Abstract
Background:
Compared to adult studies, studies which involve the treatment of pediatric congenital hypogonadotropic hypogonadism (CHH) are limited and no universal treatment regimen is available. The aim of this study was to evaluate the feasibility of human chorionic gonadotropin (hCG)/human menopausal gonadotropin (hMG) therapy for treating male adolescents with CHH.
Methods:
Male adolescent CHH patients were treated with hCG/hMG (n = 20) or a gonadotropin-releasing hormone (GnRH) pump (n = 21). The treatment was divided into a study phase (0–3 months) and a follow-up phase (3–12 months). The testicular volume (TV), penile length (PL), penis diameter (PD), and sex hormone levels were compared between the two groups. The TV and other indicators between the groups were analyzed using a t-test (equal variance) or a rank sum test (unequal variance).
Results:
Before treatment, there was no statistical difference between the two groups in terms of the biochemistry, hormones, and other demographic indicators. After 3 months of treatment, the TV of the hCG/hMG and GnRH groups increased to 5.1 ± 2.3 mL and 4.1 ± 1.8 mL, respectively; however, the difference was not statistically significant (P > 0.05, t = 1.394). The PL reached 6.9 ± 1.8 cm and 5.1 ± 1.6 cm (P < 0.05, t = 3.083), the PD reached 2.4 ± 0.5 cm and 2.0 ± 0.6 cm (P < 0.05, t = 2.224), respectively, in the two groups. At the end of 6 months of treatment, biomarkers were in normal range in the two groups. Compared with the GnRH group, the testosterone (T) level and growth of PL and PD were significantly greater in the hCG/hMG group (all P < 0.05). While the TV of both groups increased, the difference was not statistically significant (P > 0.05, t = 0.314). After 9 to 12 months of treatment, the T level was higher in the hCG/hMG group. Other parameters did not exhibit a statistical difference.
Conclusions:
The hCG/hMG regimen is feasible and effective for treating male adolescents with CHH. The initial 3 months of treatment may be a window to optimally observe the strongest effects of therapy. Furthermore, results from the extended time-period showed positive outcomes at the 1-year mark; however, the long-term effectiveness, strengths, and weaknesses of the hCG/hMG regimen require further research.
Trial Registration:
ClinicalTrials.gov, NCT02880280; https://clinicaltrials.gov/ct2/show/NCT02880280.
Keywords: Congenital hypogonadotropic hypogonadism, Gonadotropin-releasing hormone, Human chorionic gonadotropin, Human menopausal gonadotropin, Treatment regime
Introduction
Congenital hypogonadotropic hypogonadism (CHH) is a disorder that results from the insufficient release and secretion of gonadotropin-releasing hormone (GnRH).[1] Two subtypes of CHH exist; they are Kallmann syndrome (KS), which is associated with olfactory dysfunction, and normosmic idiopathic hypogonadotropich hypogonadism (nIHH), which retains intact olfactory function without other intracranial pathologies. The incidence of CHH is in the range of 1 to 10/100,000 and the male to female ratio is 5:1.[2] There are many clinical treatment options for CHH, the most common among which involves the usage of a GnRH pump, the combination of human chorionic gonadotropin (hCG)/human menopausal gonadotropin (hMG) (follicle stimulating hormone [FSH]) and testosterone replacement.[3] Notably, testosterone replacement can promote the development of secondary sexual characteristics in patients, but unfortunately cannot improve fertility. Obtaining fertility is a very high priority for Chinese patients; however, our previous therapeutic experience indicates that the application of hCG alone is not sufficient to ensure this outcome.[4] On the other hand, hCG/hMG injection and GnRH pump treatment can promote the production of testosterone and sperm in the testes.[5,6] Compared to patients with acquired hypogonadism, gonadotropin deficiency occurs in the fetal period for CHH patients, and abnormalities such as cryptorchidism, a small penis, and scrotal dysplasia can be observed at birth.[7] CHH patients also usually show a poor response to the application of spermatogenic therapy when they are in adulthood.[8] Nevertheless, the dose of hCG for CHH pre-treatment may also predict future therapeutic effects.[9] Recombinant human follicle-stimulating hormone (r-hFSH) has been approved for CHH-treatment in many countries because exogenous FSH can stimulate Sertoli cells and spermatogonia, increase levels of anti-Müllerian hormone (AMH), and elevate testicular volume (TV) and sperm density.[1,5,10] However, there may still be testicular dysfunction in children with CHH, and if FSH is initially applied, the testes may not develop in some children. Considering the high cost of FSH in China, we chose to use hCG pre-treatment in the clinic and combined it with monitoring of testosterone levels. If testicular function appears normal, hMG (or FSH) may be applied to further induce puberty.
Compared to adult studies, those involving the treatment of pediatric CHH are limited and no universal treatment regimen is available. A multicenter study in Germany in 60 children aged 14 to 22 years indicated that subcutaneous injection of hCG/r-hFSH may be a safe and valid treatment option based on the positive therapeutic effects, compared to testosterone treatment.[11] Despite these positive results, the details of the same therapy in each treatment center are generally dissimilar.[11] Some scholars propose a treatment protocol that begins with low-doses of gonadotropin at a timing which is closer to the onset of puberty (namely, a timing which coincides with entrance into junior high school).[12] However, this idea is not easily adherent because puberty is a complicated and long process, and proof for the effectiveness of treatments which involve low-dose gonadotropin-administration cannot be expected in a short time-period. In addition, it is not easy to effectively compare or summarize treatment effects because the treatment regimen varies across patients according to individual needs.
Regarding the pituitary hormone pump, the cost is high, it is inconvenient and difficult to conceal, and it does not offer much privacy. For these reasons, many adolescent patients prefer hCG/hMG injection over the pituitary hormone pump. Therefore, the goal of this study was to establish a standardized, simple hCG/hMG treatment scheme for adolescent males with CHH. This regimen is simple and effective over a short time-period and can aid in increasing adherence.
Furthermore, puberty development in boys begins at the age of 9 to 14 years, with 12 years being the median age for full development. Patients with CHH at this age have been subject to preliminary study at the Beijing Children's Hospital, National Center for Children's Health, Beijing.[4] We hope that through therapeutic research, the treatment age of CHH can be extended to 12 years old; doing so would ensure that penile development in similarly-aged children is similar and can reduce the psychological burden of such children and their parents. In this retrospective cohort study, we therefore also compared the efficacy and safety, as well as the impact on growth and development, of the two previously mentioned treatment regimens in an adolescent population.[13,14]
Methods
Ethical approval
The study was approved by the Ethics Committee of Capital Medical University (No. 2016-Y-005-D). All patients provided written informed consent.
Patients
A total of 41 CHH patients aged between 12 and 18 years were enrolled in the current study from 2008 to 2019 in the Beijing Children's Hospital. The GnRH pump therapy has been used since 2008 and the hCG/hMG regimen started in 2016. We developed the treatment regimen by considering the average age of Chinese juvenile spermatorrhea (13–14 years)[15,16] as well as the expert consensus on the diagnosis and treatment of idiopathic hypogonadotropic hypogonadism.[5] Thus, administration of hCG/hMG began at an age older than 12 years and at a bone age (BA) of 12 years.
Inclusion criteria
The inclusion and exclusion criteria used in the current study were similar to those previously described.[17] The inclusion criteria of the older group (>14-year-old boy) were as follows: (1) no puberty development, male TV <4 mL; may be accompanied by a small penis and/or cryptorchidism; (2) BA ≥12 years; (3) baseline serum testosterone lower than that of normal adolescent males; (4) basal gonadotropin level not elevated (luteinizing hormone [LH] <0.1 IU/L, FSH at normal prepubertal level); and (5) 46 XY karyotype.
The inclusion criteria of the 12 to 14-years-old group were children who were diagnosed with KS or nIHH, combined with the following: (1) conditions 2 to 5 of the older group; (2) small penis and/or cryptorchidism, as well as olfactory abnormalities, ascertained from imaging findings of olfactory bulb, olfactory bundle, or other dysplasia; and (3) the luteinizing hormone releasing hormone test showing hypothalamic-pituitary-gonadal axis silence when BA >12 years. Due to the different phenotypes of KS, some patients who experienced puberty development, who exhibited olfactory abnormalities, who produced images with clear olfactory bulb, olfactory bundle, or other dysplasia, or who had a TV >4 mL or a basic testosterone level <100 ng/L (but no puberty progression for half of a year) were included in this study as well.
Exclusion criteria
Those patients who had (1) clear causes (such as chromosomal abnormalities, trauma surgery, etc) or other diseases caused by various types of high gonadotropia or sexual dysplasia (such as Klinefelter syndrome, etc), (2) chronic systemic diseases (such as uremia, thalassemia, or poorly controlled diabetes), (3) protein-energy malnutrition or eating disorders (including anorexia nervosa and appetite hyperactivity), (4) CHH and had been discontinuously administered androgen replacement therapy or other gonadotropin treatment for <1 month, or (5) intracranial space-occupying lesions or pituitary tumors with incomplete treatment of the primary disease were excluded in this study.
Treatment regimen
Patients in the two groups were followed up every 3 months. Height and body weight were recorded every 6 months during the course of treatment. Treatment effectiveness was assessed with TV, sex hormones, occurrence of nocturnal emission, penile length (PL), and change of scrotal skin. The treatment regimen was designed and modified based on “Expert consensus on the diagnosis and treatment of idiopathic hypogonadotropic hypogonadism.”[5] We referred to studies which recommended adult hCG doses in the range of 4000 to 6000 U per week, combined with 75 to 150 U of hMG 2 to 3 times per week.[1,4,5] The actual total dose was less than the recommended dose for adults because multiple low doses were more in line with physiological needs. For the hCG/hMG group, hCG was injected muscularly as follows: for the first 3 months (pre-treatment phase), 1000 to 2000 U of hCG was injected intramuscularly once every other day or twice per week. This injection volume was started at a low dose and was gradually increased until the testosterone level reached 200 ng/dL. Following this, and to start the formal therapeutic stage, hMG containing 75 U of FSH and 75 U of LH was administered once per day. In practice, the dosage of hCG was adjusted to maintain the testosterone value at 200 to 500 ng/dL to the extent possible.
The GnRH group was continuously treated with a GnRH pump. The dose of GnRH was chosen according to adult hypogonadotropic hypogonadism (HH) guidelines and our previous research.[1,4] Briefly, 8 to 10 μg of GnRH (200 μg/mL) were subcutaneously injected every 90 min via an injection pump. If side effects (described below) occurred, the dose of the GnRH was reduced.
During treatment, we carefully monitored adverse reactions, such as breast development, self-perceived excessive acne, frequent (>1 time per day) penile erection, erectile pain, skin infection and discomfort at the injection site, liver dysfunction, renal dysfunction, electrolyte abnormalities, and other autonomic reactions.
Additional treatment
We recommended a normal balanced diet and regular physical exercise during the course of treatment. If patients suffered from other acute sicknesses during treatment, such as respiratory infections, they were advised to follow the recommended medical order without stopping the hCG/hMG or the GnRH pump treatment.
Physical signs and parameters of therapy efficacy
The results of the physical examinations performed by the same clinician were recorded by following similar protocols as are described in the literature (room temperature of 22 to 28°C, no external interference) for all patients.[4] The times of occurrence of voice-change, height, weight, pubic hair, armpit hair, and external genital development, including the volume of testes, were also recorded. Sex hormones, AMH, Inhibin B (Inh-B), blood and urine routines, and other laboratory tests were performed. The volume of the testes was measured by an orchidometer, which contained a series of beads with different sizes. The Prader testicular model was used to measure the testes’ volume, including the scrotum. The measurement was made when the scrotal skin was squeezed tight. Following this, the testes were compared to the beads until a similar size of the bead was found, the volume of which was used to estimate the size of the testes. After emptying the bladder, measurement of the PL was performed as the length from the pubic symphysis to the tip of the glans in a standing position with penile erection.[10]
The TV was selected as the major efficacy indicator and PL, penile diameter (PD), testosterone (T), LH, FSH, AMH, and Inh-B concentration were considered as secondary efficacy indicators.
Indicators of therapy safety
The therapeutic and side effects, as described above, were monitored during the entire period. At the end of the study, in addition to these indicators, tumor indicators, thyroid function, insulin-like growth factor (IGF)-1, IGF-binding proteins 3, BA, chest X-ray, and electrocardiogram were examined as well.
Compliance evaluation
Compliance was evaluated according to the follow-up data and the number of interruptions during the 3-month treatment period. Treatment interruptions in the range of 0 to 18 days (<20% of the 90 days) of the total 3-month period were considered as good compliance. However, >18 days of interruption was considered poor compliance.
Quality control
The quality control of this study was the responsibility of various separate experimenters. For example, the experimental design was completed by the corresponding author and the first author, the outpatient service was processed by the corresponding author and trained research fellows, and the follow-up sessions were performed by the first author via telephone communication.
The follow-up data were collected from the outpatient system and recorded in Excel, manuscript writing was completed by the first author under the guidance of the tutor, and data input was double-checked by two members of the research group.
Statistical analysis
All data were managed using Excel and were double-checked by two persons. According to the preliminary experiments, the margin of non-inferiority was defined as −2.0 mL (the difference between the two groups). The estimated sample size required for this study was at least 22 participants (11 in each arm) as analyzed by the PASS software (NCSS LLC, Chicago, IL, USA). This sample size would provide 90% power at the significance level of 0.025. Considering the feasibility of the study, we planned to enlarge the sample size to 20 in each group.
All statistical analysis was performed using the SPSS v23.0 software package (IBM Corp, Armonk, NY, USA). The normally distributed dose data is expressed as the mean ± standard deviation. Homogeneous data were analyzed using an analysis of variance or a Student's t test. A paired analysis was used to compare the before- and after-treatment data of the same group. Non-normally distributed data were analyzed using a rank-sum test. The δ-value for the non-inferiority test was set according to the difference of 2 mL of TV growth between the two treatment groups after 3 months of treatment. When α = 0.05, a two-tailed Student's t test was used. When the difference in TV growth between the two groups was <2 mL, along with P < 0.05, the hCG/hMG group was considered to be not significantly different from the GnRH group.
Results
Baseline data
Two therapeutic schemes (hCG/hMG and GnRH pump) were provided to CHH patients. Before 2016, patients could only use the GnRH pump for their treatment because hMG was not available. However, all patients could choose any one of the two therapeutic options for treatment after 2016. Among them, 20 patients joined in the hCG/hMG group, including 10 cases of KS, 6 cases of nIHH, and 4 cases of multiple pituitary dysfunction (MPHD). In the hCG/hMG group, 9 patients had a history of cryptorchidism. The GnRH group contained a total of 21 patients (14 cases of KS, six cases of nIHH, and one case of MPHD). In the GnRH group, 8 patients had a history of cryptorchidism. Before treatment, there was no statistical difference between the two groups in terms of the biochemistry, hormones, and other demographic indicators. The TV and PL were 2.2 ± 1.1 mL vs. 2.6 ± 1.5 mL and 4.7 ± 1.5 cm vs. 4.7 ± 1.2 cm, respectively, for the hCG/hMG and GnRH groups [Table 1].
Table 1.
Baseline parameters in the hCG/hMG and GnRH groups before treatment.
| Items | hCG/hMG group (n=20) | GnRH group (n=21) | t (U) value | P value |
| KS/nIHH/MPHD | 10/6/4 | 14/6/1 | ||
| Age (years) | 14.9 ± 1.9 | 14.2 ± 1.3 | 1.355 | 0.183 |
| H (cm) | 160.6 ± 8.4 | 160.0 ± 10.4 | 0.228 | 0.821 |
| BW (kg) | 55.9 ± 10.5 | 59.6 ± 17.0 | −0.831 | 0.411 |
| BMI (kg/m2) | 21.6 ± 3.2 | 23.0 ± 4.9 | −1.126 | 0.268 |
| Cryptorchidism (n) | 9 | 8 | ||
| BA (years) | 13.0 ± 1.5 | 13.0 ± 1.0 | 0.009 | 0.993 |
| BA/CA | 0.9 ± 0.1 | 0.9 ± 0.1 | −1.071 | 0.293 |
| TV (mL) | 2.2 ± 1.1 | 2.6 ± 1.5 | −0.806 | 0.425 |
| PL (cm) | 4.7 ± 1.5 | 4.2 ± 1.4 | 1.073 | 0.290 |
| PD (cm) | 1.7 ± 0.5 | 1.5 ± 0.5 | 0.767 | 0.448 |
| LH (mIU/L) | 0.5 ± 0.7 | 0.5 ± 0.9 | 0.119 | 0.906 |
| FSH (mIU/L) | 1.2 ± 1.3 | 1.3 ± 1.7 | −0.202 | 0.841 |
| LH/FSH | 0.5 ± 0.2 | 0.4 ± 0.3 | 0.710 | 0.483 |
| T (ng/dL) | 19.7 ± 1.7 | 19.6 ± 3.3 | 0.070 | 0.945 |
| AMH (ng/mL) | 14.7 ± 7.3 | 14.6 ± 9.2 | 0.011 | 0.991 |
| Inh-B (pg/mL) | 32.5 ± 20.1 | 34.8 ± 12.1 | −0.319 | 0.753 |
Data are expressed as mean ± standard deviation or n.
AMH: Anti-Müllerian hormone; BA: Bone age; BMI: Body mass index; BW: Body weight; CA: Chronological age; FSH: Follicle stimulating hormone; H: Height; Inh-B: Inhibin B; KS: Kallmann syndrome; LH: Luteinizing hormone; MPHD: Multiple pituitary dysfunction; nIHH: Normosmic idiopathic hypogonadotropic hypogonadism; PL: Penile length; PD: Penile diameter; T: Testosterone; TV: Testicular volume.
Therapeutic effects
Based on the 3 to 12 month follow-up data, both groups significantly improved in male secondary sexual characteristics after treatment. All indices of the major and secondary efficacy indicators increased in both groups after treatment, compared to the levels ascertained before treatment [Table 2]. During the treatment period, 9 patients in the hCG/hMG group received 1000 U of hCG every other day to maintain T levels at approximately 200 ng/dL, whereas 11 patients required 2000 U of hCG every other day or twice per week to maintain the same T levels.
Table 2.
Therapeutic effects in the hCG/hMG and GnRH groups after 3–12 months of treatment.
| Items | Groups | Pre-treatment | 3 months | 6 months | 9 months | 12 months |
| TV (mL) (n) | hCG/hMG | 3.9 ± 1.6 (20) | 5.1 ± 2.3 (20) | 6.2 ± 3.4 (11) | 6.6 ± 2.4 (9) | 8.8 ± 3.6 (5) |
| GnRH | 4.1 ± 1.8 (21) | 4.1 ± 1.8 (21) | 5.7 ± 3.0 (14) | 7.7 ± 3.2 (9) | 8.6 ± 4.3 (10) | |
| t (U) value | −0.494 | 1.394 | 0.314 | −0.843 | 0.131 | |
| P value | 0.624 | 0.172 | 0.757 | 0.414 | 0.898 | |
| ΔTV (mL) | hCG/hMG | 1.3 ± 1.3 | 2.9 ± 2.0 | 4.3 ± 3.7 | 4.2 ± 2.2 | 6.6 ± 4.4 |
| GnRH | 1.8 ± 1.7 | 1.8 ± 1.7 | 3.2 ± 2.3 | 5.3 ± 2.5 | 6.5 ± 3.4 | |
| t (U) value | −0.923 | 1.782 | 0.811 | −0.948 | 0.041 | |
| P value | 0.362 | 0.084 | 0.426 | 0.359 | 0.968 | |
| PL (cm) | hCG/hMG | 6.0 ± 1.4 | 6.9 ± 1.8 | 7.1 ± 1.1 | 7.7 ± 2.3 | 8.1 ± 2.1 |
| GnRH | 5.1 ± 1.6 | 5.1 ± 1.6 | 5.9 ± 1.1 | 6.4 ± 1.6 | 6.7 ± 1.8 | |
| t (U) value | 1.796 | 3.083 | 2.464 | 1.242 | 1.316 | |
| P value | 0.081 | 0.004 | 0.022 | 0.238 | 0.211 | |
| ΔPL (cm) | hCG/hMG | 1.3 ± 1.1 | 2.2 ± 1.6 | 2.7 ± 1.7 | 3.0 ± 1.7 | 3.2 ± 1.4 |
| GnRH | 1.0 ± 0.8 | 1.0 ± 0.8 | 1.9 ± 1.0 | 2.1 ± 1.1 | 2.4 ± 1.0 | |
| t (U) value | 0.750 | 2.823 | 1.492 | 1.172 | 1.278 | |
| P value | 0.458 | 0.008 | 0.150 | 0.264 | 0.224 | |
| PD (cm) | hCG/hMG | 2.3 ± 0.8 | 2.4 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.3 |
| GnRH | 2.0 ± 0.6 | 2.0 ± 0.6 | 2.0 ± 0.4 | 2.3 ± 0.5 | 2.4 ± 0.4 | |
| t (U) value | 1.319 | 2.224 | 2.942 | 949 | 0.828 | |
| P value | 0.197 | 0.034 | 0.008 | 0.363 | 0.425 | |
| ΔPD (cm) | hCG/hMG | 0.7 ± 0.8 | 0.9 ± 0.5 | 1.1 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.4 |
| GnRH | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.5 ± 0.4 | 0.5 ± 0.3 | 0.7 ± 0.4 | |
| t (U) value | 0.920 | 2.311 | 2.970 | 1.743 | 0.713 | |
| P value | 0.365 | 0.028 | 0.007 | 0.109 | 0.491 | |
| T (ng/dL) | hCG/hMG | 285.7 ± 73.7 | 443.8 ± 158.3 | 394.8 ± 162.6 | 414.0 ± 176.6 | 584.3 ± 180.5 |
| GnRH | 102.2 ± 98.8 | 102.2 ± 98.8 | 109.5 ± 61.3 | 178.3 ± 114.3 | 180.1 ± 89.2 | |
| t (U) value | 7.372 | 8.039 | 5.897 | 3.541 | 5.811 | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| ΔT (ng/dL) | hCG/hMG | 276.2 ± 78.9 | 435.2 ± 157.7 | 374.7 ± 162.6 | 393.8 ± 176.4 | 564.1 ± 180.5 |
| GnRH | 82.6 ± 100.0 | 82.6 ± 100.0 | 89.5 ± 61.3 | 158.3 ± 114.3 | 161.5 ± 90.4 | |
| t (U) value | 6.972 | 7.959 | 5.897 | 3.541 | 5.763 | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| AMH (ng/mL) | hCG/hMG | 13.1 ± 7.6 | 12.2 ± 8.1 | 9.7 ± 8.5 | 8.1 ± 6.2 | 4.6 ± 1.3 |
| GnRH | 14.5 ± 8.7 | 14.5 ± 8.7 | 10.4 ± 8.3 | 4.1 ± 2.8 | 4.8 ± 0.6 | |
| t (U) value | −0.378 | −0.625 | −0.144 | 1.043 | −0.221 | |
| P value | 0.710 | 0.539 | 0.888 | 0.373 | 0.836 | |
| Inh-B (pg/mL) | hCG/hMG | 53.6 ± 26.6 | 80.9 ± 45.4 | 76.5 ± 37.4 | 79.1 ± 38.7 | 100.8 ± 30.3 |
| GnRH | 90.5 ± 92.9 | 90.5 ± 92.9 | 54.7 ± 28.0 | 74.1 ± 44.2 | 119.8 ± 36.5 | |
| t (U) value | −1.301 | −0.332 | 1.113 | 0.149 | −0.686 | |
| P value | 0.209 | 0.743 | 0.289 | 0.889 | 0.530 | |
| H (cm) | hCG/hMG | 164.11 ± 7.6 | 166.5 ± 5.4 | 166.7 ± 5.2 | 168.7 ± 4.3 | |
| GnRH | 159.6 ± 10.6 | 163.8 ± 11.6 | 163.9 ± 12.2 | 164.4 ± 10.7 | ||
| t (U) value | 1.431 | 0.679 | 0.573 | 0.916 | ||
| P value | 0.162 | 0.505 | 0.576 | 0.376 | ||
| BW (Kg) | hCG/hMG | 61.3 ± 13.7 | 59.5 ± 11.0 | 56.8 ± 8.3 | 60.3 ± 9.8 | |
| GnRH | 60.6 ± 18.1 | 63.4 ± 17.1 | 64.7 ± 17.9 | 64.6 ± 16.2 | ||
| t (U) value | 0.136 | −0.627 | −1.070 | −0.587 | ||
| P value | 0.893 | 0.538 | 0.304 | 0.567 | ||
| BMI | hCG/hMG | 22.6 ± 3.9 | 21.4 ± 3.3 | 20.4 ± 2.6 | 21.1 ± 3.0 | |
| GnRH | 23.4 ± 5.1 | 23.4 ± 5.0 | 24.8 ± 5.0 | 23.4 ± 4.8 | ||
| t (U) value | −0.525 | −1.121 | −1.627 | −1.026 | ||
| P value | 0.604 | 0.276 | 0.128 | 0.324 |
Data are expressed as mean ± standard deviation or n.
AMH: Anti-Müllerian hormone; BMI: Body mass index; BW: Body weight; FSH: Follicle stimulating hormone; GnRH: Gonadotropin-releasing hormone; H: Height; hCG: Human chorionic gonadotropin; hMG: Human menopausal gonadotropin; Inh-B: Inhibin B; LH: Luteinizing hormone; PD: Penile diameter; PL: Penile length; T: Testosterone; TV: Testicular volume; ΔPL (increased penile length): Penile length after treatment minus that before treatment; ΔPD (increased value of penile diameter): Penile diameter after treatment minus that before treatment; ΔT (increased value of testosterone): Value of testosterone after treatment minus that before treatment; ΔTV (increased testicular volume): Testicular volume after treatment minus that before treatment.
After 3 months of treatment, the TV of the hCG/hMG and GnRH groups increased to 5.1 ± 2.3 mL vs. 4.1 ± 1.8 mL, respectively (P > 0.05, t (U) = 1.394). The PL and PD reached 6.9 ± 1.8 cm vs. 5.1 ± 1.6 cm (P < 0.05, t (U) = 3.083) and 2.4 ± 0.5 cm vs. 2.0 ± 0.6 cm (P < 0.05, t U) = 2.224) in the hCG/hMG and GnRH groups, respectively. Furthermore, the T level was 443.8 ± 158.3 vs. 102.2 ± 98.8 ng/dL in the two groups. Overall, the PL, PD, and testosterone levels of the hCG/hMG group were significantly higher than those of the GnRH group [Table 2]. In addition, the T values, PD, penile diameter after treatment minus that before treatment (ΔPD), PL and penile length after treatment minus that before treatment (ΔPL) of the hCG/hMG group were significantly higher than the GnRH group after 3 months. However, the TV, testicular volume after treatment minus that before treatment (ΔTV), AMH, Inh-B, height, body weight, and body mass index (BMI) did not show significant differences [Table 2]. The difference in TV after treatment between the two groups was −1.09 mL (95% confidence interval: −0.15, 2.33) and was within the non-inferiority margin (δ = −2 mL, P < 0.05). It was inferred that the GnRH treatment was not significantly advantageous compared to the hCG/hMG treatment overall.
Furthermore, we compared the data of hCG/hMG group patients who completed 6 to 21 months of treatment to that of GnRH group patients who completed 6 to 26 months of treatment. After 6 months of treatment, no abnormal blood biomarkers were observed in either group during treatment. Compared with the GnRH group, the T level and growth of PL and PD were significantly increased in the hCG/hMG group (all P < 0.05). However, even though the TV and ΔTV of both groups increased, the difference was not statistically significant [Figure 1]. For the 9 and 12-month treatment time points, the T level of the hCG/hMG group was significantly higher than that of the GnRH group (all P < 0.05, t (U) = 5.897, 3.541, 5.811). Yet other parameters, such as TV, ΔTV, PL, ΔPL, PD, and ΔPL did not exhibit a significant difference (all P > 0.05) [Table 2 and Figure 1].
Figure 1.
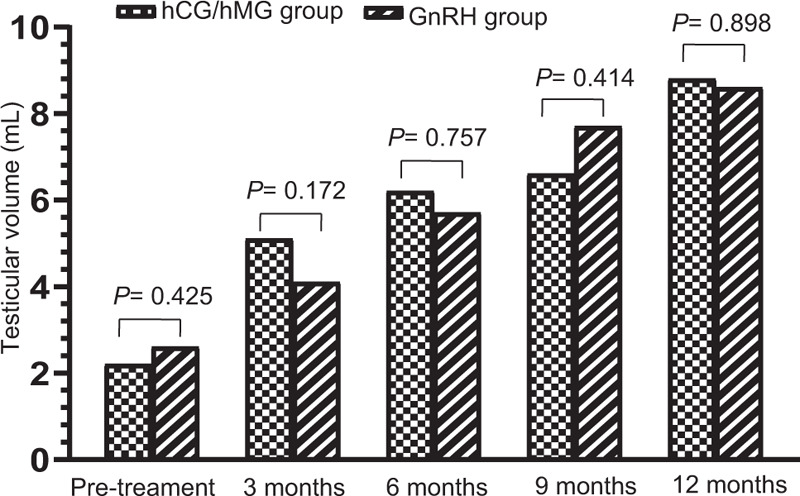
Alteration of TV during 0 to 12 months of treatment. The TV of hCG/hMG and GnRH patients at various time intervals (months) after treatment onset. Bars represent mean ± standard deviation. The P value of the comparison at each time point is displayed at the top of the corresponding pairs of bars. GnRH: Gonadotropin-releasing hormone; H: Height; hCG: Human chorionic gonadotropin; hMG: Human menopausal gonadotropin; TV: Testicular volume.
Eight patients in the hCG/hMG group had spermatorrhea at 4 (TV = 10 mL), 6 (TV = 6 mL), 9 (TV = 5 mL), 10 (TV = 6 mL), 12 (TV = 4 mL), 16 (TV = 8 mL), 16 (TV = 9 mL), and 18 (TV = 6 mL) months, respectively, after the commencement of treatment. In the GnRH group, twelve children had spermatorrhea at 9 (TV = 8 mL), 9 (TV = 7 mL), 10 (TV = 7 mL), 11 (TV = 9 mL), 12 (TV = 10 mL), 12 (TV = 11 mL), 14 (TV = 9 mL), 14 (TV = 7 mL), 15 (TV = 8 mL), 20 (TV = 11 mL), 24 (TV = 10 mL), and 26 (TV = 20 mL) months, respectively. On account of the applicable laws and regulations, doctors in Beijing hospitals refuse to do a semen examination for individuals who are <18 years old. Therefore, we can only record such semen-related results according to the children's private complaints and based on their consent. In this study, only seven patients were examined in other provinces. The sperm motility was improved gradually over the course of treatment.
Safety
No abnormal blood biomarkers were observed in either treatment group during treatment. Most of the children did not show adverse reactions which may be caused by a high T level. After acne and penile erection occurred frequently in a few children, the hCG dose was reduced and the adverse reactions were significantly ameliorated. Two children in the hCG/hMG group developed acne within 3 months of treatment onset. One patient lost hair and had a testosterone level of 559 ng/dL. We; therefore, reduced the dose of hCG from 2000 U once every other day to 2000 U twice weekly because we suspected that these effects were related to increased androgen. The T level in this patient dropped to 302 ng/dL and the adverse reactions were ameliorated after 3 months. Another patient suffered acne, especially on his back when the T level reached 422 ng/dL after 2 months of hCG/hMG treatment. After reducing the dose of hCG from 2000 U once every other day to 2000 U twice weekly, the T level dropped to 226 ng/dL and the acne cleared. Two other children were treated with metformin (0.5 g three times a day) because of their obesity and hyperinsulinemia. One patient stopped the medication 1 month later, but the obesity situation did not improve. The other patient received the medication for 3 months based on the doctor's instructions, and his obesity situation improved and was accompanied by a decrease of his BMI from 30.1 to 28.6 kg/m2. A skin allergy was observed in three patients in the GnHR group. No adverse reaction was observed at the injection site in the hCG/hMG group.
Compliance evaluation
During the 3-month treatment period, both groups were subject to follow-up at the proper time and the compliance was observed to be good. The long-term compliance needs further observation.
Discussion
Puberty induction therapy in CHH patients is needed according to the evidence that (1) Sertoli cell proliferation occurs in early life (a few months after birth) and early adolescence[18]; (2) animal experiments have shown that FSH, LH, and even T can stimulate the proliferation of Sertoli cells (which are directly related to spermatogenic ability)[19,20]; (3) some studies have shown that gonadotropin-treatment in adolescence and early adulthood can reduce the treatment time which is required to normalize sperm production after marriage for reproduction[21]; and (4) induction of puberty development at the appropriate age of puberty is more in line with human physiological characteristics. The early diagnosis of CHH and timely administration of hormone replacement therapy not only helps the development of the reproductive system, but also promotes or preserves adult reproductive function.[22]
We created a simple and feasible treatment regimen for CHH and chose TV as the main research indicator. The result of the TV analysis indicated that both groups were similar after 3 months of treatment. All children responded well; the T level, PL, and PD values of the hCG/hMG group were significantly higher than those of the GnRH group after 3 months of treatment, indicating that the proposed regime is effective. The first 3 months may be an appropriate window to observe the effectiveness of treatment; after this time-period, the therapeutic effect in the hCG/hMG group was not worse than that of the GnRH group. To further confirm this conclusion, we collected extended time-period data in a few cases, which showed that after treatment for 6 months, there was no significant difference in TV growth between the two groups. There were still statistical differences in T levels between the two groups after 9 to 12 months of treatment; however, there was no statistical difference in TV, PL, PD, Inh-B, AMH, and so on. We assumed that these results were directly related to the adjustments made to the hCG/hMG treatment based on the T levels. Results from the cases which pertain to the extended time-periods also showed satisfactory outcomes after 1 year.
Indeed, treatment with our regimen showed positive results regarding TV, which is more representative of the puberty development process compared to other indicators. Since hCG was used for pre-treatment, the patients in the hCG/hMG group had a higher PL at early time points, but the difference disappeared after 6 to 9 months.
After 6 months of treatment, a patient complained that a “white sticky secretion” was found in his underwear when his TV was 4 to 5 mL. This substance was attributed to spermatorrhea due to the enlargement of the testes after the treatment, although a semen test was not conducted. Nevertheless, spermatorrhea has been previously reported when TV reached 4 to 6 mL.[23] For some CHH patients who exhibited a dual form of the condition (such as HH + cryptorchidism), the initial treatment could be effective because the testicular function is preserved. However, according to our experience, those patients would experience a decline in testicular function in the near future.
The children in the hCG/hMG group who observed spermatorrhea when their TV reached 4 to 5 mL may be at risk of premature testicular failure. Consequent to the laws in Beijing, we could only perform a semen test if consent was provided. This is a limitation of the current work and any difference between the time of the first spermatorrhea and the subsequent incidence of spermatorrhea between the two groups needs further study.
The hCG stimulation test is usually applied in childhood. Evaluation of testicular function is important, since the provocation test only fails to stimulate patients with poor testicular function, while those who retained some functions usually have a normal T value. The patients with normal testes, namely mild CHH patients, only present with delayed puberty onset and can often have puberty induced via stimulation. Therefore, for these patients, testosterone levels will gradually increase over the course of 2 to 3 years until it completely reaches the adult level.
In theory, the possibility of side effects was small under the premise of ensuring a therapeutic effect. The height and weight of both groups increased linearly and there were no significant differences in other safety indexes including liver and kidney functions, insulin-like growth factor-1, insulin-like growth factor-1/ IGF-binding proteins 3, and alkaline phosphatase, excessive T, acne, and male breast development, etc. between the two groups. We noted that the patients in the hCG/hMG group did not experience any skin allergies, but do not believe that this is related to the treatment difference. Therefore, we conclude that treatment with 1000 to 2000 U of hCG (once every other day or 2–3 times a week) combined with 75 U of hMG (once a day) can be implemented as a safe, effective, and suitable treatment plan for most adolescents, that can act as a therapeutic alternative for patients with CHH. Some people believe that it is better to use FSH first; however, we did not compare such regimens. Our study suggested that the application of hCG as a pre-treatment can screen and exclude patients who have abnormal testicular function and do not respond to hCG at all.
In short, 3 months of treatment with hCG/hMG provides a safe and effective treatment window for inducing puberty in male adolescent CHH patients. It can be used as an alternative option to hCG for inducing puberty and fertility, especially for those patients who have pituitary lesions. Treatment using a GnRH pump is still effective for patients with MPHD; however, it is thought that such patients still retain some pituitary gonadotropin cells that can be stimulated by GnRH. Thus, this treatment may be able to restart pituitary testicular axis function.
The price of the pituitary hormone pump is still high and inconvenient. For privacy protection, many adolescent patients prefer hCG/hMG injection instead of wearing a pituitary hormone pump. This study suggests a different treatment scheme for adolescent CHH, that has similar efficacy and safety as the hormone pump and that provides more data and choice for clinicians to treat various patients.
This study compared the therapeutic effect and safety, as well as the impact on growth and development, of two treatment options in an adolescent CHH population. The target population of this study was adolescents, since similar work in adults has been previously reported. While the ultimate goal of this work is to help CHH patients simulate normal puberty development and to fill their psychological and physiological needs, various limitations of the current work must be addressed in the future. First, the observation duration period of the current study was short and without semen analysis. Second, the sample size was small and an extended phase is necessary to validate these results. These data will be gradually supplemented and improved in future research.
Acknowledgements
The authors wish to thank Ferring Pharmaceuticals, which donated highly purified menotrophin (MENOPUR).
Funding
This work was supported by a grant from Jin Lei Pediatric Endocrinology Growth Research Fund for Young Physicians (PEGRF) (No. PEGRF201809006).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu Y, Ren XY, Peng YG, Chen SK, Cheng XR, Qin M, Wang XL, Song YN, Fan LJ, Gong CX. Efficacy and safety of human chorionic gonadotropin combined with human menopausal gonadotropin and a gonadotropin-releasing hormone pump for male adolescents with congenital hypogonadotropic hypogonadism. Chin Med J 2021;134:1152–1159. doi: 10.1097/CM9.0000000000001419
Ying Liu and Xiao-Ya Ren contributed equally to this work.
References
- 1.Boehm U, Bouloux P-M, Dattani MT, de Roux N, Dodé C, Dunkel L, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism – pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 2015; 11:547–564. doi: 10.1038/nrendo.2015.112. [DOI] [PubMed] [Google Scholar]
- 2.Bianco SDC, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 2009; 5:569–576. doi: 10.1038/nrendo.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacques Y, Cheng X, Papadakis GE, Acierno JS, Maione L, Hietamäki J, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev 2019; 40:669–710. doi: 10.1210/er.2018-00116. [DOI] [PubMed] [Google Scholar]
- 4.Gong C, Liu Y, Qin M, Wu D, Wang X. Pulsatile GnRH Is superior to hCG in therapeutic efficacy in adolescent boys with hypogonadotropic hypogonadodism. J Clin Endocrinol Metab 2015; 100:2793–2799. doi: 10.1210/jc.2015-1343. [DOI] [PubMed] [Google Scholar]
- 5.Department of Endocrinology, Chinese Medical Association, Department of Gonadology. Expert consensus on the diagnosis and treatment of idiopathic hypogonadotropin hypogonadism (in Chinese). Chin J Intern Med 2015; 54:739–744. doi: 10.3760/cma.j.issn.0578-1426.2015.08.021. [Google Scholar]
- 6.Gong CX, Li LL. Procedure of diagnosis and treatment for disorders of sex development: based on the abundant clinical experiences and more than 400 cases of 46,XY disorders of sex development. Chin J Appl Clin Pediatr 2017; 32:1521–1525. doi: 10.3760/cma.j.issn.2095-428X.2017.20.001. [Google Scholar]
- 7.Papadimitriou DT, Chrysis D, Nyktari G, Zoupanos G, Liakou E, Papadimitriou A, et al. Replacement of male mini-puberty. J Endocr Soc 2019; 3:1275–1282. doi: 10.1210/js.2019-00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF, Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2002; 87:4128–4136. doi: 10.1210/jc.2002-020518. [DOI] [PubMed] [Google Scholar]
- 9.Warne DW, Decosterd G, Okada H, Yano Y, Koide N, Howles CM. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril 2009; 92:594–604. doi: 10.1016/j.fertnstert.2008.07.1720. [DOI] [PubMed] [Google Scholar]
- 10.Zacharin M, Sabin MA, Nair VV, Dagabdhao P. Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertil Steril 2012; 98:836–842. doi: 10.1016/j.fertnstert.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Rohayem J, Hauffa BP, Zacharin M, Kliesch S, Zitzmann M. the “German Adolescent Hypogonadotropic Hypogonadism Study Group”. Testicular growth and spermatogenesis: new goals for pubertal hormone replacement in boys with hypogonadotropic hypogonadism? – a multicentre prospective study of hCG/rFSH treatment outcomes during adolescence. Clin Endocrinol (Oxf) 2017; 86:75–87. doi: 10.1111/cen.13164. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Hasegawa T, Hasegawa Y, Arisaka O, Ozono K, Amemiya S, et al. Treatment situation of male hypogonadotropic hypogonadism in pediatrics and proposal of testosterone and gonadotropins replacement therapy protocols. Clin Pediatr Endocrinol 2015; 24:37–49. doi: 10.1297/cpe.24.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schopohl J. Pulsatile gonadotrophin releasing hormone versus gonadotrophin treatment of hypothalamic hypogonadism in males. Hum Reprod 1993; 8: (Suppl 2): 175–179. doi: 10.1093/humrep/8.suppl_2.175. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Banks SM, Barnes KM, Sherins RJ. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 1988; 67:1140–1145. doi: 10.1210/jcem-67-6-1140. [DOI] [PubMed] [Google Scholar]
- 15.Zhou XW, Wang N, Li YD. Evaluation of the age of the first spermatorrhea in China from 1980 to 2013 (in Chinese). China Public Health 2017; 33:1408–1413. doi: 10.11847/zgggws2017-33-09-29. [Google Scholar]
- 16.Liang JP, Mai JC, Yang JW, Lin JL, Yao ZJ. Analysis of the age change trend of first-time spermatorrhea or menarche in Guangzhou from 1985 to 2014 (in Chinese). Chin J School Health 2016; 37:1670–1672. doi: 10.16835/j.cnki.1000-9817.2016.11.022. [Google Scholar]
- 17.Gong C. Response to the Letter by Raivio T. et al. J Clin Endocrinol Metab 2015; 100:L75.doi: 10.1210/jc.2015-3030. [DOI] [PubMed] [Google Scholar]
- 18.Cortes D, Müller J, Skakkebæk NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl 1987; 10:589–596. doi: 10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta). Biol Reprod 2000; 63:82–88. doi: 10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- 20.Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human sertoli cell population: Its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 1984; 31:785–795. doi: 10.1095/biolreprod31.4.785. [DOI] [PubMed] [Google Scholar]
- 21.Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, Handelsman DJ. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Hum Reprod 2002; 17:625–633. doi: 10.1093/humrep/17.3.625. [DOI] [PubMed] [Google Scholar]
- 22.Raivio T, Wikstrom AM, Dunkel L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur J Endocrinol 2007; 156:105–111. doi: 10.1530/eje.1.02315. [DOI] [PubMed] [Google Scholar]
- 23.Peking Union Medical College Hospital, Liu ZX. Identification of genetic pathology for patients with 46,XY disorders of sex development and efficacy and outcome predictors of gonadotropin-induced spermatogenesis for male patients with congenital hypogonadotropic hypogonadism. 2016. [Google Scholar]


