Abstract
Background:
Despite almost two decades of well-funded and comprehensive response efforts by the Chinese Government, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) remains a major problem in China. Yet, few studies have recently examined long-term trends in HIV/AIDS prevalence, incidence, and mortality at the national level. This study aimed to determine the prevalence, incidence, and mortality trends for HIV/AIDS over the past 28 years in China.
Methods:
We conducted a descriptive, epidemiological, secondary analysis of the Global Burden of Diseases, Injuries, and Risk Factors Study 2017 data. To evaluate trends in prevalence, incidence, and mortality over the study period from 1990 to 2017, we calculated values for annual percentage change (APC) and corresponding 95% confidence intervals (CIs) using joinpoint regression analysis.
Results:
A significant increase in HIV/AIDS prevalence was observed for 1990 to 2009 (APC: 10.7; 95% CI: 10.4, 11.0; P < 0.001), and then remained stable for 2009 to 2017 (APC: 0.7; 95% CI: −0.3, 1.7; P = 0.1). A significant increase in HIV incidence was also observed for 1990 to 2005 (APC: 13.0; 95% CI: 12.6, 13.4; P < 0.001), and then a significant decrease was detected for 2005 to 2017 (APC: −6.5; 95% CI: −7.0, −6.1; P < 0.001). A significant increase in AIDS-related mortality rate was detected for 1990 to 2004 (APC: 10.3; 95% CI: 9.3, 11.3; P < 0.001), followed by a period of stability for 2004 to 2013 (APC: 1.3; 95% CI: −0.7, 3.3; P = 0.2), and then another significant increase for 2013 to 2017 (APC: 15.3; 95% CI: 8.7, 22.2; P < 0.001).
Conclusions:
Although prevalence has stabilized and incidence has declined, AIDS-related mortality has risen sharply in recent years. These findings suggest more must be done to bring people into treatment earlier, retain them in treatment more effectively, actively seek to reenter them in treatment if they dropout, and improve the quality of treatment and care regimens.
Keywords: Prevalence, Incidence, Mortality, HIV, Trend, China, Acquired immunodeficiency syndrome
Introduction
The human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) pandemic has been devastating on a global scale. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), 75.7 (55.9–100) million people have become infected with HIV, and 32.7 (24.8–42.2) million people have died from AIDS and AIDS-related causes worldwide.[1] Annual new infections peaked globally in 1997 at 2.9 (2.3–3.8) million and have since gradually declined to 1.7 (1.2–2.2) million in 2019. Global AIDS-related deaths have also declined from a peak of 1.7 (1.3–2.4) million in 2004 to 0.69 (0.50–0.97) million in 2019.[1,2]
Despite almost two decades of well-funded and comprehensive response efforts by the Chinese Government, HIV/AIDS remains a major problem in China.[3] Although the national-level prevalence of HIV/AIDS in China is a very low 0.09%, for the world's most populous country with 1.4 billion citizens, this translates to a very large number of affected persons — an estimated 1.25 million people living with HIV (PLWH) in 2018.[3] HIV/AIDS became the leading cause of death by infectious disease in China in 2008.[4] By 2019, nearly 21,000 PLWH were dying each year from AIDS in China, >5 times the number from all other infectious diseases combined.[5]
However, few studies have recently examined long-term trends in HIV/AIDS prevalence, incidence, and mortality at the national level in China.[3,6–8] Yet, these trends can serve as important evidence upon which policymakers, funders, program leaders, and others base decisions about how to move forward toward HIV prevention and control. Thus, we used the Global Burden of Diseases (GBD), Injuries, and Risk Factors Study 2017 data to determine the prevalence, incidence, and mortality trends for HIV/AIDS over the past 28 years in China.
Methods
Study design and ethical approval
This study was a descriptive, epidemiological, secondary analysis of GBD Study 2017 data for the mainland of China. Outcomes of interest were prevalence, incidence, and mortality over the period 1990 to 2017, a total of 28 years. Since no individual-level primary data were included in the study, informed consent was not required. This study was approved by the Institutional Review Board of the Chinese Center for Disease Control and Prevention (China CDC; approval number KX201202626).
Data source
This study was based on the data and measures of the GBD study 2017. Conducted by the Institute for Health Metrics and Evaluation (IHME), the GBD study provides a comprehensive account of morbidity and mortality caused by 354 diseases and injuries, as well as risk factors to health in 195 countries from 1990 to 2017 (ie, the most current available dataset). GBD 2017 study protocol information and data are available on the IHME website (www.healthdata.org/gbd/gbd-2017-resources). The rigorous approach and standardized methodology used annually by the >3600-researcher consortium representing >145 countries to analyze >1 billion data points results in a highly reliable picture of what ails and kills people across the world and over time by age and by sex. GBD 2017 measures included deaths, years of life lost, years lived with disability, disability-adjusted life years (DALYs), prevalence, incidence, life expectancy, probability of death, healthy life expectancy, maternal mortality ratio, and summary exposure value.
The methods used for producing global, regional, and national HIV incidence, prevalence, and mortality using GBD 2017 data have been previously described.[9] In brief, for countries like China, which was assessed as only having some vital registration data available, a process they called “cohort incidence bias adjustment” (CIBA) was used to estimate incidence and prevalence using mortality data. The first stage spectrum (ie, the UNAIDS compartmental model that applies HIV incidence, progression, and mortality to a population that ages over time to generate age-sex-specific HIV incidence, prevalence, and mortality[10]) was run to produce initial incidence, prevalence, and mortality curves. Next, the incidence was adjusted using spectrum cohort survival estimates and calculated bias between spectrum mortality estimates and smoothed vital registration data for each year. Then, the second stage spectrum was run to generate age–sex-specific incidence and prevalence estimates using adjusted incidence. Finally, to estimate mortality, smoothed vital registration data were used to inform spectrum estimated mortality through the CIBA process.[9] Each step was repeated and stored in 1000 draws and final estimates were computed by taking the mean estimate across 1000 draws. Additionally, 95% confidence intervals (CIs) were calculated as the 2.5th and 97.5th percentile of all 1000 draw values for each of the measures.[9]
Analysis
For HIV/AIDS cases, total cases are presented as an estimated number, and the prevalence is calculated as the estimated number of cases (numerator) divided by the total population (denominator) and presented as per 100,000. For new HIV infections, new cases are presented as an estimated number while incidence is calculated as the estimated number of new infections (numerator) divided by the total population (denominator) and presented as per 100,000. Finally, for AIDS-related deaths, total deaths are presented as an estimated number, and the mortality rate is calculated as the estimated number of deaths (numerator) divided by the total population (denominator) and presented as per 100,000.
To evaluate trends in prevalence, incidence, and mortality over the study period 1990 to 2017, we calculated values for annual percentage change (APC) and corresponding 95% CIs using the Joinpoint Regression Program (v.4.8.0.1, National Cancer Institute, Bethesda, MD, USA; surveillance.cancer.gov/joinpoint/). Model selection was performed by the Monte Carlo permutation test, which determines the P value from the permutation distributions derived from F-statistic as a goodness-of-fit measure.[11] The Z test was used to assess whether an APC was significantly different from 0 at the α = 0.05 level. In describing trends, we used the terms “increase” or “decrease” when the P value was <0.05, and we used the term “stable” to indicate no significant change when the P value was ≥0.05.
Results
Total cases, new infections, and deaths
As shown in Table 1, total HIV/AIDS cases increased from 76,134 (95% CI: 56,869, 96,289) in 1990 to 642,278 (95% CI: 332,101, 1,171,749) in 2015 and then fell slightly to 633,923 (95% CI: 312,439, 1,209,871) in 2017. Total new HIV infections (ie, new cases) increased from 11,267 (95% CI: 7496, 19,908) in 1990 to 71,368 (95% CI: 46,485, 107,199) in 2005, and then decreased to 33,329 (95% CI: 13,802, 57,284) in 2017. Total deaths increased from 3337 (95% CI: 2502, 4034) in 1990 to 34,800 (95% CI: 32,582, 36,553) in 2017.
Table 1.
HIV/AIDS total cases and prevalence, new cases, and incidence, and deaths and mortality for China, 1990 to 2017, from the GBD, Injuries, and Risk Factors Study 2017 data.
| HIV/AIDS cases | New HIV infections | AIDS-related deaths | ||||
| Year | Total cases, n (95% CI) | Prevalence rate, per 100,000 (95% CI) | Total new cases, n (95% CI) | Incidence rate, per 100,000 (95% CI) | Total deaths, n (95% CI) | Mortality rate, per 100,000 (95% CI) |
| 1990 | 76,134 (56,869, 96,289) | 6.36 (4.75, 8.04) | 11,267 (7496, 19,908) | 0.94 (0.63, 1.66) | 3337 (2502, 4034) | 0.28 (0.21, 0.34) |
| 1991 | 85,699 (65,338, 106,638) | 7.07 (5.39, 8.80) | 12,732 (8846, 20,744) | 1.05 (0.73, 1.71) | 4247 (3435, 4888) | 0.35 (0.28, 0.40) |
| 1992 | 96,262 (75,468, 120,166) | 7.87 (6.17, 9.82) | 14,455 (10,318, 22,203) | 1.18 (0.84, 1.81) | 5037 (4276, 5561) | 0.41 (0.35, 0.45) |
| 1993 | 107,640 (85,103, 136,652) | 8.71 (6.89, 11.06) | 16,399 (11,707, 24,224) | 1.33 (0.95, 1.96) | 5885 (5232, 6290) | 0.48 (0.42, 0.51) |
| 1994 | 119,312 (93,075, 156,467) | 9.57 (7.47, 12.55) | 18,424 (13,015, 27,702) | 1.48 (1.04, 2.22) | 6518 (5979, 6835) | 0.52 (0.48, 0.55) |
| 1995 | 131,068 (100,371, 179,438) | 10.43 (7.99, 14.28) | 20,410 (13,947, 30,999) | 1.62 (1.11, 2.47) | 7574 (7142, 7879) | 0.60 (0.57, 0.63) |
| 1996 | 144,042 (108,378, 200,637) | 11.38 (8.56, 15.85) | 23,115 (15,522, 35,179) | 1.83 (1.23, 2.78) | 8103 (7733, 8413) | 0.64 (0.61, 0.66) |
| 1997 | 159,454 (117,114, 225,211) | 12.51 (9.19, 17.67) | 27,102 (17,633, 41,565) | 2.13 (1.38, 3.26) | 8768 (8455, 9082) | 0.69 (0.66, 0.71) |
| 1998 | 176,795 (125,942, 256,030) | 13.78 (9.82, 19.95) | 31,828 (20,015, 49,471) | 2.48 (1.56, 3.86) | 9769 (9455, 10,118) | 0.76 (0.74, 0.79) |
| 1999 | 195,289 (134,271, 289,920) | 15.13 (10.40, 22.46) | 36,712 (22,362, 58,394) | 2.84 (1.73, 4.52) | 10,580 (10,248, 10,966) | 0.82 (0.79, 0.85) |
| 2000 | 214,263 (142,915, 322,802) | 16.50 (11.00, 24.86) | 41,066 (24,230, 65,897) | 3.16 (1.87, 5.07) | 11,428 (11,061, 11,872) | 0.88 (0.85, 0.91) |
| 2001 | 242,064 (157,525, 366,078) | 18.53 (12.06, 28.03) | 46,316 (27,785, 73,062) | 3.55 (2.13, 5.59) | 12,664 (12,250, 13,131) | 0.97 (0.94, 1.01) |
| 2002 | 282,952 (177,989, 435,711) | 21.55 (13.56, 33.18) | 53,476 (32,747, 82,283) | 4.07 (2.49, 6.27) | 13,850 (13,331, 14,450) | 1.05 (1.02, 1.10) |
| 2003 | 329,400 (202,015, 515,780) | 24.95 (15.30, 39.07) | 61,159 (38,364, 93,453) | 4.63 (2.91, 7.08) | 14,890 (14,231, 15,699) | 1.13 (1.08, 1.19) |
| 2004 | 373,075 (223,569, 597,148) | 28.12 (16.85, 45.00) | 67,767 (43,403, 103,056) | 5.11 (3.27, 7.77) | 15,763 (15,277, 16,347) | 1.19 (1.15, 1.23) |
| 2005 | 404,644 (238,354, 661,768) | 30.34 (17.87, 49.62) | 71,368 (46,485, 107,199) | 5.35 (3.49, 8.04) | 17,577 (17,088, 18,153) | 1.32 (1.28, 1.36) |
| 2006 | 435,436 (256,339, 723,873) | 32.48 (19.12, 54.00) | 70,999 (47,468, 107,111) | 5.30 (3.54, 7.99) | 18,368 (17,680, 19,260) | 1.37 (1.32, 1.44) |
| 2007 | 478,302 (278,616, 798,666) | 35.50 (20.68, 59.27) | 67,827 (46,229, 102,571) | 5.03 (3.43, 7.61) | 18,508 (17,913, 19,371) | 1.37 (1.33, 1.44) |
| 2008 | 525,031 (300,501, 886,550) | 38.77 (22.19, 65.46) | 63,184 (43,751, 98,049) | 4.67 (3.23, 7.24) | 19,531 (19,080, 20,126) | 1.44 (1.41, 1.49) |
| 2009 | 567,034 (314,173, 967,395) | 41.66 (23.08, 71.08) | 58,696 (40,415, 93,026) | 4.31 (2.97, 6.84) | 20,348 (19,805, 21,117) | 1.50 (1.46, 1.55) |
| 2010 | 593,487 (314,702, 1,014,572) | 43.41 (23.02, 74.21) | 55,710 (38,323, 89,621) | 4.07 (2.80, 6.55) | 19,893 (19,401, 20,512) | 1.46 (1.42, 1.50) |
| 2011 | 607,907 (321,602, 1,048,495) | 44.28 (23.43, 76.38) | 53,692 (36,830, 85,397) | 3.91 (2.68, 6.22) | 19,640 (19,119, 20,202) | 1.43 (1.39, 1.47) |
| 2012 | 620,684 (328,098, 1,083,056) | 45.03 (23.81, 78.58) | 51,245 (34,198, 80,731) | 3.72 (2.48, 5.86) | 18,532 (17,807, 19,259) | 1.34 (1.29, 1.40) |
| 2013 | 631,128 (332,162, 1,117,080) | 45.58 (23.99, 80.68) | 48,440 (31,608, 76,832) | 3.50 (2.28, 5.55) | 19,637 (18,778, 20,455) | 1.42 (1.36, 1.48) |
| 2014 | 638,639 (333,318, 1,142,642) | 45.90 (23.95, 82.12) | 45,174 (27,315, 72,066) | 3.25 (1.96, 5.18) | 23,889 (22,695, 24,835) | 1.72 (1.63, 1.78) |
| 2015 | 642,278 (332,101, 1,171,749) | 45.97 (23.77, 83.87) | 41,408 (23,387, 67,620) | 2.96 (1.67, 4.84) | 28,950 (27,826, 29,828) | 2.07 (1.99, 2.14) |
| 2016 | 640,944 (325,469, 1,194,440) | 45.66 (23.18, 85.09) | 37,505 (18,440, 62,693) | 2.67 (1.31, 4.47) | 32,879 (31,475, 34,068) | 2.34 (2.24, 2.43) |
| 2017 | 633,923 (312,439, 1,209,871) | 44.88 (22.12, 85.66) | 33,329 (13,802, 57,284) | 2.36 (0.98, 4.06) | 34,800 (32,582, 36,553) | 2.46 (2.31, 2.59) |
CI: Confidence interval; GBD: Global Burden of Diseases; HIV/AIDS: Human immunodeficiency virus/acquired immunodeficiency syndrome.
Prevalence, incidence, and mortality
HIV/AIDS prevalence increased from 6.36 per 100,000 (95% CI: 4.75, 8.04) in 1990 to 45.97 per 100,000 (95% CI: 23.77, 83.87) in 2015, and then declined slightly to 44.88 per 100,000 (95% CI: 22.12, 85.66) in 2017. HIV incidence increased from 0.94 per 100,000 (95% CI: 0.63, 1.66) in 1990 to 5.35 per 100,000 (95% CI: 3.49, 8.04) in 2005, and then decreased to 2.36 per 100,000 (95% CI: 0.98, 4.06) in 2017. AIDS-related mortality rate increased over the entire 28-year study period, from 0.28 per 100,000 (95% CI: 0.21, 0.34) in 1990 to 2.46 per 100,000 (95% CI: 2.31, 2.59) in 2017 [Table 1].
Sex differences
As shown in Figure 1, HIV/AIDS prevalence was higher and increased more among males than females during the study period. Prevalence increased from 8.52 per 100,000 (95% CI: 6.33, 10.77) in 1990 to 65.05 per 100,000 (95% CI: 32.29, 123.71) in 2017 among males, and from 4.05 per 100,000 (95% CI: 3.07, 5.05) in 1990 to 23.84 per 100,000 (95% CI: 11.44, 45.46) in 2017 among females. HIV incidence peaked in 2005 for both males and females. For males, HIV incidence increased from 1.24 per 100,000 (95% CI: 0.83, 2.21) in 1990 to 7.71 per 100,000 (95% CI: 4.97, 11.56) in 2005, and then declined to 3.67 per 100,000 (95% CI: 1.51, 6.37) in 2017. For females, incidence increased from 0.62 per 100,000 (95% CI: 0.41, 1.08) in 1990 to 2.86 per 100,000 (95% CI: 1.91, 4.37) in 2005, and then it declined to 0.99 per 100,000 (95% CI: 0.44, 1.65) in 2017. AIDS-related mortality rate among males increased from 0.36 per 100,000 (95% CI: 0.26, 0.44) in 1990 to 3.63 per 100,000 (95% CI: 3.40, 3.82) in 2017, and among females increased from 0.19 per 100,000 (95% CI: 0.15, 0.23) in 1990 to 1.25 per 100,000 (95% CI: 1.17, 1.32) in 2017.
Figure 1.
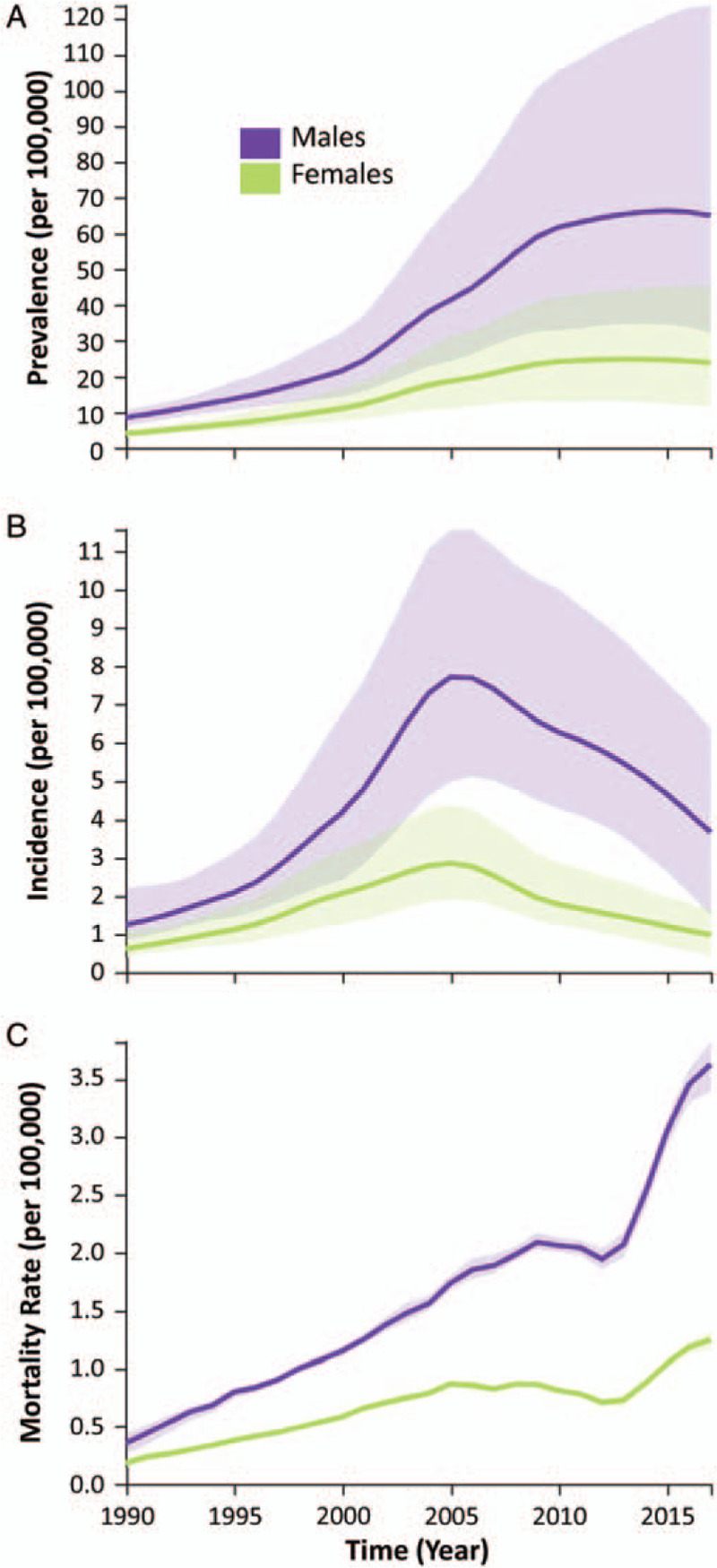
HIV/AIDS prevalence (A), incidence (B), and mortality rate (C) by sex, for all ages, in China, 1990 to 2017, from the GBD, Injuries, and Risk Factors Study 2017 data. Estimated values are shown as solid lines, while 95% CIs are indicated by the shaded regions. CIs: Confidence intervals; GBD: Global Burden of Diseases; HIV/AIDS: Human immunodeficiency virus/acquired immunodeficiency syndrome.
Trends
As shown in Figure 2, joinpoint regression analysis identified trends in prevalence, incidence, and mortality rate during the 28-year study period that were significantly different from 0. For HIV/AIDS prevalence, a significant increase was detected for 1990 to 2009 (APC: 10.7; 95% CI: 10.4, 11.0; P < 0.001), while the rate remained stable for 2009 to 2017 (APC: 0.7; 95% CI: −0.3, 1.7; P = 0.1). For HIV incidence, a significant increase was detected for 1990 to 2005 (APC: 13.0; 95% CI: 12.6, 13.4; P < 0.001), and then a significant decrease was detected for 2005 to 2017 (APC: −6.5; 95% CI: −7.0, −6.1; P < 0.001). For AIDS-related mortality rate, a significant increase was detected for 1990 to 2004 (APC: 10.3; 95% CI: 9.3, 11.3; P < 0.001), followed by a period of stability for 2004 to 2013 (APC: 1.3; 95% CI: −0.7, 3.3; P = 0.2), and then another significant increase for 2013 to 2017 (APC: 15.3; 95% CI: 8.7, 22.2; P < 0.001).
Figure 2.
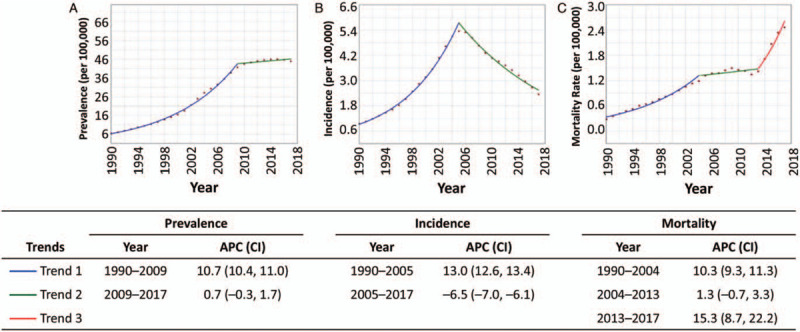
Trends in HIV/AIDS prevalence (A), incidence (B), and mortality rate (C) in China, 1990 to 2017, from the GBD, Injuries, and Risk Factors Study 2017 data. APC was significantly different from 0 at the α = 0.05 level for trend 1 (blue) prevalence (A), trend 1 (blue) and trend 2 (green) for incidence (B), and trend 1 (blue) and trend 3 (red) for mortality (C). APC: Annual percentage change; CI: Confidence interval; GBD: Global Burden of Diseases; HIV/AIDS: Human immunodeficiency virus/acquired immunodeficiency syndrome.
Discussion
The main findings of this study were the increasing (1990–2009) and then stabilizing (2009–2017) trends in HIV/AIDS prevalence, the increasing (1990–2005) and then decreasing (2005–2017) trends in HIV incidence, and the increasing (1990–2004), then stabilizing (2004–2013), and then increasing (2013–2017) trends in AIDS-related mortality rate [Figure 2]. This finding is enriched by our observations of notably large differences in prevalence, incidence, and mortality rate between males and females. Although females generally display the same shapes in the curves of the three metrics over time, males have much higher amplitude effects. This is particularly evident for the incidence, which peaked in 2005 at 7.71 per 100,000 for males vs. 2.86 per 100,000 for females, and mortality rate, which climbed to 3.63 per 100,000 in males vs. 1.25 per 100,000 in females in 2017 [Figure 1].
China has made substantial progress in the development and implementation of effective intervention strategies through its comprehensive National HIV/AIDS Response Program over the past 17 years.[3,12] Undoubtedly, these massive efforts have positively impacted the three metrics of interest examined in this study — prevalence, incidence, and mortality rate. However, in comparison to the global picture,[9] China is clearly falling behind with respect to AIDS-related mortality, particularly among men. This finding is supported by both government data and results of observational studies,[4–6,8,12,13] indicating that there are several aspects of China's National HIV/AIDS Response Program that must be improved to combat high and rising AIDS-related mortality.
First, China must find and diagnose PLWH earlier and faster. The unfortunate problem of late presentation (ie, diagnosis when CD4 counts are already very low or clinical signs of AIDS are already present) persists in China and contributes significantly to AIDS-related mortality.[14,15] One recent study reported that the mean duration of infection at the time of diagnosis for nearly 6000 Chinese PLWH newly diagnosed between 2008 and 2015 was 6.3 years.[16] Another new study reported that among >45,000 PLWH newly diagnosed between 2012 and 2016, 70% presented late and 45% had advanced HIV disease.[17] Earlier case finding and rapid diagnosis depends heavily on both the availability of quality testing services in a variety of formats and on the uptake of testing services by those who need them.[12] Rapid scale-up of testing services,[3,12] and streamlining of the process from screening to diagnosis to treatment initiation,[18,19] has been ongoing in China for some years, yet an enormous number of PLWH remain undiagnosed—an estimated 360,000 at the end of 2018.[3] Case finding and linking to care must be a focus of a renewed agenda within the National HIV/AIDS Response Program.
Second, China must rapidly get PLWH into treatment and durably retain them there through the provision of quality treatment services, monitoring, and support. The many important individual and community benefits of early treatment (ie, before disease progression) are now well accepted internationally.[20] However, in settings like China, where late presentation is still common, the focus must also be on immediate treatment (ie, without delay after diagnosis).[21–23] Although shortening of time from screening to treatment has proven benefits in the China setting that include reductions in mortality rates,[18,19,23] implementation of these procedural changes in China's hospitals and clinics remains incomplete and PLWH still slip through the cracks, still too often failing to initiate treatment quickly or at all. Faster treatment starts must also be an area of focus for the future of China's National HIV/AIDS Response Program.
Third, China must deal with the constant challenge of treatment retention and must finally develop a program for treatment “recapture.” The first investigation into the performance of China's HIV care continuum found that in 2015, only 67% of PLWH who were diagnosed were on treatment.[24] While some of these untreated individuals never initiated treatment, some initiated and then dropped out or discontinued treatment.[13,25,26] Since without treatment, all PLWH will eventually progress to AIDS and die, it is of vital importance to improve treatment retention. While this will require fundamental changes to the ways in which treatment is delivered, it must also include planful, deliberate methods for supporting PLWH on antiretroviral therapy over long periods. Less well recognized is the similarly urgent need to implement an effective mechanism to identify and actively reach out to PLWH who were lost to follow-up or who dropped out or ceased treatment, to try and get them re-started. China must reconsider how best to keep as many PLWH on treatment as long as possible with as few interruptions as possible if it is to improve the current trend in mortality rates.
Fourth, China must urgently work to optimize HIV treatment, care, and monitoring to improve the effectiveness of treatment and slow the growing of drug resistance. In 2015, only 65% of diagnosed PLWH on treatment were virally suppressed.[24] Some of this gap is known to be caused by poor adherence to treatment whereas some have been attributed to outdated drugs, formulations, and regimens.[27,28] Although improving, viral load and drug resistance testing for monitoring the effectiveness of treatment are still sub-optimal and virological failure continues to be under-diagnosed as well as under-treated since options for the second- and third-line therapies are still limited.[12,27–29] All aspects of treatment, care, and monitoring must also be central to meaningful evaluation and improvement of China's National HIV/AIDS Response Program.
Finally, special attention must be paid to the unique barriers to care experienced by different subgroups of Chinese men: men who have sex with men, men who self-identify as heterosexual, older men, transgender men, migrant worker men, and others.[3,12] These issues must be thoroughly researched and interventions that aim to reduce barriers to all steps along the HIV care continuum must be designed, tested, and broadly implemented. Some evidence from other settings suggests that female PLWH benefit more from treatment than male PLWH, which then contributes to sex differences in AIDS-related mortality rates.[30,31] This observation must be evaluated in the China setting and if sex differences in the effectiveness of treatment are confirmed, then the reasons behind it must be investigated. Modernization of recognized high-risk groups, thorough and current understanding of their barriers to success in the HIV care continuum, and interventions to facilitate their achievement of sustained viral suppression must also be prioritized if China is to bring down AIDS-related mortality rates.
Several limitations of our study should be noted. First, we only included GBD 2017 data in our study. The GBD estimates are based on aggregated data and are adjusted via a back-estimation from mortality data. These inherent limitations in the data cause some uncertainties in the estimates that are larger in more recent years.[9] However, we have presented estimates of these CIs for all measures included in our study. Furthermore, by limiting our study to GBD 2017 data only, we did not account for other sources of data, which could have improved our understanding of these trends. Second, we did not include all GBD 2017 measures in our study. For example, we excluded GBD 2017 estimates of HIV/AIDS-associated DALYs.[9] As HIV/AIDS has evolved into a chronic disease that is manageable with treatment over the long-term, DALYs has become an increasingly relevant measure of the burden of HIV/AIDS and should be included in future research.
In conclusion, although our results show that prevalence has stabilized and incidence has declined, we have found that AIDS-related mortality has risen sharply in recent years. This evidence indicates that more must be done to bring people into treatment earlier and faster, retain them in treatment more effectively, actively seek to restart them on treatment if they dropout, and revise their treatment if it fails. Furthermore, the quality of treatment and care regimens as well as treatment monitoring must be improved. China needs to intensify its efforts if it is to meaningfully reduce AIDS-related mortality and bring its HIV/AIDS epidemic under control.
Funding
This work was supported by a grant from the National Health Commission of the People's Republic of China (No. 2018ZX10721102).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu XJ, McGoogan JM, Wu ZY. Human immunodeficiency virus/acquired immunodeficiency syndrome prevalence, incidence, and mortality in China, 1990 to 2017: a secondary analysis of the Global Burden of Disease Study 2017 data. Chin Med J 2021;134:1175–1180. doi: 10.1097/CM9.0000000000001447
References
- 1.UNAIDS. Global HIV & AIDS Statistics — 2020 Fact Sheet. Geneva: Joint United Nations Programme on HIV/AIDS; 2020. Available from: https://www.unaids.org/en/resources/fact-sheet. [Accessed September 15, 2020] [Google Scholar]
- 2.UNAIDS. UNAIDS Data 2019. Geneva: Joint United Nations Programme on HIV/AIDS, 2019. Available from: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data. [Accessed September 15, 2020] [Google Scholar]
- 3.Wu Z, McGoogan JM, Detels R. The enigma of the HIV epidemic in China. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa835. [Ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Chen J, Scott SR, McGoogan JM. History of the HIV epidemic in China. Curr HIV/AIDS Rep 2019; 16:458–466.doi: 10.1007/s11904-019-00471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summary of 2019 Notifiable Diseases in China (in Chinese). Beijing: National Health Commission; 2020. Available from: http://www.nhc.gov.cn/jkj/s3578/202004/b1519e1bc1a944fc8ec176db600f68d1.shtml. [Accessed September 15, 2020] [Google Scholar]
- 6.Gao D, Zou Z, Dong B, Zhang W, Chen T, Cui W, et al. Secular trends in HIV/AIDS mortality in China from 1990 to 2016: gender disparities. PLoS One 2019; 14:e0219689.doi: 10.1371/journal.pone.0219689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Wang L, Burgner DP, Miller JE, Song Y, Ren X, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ 2020; 369:m1043.doi: 10.1136/bmj.m1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Shi O, Yan Q, Fang Q, Zuo J, Chen Y, et al. Changing epidemiological patterns of HIV and AIDS in China in the post-SARS era identified by the nationwide surveillance system. BMC Infect Dis 2018; 18:700.doi: 10.1186/s12879-018-3551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2017 HIV Collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019; 6:E831–E859. doi: 10.1016/S2352-3018(19)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover J, Brown T, Marston M. Updates to the spectrum/estimation and projection package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect 2012; 88:i11–i16. doi: 10.1136/sextrans-2012-050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19:335–351.doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Wang Y, Detels R, Bulterys M, McGoogan JM. Springer Nature, HIV/AIDS in China — Epidemiology, Prevention and Treatment. Singapore: 2020. [Google Scholar]
- 13.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med 2009; 151:241–251. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Tang W, Han Z, Tangthanasup TM, Zhong F, Qin F, et al. Late presentation of HIV infection: prevalence, trends, and the role of HIV testing strategies in Guangzhou, China, 2008–2013. Biomed Res Int 2016; 2016:1631878.doi: 10.1155/2016/1631878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J, Hsieh E, Sun M-Q, Wang H-L, Lv W, Fan H-W, et al. Delays in HIV diagnosis and associated factors among patients presenting with advanced disease at a tertiary care hospital in Beijing, China. PLoS One 2017; 12:e0182335.doi: 10.1371/journal.pone.0182335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A-H, Wu Z-Y, Jiang Z, McGoogan JM, Zhao Y, Duan S. Duration of human immunodeficiency virus infection at diagnosis among new human immunodeficiency virus cases in Dehong, Yunnan, China, 2008–2015. Chin Med J 2018; 131:1936–1943.doi: 10.4103/0366-6999.238152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Liang B, Zhou C, Jiang J, Huang J, Ning C, et al. HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther 2019; 16:6.doi: 10.1186/s12981-019-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Zhao Y, Ge X, Mao Y, Tang Z, Shi CX, et al. Simplified HIV testing and treatment in China: analysis of mortality rates before and after a structural intervention. PLoS Med 2015; 12:e1001874.doi: 10.1371/journal.pmed.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Tang Z, Mao Y, Van Veldhuisen P, Ling W, Liu D, et al. Testing and linkage to HIV care in China: a cluster-randomised trial. Lancet HIV 2017; 4:E555–E565. doi: 10.1016/S2352-3018(17)30131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd Edn. Geneva: World Health Organization; 2016. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/. [Accessed September 15, 2020] [PubMed] [Google Scholar]
- 21.Zhao Y, Wu Z, McGoogan JM, Shi CX, Li A, Dou Z, et al. Immediate antiretroviral therapy decreases mortality among patients with high CD4 counts in China: a nationwide, retrospective cohort study. Clin Infect Dis 2018; 66:727–734. doi: 10.1093/cid/cix878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Wu Z, McGoogan JM, Sha Y, Zhao D, Ma Y, et al. Nationwide cohort study of antiretroviral therapy timing: treatment dropout and virological failure in China, 2011–2015. Clin Infect Dis 2019; 68:43–50. doi: 10.1093/cid/ciy400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, McGoogan JM, Wu Z. The benefits of immediate ART. J Int Assoc Provid AIDS Care 2019; 18:2325958219831714.doi: 10.1177/2325958219831714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Dou Z, Guo W, Mao Y, Zhang F, McGoogan JM, et al. The human immunodeficiency virus care continuum in China: 1985–2015. Clin Infect Dis 2018; 66:833–839. doi: 10.1093/cid/cix911. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Napravnik S, Eron J, Cole S, Ma Y, Wohl D, et al. Attrition among human immunodeficiency virus (HIV)-infected patients initiating antiretroviral therapy in China, 2003–2010. PLoS One 2012; 7:e39414.doi: 10.1371/journal.pone.0039414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu D, Mao Y, Tang Z, Montaner J, Shen Z, Zhu Q, et al. Loss to follow-up from HIV screening to ART initiation in rural China. PLoS One 2016; 11:e0164346.doi: 10.1371/journal.pone.0164346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Wang N, Vermund SH, Zou H, Li X, Zhang F, et al. Interventions to improve the HIV continuum of care in China. Curr HIV/AIDS Rep 2019; 16:448–457. doi: 10.1007/s11904-019-00469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining challenges. Curr HIV/AIDS Rep 2020; 17:26–34. doi: 10.1007/s11904-019-00478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Dou Z, McGoogan JM, Wu Z. Reply to Zhang et al. Clin Infect Dis 2018; 67:809–810. doi: 10.1093/cid/ciy164. [DOI] [PubMed] [Google Scholar]
- 30.Takarinda KC, Harries AD, Shiraishi RW, Mutasa-Apollo T, Abdul-Quader A, Mugurungi O. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. Int J Infect Dis 2015; 30:98–105. doi: 10.1016/j.ijid.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med 2015; 12:e1001905.doi: 10.1371/journal.pmed.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]


