative (MRD−).[11,46]
Prevention and treatment of relapse
Once MRD turned positive or hematological relapse occurred, some measures were taken to prevent or treat relapse, including immunosuppression tapering, targeted drugs (such as tyrosine kinase inhibitors), interferon, donor lymphocyte infusion (DLI) with previous chemotherapy, and chimeric antigen receptor T cell immunotherapy.[46,60]
Definitions and assessments
The diagnostic criteria for ALL followed the World Health Organization 2008 criteria.[61] The primary study endpoint was the cumulative incidence of leukemia relapse. The secondary endpoints were the cumulative incidences of non-relapse mortality (NRM), the probabilities of leukemia-free survival (LFS), and overall survival (OS). Neutrophil engraftment was defined as an absolute neutrophil count ≥0.5 × 109/L for three consecutive days, and platelet engraftment was defined as ≥20 × 109/L for seven consecutive days without platelet transfusion. aGVHD, cGVHD, NRM, relapse, LFS, and OS were defined as described previously.[62,63] Patients with confirmed MRD were not classified as having a relapse.
Statistical analysis
Descriptive statistics, including the frequency (proportions) for categorical variables and the median (range) for quantitative variables, were used to describe the patient demographic and clinical characteristics. LFS, OS, NRM, and relapse incidence curves were estimated with the Kaplan–Meier method. Separate analyses were performed for six landmarks, namely samples from days +30, +60, +90, +120, and +180 or more after allo-HSCT. In each landmark analysis, time was measured from the date of sampling. Cumulative incidence curves were used in a competing risk setting, with relapse treated as a competing event, to calculate NRM probabilities. Only variables with P < 0.1 were included in a Cox proportional hazards model with time-dependent variables. Only variables with P < 0.1 were included in a Cox proportional hazards model with time-dependent variables, while the P < 0.05 was defined as significant in the multivariate analysis.
Finally, the risk score system included three significant prognostic factors, disease status, post-MRD, and cGVHD status, which, based on the results of the multivariate analysis, would be established. This risk score system ranged from 0 to 3 (0 for no risk factor, 1 for any one of the three risk factors, 2 for any two of the three risk factors, and 3 for all three risk factors). Calculations were performed using the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The characteristics and outcomes of the 477 patients with B-ALL who underwent allo-HSCT are outlined in Table 1. There were 163 (34.2%) patients with positive Philadelphia chromosome (Ph) and 314 (65.8%) patients with negative Ph. The median age of the patients was 26 years, ranging from 2.5 to 63.0 years. Of all patients, 423 (88.7%) achieved CR1 before transplantation, and 54 (11.3%) achieved CR2 or CR3. There were 118 patients with pre-MRD+ and 77 patients with post-MRD+, and the subsequent pre-emptive interventions are shown in Figure 1.
Table 1.
Demographic and clinical characteristics of patients with B-ALL underwent allo-SCT (n = 477).
| Parameters | Values |
| Age (years) | 26 (2.5–63.0) |
| Gender | |
| Male | 267 (56.0) |
| Female | 210 (44.0) |
| ALL type | |
| Ph positive | 163 (34.2) |
| Ph negative | 314 (65.8) |
| Disease status | |
| CR1 | 423 (88.7) |
| CR >1 | 54 (11.3) |
| Transplant type | |
| HBMT | 340 (71.3) |
| MSDT | 127 (26.6) |
| MUDT | 10 (2.1) |
| Conditioning regimen | |
| MA | 477 (100) |
| Donor-recipient sex match | |
| Male-male | 170 (35.6) |
| Male-female | 130 (27.3) |
| Female-male | 96 (20.1) |
| Female-female | 81 (17.0) |
| ABO matched | |
| Matched | 259 (54.3) |
| Major mismatched | 98 (20.5) |
| Minor mismatched | 93 (19.5) |
| Bidirectional mismatched | 27 (5.7) |
| Infused cell doses | |
| MNC (×106/kg) | 7.9 (2.5–20.1) |
| CD34+ cells (×106/kg) | 2.5 (0.4–12.7) |
| Engraftment (yes or no) | |
| Neutrophil | 477 (100) |
| Platelet | 455 (95.4) |
| Engraftment (days) | |
| Neutrophil | 13 (9–26) |
| Platelets | 14 (7–506) |
| Acute GVHD grades | |
| I | 128 (26.8) |
| II | 95 (19.9) |
| III | 19 (4.0) |
| IV | 9 (1.9) |
| Chronic GVHD (n = 197) | |
| Clinical extensive | 44 (9.2) |
| Median follow-up for surviving patients (days) | 1816 (1277–3107) |
Data are presented as median (range), or n (%). ALL: Acute lymphoblastic leukaemia; allo-SCT: allogeneic stem cell transplantation; CR: Complete remission; CR1: First complete remission; GVHD: graft-versus-host disease; HBMT: Haploidentical blood and marrow transplantation; MA: Myeloablative conditioning regimen; MNC: Mononuclear cell; MSDT: HLA-matched sibling donor transplantation; MUDT: HLA-matched unrelated donor transplantation; Ph: Philadelphia chromosome.
Figure 1.
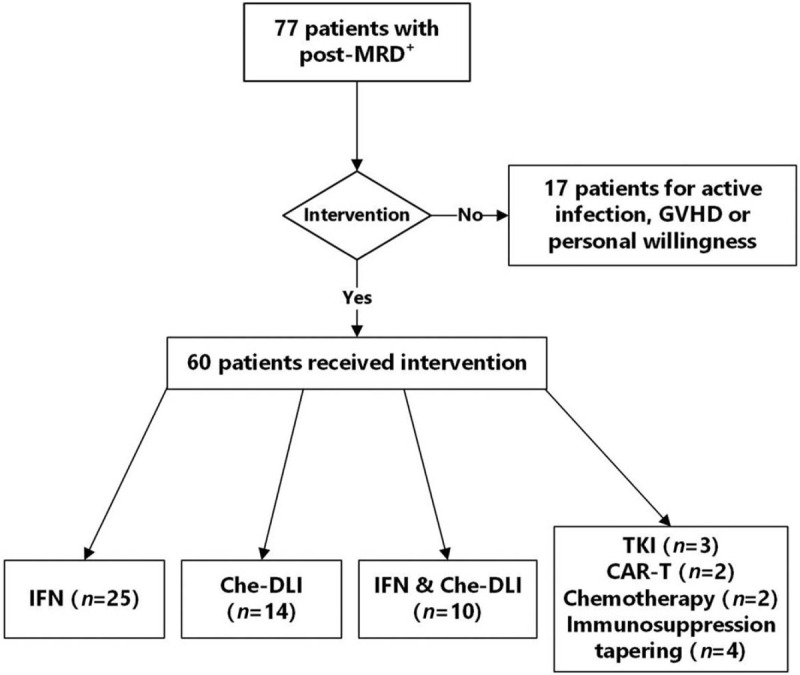
Pre-emptive intervention for patients with positive post-MRD after allo-SCT. allo-SCT: Allogeneic stem cell transplantation; CAR-T: Chimeric antigen receptor T cell immunotherapy; Che-DLI: DLI with previous chemotherapy; DLI: Donor lymphocyte infusion; GVHD: Graft-versus-host disease; IFN: Interferon; MRD: Minimal residual disease; TKI: Tyrosine kinase inhibitor.
By the end of the follow-up time, June 30, 2019, there was a median follow-up duration of 1570 (24–3107) days for all patients and 1816 (1277–3107) days for surviving patients; a total of 143 patients died, and the causes of death were relapse (53.8%), infection (28.7%), poor graft function (4.9%), GVHD (2.8%), and other causes (9.8%). All patients achieved neutrophil engraftment, and 95.4% of patients achieved platelet engraftment. The 100-day cumulative incidence of Grades III–IV aGVHD was 5.9% (95% confidence interval [CI] 3.7%–8.1%), while the 5-year cumulative incidence of cGVHD was 48% (95% CI 43.1%–52.9%); the transplant outcomes are listed in Table 2.
Table 2.
Transplant outcomes for B-ALL patients who underwent allo-SCT in different sub-group cases.
| Parameters | n | 5-year relapse | 5-year OS | 5-year LFS | 5-year NRM |
| Total patients | 477 | 20.7 (17.0–24.4) | 70.4 (66.3–74.5) | 65.6 (61.3–69.9) | 13.9 (10.8–17.0) |
| Pre-MRD | |||||
| Negative | 359 | 15.5 (12.3–18.7)∗ | 73.3 (68.6–78.0)∗ | 69.0 (64.1–73.9)∗ | 16.1 (12.1–20.1) |
| Positive | 118 | 36.4 (27.7–45.1) | 61.8 (53.0–70.6) | 55.3 (46.1–64.5) | 7.6 (2.8–12.4) |
| Post-MRD | |||||
| Negative | 400 | 11.9 (8.7–15.1)† | 76.0 (71.7–80.3)† | 72.8 (68.3–77.3)† | 15.5 (11.9–19.1) |
| Positive | 77 | 66.6 (61.1–72.1) | 41.5 (52.5–30.5) | 28.2 (17.6–38.8) | 5.2 (0.2–10.2) |
| Risk score system | |||||
| 0 | 159 | 6.3 (2.6–10.0)‡ | 85.7 (80.0–91.4)‡ | 83.2 (77.1–89.3)‡ | 10.6 (5.5–15.7) |
| 1 | 235 | 16.6 (11.7–21.5)‡ | 69.2 (62.7–75.7)‡ | 66.6 (60.5–72.7)‡ | 17.1 (12.2–22.0) |
| 2 | 72 | 55.9 (43.9–67.5) | 36.6 (24.8–48.4) | 31.8 (20.8–42.8) | 12.3 (4.7–19.9) |
| 3 | 11 | 81.8 (55.3–100.0) | 27.3 (1.0–53.6) | 18.2 (0–40.9) | 0 |
Data are presented as percentage of incidence (95% confidence interval).
Indicates P < 0.05 compared with patients with pre-MRD positive.
Indicates P < 0.05 compared with patients with pre-MRD positive.
Indicates P < 0.05 compared with patients with the other three risk scores. allo-SCT: Allogeneic stem cell transplantation; B-ALL: B cell acute lymphocytic leukemia; CI: Confidence interval; HR: Hazard ratio; LFS: Leukemia-free survival; MRD: Minimal residual disease; NRM: Non-relapse mortality; OS: Overall survival.
Variables associated with outcomes
Univariate analysis showed the following: (i) disease status (CR1 vs. CR ≥2) was related to CIR, LFS, and OS; (ii) pre-MRD (positive vs. negative), post-MRD (positive vs. negative), and cGVHD (yes or no) were associated with CIR, LFS, OS, and NRM; (iii) platelet engraftment (yes or no) and aGVHD III–IV (yes or no) were associated with LFS, OS, and NRM; while aGVHD (yes or no) was related to CIR. The multivariate analysis showed that patients with post-MRD+ suffered higher CIR (66.6% vs. 11.9%, P < 0.001), lower LFS (28.2% vs. 72.8%, P < 0.001), and OS (41.5% vs. 76.0%, P < 0.001) than those with post-MRD−. Patients with post-MRD+ showed a trend of higher NRM than those with post-MRD− (5.2% vs. 15.5%, P = 0.057) in the univariate analysis, but there was no significance in the multivariate analysis. The landmark analysis showed that post-MRD at all time points (days +30, +60, +90, +120, and +180 or more) was inversely correlated with OS as well as LFS and positively correlated with CIR, but there was no relationship with NRM [Table 3].
Table 3.
Transplant outcomes for B-ALL patients at different time points after allo-SCT.
| MRD status at different time points | 5-year relapse | P value | 5-year OS | P value | 5-year LFS | P value | 5-year NRM | P value |
| +30 days | <0.001 | <0.001 | <0.001 | 0.416 | ||||
| MRD− | 21.7 (17.6–25.8) | 72.6 (68.5–76.7) | 67.7 (63.4–72.0) | 13.5 (10.4–16.6) | ||||
| MRD+ | 52.3 (28.2–76.4) | 36.8 (15.0–58.6) | 36.8 (15.0–58.6) | 18.9 (0–38.5) | ||||
| +60 days | <0.001 | <0.001 | <0.001 | 0.792 | ||||
| MRD− | 21.6 (17.5–25.7) | 73.3 (69.2–77.4) | 68.3 (64.0–72.6) | 12.6 (9.5–15.7) | ||||
| MRD+ | 61.1 (35.8–86.4) | 33.3 (9.4–57.2) | 33.3 (9.4–57.2) | 11.1 (0–31.7) | ||||
| +90 days | <0.001 | 0.002 | <0.001 | 0.230 | ||||
| MRD− | 20.4 (16.5–24.3) | 75.4 (71.3–79.5) | 70.7 (66.4–75.0) | 11.0 (8.1–13.0) | ||||
| MRD+ | 70.0 (46.9–93.1) | 42.8 (18.5–67.1) | 30.0 (6.9–53.1) | 0 | ||||
| +120 days | <0.001 | <0.001 | <0.001 | 0.285 | ||||
| MRD− | 19.8 (15.9–23.7) | 76.8 (72.9–80.7) | 71.5 (67.2–75.8) | 10.7 (7.8–13.6) | ||||
| MRD+ | 73.3 (51.0–95.6) | 26.7 (4.4–49.0) | 26.7 (4.4–49.0) | 0 | ||||
| +180 or more days | <0.001 | <0.001 | <0.001 | 0.066 | ||||
| MRD− | 13.9 (10.2–17.6) | 82.8 (79.1–86.0) | 79.1 (75.0–83.2) | 8.2 (5.5–10.9) | ||||
| MRD+ | 75.0 (60.7–89.3) | 33.6 (14.8–52.4) | 25.0 (10.7–39.3) | 0 |
Data are presented as percentage of incidence (95% confidence interval). allo-SCT: Allogeneic stem cell transplantation; B-ALL: B cell acute lymphocytic leukaemia; LFS: Leukemia-free survival; MRD: Minimal residual disease; NRM: Non-relapse mortality; OS: Overall survival.
In this study, a total of 77 patients had positive post-MRD, of which 60 were treated with pre-emptive interventions [Figure 1]. The CIR of the intervention group showed a lower incidence than that of the non-intervention group (68.1% vs. 75.2%), but the difference was not statistically significant (P = 0.394). Meanwhile, the OS (41.4% vs. 11.8%, P = 0.002) and LFS (31.9% vs. 15.7%, P = 0.039) were better and the TRM was lower (0% vs. 29.8%, P < 0.001) in the intervention group than those in the non-intervention group.
The multivariate analysis also showed that higher disease status (≥CR2 vs. CR1) and the occurrence of cGVHD (without cGVHD vs. with cGVHD) were associated with higher CIR (P = 0.004, P < 0.001), worse OS (P = 0.009, P < 0.001), and LFS (P = 0.017, P < 0.001). Patients without platelet (PLT) engraftment and with Grades III–IV aGVHD had higher NRM (P < 0.001, P < 0.001), lower OS (P < 0.001, P = 0.005), and LFS (P < 0.001, P = 0.022) than patients with PLT engraftment and without Grades III–IV aGVHD.
Improving relapse risk stratification with post-MRD, CR, and cGVHD status
Considering the effects of disease status, post-MRD, and cGVHD on relapse, we performed sub-group analyses to confirm whether the combination of the above risk factors could achieve further stratification of relapse and survival.
First, as shown in Table 4, the combination of CR and post-MRD status could stratify patients with different incidences of relapse. For patients with post-MRD+, there was a tendency for patients transplanted in ≥CR2 to have a higher CIR than those in CR1 (80.0% vs. 63.3%, P = 0.096). Similar results were obtained in patients with post-MRD−, in which patients transplanted in ≥CR2 had a higher CIR than those transplanted in CR1 (25.3% vs. 10.5%, P = 0.007).
Table 4.
The effects of pairwise combination of post-MRD, disease status, and cGVHD on transplant outcomes.
| Sub-groups | 5-year relapse | P value | 5-year OS | P value | 5-year LFS | P value | 5-year NRM | P value |
| Status of post-MRD and CR | <0.001 | <0.001 | <0.001 | 0.408 | ||||
| Post-MRD+ and CR1 | 63.3 (50.4–76.2) | 36.5 (21.8–51.2) | 30.3 (18.3–42.3) | 6.5 (0.2–12.8) | ||||
| Post-MRD+ and ≥CR2 | 80.0 (57.7–100.0) | 25.0 (2.3–47.7) | 20.0 (0–40.2) | 0 | ||||
| Post-MRD− and CR1 | 10.5 (7.4–13.6) | 77.5 (73.2–81.8) | 74.4 (69.9–78.9) | 14.9 (11.2–18.6) | ||||
| Post-MRD− and ≥CR2 | 25.3 (10.0–40.6) | 55.4 (37.6–73.2) | 56.8 (40.1–73.5) | 18.0 (5.8–30.2) | ||||
| Status of post-MRD and cGVHD | <0.001 | <0.001 | <0.001 | 0.004 | ||||
| Post-MRD+ and cGVHD | 61.3 (32.9–89.7) | 54.5 (32.5–76.5) | 33.8 (8.7–58.9) | 5.0 (0–14.8) | ||||
| Post-MRD+ and no cGVHD | 69.2 (56.7–81.7) | 33.5 (20.6–46.4) | 25.6 (14.0–37.2) | 5.3 (0–11.2) | ||||
| Post-MRD− and cGVHD | 6.3 (2.8–10.0) | 85.7 (80.2–91.2) | 83.5 (77.8–89.2) | 10.3 (5.6–15.0) | ||||
| Post-MRD− and no cGVHD | 16.3 (11.4–21.2) | 68.4 (62.3–74.5) | 64.5 (58.2–70.8) | 19.6 (14.3–24.9) | ||||
| Status of CR and cGVHD | <0.001 | <0.001 | <0.001 | 0.050 | ||||
| CR1 and cGVHD | 10.5 (5.8–15.2) | 82.1 (75.8–88.4) | 79.3 (73.0–85.6) | 10.2 (5.5–14.9) | ||||
| CR1 and no cGVHD | 23.7 (18.4–29.0) | 64.2 (58.1–70.3) | 60.0 (53.9–66.1) | 16.6 (11.9–21.3) | ||||
| ≥CR2 and cGVHD | 21.1 (2.1–40.1) | 73.3 (53.3–93.3) | 73.7 (53.9–93.5) | 5.3 (0–15.7) | ||||
| ≥CR2 and no cGVHD | 50.4 (32.4–68.4) | 33.3 (15.2–51.3) | 32.5 (16.0–49.0) | 17.1 (4.4–29.8) |
Data are presented as percentage of incidence (95% confidence interval). cGVHD: Chronic graft-versus-host disease; CI: Confidence interval; CR: Complete remission; LFS: Leukemia-free survival; MRD: Minimal residual disease; NRM: Non-relapse mortality; OS: Overall survival.
Second, we made four sub-groups based on the post-MRD and cGVHD status of all patients. As shown in Table 4, patients with post-MRD− who developed cGVHD had a lower CIR than patients without cGVHD (6.3% vs. 16.3%, P < 0.001); meanwhile, patients with post-MRD+ who developed cGVHD had similar CIR compared to patients without cGVHD (61.3% vs. 69.2%, P = 0.159).
Third, the results of our study showed that patients transplanted in ≥CR2 had higher CIR than those in CR1, and further stratification analysis showed that patients transplanted in ≥CR2 who developed cGVHD could achieve the same relapse rate as patients transplanted in CR1 without cGVHD (21.1% vs. 23.7%, P = 0.631) [Table 4].
Risk score for relapse in the entire cohort
We chose three factors (including disease status, post-MRD status, and cGVHD status) to formulate a risk score according to the results of the multivariate analysis: 0 corresponded to patients in CR1, with post-MRD− and with cGVHD, while 1 corresponded to patients in ≥CR2, with post-MRD+ but without cGVHD. All patients were divided into four sub-groups based on their score (0, 1, 2, or 3).
As expected, our study concluded that the 5-year CIR increased with increasing risk score: 6.3% (score 0), 16.6% (score 1), 55.9% (score 2), and 81.8% (score 3); the 5-year OS (85.7% vs. 69.2% vs. 36.6% vs. 27.3%) and 5-year LFS (83.2% vs. 66.6% vs. 31.8% vs. 18.2%) decreased with increasing risk score, and all relationships were statistically significant. Multivariate analysis showed that the risk score was an independent risk factor associated with CIR, LFS, and OS [Tables 2 and 5, and Figure 2].
Table 5.
Univariate and multivariate analysis of variables related to transplant outcomes among all patients.∗
| Univariate analysis | Multivariate analysis | |||||
| Covariates | HR | 95% CI | P value | HR | 95% CI | P value |
| Relapse | ||||||
| Pre-MRD (positive vs. negative) | 2.724 | 1.824–4.068 | <0.001 | 1.576 | 1.031–2.409 | 0.036 |
| Risk score system | ||||||
| 0 | 1 | 1 | ||||
| 1 | 3.348 | 1.616–6.938 | 0.001 | 3.062 | 1.525–6.148 | 0.002 |
| 2 | 15.707 | 7.596–32.479 | <0.001 | 12.195 | 5.979–24.870 | <0.001 |
| 3 | 31.916 | 12.598–80.854 | <0.001 | 25.356 | 10.135–63.435 | <0.001 |
| Acute GVHD (yes or no) | 0.650 | 0.431–0.980 | 0.040 | |||
| LFS | ||||||
| Pre-MRD (positive vs. negative) | 1.605 | 1.153–2.234 | 0.005 | |||
| Risk score system | ||||||
| 0 | 1 | 1 | ||||
| 1 | 2.411 | 1.533–3.792 | <0.001 | 2.094 | 1.335–3.284 | 0.001 |
| 2 | 6.556 | 4.036–10.649 | <0.001 | 5.279 | 3.244–8.591 | <0.001 |
| 3 | 10.246 | 4.765–22.031 | <0.001 | 11.070 | 5.158–23.761 | <0.001 |
| Platelet engraftment (yes or no) | 0.060 | 0.037–0.098 | <0.001 | 0.079 | 0.048–0.129 | <0.001 |
| Acute GVHD III–IV (yes or no) | 1.751 | 0.993–3.091 | 0.053 | 1.854 | 1.048–3.282 | 0.034 |
| NRM | ||||||
| Pre-MRD (positive vs. negative) | 0.507 | 0.251–1.026 | 0.059 | |||
| Risk score system | ||||||
| 0 | 1 | |||||
| 1 | 1.856 | 1.037–3.322 | 0.037 | |||
| 2 | 0.256 | |||||
| 3 | 0.962 | |||||
| Platelet engraftment (yes or no) | 0.024 | 0.013–0.044 | <0.001 | 0.025 | 0.014–0.045 | <0.001 |
| Acute GVHD III–IV (yes or no) | 4.022 | 2.099–7.705 | <0.001 | 3.502 | 1.797–6.826 | <0.001 |
| OS | ||||||
| Pre-MRD (positive vs. negative) | 1.536 | 1.081–2.182 | 0.017 | |||
| Risk score system | ||||||
| 0 | 1 | 1 | ||||
| 1 | 2.311 | 1.425–3.748 | 0.001 | 2.109 | 1.295–3.433 | 0.003 |
| 2 | 6.565 | 3.936–10.949 | <0.001 | 5.262 | 3.118–8.880 | <0.001 |
| 3 | 8.412 | 3.735–18.949 | <0.001 | 9.533 | 4.221–21.527 | <0.001 |
| Platelet engraftment (yes or no) | 0.041 | 0.025–0.069 | <0.001 | 0.055 | 0.033–0.093 | <0.001 |
| Acute GVHD III–IV (yes or no) | 2.064 | 1.166–3.654 | 0.013 | 2.189 | 1.231–3.894 | 0.008 |
Results were calculated by Cox proportional hazards model.
All variables were first included in the univariate analysis; only variables with P < 0.1 were included in the Cox proportional hazards model with time-dependent variables. CI: Confidence interval; GVHD: Chronic graft-versus-host disease; HR: Hazard ratio; LFS: Leukemia-free survival; MRD: Minimal residual disease; NRM: Non-relapse mortality; OS: Overall survival.
Figure 2.
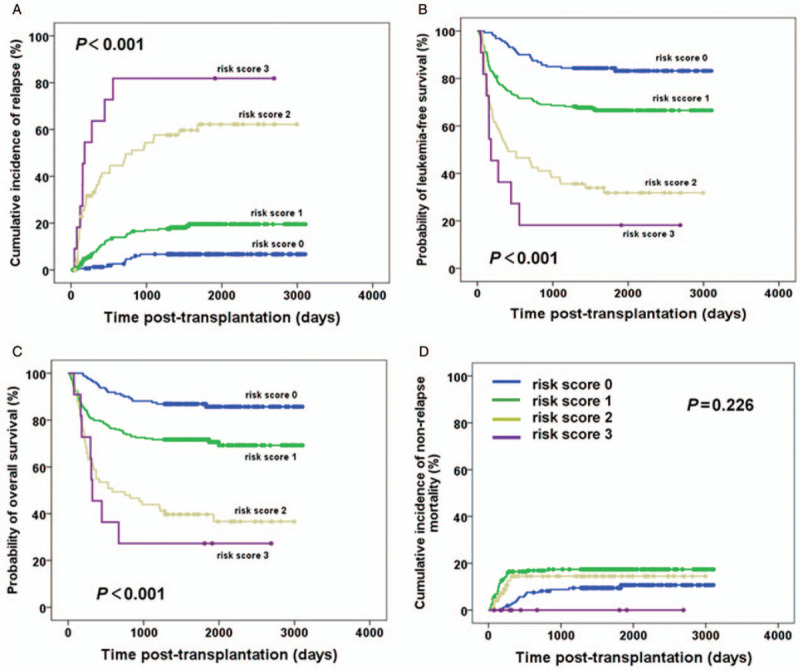
The effect of risk score on relapse (A), LFS (B), OS (C), and NRM (D) in all patients with B-ALL after allo-SCT. allo-SCT: Allogeneic stem cell transplantation; B-ALL: B cell acute lymphocytic leukemia; LFS: Leukemia-free survival; NRM: Non-relapse mortality; OS: Overall survival.
Discussion
Our study analyzed the prognostic factors associated with transplant outcomes in patients with B-ALL who received allografts, and the results were as follows: (i) the factors associated with increased CIR and inferior survival were disease status, post-MRD, and cGVHD status; and (ii) a scoring system that could predict relapse and survival was established. These results added further evidence to the inferences that disease status, post-MRD status, and cGVHD status can predict relapse in patients with B-ALL receiving allo-SCT, and that the onset of cGVHD could improve the outcomes of patients in ≥CR2 and/or those with post-MRD+. Moreover, the scoring system could stratify patients with different risks of relapse more precisely than using one indicator.
Consistent with previous studies,[14,16–20,23,25,64] our study showed that patients with post-MRD+ suffered higher CIR and poorer survival than post-MRD− patients. In addition, we also found that post-MRD+ at different time points was related to a higher incidence of relapse and a lower probability of LFS and OS, which was consistent with the results of Bader et al.[18] The above results suggested that patients with positive post-MRD had a poor prognosis. Previous studies[57,65] have illustrated that pre-emptive interventions (such as DLI and interferon) are effective treatments to reduce the CIR for patients with acute leukemia or myelodysplastic syndrome who become post-MRD positive after allo-SCT. Our study showed that pre-emptive interventions had a tendency to reduce the CIR for patients with positive post-MRD compared to no intervention, but the difference was not statistically significant because the non-intervention patients could not receive interventions to prevent relapse for active infection or GVHD, or were not willing to receive the interventions. In addition, patients died for other reasons before hematological relapse. Therefore, for ALL patients with positive post-MRD, aggressive measures should be taken to remove the MRD to reduce relapse.
Previous studies have demonstrated that disease status pre-transplant[26–30,32,34,66] is related to outcomes in patients with ALL after allo-SCT. The same results were arrived at in our study: patients transplanted in ≥CR2 had a higher CIR than those in CR1. Moreover, patients transplanted in ≥CR2 were more likely to be post-MRD positive than those in CR1 (P = 0.016) in this study, which might be a reason why being in ≥CR2 was related to a higher CIR. Previous studies[26,32,37,38,40,41,66–68] also demonstrated that GVHD had a graft-versus-leukemia effect, but Grades III–IV aGVHD were accompanied by a higher NRM than cases without Grades III–IV aGVHD.[39,69] We also concluded that patients with Grades III–IV aGVHD had a higher NRM and poorer OS and LFS than those without Grades III–IV aGVHD, but there was no correlation with relapse. The results revealed that cGVHD instead of aGVHD could reduce the CIR and improve OS and LFS. A possible reason might be that the time of relapse for most patients was >100 days after transplantation, with a median time of relapse of 322 (45–1678) days after transplantation.
Considering the above results, we formulated a scoring system to predict transplant outcomes. The model consisted of three parameters: disease status, post-MRD status, and cGVHD status. The results indicated that the higher the score was, the higher the CIR, and the lower the LFS and OS, but there was no correlation with NRM [Figure 2]. Overall, all patients in this study had B-ALL, and the study contained a large sample size of patients undergoing HBMT. Some new insights can be drawn from these findings. First, the scoring system together with findings from our previous study[11] could help predict transplant outcomes precisely in ALL patients receiving allo-SCT. Second, for patients in ≥CR2 pre-transplant and/or with post-MRD+, we can reduce relapse by inducing cGVHD. However, it is important to undertake some caution in interpreting the scoring system. First, these data were derived from an allo-SCT setting, including HBMT, MSDT, and few MUDT cases, whereas further evidence under different transplant modalities is required to confirm the results. Second, we focused on total cGVHD in this study, but cGVHD may occur before, during, or after the post-MRD turns positive; as such, the effect of cGVHD occurring at different time points on outcomes needs to be further explored.
There are some limitations to this study. First, post-MRD was detected by MFC in this study, but specific biomarkers in patients with B-ALL, such as BCR/ABL, were not taken into account. Second, clinical intervention while MRD turned positive in some patients would affect clinical outcomes, which was an interfering factor for the risk score system. Finally, this was a single-center and retrospective study, and a multicenter study is needed to provide a large sample study with adequate statistical power.
In conclusion, our study indicated that disease status, post-MRD status, and cGVHD status were related to transplant outcomes in patients with B-ALL receiving allo-SCT. The most important accomplishment of this study was the formulation of a new scoring system with the three variables mentioned above; this scoring system could further stratify patients with different risks of relapse and guide treatment more precisely.
Funding
This work was partly supported by grants from the Beijing Municipal Science and Technology Commission (No. Z181100009618032), the National Key Research and Development Program of China (No. 2017YFA0104500), the National Natural Science Foundation of China (Nos. 81670186, 82070185), and the Peking University Clinical Scientist Program (No. BMU2019LCKXJ003).
Conflicts of interest
None.
Footnotes
How to cite this article: Cao LQ, Zhou Y, Liu YR, Xu LP, Zhang XH, Wang Y, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Fan QZ, Chang YJ, Huang XJ. A risk score system for stratifying the risk of relapse in B cell acute lymphocytic leukemia patients after allogenic stem cell transplantation. Chin Med J 2021;134:1199–1208. doi: 10.1097/CM9.0000000000001402
References
- 1.Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 2011; 29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 3.Jaime-Perez JC, Pinzon-Uresti MA, Jimenez-Castillo RA, Colunga-Pedraza JE, Gonzalez-Llano O, Gomez-Almaguer D. Relapse of childhood acute lymphoblastic leukemia and outcomes at a reference center in Latin America: organomegaly at diagnosis is a significant clinical predictor. Hematology 2018; 23:1–9. doi: 10.1080/10245332.2017.1333294. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Thomas D, Ravandi F, Faderl S, Jabbour E, Garcia-Manero G, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer 2010; 116:5568–5574. doi: 10.1002/cncr.25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachanova V, Burke MJ, Yohe S, Cao Q, Sandhu K, Singleton TP, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: Effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant 2012; 18:963–968. doi: 10.1016/j.bbmt.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM study Group. J Clin Oncol 2009; 27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 7.Elorza I, Palacio C, Dapena JL, Gallur L, Sanchez de Toledo J, Diaz de Heredia C. Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica 2010; 95:936–941. doi: 10.3324/haematol.2009.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandemer V, Pochon C, Oger E, Dalle JH, Michel G, Schmitt C, et al. Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol 2014; 165:392–401. doi: 10.1111/bjh.12749. [DOI] [PubMed] [Google Scholar]
- 9.Gokbuget N, Dombret H, Giebel S, Bruggemann M, Doubek M, Foa R, et al. Minimal residual disease level predicts outcome in adults with Ph-negative B-precursor acute lymphoblastic leukemia. Hematology 2019; 24:337–348. doi: 10.1080/16078454.2019.1567654. [DOI] [PubMed] [Google Scholar]
- 10.Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol 2015; 168:395–404. doi: 10.1111/bjh.13142. [DOI] [PubMed] [Google Scholar]
- 11.Zhao XS, Liu YR, Xu LP, Wang Y, Zhang XH, Chen H, et al. Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with ALL receiving unmanipulated haploidentical allografts. Am J Hematol 2019; 94:512–521. doi: 10.1002/ajh.25417. [DOI] [PubMed] [Google Scholar]
- 12.Ding Z, Han MZ, Chen SL, Ma QL, Wei JL, Pang AM, et al. Outcomes of adults with acute lymphoblastic leukemia after autologous hematopoietic stem cell transplantation and the significance of pretransplantation minimal residual disease: Analysis from a single center of China. Chin Med J (Engl) 2015; 128:2065–2071. doi: 10.4103/0366-6999.161365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balduzzi A, Di Maio L, Silvestri D, Songia S, Bonanomi S, Rovelli A, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol 2014; 164:396–408. doi: 10.1111/bjh.12639. [DOI] [PubMed] [Google Scholar]
- 14.Lovisa F, Zecca M, Rossi B, Campeggio M, Magrin E, Giarin E, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol 2018; 180:680–693. doi: 10.1111/bjh.15086. [DOI] [PubMed] [Google Scholar]
- 15.Rossi G, Carella AM, Minervini MM, Savino L, Fontana A, Pellegrini F, et al. Minimal residual disease after allogeneic stem cell transplant: a comparison among multiparametric flow cytometry, Wilms tumor 1 expression and chimerism status (complete chimerism versus low level mixed chimerism) in acute leukemia. Leuk Lymphoma 2013; 54:2660–2666. doi: 10.3109/10428194.2013.789508. [DOI] [PubMed] [Google Scholar]
- 16.Terwey TH, Hemmati PG, Nagy M, Pfeifer H, Gokbuget N, Bruggemann M, et al. Comparison of chimerism and minimal residual disease monitoring for relapse prediction after allogeneic stem cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2014; 20:1522–1529. doi: 10.1016/j.bbmt.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Zhao XS, Liu YR, Zhu HH, Xu LP, Liu DH, Liu KY, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol 2012; 91:183–192. doi: 10.1007/s00277-011-1285-1. [DOI] [PubMed] [Google Scholar]
- 18.Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol 2015; 33:1275–1284. doi: 10.1200/JCO.2014.58.4631. [DOI] [PubMed] [Google Scholar]
- 19.Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment 2014; 2014:421723.doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortuza FY, Papaioannou M, Moreira IM, Coyle LA, Gameiro P, Gandini D, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol 2002; 20:1094–1104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- 21.Pochon C, Oger E, Michel G, Dalle JH, Salmon A, Nelken B, et al. Follow-up of post-transplant minimal residual disease and chimerism in childhood lymphoblastic leukaemia: 90 d to react. Br J Haematol 2015; 169:249–261. doi: 10.1111/bjh.13272. [DOI] [PubMed] [Google Scholar]
- 22.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll W, et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant 2015; 50:1173–1179. doi: 10.1038/bmt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli O, Peruta B, Tosi M, Guerini V, Salvi A, Zanotti MC, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica 2007; 92:612–618. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- 24.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol 2013; 162:147–161. doi: 10.1111/bjh.12358. [DOI] [PubMed] [Google Scholar]
- 25.Wang XY, Fan QZ, Xu LP, Wang Y, Zhang XH, Chen H, et al. The quantification of minimal residual disease pre- and post-unmanipulated haploidentical allograft by multiparameter flow cytometry in pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom 2020; 98:75–87. doi: 10.1002/cyto.b.21840. [DOI] [PubMed] [Google Scholar]
- 26.Cho BS, Lee S, Kim YJ, Chung NG, Eom KS, Kim HJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia 2009; 23:1763–1770. doi: 10.1038/leu.2009.102. [DOI] [PubMed] [Google Scholar]
- 27.Dalle JH, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J, et al. Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 international BFM studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant 2018; 24:1848–1855. doi: 10.1016/j.bbmt.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Esperou H, Boiron JM, Cayuela JM, Blanchet O, Kuentz M, Jouet JP, et al. A potential graft-versus-leukemia effect after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: results from the French Bone Marrow Transplantation Society. Bone Marrow Transplant 2003; 31:909–918. doi: 10.1038/sj.bmt.1703951. [DOI] [PubMed] [Google Scholar]
- 29.Hamidieh A, Kargar M, Jahani M, Alimoghaddam K, Bahar B, Mousavi SA, et al. The outcome of allogeneic hematopoietic stem cell transplants without total body irradiation in pediatric patients with acute lymphoblastic leukemia: single centre experience. J Pediatr Hematol Oncol 2012; 34:101–107. doi: 10.1097/MPH.0b013e31824435a1. [DOI] [PubMed] [Google Scholar]
- 30.Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol 2004; 22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 31.Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood 2008; 112:903–909. doi: 10.1182/blood-2008-03-143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Kim DW, Kim YJ, Park YH, Min CK, Lee JW, et al. Influence of karyotype on outcome of allogeneic bone marrow transplantation for adults with precursor B-lineage acute lymphoblastic leukaemia in first or second remission. Br J Haematol 2002; 117:109–118. doi: 10.1046/j.1365-2141.2002.03403.x. [DOI] [PubMed] [Google Scholar]
- 33.Ram R, Storb R, Sandmaier BM, Maloney DG, Woolfrey A, Flowers ME, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica 2011; 96:1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia 2012; 26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 35.Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E, et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transplant 2011; 17:1505–1511. doi: 10.1016/j.bbmt.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Balduzzi A, Dalle JH, Wachowiak J, Yaniv I, Yesilipek A, Sedlacek P, et al. Transplantation in children and adolescents with acute lymphoblastic leukemia from a matched donor versus an HLA-identical sibling: is the outcome comparable? Results from the International BFM ALL SCT 2007 study. Biol Blood Marrow Transplant 2019; 25:2197–2210. doi: 10.1016/j.bbmt.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Gu B, Wu X, Chen G, Ma X, Jin Z, Tang X, et al. Haploidentical allogeneic hematopoietic stem cell transplantation compared to matched unrelated transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Res 2017; 59:41–46. doi: 10.1016/j.leukres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Medd PG, Peniket AJ, Littlewood TJ, Pearce R, Perry J, Kirkland KE, et al. Evidence for a GVL effect following reduced-intensity allo-SCT in ALL: a British Society of Blood and Marrow Transplantation study. Bone Marrow Transplant 2013; 48:982–987. doi: 10.1038/bmt.2012.261. [DOI] [PubMed] [Google Scholar]
- 39.Yeshurun M, Weisdorf D, Rowe JM, Tallman MS, Zhang MJ, Wang HL, et al. The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv 2019; 3:670–680. doi: 10.1182/bloodadvances.2018027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferra C, Sanz J, de la Camara R, Sanz G, Bermudez A, Valcarcel D, et al. Unrelated transplantation for poor-prognosis adult acute lymphoblastic leukemia: long-term outcome analysis and study of the impact of hematopoietic graft source. Biol Blood Marrow Transplant 2010; 16:957–966. doi: 10.1016/j.bbmt.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Terwey TH, Le Duc TM, Hemmati PG, le Coutre P, Nagy M, Martus P, et al. NIH-defined graft-versus-host disease and evidence for a potent graft-versus-leukemia effect in patients with acute lymphoblastic leukemia. Ann Oncol 2013; 24:1363–1370. doi: 10.1093/annonc/mds615. [DOI] [PubMed] [Google Scholar]
- 42.Tomblyn MB, Arora M, Baker KS, Blazar BR, Brunstein CG, Burns LJ, et al. Myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia: analysis of graft sources and long-term outcome. J Clin Oncol 2009; 27:3634–3641. doi: 10.1200/JCO.2008.20.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remberger M, Mattsson J, Hentschke P, Aschan J, Barkholt L, Svennilson J, et al. The graft-versus-leukaemia effect in haematopoietic stem cell transplantation using unrelated donors. Bone Marrow Transplant 2002; 30:761–768. doi: 10.1038/sj.bmt.1703735. [DOI] [PubMed] [Google Scholar]
- 44.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood 2002; 100:1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 45.Mo XD, Kong J, Zhao T, Xu LP, Zhang XH, Liu DH, et al. Extramedullary relapse of acute leukemia after haploidentical hematopoietic stem cell transplantation: Incidence, risk factors, treatment, and clinical outcomes. Biol Blood Marrow Transplant 2014; 20:2023–2028. doi: 10.1016/j.bbmt.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Yan CH, Wang Y, Wang JZ, Chen YH, Chen Y, Wang FR, et al. Minimal residual disease- and graft-vs.-host disease-guided multiple consolidation chemotherapy and donor lymphocyte infusion prevent second acute leukemia relapse after allotransplant. J Hematol Oncol 2016; 9:87.doi: 10.1186/s13045-016-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv 2019; 3:3393–3405. doi: 10.1182/bloodadvances.2019000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol 2019; 20:e142–e154. doi: 10.1016/S1470-2045(19)30031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer 2015; 121:2517–2528. doi: 10.1002/cncr.29383. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119:978–985. doi: 10.1002/cncr.27761. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015; 125:3956–3962. doi: 10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 52.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107:3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol 2018; 11:33.doi: 10.1186/s13045-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122:1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 2011; 29:1190–1197. doi: 10.1200/jco.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol 2013; 999:123–136. doi: 10.1007/978-1-62703-357-2_8. [DOI] [PubMed] [Google Scholar]
- 57.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119:3256–3262. doi: 10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]
- 58.Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY, et al. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol 2013; 92:1111–1119. doi: 10.1007/s00277-013-1733-1. [DOI] [PubMed] [Google Scholar]
- 59.Liu YR, Zhang LP, Chang Y, Cheng YF, Fu JY, Li LD, et al. Clinical significance for minimal residual disease detection by 4 color flow cytometry in adult and childhood B lineage acute lymphoblastic leukemia. Chin J Hema 2006; 27:302–305. doi: 10.1007/s11769-006-0026-1. [PubMed] [Google Scholar]
- 60.Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett 2018; 438:63–75. doi: 10.1016/j.canlet.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 61.Swerdlow. International Agency for Research on Cancer, Steven H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. [Google Scholar]
- 62.Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 2014; 49:426–433. doi: 10.1038/bmt.2013.191. [DOI] [PubMed] [Google Scholar]
- 63.Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol 2016; 34:1855–1863. doi: 10.1200/JCO.2015.63.8817. [DOI] [PubMed] [Google Scholar]
- 64.Bourlon C, Lacayo-Lenero D, Inclan-Alarcon SI, Demichelis-Gomez R. Hematopoietic stem cell transplantation for adult Philadelphia-negative acute lymphoblastic leukemia in the first complete remission in the era of minimal residual disease. Curr Oncol Rep 2018; 20:36.doi: 10.1007/s11912-018-0679-9. [DOI] [PubMed] [Google Scholar]
- 65.Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Interferon-alpha: a potentially effective treatment for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21:1939–1947. doi: 10.1016/j.bbmt.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Mohty M, Labopin M, Tabrizzi R, Theorin N, Fauser AA, Rambaldi A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica 2008; 93:303–306. doi: 10.3324/haematol.11960. [DOI] [PubMed] [Google Scholar]
- 67.Lee S, Cho BS, Kim SY, Choi SM, Lee DG, Eom KS, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007; 13:1083–1094. doi: 10.1016/j.bbmt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Zikos P, Van Lint MT, Lamparelli T, Gualandi F, Occhini D, Bregante S, et al. Allogeneic hemopoietic stem cell transplantation for patients with high risk acute lymphoblastic leukemia: favorable impact of chronic graft-versus-host disease on survival and relapse. Haematologica 1998; 83:896–903. doi: 10.1177/107602969800400413. [PubMed] [Google Scholar]
- 69.Kanda J, Morishima Y, Terakura S, Wake A, Uchida N, Takahashi S, et al. Impact of graft-versus-host disease on outcomes after unrelated cord blood transplantation. Leukemia 2017; 31:663–668. doi: 10.1038/leu.2016.288. [DOI] [PubMed] [Google Scholar]


