Background:
Neuromas are an under-recognized contributor to chronic abdominal pain. Other than after mesh inguinal hernia repair, surgical management of painful abdominal wall neuromas has not been well established in the literature.
Methods:
All patients who underwent surgical treatment for painful abdominal wall neuromas by the senior author at Northwestern Memorial Hospital were reviewed. Patients were treated with neuroma excision and allograft nerve reconstruction and/or with targeted muscle reinnervation (TMR). Follow-up pain surveys were issued to assess pain levels, activities of daily living, and pain medication usage.
Results:
Twenty patients met inclusion criteria. Eighteen (90%) patients reported improvement in neuropathic pain postoperatively. Two (10%) patients had TMR following failed nerve allograft reconstruction, which led to complete pain resolution. Twenty-seven nerves were treated surgically, the majority of which were abdominal intercostal (13), followed by ilioinguinal (10), genitofemoral (3), and iliohypogastric (1). Nerve allograft reconstruction was used alone for 18 procedures, in combination with TMR for 2, and TMR was used alone in 8. In all cases of TMR, the freshened nerve ending after neuroma excision was coapted to a motor nerve of the internal oblique. The mean length of follow-up was 18.9 months (SD ±14.5).
Conclusions:
This retrospective review demonstrated that 90% (18) of the patients had significant improvement in abdominal neuroma pain postoperatively. These results may help guide providers toward effective management of abdominal wall neuropathic pain.
INTRODUCTION
Neuromas are an under-recognized significant contributor to chronic abdominal pain because healthcare providers are typically unfamiliar with the evaluation and/or treatment of neuropathic pain. The few reports on surgical treatment for postoperative abdominal wall neuromas have mainly espoused neuroma excision and nerve implantation into local tissues. Several of the most frequently performed operative procedures in the United States include ventral, umbilical and inguinal hernia repairs, appendectomy, cholecystectomy, and cesarean section. Over 400,000 ventral hernia repairs are performed annually in the United States.1 Worldwide, over 20 million patients undergo inguinal hernia repairs annually.2 Because these procedures are abdominally based (from incision to closure), the nerves innervating the abdominal wall are vulnerable to injury.
Estimates of 5% of patients suffer from groin pain after mesh inguinal hernia repair, due to scarring and irritation of the ilioinguinal nerve.3,4 Patients are at risk of peripheral nerve injury due to direct surgical trauma laparoscopic portals. In the absence of a surgical incision near an abdominal nerve, even self-retaining retractors (such as a Balfour retractor) used with short midline laparotomy incisions can cause a remote intercostal nerve injury just medial to the semilunar line. For unclear reasons, chronic local pain of the abdominal wall is seen not infrequently in weight-loss patients.5 Blunt injuries from trunk harnesses such as due to kitesurfing have been associated with (symmetric) chronic unremitting abdominal wall pain.6 These patients with chronic neuropathic pain of the abdominal wall are most commonly sent to pain management, as there is little thought for a surgical resolution. The primary aim of this study was to review the outcomes of surgical intervention for painful abdominal wall neuromas. Our hypothesis is that surgical procedures, specifically targeted muscle reinnervation (TMR) and nerve reconstruction with allograft, are effective lasting treatments for abdominal wall neuropathic pain.
METHODS
Diagnosis
The clinical diagnosis of painful abdominal wall neuroma is made as a combination of history of inciting event, a lack of visceral findings, and a reproduceable physical examination of localized pain. A thorough past medical and surgical history will help rule out intraabdominal causes. Careful examination, looking for skin incisions of laparoscopy portals near the area of pain, should be performed. Abdominal CT imaging is useful to rule out a hernia or other abdominal pathology. Imaging, including ultrasound, is usually negative for patients with abdominal wall neuropathic pain.
During the physical examination, patients can readily identify the site of greatest discomfort. The examiner initially palpates in nontender areas of the abdomen and then moves to the area of tenderness. Typically, the pain will be greatest either just medial or just lateral to the semilunar line, and often 1–2 cm medial to the costal cartilages. Then, in the office, the area of pain is injected with 3cm3 of Xylocaine with epinephrine using a 25-gauze needle. As this is a “blind” injection, this can be repeated for lack of effect to determine if pain relief can be obtained during the office visit. A negative CT scan, a reproduceable physical examination as to the location of pain, and the ability to relieve pain with a lidocaine injection nerve block is enough to offer the patient a surgical exploration. All patients received at least 1 diagnostic nerve block as part of their evaluation before surgery.
Surgical Technique
Targeted Muscle Reinnervation
The application of TMR for abdominal wall neuroma pain is done primarily for inguinal nerve pain. In the preoperative holding area with the patient supine, the area of greatest tenderness is marked. An inguinal incision curving one finger breadth toward the anterior superior iliac spine is made. After opening the external oblique fascia, the neuroma is identified, and the nerve dissected laterally to reach healthy fascicles before transfer and coaptation. Just before the ilioinguinal nerve emerges from the internal oblique muscle, it consistently gives off a 0.5-mm motor fascicle to the internal oblique muscle and transversus abdominis, and this branch is confirmed with a Checkpoint nerve stimulator (Checkpoint Surgical, Beechwood Ohio). (See Video 1 [online], which demonstrates direct nerve stimulation of the motor nerve to the internal oblique)
Video 1. Video 1 from “Surgical Treatment of Abdominal Wall Neuromas”.
The ilioinguinal nerve is divided and coapted to the newly divided distal segment of the local internal oblique motor nerve using loupe magnification and two 7-0 polypropylene sutures (Figs. 1, 2). Surgery typically takes about 60 minutes to complete skin-to-skin.
Fig. 1.
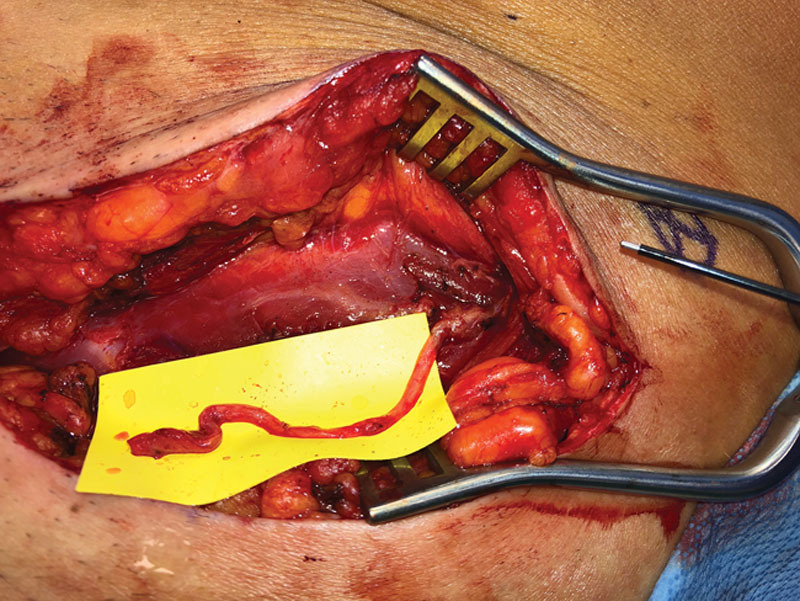
Image of the left groin in a female patient with chronic inguinal neuropathic pain due to an ilioinguinal neuroma after laparoscopy. The patient underwent left groin exploration, and the discovered neuroma in-continuity of the ilioinguinal nerve was excised and reconstructed with a nerve allograft. The patient's pain initially improved, but then returned and became intolerable. At re-exploration 1 year after her allograft procedure, a neuroma was found at the proximal coaptation of the nerve allograft site. The motor nerve to the internal oblique is seen as a small transverse white structure that stimulated easily and is touching the upper right corner of the yellow background. Please see video (Video 1, Intraoperative video demonstrating direct nerve stimulation of the motor nerve to the internal oblique) accompanying this image which demonstrates intraoperative nerve stimulation of the motor nerve to the internal oblique.
Fig. 2.
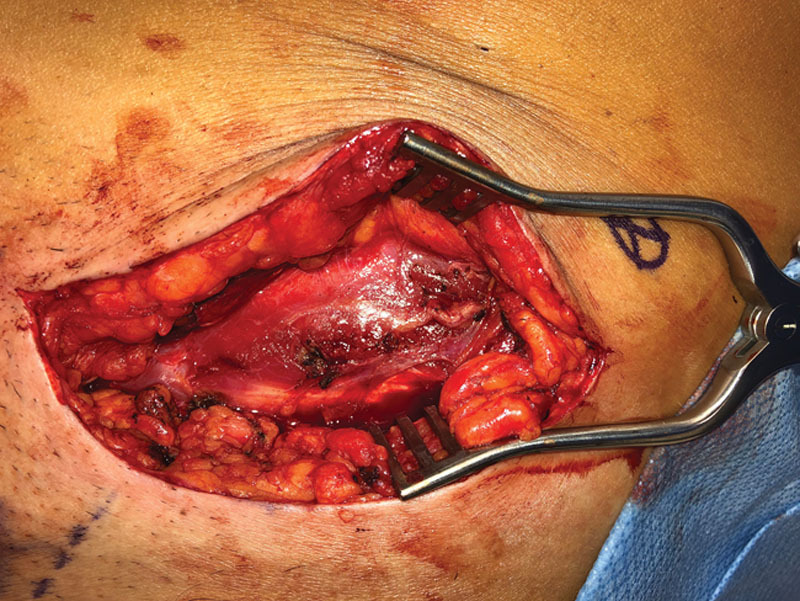
In the same patient as in Figure 1, the ilioinguinal nerve was shortened, and targeted muscle reinnervation (TMR) was performed on the motor nerve of the internal oblique.
Nerve Allograft Technique
The application of nerve allograft reconstruction for abdominal wall neuroma pain as done by the senior author involves resection of the intercostal nerve neuroma, with reconstruction using a nerve allograft (Avance Nerve Allograft, Axogen Co., Alachua, Fla.).7 An oblique incision about 8-cm long is made over the area of greatest pain, centered either lateral or just medial to the semilunar line depending on prior (laparoscopic) incisions and physical examination. Lateral to the semilunar line, the nerve stimulator is used to stimulate through the external oblique to pinpoint the location of the underlying intercostal nerve. The internal oblique is either split along its fibers or, more rarely, incised parallel to the external oblique incision to reveal the intercostal nerve (Figs. 3–5). For incisions medial to the semilunar line, an incision either transversely or obliquely through the anterior rectus fascia allows the rectus muscle to be pulled medially to identify the intercostal nerve as it emerges between layers of the posterior rectus fascia. Invariably, and in each case, an intercostal nerve was found immediately under the area of greatest tenderness. Typically, the painful nerve is reddened, firm to palpation, and scarred to surrounding muscle, and the majority of these painful nerves are neuromas-in-continuity. The neuroma is excised and resulting gap is reconstructed with a <3 cm long nerve allograft. A similar procedure is performed for neuromas medial to the semilunar line, where the anterior rectus fascia is incised, and the rectus muscle retracted medially to identify the intercostal nerves. The semilunar line fascia can be incised as necessary, but closure is much more difficult to prevent a Spigelian type incisional hernia. Occasionally when TMR is performed, there is inadequate length between the end of the newly excised neuroma and the smaller motor nerve target. Allografts can also be used to help bridge the gap, to give the newly divided nerve “somewhere to go and something to do.”
Fig. 3.
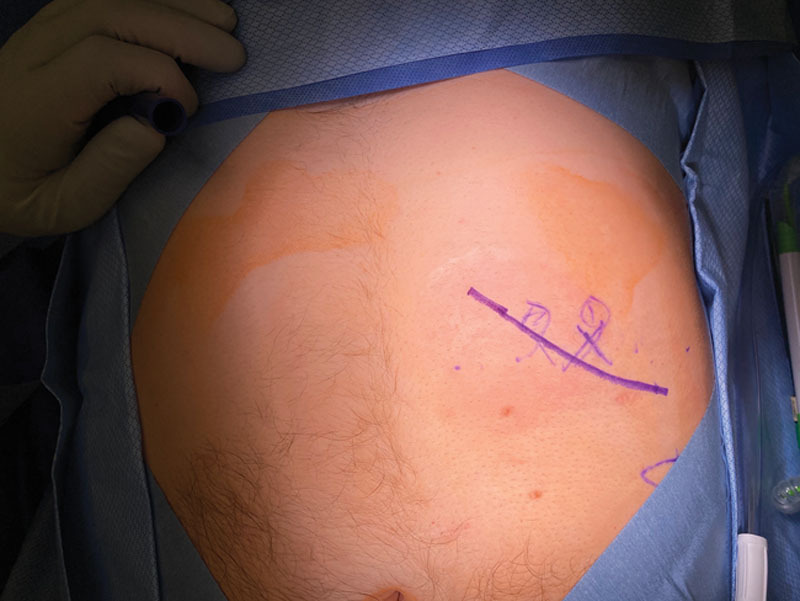
Patient with left abdominal intercostal pain from an intercostal neuroma-in-continuity. The X's mark the area of greatest tenderness. The drawn line represents the incision to be performed, and just lateral to this line is the costal margin.
Fig. 5.
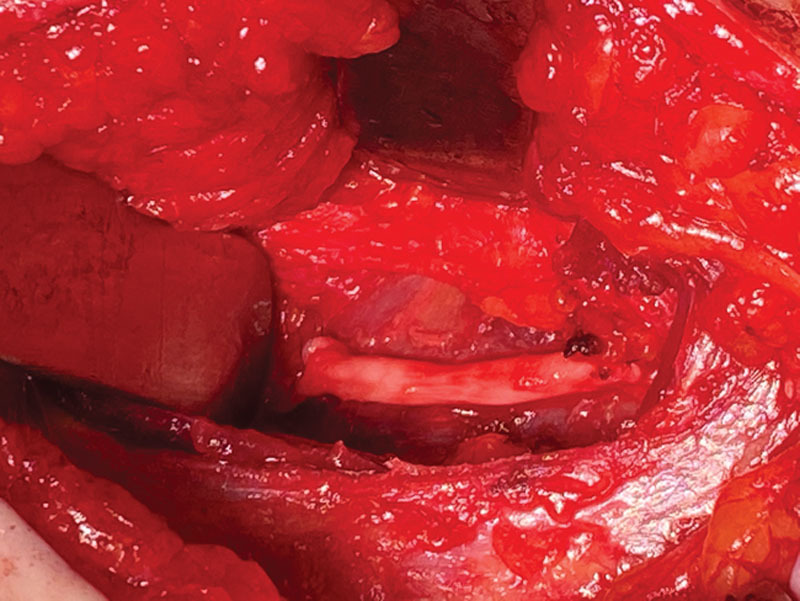
The uppermost aspect of the internal oblique muscle is released, the intercostal neuroma-in-continuity is resected, and the nerve reconstructed with a 3 cm long allograft.
Fig. 4.
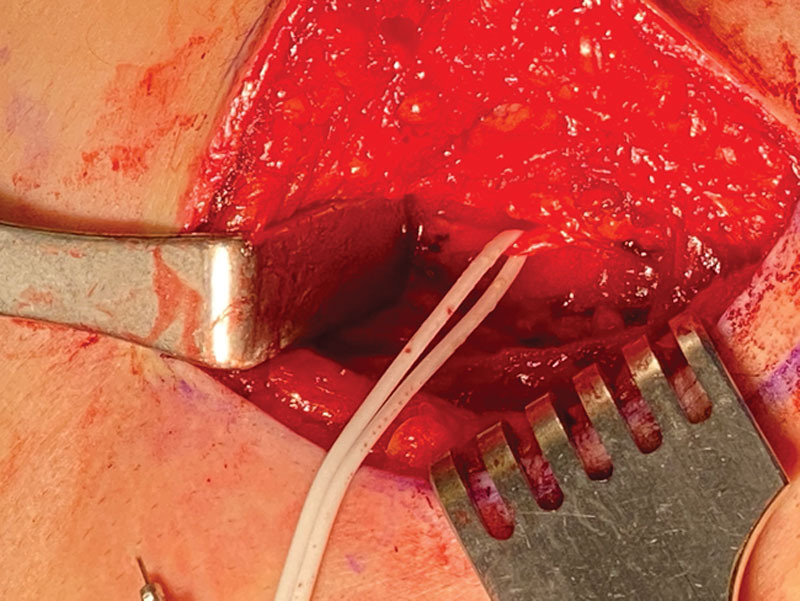
A vessel loop surrounds an intercostal nerve as it emerges from underneath the ribs to innervate the rectus abdominis.
In the upper abdomen, the intercostal nerve can be compressed or irritated as it passes by the linear fascia of the internal oblique muscle. This should be explored and released when the patient’s pain seems immediately adjacent to the costal cartilages. Surgery typically takes 60 minutes to complete skin-to-skin.
Study Design and Setting
Using the Northwestern Medicine Electronic Data Warehouse (EDW), we identified 359 patients who received a neuroma diagnosis and/or underwent neuroma related surgery, based on International Classification of Disease Ninth and Tenth Revision and Current Procedural Terminology codes. A manual retrospective chart review of the captured patients was conducted by the research team to identify patients that met inclusion criteria. Eligible patients were also added prospectively during the study period. All patients had a clinical diagnosis of painful neuroma of the inguinal and/or abdominal intercostal regions that was treated surgically. Thoracic intercostal neuromas were not included in the study. This study received IRB approval from Northwestern University. (See Supplemental Digital Content 1, which displays (A) Search strategy with codes provided for Electronic Database Warehouse analyst at Northwestern University. (B) Inclusion criteria. (C) Flow diagram depicting patient selection process for the review and final analysis. (D) Postoperative follow-up survey. http://links.lww.com/PRSGO/B650.)
The 20 patients who met the inclusion criteria were contacted via email and invited to complete an online prospective pain survey. Patients who agreed to participate in the survey via the online consent form were provided with a link to the pain survey. The survey data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools.8
The primary study end points were postsurgical intervention patient reported pain relief at follow-up (clinic notes and/or survey responses), number of revision surgeries after TMR or allograft nerve reconstruction for neuropathic pain, postoperative analgesic medication requirements, nerve transfers used for TMR, nerves reconstructed with allograft, interference of activities of daily living (ADLs) due to abdominal neuroma pain, and follow-up period (months). Secondary study end points include number and type of postoperative complications and specific abdominal wall event preceding painful neuroma development.
Patient-reported Outcomes
Postoperative pain outcomes were first reviewed based on review of clinical notes. The survey instrument was used to provide a standardized measure of postoperative pain levels in this patient population. The specific survey measurements included 8 questions on a 0–10 scale. (See Supplemental Digital Content 1, http://links.lww.com/PRSGO/B650.) The 8 questions covered dimensions of pain, pain medication, mood, mobility, return to work, and sleep. These questions were adapted from validated existing questionnaires.9 Questions to elicit the intensity of pain were based on the Patient-Reported Outcomes Measurement Information System Pain Intensity Short Form version 1.0. Patient-Reported Outcomes Measurement Information System is a validated toolbox of patient-reported outcomes measures developed with modern psychometric techniques to allow for use across conditions.9 Our research team has previously used Patient-Reported Outcomes Measurement Information System to measure pain intensity, behavior, and interference in amputees.10,11
Statistical Analysis
Data from the retrospective chart review and the follow-up pain survey were transferred to Microsoft Excel (2016) worksheets for comprehensive analysis. Analysis of survey results were means (±SDs) for the data points involving patient ratings. Long term follow-up (mean, ±SD, median) was calculated based on last clinic visit or date of pain survey response entered. Occupation status and current analgesic medication use were reported in terms of % (N).
RESULTS
Twenty patients were included in this retrospective review. Patient demographics and major surgical characteristics are shown in Table 1. The majority [65% (13)] were women and 35% (7) were men. The mean age was 46 years and mean BMI 28.5 (SD ±6.2). Two patients (10%) met criteria for history of massive weight loss.12 One patient (5%) at the time of preoperative diagnosis and surgery was a current smoker. None of the patients had a diagnosis of diabetes mellitus. One patient (5%) had a diagnosis of peripheral vascular disease. Eight patients (40%) reported and/or had an active prescription for narcotic pain medication at preoperative consultation. The mean length of follow-up was 18.9 months (SD±14.5), and median length of follow-up was 14.6 months.
Table 1.
Demographics
| n = 20 | |
|---|---|
| Age, mean (SD) | 46.5 (14.4) |
| Gender | |
| Men | 7 (35%) |
| Women | 13 (65%) |
| BMI, mean (SD) | 28.5 (6.4) |
| Smoking status | |
| Never | 13 (65%) |
| Former | 6 (30%) |
| Current | 1 (5%) |
| DM | |
| Yes | 0 (0%) |
| No | 20 (100%) |
| PVD | |
| Yes | 1 (5%) |
| No | 19 (95%) |
| History of massive weight loss | |
| Yes | 2 (10%) |
| No | 18 (90%) |
| Use of narcotics preoperatively | |
| Yes | 8 (40%) |
| No | 12 (60%) |
| Nerve affected | |
| Intercostal | 13 |
| Ilioinguinal | 10 |
| Genitofemoral | 3 |
| Iliohypogastric | 1 |
| Surgical treatment | |
| Allograft | 18 |
| TMR | 8 |
| TMR + allograft | 2 |
| Length of follow-up, months | |
| Mean (SD) | 18.9 (14.5) |
| Median | 14.6 |
Regarding preceding abdominal wall surgeries, inguinal hernia repairs were the most frequently observed 27% (10), followed by hysterectomy 22% (8), and cholecystectomy 19% (7).
Eight patients (40%) had a cumulative total of 19 prior procedures to attempt to treat abdominal wall neuroma pain. The most frequently used procedure in these patients was ilioinguinal nerve blocks 47% (8/19), followed by regenerative peripheral nerve interfaces (RPNI) 16% (3/19) and anterior subcutaneous neurectomy 16% (3/19).
Twenty-seven nerves were treated surgically. The highest incidence of neuromas was found in intercostal nerves 48% (13), followed by ilioinguinal 37% (10), genitofemoral 11% (3), and iliohypogastric 4% (1). There were 28 procedures performed. Nerve allograft reconstruction was used alone for 18 procedures, TMR was used alone for 8 procedures, and the 2 techniques were used in combination for 2 procedures (multiple nerves on a single patient). There were no reported postoperative complications, other than the 3 patients who required additional surgery for ongoing pain (Table 2). No patient required a later hernia repair.
Table 2.
Surgical Characteristics
| Patient | Type of Abdominal Neuroma Surgery | Nerve Reconstructed | Postoperative Complications | First Postoperative Visit | Most Recent Postoperative Visit | Responded to Survey (See Table 3) | Length of Follow-up (mo) |
|---|---|---|---|---|---|---|---|
| 1 | Allograft 3cm | Intercostal | None | Pain is completely changed from preoperatively | N/A | Yes | 54.16 |
| 2 | 1. Allograft 2 cm 2. Allograft 1.5 cm | 1. Intercostal 2. Intercostal (1 interspace below previous) | None | Pain improved and different from preoperatively | No pain at the Avance nerve grafting site (neuroma pain is resolved) | No | 23.53 |
| 3 | Allograft 2.5 cm | Intercostal | None | Pain much different than it was preoperatively | Almost no pain | Yes | 44.77 |
| 4 | Allograft 5 cm and TMR (coaptation to motor point of internal oblique) | Ilioinguinal | None | Complete resolution of pain | Patient had recurring neuroma pain and subsequently had peripheral nerve stimulator placed. Pain improved during last functional neurosurgery visit. | Yes | 43.65 |
| 5 | Allograft 6 cm | Ilioinguinal | None | Pain improved and different from preoperatively | Pain has returned in former region | Yes | 33.13 |
| 6 | 1. TMR to motor nerve of internal oblique muscle 2. Allograft 3 cm | 1. Ilioinguinal 2. Intercostal | None | 1. Pain improved and different than preoperatively 2. Pain improved and different than preoperatively | 1. Pain is all resolved 2. Occasional dull ache but resolved | No | 18.19 |
| 7 | 1. Allograft 2cm 2. TMR (coaptation to motor nerve to internal oblique)- performed after failed allograft reconstruction | Ilioinguinal | None | Pain different from preoperatively, but continued discomfort | After TMR surgery for failed reconstruction, patient is pain free at that site | No | 5.45 |
| 8 | Allograft 2 cm | Intercostal | None | N/A | N/A | No | 22.00 |
| 9 | TMR (coaptation to motor nerve to internal oblique) | Ilioinguinal | None | Pain improved and different from preoperatively | Pain improved significantly | Yes | 20.06 |
| 10 | TMR (coaptation to motor nerve to internal oblique) | Ilioinguinal | None | Patient states improvement and change in quality of pain compared with preoperatively | N/A | Yes | 18.48 |
| 11 | 1. Allograft 4 cm 2. Allograft 2.5 cm | 1. Genitofemoral 2. Ilioinguinal | None | Improvement in pain and different from preoperatively | Significantly improved her pain and different from preoperatively | Yes | 14.42 |
| 12 | Allograft 4 cm and TMR (coaptation to motor nerve to internal oblique) | Genitofemoral | None | Patient still with pain but different from preoperatively | Bad incisional pain that is different from the pain before surgery | Yes | 14.81 |
| 13 | Allograft 3 cm | Genitofemoral | None | States the pain is improved and completely different | Pain much improved from surgery | Yes | 13.03 |
| 14 | Allograft 2.4 cm | Intercostal | None | States pain feels completely different than it did preoperatively | No pain | Yes | 9.42 |
| 15 | 1. Allograft 2 cm 2. Allograft 2cm | 1. Left intercostal 2. Right intercostal | None | N/A | N/A | Yes | 9.48 |
| 16 | Allograft 3 cm | Intercostal | None | Pain improved and is definitely different from before surgery | Pain is manageable | Yes | 7.87 |
| 17 | 1. Allograft 2 cm 2. Allograft 2 cm | 1. Left intercostal 2. Right intercostal | None | Patient states pain feels much improved | States completely without pain on left abdominal wall. On the right side, pain is much improved. | Yes | 5.84 |
| 18 | Allograft 2 cm | Intercostal | None | Pain improved after surgery and different from preoperatively | N/A | No | 1.58 |
| 19 | R ilioinguinal- TMR to motor nerve of internal oblique, L ilioinguinal- TMR to motor nerve of internal oblique | Bilateral ilioinguinal nerves | None | Some new dysesthesias in lower abdominal skin | 3.11.20 clinic visit, much improved and no longer on preoperative pain medications | Yes | 6.94 |
| 20 | TMR of right ilioinguinal and iliohypogastric nerves to motor branches of internal oblique | Ilioinguinal and iliohypogastric neuromas | None | No pain and just on tylenol | No pain at last clinic visit | Yes | 10.71 |
From the postoperative clinic notes, 85% (17) of patients reported significant improvement in preoperative pain at first postoperative visit. Three patients (15%) required further surgical treatment due to recurrent pain. Of note, 2 of these patients underwent TMR following a failed nerve allograft reconstruction, which led to complete resolution of pain. The third patient had a peripheral nerve stimulator placed by Neurosurgery.
The survey was completed by 75% (15) of the study patients (Table 3). The mean level of abdominal pain over the past week was 2.8 (SD ±2.3), on a 0–10 scale with 0 being no pain and 10 being severe pain. The most severe abdominal pain over the past week had a mean of 4.6 (SD ±2.9). Respondents had a mean current level of abdominal pain while taking the survey of 2.5 (SD ±2.1). Respondents also had a mean score of 4.3 (SD ±3.2) for interference of abdominal pain in activities of daily living (0 being no interference and 10 severe interference). In terms of analgesic medication use, 4/15 (26.7%) reported using narcotics (oxycodone and acetaminophen), 2/15 (13.3%) NSAIDs, 1/15 (6.7%) acetaminophen, and 2/15 (13.3%) topical agents (including lidocaine and CBD ointment).
Table 3.
Abdominal Wall Neuroma Pain Survey Responses
| n = 15 | |
|---|---|
| Survey question responses, rated on a scale from 0 to 10 | |
| Worst abdominal pain in the past week (0 = none), mean (SD) | 4.6 (2.9) |
| Average abdominal pain in the past week (0 = none), mean (SD) | 2.8 (2.3) |
| Current abdominal pain (0 = none), mean (SD) | 2.5 (2.1) |
| Frequency of feeling emotionally upset in the past week (0 = never), mean (SD) | 2.4 (2.2) |
| Sleep quality in the past week (0 = worst), mean (SD) | 6.7 (2.6) |
| Interference of abdominal pain in ability to do ADLs/activities of enjoyment (0 = none), mean (SD) | 4.3 (3.2) |
| Current pain medications | |
| Opioids | 4 (26.7%) |
| NSAIDs | 2 (13.3%) |
| Tylenol | 1 (6.7%) |
| Topical agents (lidocaine, CBD) | 2 (13.3%) |
| Occupation status | |
| Working full-time | 8 (53.3%) |
| Retired | 5 (33.3%) |
| Unable to work | 2 (13.3%) |
Patients who responded to the survey also reported a mean level of emotional upset (including depression and anxiety symptoms) of 2.4 (SD ±2.2). This suggests a beneficial outcome, as emotional upset was evaluated based on a 0–10 scale, with 0 indicating none and 10 indicating severe emotional upset. Additionally, sleep quality over the past week had a mean rating of 6.7 (SD ±2.6) among the respondents (0 being no sleep and 10 being excellent sleep).
Over half of the respondents (8/15) reported working full time. Five of the respondents were retired. Two of the respondents reported being unable to work; however, the reason was not documented.
DISCUSSION
Patients with abdominal wall neuroma pain are clinical challenges to their primary care physicians, internal medicine specialists, and surgeons. Arguably, this is due to the lack of research and literature on abdominal wall pain neuroma pain, as well as the fact that the general diagnostic mindset of clinicians is still “viscerally” based for the abdomen.13 However, because abdominal wall neuropathic pain can mimic a variety of acute and chronic, and sometimes urgent, conditions, it must remain a diagnosis of exclusion.14 Unlike most things in modern medicine, imaging has not been overly helpful other than for ruling out other diagnoses, and this remains a clinical diagnosis.
To date, no gold standard for treatment of chronic abdominal wall neuroma pain exists. Existing surgical treatments for related conditions, such as ilioinguinal neuroma, intercostal neuralgia and anterior cutaneous nerve entrapment syndrome, include simple excision of the inflamed peripheral nerve/neuroma, and excision and implantation of the freshened proximal nerve segment into local muscle.13,15–20 Lee et al in 2000 conducted a study on surgical treatment of chronic groin pain in 54 patients with neuromas of the lateral femoral cutaneous, ilioinguinal, iliohypogastric, and genitofemoral nerves.21 The techniques employed included either neurolysis or resection and burying, and they found that 68% of patients had excellent relief of pain. In their study, the lowest success rate occurred in genitofemoral neuromas (only 50% reported excellent relief of pain).21 Then, Dellon et al in 2014 described in 8 patients the treatment of intercostal neuroma pain after laparoscopic cholecystectomy with surgical resection of the neuroma and then implantation of the proximal nerve segment into local muscle (serratus or latissimus).17 Overall results demonstrated majority of the patients had excellent results (63%), meaning significantly decreased pain after surgical treatment.17 Similarly, a retrospective review of 13 patients treated with a 2-team, open and endoscopic approach to identify and transect a peripheral nerve groin neuroma (then implant the proximal end into local muscle) resulted in 77% (10) with excellent/good pain relief.22 Yet, these are limited case series and recent larger comparison studies have shown implanting the proximal segment into local muscle is unpredictable.10
The ideal treatment of genitofemoral (GF) neuroma is controversial. This is due in part to the anatomic variability of the GF nerve, and its sensory territories.23 Some argue that the best approach is to resect and allow the freshened proximal end to drop into the pelvis. Ducic et al described the anatomy of the genitofemoral nerve and treatment of neuropathic testicular pain with the method of GF identification within the inguinal canal, dissection, and then proximal resection and placement behind the peritoneum.24 The 4-patient case series demonstrated relief of preoperative pain with this approach.24 We believe that TMR or allograft has a role in GF neuroma pain because leaving the proximal end of a divided nerve without a target is less predictable than reconstructing and/or providing direct nerve-to-nerve healing.
Our retrospective case series of 20 patients demonstrated that 90% (18) patients had significant improvement in abdominal wall neuropathic pain after surgical management. Therefore, this article supports a paradigm shift for these patients. Localized pain that seems localized to the abdominal wall on physical examination can be temporarily anesthetized with local numbing medicine, and is intolerable after all other treatment modalities should be referred to a surgeon competent in the handling of painful nerves. To communicate our results to patients simply, a patient with abdominal wall neuropathic pain is told that 80% of patients get about 80% better with surgery. Before surgery, 40% of patients (8 of 20 patients) had documented narcotic pain medication prescriptions, and this dropped to 26% (4 of 15 survey respondents) after surgery. The survey data also revealed that over half of the survey respondents returned to work. Although 2 patients (13.3%) were unable to return to work, it is important to note that the specific details of inability to return to work were not captured by the survey instrument. However, these are the same 2 patients that reported persistent neuropathic pain after surgical treatment; so pain is a likely contributor.
In the literature, there is little consensus for management and treatment of these patients who are typically bounced from specialist to specialist. When a symptomatic neuroma is finally diagnosed and the neuroma pain is felt to be intolerable, treatments include excision ± implantation, nerve excision and allograft reconstruction, RPNIs, and TMR. Our center has helped reestablish the concept that optimal management of newly divided nerve endings should entail active treatment of the end of the resected nerve, instead of burying or hiding the nerve ending.25–27 Nerve allografts conceptually are the optimal treatments of these injured intercostal nerves, by channeling reinnervation into the same receptor bed that the nerve had originally served. RPNIs similarly work to provide nerve fascicles with terminal receptors in the form of muscle grafts wrapped around the freshened nerve endings.28–30 However, RPNIs would render the abdominal muscle downstream of the divided nerve permanently denervated, and this denervated abdominal wall muscle could stretch and bulge over time. The same could be true of TMR used for the ilioinguinal nerve with potential resultant bulging of the internal oblique muscle. Fortunately, though with limited follow-up, that has not been seen with any of our patients to date.
A logical question is why our center uses TMR for the ilioinguinal nerve, but allografts are used for the other intercostal nerves. Our clinical observation is that the quality of pain relief seems more reliable and complete for TMR than for nerve allografts. Two patients with failed allografts had nearly complete pain relief with TMR. In addition, the 2 anatomic locations are different, with the higher nerves surrounded by muscle, but with the ilioinguinal nerve being covered by external oblique fascia. Maybe, using the reasoning of RPNIs, the allograft surrounded by muscle does better than an allograft with muscle only on its deep surface. Although TMR for the higher intercostal nerves is possible, it would require a longer dermatomal incision through the external oblique and internal oblique musculature to find a suitable motor nerve. The internal oblique would require a division of muscle fibers, which would be difficult to re-suture without a mesh. The resulting complexity of the closure and the possible bulges that could result have led the senior author to prefer a muscle splitting dissection to place an allograft, rather than TMR. For these reasons, TMR is performed for the ilioinguinal nerve, and allografts are used for higher intercostal nerves. Nerve allografts are well described in the treatment of neuroma pain in the upper and lower extremity.7,31,32 Of note, processed nerve allografts have been shown to be effective for nerve reconstruction of gaps up to 3 cm,33–36 and all of our cases used an allograft this length or shorter. Based on the senior author’s experience for painful nerves in the groin and the extremities, TMR is a more effective pain treatment than allograft reconstruction, and so the inguinal area now receives this procedure preferentially. In the upper abdomen, the senior author has not figured out a means to achieve TMR without denervating a large amount of functional muscle, and so relies on allografts. When allografts fail in the upper abdomen, an RPNI is attempted for salvage. We have not ever used a sensory nerve (sural) autograft to reconstruct a painful intercostal nerve for fear of creating donor site morbidity. Neurolysis of painful nerves was tried in the extremities and soon-after abandoned by the senior author for lack of efficacy. It has not been tried for intercostal nerves.
Strengths of the study include the long follow-up period, with a mean of 18.9 months, and our approach, with either TMR or allograft reconstruction, does not have a risk of iatrogenic hernias. Additionally, the diagnostic workup, involving lidocaine injection and office workups while eschewing radiography, is cost-effective. A significant limitation is that we have not demonstrated that nerve allografts channel motor nerves to reinnervate abdominal wall muscle, though the literature is replete with these observations. Other limitations include the absence of a control group and no standardized preoperative pain, activities of daily living, mood, and sleep evaluation assessments. Our results are dependent on the preoperative consultation notes with described severity of pain and daily disability that caused these patients to seek surgical treatment. Finally, surveys are at risk of recall and selection biases. One way these biases were mitigated were to use postoperative clinic notes (when available) to assess concurrency between clinic reports and the survey responses. In addition, the survey instrument was based on prior studies10,11 from our research team that created patient chronic pain related surveys from validated chronic pain assessment tools.9 A weakness of the study is that there is an element of “expert opinion” by the senior author. He has seen a few groin allografts fail and need to be converted to TMR (all successful). The TMRs of the groin as well as the allografts of the abdomen have generally worked, though the unmasking phenomenon on occasion requires the treatment of an adjacent intercostal nerve not addressed in the primary surgery.
The authors acknowledge that this is an initial report of their findings using established neuroma techniques of nerve grafting and TMR for management of abdominal wall pain—giving the nerve somewhere to go and something to do. Future studies using improved pain outcomes tools horizontally applied to all patients preoperatively and postoperatively and with all patients having >12-month follow-up will be the next goal for these investigations.
CONCLUSIONS
This case series with postoperative pain outcome surveys demonstrated significant improvement in abdominal neuroma pain after TMR and/or nerve allograft reconstruction. Surgical techniques and principles from TMR may apply to successfully treat abdominal wall neuroma pain and directly improve quality of life after necessary abdominal surgical procedures.
Supplementary Material
Footnotes
Published online 24 May 2021.
Presented as a poster at the January 2020 American Peripheral Nerve Society Meeting (ASPN), in Fort Lauderdale, Fla.
Disclosure: Dr. Dumanian is a consultant for Checkpoint Surgical and is the founder of Advanced Suture Inc. and Mesh Suture Inc. The other authors have no financial interest to disclose in relation to the content of this article. This study was supported by a Northwestern Medicine Electronic Database Warehouse Pilot Data Program 2018–2019 grant.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: Making the case for hernia research. Hernia. 2012; 16:179–183. [DOI] [PubMed] [Google Scholar]
- 2.HerniaSurge Group. International guidelines for groin hernia management. Hernia. 2018; 22:1–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoshmohabat H, Panahi F, Alvandi AA, et al. Effect of ilioinguinal neurectomy on chronic pain following herniorrhaphy. Trauma Mon. 2012; 17:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroori S, Spence RA. Chronic pain after hernia surgery—an informed consent issue. Ulster Med J. 2007; 76:136–140. [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen CMAH, Bonouvrie DS, Jacobs MLYE, et al. Chronic abdominal pain after previous bariatric surgery: Consider the abdominal wall. Obes Surg. 2020; 30:2942–2948. [DOI] [PubMed] [Google Scholar]
- 6.Stoehr JR, Chappell AG, Dumanian GA. Kiteboarding induced abdominal wall pain: intercostal neuroma versus anterior cutaneous nerve entrapment (ACNES). Plast Reconstr Surg Glob Open. 2021; 9:e3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi A, Park E, Dumanian GA. Treatment of painful nerves in the abdominal wall using processed nerve allografts. Plast Reconstr Surg Glob Open. 2018; 6:e1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010; 63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: A randomized clinical trial. Ann Surg. 2019; 270:238–246. [DOI] [PubMed] [Google Scholar]
- 11.Valerio IL, Dumanian GA, Jordan SW, et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019; 228:217–226. [DOI] [PubMed] [Google Scholar]
- 12.Shermak MA, Chang D, Magnuson TH, et al. An outcomes analysis of patients undergoing body contouring surgery after massive weight loss. Plast Reconstr Surg. 2006; 118:1026–1031. [DOI] [PubMed] [Google Scholar]
- 13.Scheltinga MR, Roumen RM. Anterior cutaneous nerve entrapment syndrome (ACNES). Hernia. 2018; 22:507–516. [DOI] [PubMed] [Google Scholar]
- 14.Carnett J. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet. 1926; 42:625–632. [Google Scholar]
- 15.Williams EH, Williams CG, Rosson GD, et al. Neurectomy for treatment of intercostal neuralgia. Ann Thorac Surg. 2008; 85:1766–1770. [DOI] [PubMed] [Google Scholar]
- 16.Ducic I, West J, Maxted W. Management of chronic postoperative groin pain. Ann Plast Surg. 2008; 60:294–298. [DOI] [PubMed] [Google Scholar]
- 17.Dellon AL. Intercostal neuroma pain after laparoscopic cholecystectomy: Diagnosis and treatment. Plast Reconstr Surg. 2014; 133:718–721. [DOI] [PubMed] [Google Scholar]
- 18.Siawash M, de Jager-Kievit JW, Ten WT, et al. Prevalence of anterior cutaneous nerve entrapment syndrome in a pediatric population with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2016; 62:399–402. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin C, Gandhi A, Hamner CE. Anterior cutaneous neurectomy for chronic abdominal wall pain: A case report and review of the literature. J Pediatr Surg Case Rep. 2017; 22:44–46. [Google Scholar]
- 20.Armstrong LB, Dinakar P, Mooney DP. Neurectomy for anterior cutaneous nerve entrapment syndrome in children. J Pediatr Surg. 2018; 53:1547–1549. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Dellon AL. Surgical management of groin pain of neural origin. J Am Coll Surg. 2000; 191:137–142. [DOI] [PubMed] [Google Scholar]
- 22.George T, Williams EH, Franklin R, et al. Two-team surgical approach to improve retroperitoneal nerve identification in the treatment of groin pain. Ann Plast Surg. 2019; 82:82–84. [DOI] [PubMed] [Google Scholar]
- 23.Rab M, Ebmer And J, Dellon AL. Anatomic variability of the ilioinguinal and genitofemoral nerve: Implications for the treatment of groin pain. Plast Reconstr Surg. 2001; 108:1618–1623. [DOI] [PubMed] [Google Scholar]
- 24.Ducic I, Dellon AL. Testicular pain after inguinal hernia repair: An approach to resection of the genital branch of genitofemoral nerve. J Am Coll Surg. 2004; 198:181–184. [DOI] [PubMed] [Google Scholar]
- 25.Hijjawi JB, Kuiken TA, Lipschutz RD, et al. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006; 118:1573–1578. [DOI] [PubMed] [Google Scholar]
- 26.Kuiken TA, Dumanian GA, Lipschutz RD, et al. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004; 28:245–253. [DOI] [PubMed] [Google Scholar]
- 27.Kuiken TA, Miller LA, Lipschutz RD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: A case study. Lancet. 2007; 369:371–380. [DOI] [PubMed] [Google Scholar]
- 28.Woo SL, Urbanchek MG, Cederna PS, et al. Revisiting nonvascularized partial muscle grafts: A novel use for prosthetic control. Plast Reconstr Surg. 2014; 134:344e–346e. [DOI] [PubMed] [Google Scholar]
- 29.Kung TA, Langhals NB, Martin DC, et al. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014; 133:1380–1394. [DOI] [PubMed] [Google Scholar]
- 30.Santosa KB, Oliver JD, Cederna PS, et al. Regenerative peripheral nerve interfaces for prevention and management of neuromas. Clin Plast Surg. 2020; 47:311–321. [DOI] [PubMed] [Google Scholar]
- 31.Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012; 32:1–14. [DOI] [PubMed] [Google Scholar]
- 32.Souza JM, Purnell CA, Cheesborough JE, et al. Treatment of foot and ankle neuroma pain with processed nerve allografts. Foot Ankle Int. 2016; 37:1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res Int. 2014; 2014:698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009; 4:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safa B, Buncke G. Autograft substitutes: Conduits and processed nerve allografts. Hand Clin. 2016; 32:127–140. [DOI] [PubMed] [Google Scholar]
- 36.Zhu S, Liu J, Zheng C, et al. Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: A 3 year follow-up study. J Tissue Eng Regen Med. 2017; 11:2314–2322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


