Abstract
The coronavirus disease 2019 (COVID-19) pandemic spreading at an alarming rate has taken a heavy toll on the public healthcare systems and economies worldwide. An abnormal and overactivated inflammatory response is occasionally elicited by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and this hyperinflammation is associated with worse prognosis of COVID-19. Theoretically, one would expect patients with asthma to be at a greater risk of SARS-CoV-2 infection considering their increased susceptibility to common respiratory virus-associated exacerbations. Surprisingly, current data do not consistently suggest an increased prevalence of asthma among patients with COVID-19. Considering the high global prevalence of asthma, the characteristics of the disease and/or their conventional therapy might play a role in their potential defense against COVID-19. This may be attributed to the T helper type 2 immune response predominantly seen in patients with asthma. Likewise, asthma therapeutics, including corticosteroids and biologics, may in fact benefit the patients with asthma by alleviating the development of hyperinflammation. On the other hand, elevated IL-17 levels are characteristically seen in a subset of asthma patients with severe disease as well as in patients with COVID-19. Targeting the IL-17 pathway as a treatment strategy could plausibly alleviate acute respiratory distress syndrome (ARDS) in patients with COVID-19 and asthma demonstrating a predominant T helper type 17 response. A clinical trial including a drug targeting this pathway may thus, constitute a logical addition to the global pursuit for effective therapeutics against COVID-19. The complex interplay between the asthma endotypes and COVID-19 is not very well understood and will be discussed in this mini-review.
Keywords: asthma, COVID-19, SARS-CoV-2, Th17 asthma, Th2-high asthma
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic set off by the novel strain of coronavirus, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is spreading at an alarming rate with over 116 million confirmed cases, including 2.5 million deaths, globally as of 9th March 2021 (1). This global threat has taken a heavy toll on our public healthcare systems and economies worldwide. SARS-CoV-2 use the angiotensin-converting enzyme 2 (ACE2) receptor as their gateway for cellular entry (2). Post entry into the cell, the virus initiates its replication program, eliciting a dysregulated innate and adaptive immune response in some individuals especially the elderly over the age of 60 and those with underlying chronic comorbidities, which may lead to overactivated inflammation and related cytokine storm. This hyperinflammation is associated with worse prognosis of COVID-19. However, the young generally exhibit increased resilience, in part because their immune systems are better equipped to counter the consequences of lung injury associated with SARS-CoV-2 infection.
Although a substantial proportion of the infected patients are asymptomatic or with mild symptoms (3), pneumonia in some patients can lead to acute respiratory distress syndrome (ARDS), which is associated with a high mortality rate (4). COVID-19-related ARDS encompasses alveolar damage, pulmonary edema, and subsequent lung failure, as well as associated functional damage in other organ systems, including the kidney, heart, and liver (4). Severe presentations of COVID-19 portray an increase in absolute neutrophil count and a decrease in absolute eosinophil count (5, 6). Current treatments for COVID-19 are largely symptomatic, focused on alleviating the displayed symptoms in infected patients, and there is no specific drug cure for COVID-19. The lack of a specific treatment regimen necessitates a deeper understanding of this fast-spreading menace to direct the development of effective therapeutics, especially for those with life-threatening severe disease.
Patients with chronic respiratory diseases such as asthma would be expected to be at high risk from COVID-19 as the virus primarily targets the airways and lung parenchyma (7). Asthma is a disease of the conducting airways of the lungs and is associated with an abnormal airway repair mechanism resulting in inflammatory and structural remodeling changes to the airways. The current pathophysiological paradigm of asthma is characterized by its hallmark features, including airway inflammation, reversible airflow obstruction, airway hyperresponsiveness, and airway remodeling, which manifest as clinical symptoms of wheeze, chest tightness, cough, and dyspnea. Majority of the patients with asthma demonstrate a predominantly T helper type 2 (Th2) immune response, involving the type 2 immune cells (including Th2 cells, eosinophils, group 2 innate lymphoid cells, and mast cells) and cytokines (including IL-4, IL-5, and IL-13). Although patients with mild and moderate asthma cater to a Th2-dominated eosinophilic immune response, patients with severe asthma show signs of a Th2-low, T helper type 17 (Th17) endotype associated with a predominantly neutrophilic inflammation. Thus, fundamentally different pathophysiological mechanisms underly the various subtypes of asthma.
The current understanding of the complex interplay between asthma and COVID-19 is still in its nascent stage and needs further elucidation. Here, we examine the possible interactions between the two and how preexisting asthma and its subtypes may affect susceptibility to COVID-19 and subsequent course of the disease. We use our understanding from both common respiratory viruses, such as rhinovirus (RV), respiratory syncytial virus (RSV), and influenza virus, as well as SARS-CoV-2-related coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), to assess the risk of patients with asthma to contract COVID-19 and predict its outcomes. The recent advances that describe the contributions of asthma pathobiological features to COVID-19 pathology, the therapeutic option of conventional asthma medications, and anti-IL-17 therapy in patients with COVID-19 with a heightened IL-17 immunological profile, are also summarized.
ASSOCIATION BETWEEN PREEXISTING ASTHMA AND COVID-19
Although chronic respiratory diseases are considered a high-risk category among comorbidities in developing severe COVID-19 infection, the prevalence of asthma among patients with COVID-19 is controversial at the moment. The reported asthma prevalence among patients hospitalized with COVID-19 portrays conflicting data with regional as well as national differences (8). This discrepancy could be explained by the prevalence of asthma in the general population of a specific area and the impact of COVID-19 outbreak in that area (8). For instance, the epidemiological characteristics of the COVID-19 outbreak in China did not identify asthma as a risk factor for severity and mortality from COVID-19 illness (9–11). In the Chinese population, asthma was either underreported or entirely absent among patients with COVID-19 (9, 12). This led to the speculation that Th2 immune response in patients with asthma may reduce their susceptibility to COVID-19. There were no reports of increased asthma exacerbation on account of COVID-19 in France and Italy as well (13, 14). A similar observation was made in Russia where among 1,307 intensive care unit (ICU) patients on mechanical ventilation due to SARS‐CoV‐2 pneumonia, the prevalence of bronchial asthma was 1.8%, albeit with female predominance (15). A rapid review of evidence by Asthma Australia also reported patients with asthma to be at slightly reduced risk of acquiring COVID-19 or of hospitalization (16). However, hospitalization rendered them at a slightly higher risk of ICU admission and mechanical ventilation. Regardless, the likelihood of dying from COVID-19 was slightly lower in people with asthma than in those without asthma (17).
On the other hand, contradictory data were reported from studies in Germany and the United States, where higher asthma prevalence was noted among patients with COVID-19 (18, 19). This led the US Centers for Disease Control and Prevention (CDC) to deliberate that patients with moderate-to-severe asthma might be at an increased risk from severe COVID-19 outcomes as limited information is currently available regarding the impact of underlying asthma or asthma control on the increased risk of severe illness from COVID-19 (20). Multiple studies across the United States that characterized the patients hospitalized with COVID-19 have reported asthma as a comorbidity with prevalence varying from 9% to 17% (18, 21–23). Nevertheless, asthma diagnosis was not found to contribute to worse outcomes (23). A pertinent study that specifically investigated the prevalence and characterization of asthma among 1,526 hospitalized and nonhospitalized patients with COVID-19 in the United States identified that despite a substantial prevalence (14%), neither preexisting asthma nor the use of corticosteroids was associated with increased COVID-19-related hospitalization risk (24). In the United Kingdom, even though asthma was the fifth common (14.5%) major comorbidity recorded on admission in 20,133 hospital in-patients with COVID-19, asthma was not associated with increased hospital mortality (25). An Irish study also reported an asthma prevalence of 8.8% in 193 patients hospitalized with COVID-19 (26). A systematic review and meta-analysis assessing the prevalence and mortality risk of preexisting comorbidities among patients with COVID-19 identified asthma as one of the preexisting comorbidities in patients with COVID-19 (27). Although the mortality risk in COVID-19 patients with respiratory diseases was roughly two times higher than those without respiratory diseases, asthma was not found to be a significant contributor in this category. This inconsistency in asthma prevalence among patients with COVID-19 mandates more studies with larger cohorts to assess the true risk factor. Thus, with the current available evidence, asthma does not appear to increase the risk of poor outcomes with COVID-19.
In a UK Biobank community cohort of 269,070 participants, asthma was a common comorbidity in hospitalized COVID-19 patients (17). Interestingly, asthma was reported to be an independent risk factor for COVID-19 hospitalization in women in this study. Similar observations were made in three other studies were a large proportion of asthma patients hospitalized for COVID-19 were women (13, 23, 24). A large meta-analysis has also shown that obesity alone is an important risk factor for COVID-19 positivity, hospitalization, ICU admission, and mortality (28). A study looking at 502 adult patients with COVID-19 revealed that COVID-19 patients with asthma were more likely to be obese (29). However, they were not at increased risk for ICU admission, intubation, or COVID-19-related complications.
Despite its relatively high global prevalence, asthma not being adequately documented in patients with COVID-19 suggests that individuals with asthma may exhibit some degree of defense against COVID-19, perhaps as a result of some asthma-related features and/or conventional asthma therapy.
IMPLICATIONS OF ASTHMA PATHOBIOLOGY ON COVID-19 PATHOGENESIS
Immunoregulatory factors, such as Th2- and Th17-driven inflammation, are likely to modify the risk of COVID-19 outcomes in asthma. Allergic and antiviral immune responses appear to be regulated by a reciprocal negative regulatory loop (30). Therefore, the predisposition of atopic individuals with asthma to allergic responses renders them susceptible to common respiratory viral infections as a result of deficient antiviral response. For example, IgE cross linking has been reported to dampen the antiviral response in asthma patients, primarily by abrogating interferon (IFN) production (31). The ability of Th2 cytokines and transforming growth factor-β to suppress viral-induced IFN production is another mechanism resulting in deficient innate IFN induction in asthma (32, 33). In addition, these infections can potentially worsen asthma symptoms and exacerbations. Therefore, theoretically, one would expect patients with asthma to be at a greater risk of SARS-CoV-2 infection. It is interesting to note here that during the SARS outbreak, children with asthma were found to be less susceptible to the virus and likewise, SARS-CoV did not appear to trigger asthma exacerbations in children (34). This was further associated with a concomitant decline in the incidence of acute respiratory infections, such as that caused by RSV and influenza virus. In fact, not all respiratory viruses affect individuals with asthma equally. Compared with RV, RSV, and influenza virus, coronaviruses are not as common triggers for acute asthma exacerbations (35).
Th2-High Asthma
Eosinophilia.
Majority of patients with asthma demonstrate a predominantly Th2-high immune response. Hallmark features of asthma, such as eosinophilia and Th2 inflammation, are potentially capable of promoting viral clearance and inducing antiviral immunity, which may therefore account for the low prevalence of asthma reported among COVID-19 individuals in some studies. In addition to their secretory ribonucleases that can target viral genomic RNA (36), eosinophil activation by the TLR7-MyD88 nucleic-acid sensing pathway is known to decrease the infectivity of RSV, thus, limiting virus-induced lung dysfunction (37). In response to viral infections, pulmonary eosinophils are known to function as antigen-presenting cells for viral antigens by upregulating their expression of MHC-I, MHC-II, and costimulatory molecules to CD8+ T cells promoting the recruitment of these viral-specific T cells into the lungs, thereby promoting antiviral host cellular immunity (38). It is no surprise therefore that similar to SARS and MERS infections (39), eosinopenia is a clinical characteristic of acute respiratory deterioration in patients with COVID-19, more so in the severe than in the mild patients (6) and resolution of eosinopenia may serve as an indicator of COVID-19 improvement (40). This reduction in eosinophilic inflammation may also contribute to the decline in SARS-CoV-2-induced asthma exacerbations when compared with RV infections. In fact, a recent report demonstrated the protective nature of preexisting eosinophilia in COVID-19 patients with asthma, where COVID-19-infected patients with asthma with absolute eosinophil counts (AEC) ≥150 cells/μL were less likely to be admitted (41). Furthermore, improvement in hospital-measured AEC ≥150 cells/μL in hospitalized patients with asthma reduced the likelihood of mortality from COVID-19. Of note, COVID-19 mortality in patients with asthma alone without any comorbidities was not different from those without asthma. This is a key observation indicating Th2 asthma endotype to be an important predictor for reduced COVID-19-associated morbidity and mortality. Although eosinophils are causally related to asthma pathology, it is not certain at this point in time if this is true with regards to COVID-19 pathology too. At the same time, it also likely that eosinopenia is a secondary effect of COVID-19 pathophysiology. The ability of eosinophilia to exert both proinflammatory and antiviral effects may alter the course of COVID-19. It would therefore, be intriguing to explore the direct mechanistic role of eosinophilic inflammation on the disease course of COVID-19 and whether preexisting eosinophilia in patients with asthma alters the pathogenesis of COVID-19 by promoting viral clearance and establishing antiviral immunity in patients with asthma.
Th2 cytokines.
Type 2 cytokines, IL-4, IL-13, and IL-9, have demonstrated antiinflammatory activities, where they were found to inhibit the secretion of various proinflammatory cytokines, including IL-1α, IL-1β, TNF-α, IL-6, and IL-12 (42), that are implicated in COVID-19 pathogenesis. Therefore, we can speculate that the predominance of Th2 cytokines could potentially counteract the development of proinflammatory cytokine storm and hence, COVID-19 pathogenesis. However, Th2 cytokines, IL-4 and IL-13, have previously been shown to impair interferon (IFN-β and IFN-λ1) induction in bronchial epithelial cells in response to RV infection (33). The impact of Th2 cytokines on susceptibility to viral infections thus appears to depend on the disease mechanisms invoked by the virus. In addition, IL-4 and IL-13 have also been shown to regulate COVID-19 pathogenesis by modulating the expression levels of SARS-CoV-2 cellular entry mediators, ACE2, and transmembrane protease serine 2 (TMPRSS2).
ACE2 and TMPRSS2 expression.
ACE2 and TMPRSS2 receptor expression was found to be affected by asthma and asthmatic conditions. RNA sequencing of upper and lower airway cells from three different cohorts including children and adults identified asthma and respiratory allergies to be associated with significantly reduced ACE2 expression (43). Age and male sex are known risk factors for COVID-19 and accordingly, increased expression of ACE2 was noted in the airways of older population and males (44). In patients with asthma, the reduced gene and protein expression of ACE2 was accompanied by lower expression of furin, a protease that cleaves the spike glycoprotein, and higher expression of ADAM-17, a disintegrin that cleaves ACE2 from the surface (44). COVID-19 patients with asthma were also characterized by a reduction in ACE2 protein expression in their lower airways, when compared with COVID-19 subjects without asthma (45). Furthermore, type 2 biomarkers, including total IgE level, nasal epithelial expression of IL-13, and fractional exhaled nitric oxide, also inversely correlated with ACE2 expression, suggesting one potential explanation for the decreased susceptibility of patients with asthma to SARS-CoV-2 infection and its reduced severity. Our team recently reported the reduced expression of ACE2 and TMPRSS2 in patients with chronic rhinosinusitis with nasal polyps and this was found to be accompanied by increased expression of Th2 cytokines, IL-4, IL-5, and IL-13 in nasal polyp tissue (46). Interestingly, although eosinophilic inflammation in nasal polyp tissue reduced ACE2 and TMPRSS2, noneosinophilic inflammation increased the expression of both receptors. Bioinformatic analysis further revealed the inhibitory effect of IL-13 on ACE2 expression in both nasal and bronchial epithelium (43). Interestingly, although IL-13 reduced ACE2 expression, it increased the gene expression of SARS-COV-2 entry-mediated protein, TMPRSS2, in airway epithelial cells of Th2-high asthma when compared with Th2-low asthma (47). In addition to IL-13, IL-4 also downregulated ACE2 expression in airway epithelial cells (47). The above evidence that type 2 inflammatory processes modulate the expression of ACE2 and TMPRSS2 further supports an important role of type 2 immune regulation in COVID-19 pathogenesis. Also, another study identified ACE2 as a human interferon-stimulated gene (ISG) using airway epithelial cells in vitro, suggesting interferon-driven upregulation of ACE2 as a mechanism by which SARS-CoV-2 might exploit host antiviral defense to facilitate its entry into target cells (48). It is therefore possible that deficient IFN responses in patients with asthma likely limit ACE2 expression and viral invasion into target cells.
At the same time, RV infection in patients with asthma demonstrated increased expression of ACE2 but not that of TMPRSS2 as well as activated cytokine pathways that have been associated with COVID-19 (49). However, this viral-induced pattern was not exclusive to asthma as similar changes were induced by influenza A and RSV in normal human bronchial epithelial cells. In another study, no differences were detected in the mRNA expression of ACE2, TMPRSS2, or furin genes in human bronchial brushes and biopsies between asthmatics of varying severities and healthy controls (50). This study served as an indication that the asthmatic population of varying severities and treatment intensities were at no greater COVID-19 risk than the background population in the absence of other known risk factors such as cardiovascular diseases and diabetes. However, ACE2 expression negatively and positively correlated with Th2- and Th17-dependent epithelial signatures, respectively. Contrasting results were obtained from a similar study in which the gene expression of ACE2, TMPRSS2, and furin was elevated in severe asthma, particularly the neutrophilic phenotype, in comparison with mild-to-moderate asthma and healthy controls (51). Thus, there is a possibility that the neutrophilic phenotype in patients with severe asthma may place them at increased risk of COVID-19.
Childhood asthma.
Childhood asthma is typically considered a Th2-high endotype (52) and the type 2 immune response may drive the low susceptibility of children with asthma to COVID-19, as was seen during the SARS outbreak (34). Nevertheless, severe asthma has also been reported in children (53). The risk of COVID-19 infection and disease course in children has been reported to be different from that in adults (54–56). This could be attributed to the low expression of ACE2 and TMPRSS2, in their upper and lower airways (57). Nonetheless, nasal airway epithelium transcriptome data from 695 children revealed the upregulation of TMPRSS2 and ACE2 expression by type 2 and viral-induced IFN inflammation, respectively (58). Here again, ACE2 expression negatively correlated with type 2 inflammation. Extrapolation of these results to the clinical management of COVID-19 must be done with caution. Furthermore, severe COVID-19 infection was found to be a rare occurrence in children and young people, irrespective of underlying asthma (54, 59). On the contrary, asthma was reportedly a common underlying condition in pediatric COVID-19 cases in the United States (55), further stressing the need for differentiating the asthma phenotypes and endotypes in both children and adults.
Th2-Low/Th17 Asthma
IL-17 cytokine.
IL-17 is largely accepted as a pathogenic player in asthma as the severity of asthma positively correlates with IL-17 levels. The expression of Th17-associated cytokines (IL-17A and IL-17F) was observed to progressively increase in airway tissues with increased severity (60). Increased levels of IL-17 have also been reported in the sputum and bronchoalveolar lavage fluid of subjects with asthma (61). We have previously reviewed the pleiotropic role of IL-17 in the pathogenesis of asthma and the clinical implications of targeting IL-17 (62). We postulated a plausible dual regulatory role of IL-17 on Th2 inflammation depending on the timing of its induction.
One of the most common complications seen in patients infected with SARS-CoV-2 is ARDS leading to ICU admission (4, 63). These patients are usually pathologically characterized by cytokine storm syndrome, which manifest as heightened serum levels of cytokines, interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, IL-17, basic fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon (IFN)-γ, tumor necrosis factor (TNF-α), interferon-γ-inducible protein (IP-10), monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1α), and MIP-1β (63, 64). Several cytokines in this list, in particular IL-6, IL-1β, TNF-α, G-CSF, and GM-CSF, promote a Th17 response. In addition, IL-17, the canonical cytokine produced by Th17 cells, is largely considered as a proinflammatory cytokine that further induces the expression of IL-6, IL-1β, TNF-α (responsible for causing systemic inflammatory symptoms, including fever), G-CSF (responsible for granulopoiesis and recruitment of neutrophils), IL-8, IP-10, MIP-2A, MIP-3A (responsible for recruiting immune mediators), and matrix metalloproteinases (responsible for tissue injury and repair). IL-17 thus, drives the immunopathogenesis of ARDS and consequent lung damage through several mechanisms involving neutrophil recruitment, proinflammatory mediator production, and G-CSF-mediated apoptosis prevention (65). Interestingly, a recent report by Xie et al. (66) highlighted the association between IL-17 gene polymorphisms and ARDS prognosis, where a particular genotype associated with decreased IL-17 levels had decreased 30-day mortality, whereas another genotype was associated with increased IL-17 levels and worse prognosis. A patient with a severe case of COVID-19 displayed increased concentration of CCR6+ Th17 among CD4+ T cells, which may have contributed to severe hyperinflammation and subsequent death in this patient (67). An elevation in Th17 response has also been detected in patients with MERS and SARS (68, 69). Especially, MERS-CoV infection was found to promote IL-17A production in the lungs and sera to a significantly greater extent in patients with poor outcome (68). Thus, Th17 response appears to be an important contributor to the development of cytokine storm resulting in tissue damage in lung viral infections, including COVID-19. Noneosinophilic nasal polyp tissue in patients with chronic rhinosinusitis was characterized by increased IFN-γ and IL-17 levels as well as increased expression of ACE2 and TMPRSS2 (46). Furthermore, the upregulation of ACE2 expression by IL-17A highlights the differential regulation of ACE2 in Th2-high/eosinophilic and Th2-low/Th17/neutrophilic endotypes of asthma (45), and perhaps also indicates the differential susceptibility of these endotypes to SARS-CoV-2 infection. Th17 pathway may serve as a likely target to treat patients with COVID-19, especially those with an IL-17 dominant response. Since IL-17 levels correlate with asthma severity, it comes as little surprise that severe asthma was identified to constitute as a potential risk factor for COVID-19 severity (70). This further stress the importance of asthma subtypes in predicting their risk of susceptibility to the virus and its disease outcome.
Asthma in women.
Women are known to have higher asthma burden and are frequently associated with a more severe form of asthma (71). Furthermore, the incidence of nonatopic/nonallergic asthma is higher in women, particularly over the age of 35 yr (72). Since nonatopic asthma is characterized as a Th2-low endotype (73), women with asthma may be at an increased risk for severe COVID-19 requiring hospitalization (74). This could be attributed to the distinct underlying immunopathology of asthma in women, such as obesity.
Obesity.
Higher incidence and severity of asthma is observed in women with obesity (71, 72). In relation to the obesity-related asthma phenotype, nonatopic women with asthma show a higher prevalence of SARS-CoV-2 infection compared with men and it was suggested that obesity is the underlying issue. Obesity is associated with increased leptin concentrations, activation of the innate immune system, and release of proinflammatory Th1 cytokines (75). In fact, obesity-related asthma phenotype is associated with increased levels of TNF-α and IL-1β in the lungs even in the absence of antigenic stimulation (76). This Th2-low endotype may render obese asthmatic patients at increased risk for COVID-19 complications.
A recent multicenter study sought to identify and characterize subsets of asthma at increased risk for COVID-19 (77). This study demonstrated that patients with asthma with a Th2-low endotype displayed a distinctive gene signature in their bronchial epithelium characterized by increased coexpression of ACE2 and type I and II IFN-mediated viral response network. They constituted a higher risk subset for adverse outcomes from COVID-19 together with already-known high-risk characteristics for severe COVID-19 such as male sex, hypertension, eosinopenia, lymphopenia, a higher neutrophil-to-lymphocyte ratio in peripheral blood, and elevated BAL lymphocytes. This study further confirmed the positive upregulation of ACE2 expression by upstream IFN stimulation, suggesting that the activation of antiviral host response in some subsets of asthma can be deleterious to their COVID-19 outcomes.
It is important here to point out that significant crosstalk exists between the Th2 and Th17 immune responses in asthma. Reciprocal regulation of Th2 and Th17 inflammatory pathways was demonstrated in a murine model of allergen-induced asthma (78). At the same time, a novel subset of dual-positive Th2/Th17 cells displaying high production levels of IL-4 and IL-17 were found to characterize the more severe forms of asthma (79). Therefore, targeting both Th2 and Th17 pathologies using antibodies against IL-13 and IL-17 is a promising therapeutic approach. Phase I trial of BITS7201A, a novel humanized bispecific antibody that targets both IL-13 and IL-17, showed well tolerance (80). However, the trial had to be discontinued due to the high incidence of antidrug antibodies. Reflecting upon the above observations, a close surveillance of the T helper response in patients with asthmatic COVID-19 needs to be maintained among adults and children alike as our conclusions may change as the pandemic unfolds and the virus evolves.
IMPLICATIONS OF ASTHMA THERAPEUTIC STRATEGIES ON COVID-19 PATHOGENESIS
In addition to Th2/Th17 immune response, conventional asthma therapeutics, including corticosteroids, leukotriene antagonists, bronchodilators, and monoclonal antibodies, may also modulate the asthmatic immune response to SARS-CoV-2. Table 1 summarizes the potential effects of asthma therapeutics on COVID-19 pathogenesis.
Table 1.
Potential effects of asthma therapeutic strategies on COVID-19 pathogenesis
| Treatment | Potential Effects on COVID-19 | References |
|---|---|---|
| Inhaled corticosteroids (ICS) |
|
(81, 82) (83) (84) |
| LAMA glycopyrronium and β2-agonist formoterol |
|
(85) |
| Omalizumab (Anti-IgE mAb) |
|
(86, 87) (88, 89) (86) (90) |
| Azithromyzin |
|
(91) (92) |
| Fedratinib (JAK2 inhibitor) |
|
(93) |
ACE2, angiotensin-converting enzyme 2; CoV, coronavirus; COVID-19, coronavirus disease 2019, GM-CSF, granulocyte-macrophage colony stimulating factor; ISG, interferon-stimulated gene; JAK2, Janus kinase 2; MCP-1, monocyte chemoattractant protein-1; PGD2, prostaglandin D2; TMPRSS2, transmembrane protease serine 2; TYK2, tyrosine kinase 2.
Inhaled Corticosteroids
Low-dose inhaled corticosteroids (ICS) are a part of the routine treatment regimen for a considerable proportion of asthma patients. ICS budesonide suppressed the synthesis of GM-CSF and other inflammatory mediators (82). The ability of ICS to skew the balance toward an antiinflammatory milieu led to the speculation that it may protect the patients with asthma from early stages of SARS-CoV-2 infection by reducing airway inflammation (81). Similarly, another ICS, ciclesonide, has shown potential in blocking replication in coronoviruses, including SARS-CoV-2, SARS-CoV, and MERS-CoV, by targeting the viral RNA replication-transcription complex in multiple cultured cells (83). In another case report, ciclesonide inhalation successfully treated COVID-19 pneumonia in three patients with poor oxygenation and CT findings (94). Furthermore, the use of ICS was associated with decreased expression of both ACE2 and TMPRSS2 in asthmatic sputum cells, which may further explain the decreased susceptibility of patients with asthma taking ICS to SARS-CoV-2 infection and decreased COVID-19 morbidity (84). Considering their antiinflammatory and immune-suppressive properties, corticosteroids have been administered to critically ill patients across COVID-19, SARS, and MERS outbreaks to suppress the exuberant inflammatory response in the lungs. A meta-analysis of 7 randomized clinical trials recently revealed encouraging results where systemic corticosteroids, in particular dexamethasone, was found to reduce mortality among critically-ill patients with COVID-19, when compared with usual care or placebo groups (95). Strikingly, in a UK-based observational cohort study, patients with asthma on high-dose ICS alone were at an increased risk of COVID-19-related death compared with those on short-acting β-agonist (SABA) whereas those on low- or medium-dose were not (96). This led them to conclude that regular use ICS may not be protective in patients with asthma against COVID-19-related death. However, disease severity was not taken into consideration in this study and may plausibly explain the COVID-19-related death. Although corticosteroid usage in the management of severe COVID-19 was met with mixed responses (97), the benefits seem to outweigh the adverse effects (98).
Bronchodilators
Bronchodilators are another class of commonly used asthma medication. In addition to budesonide, β2-agonist formoterol and long-acting muscarinic antagonist (LAMA) glycopyrronium have shown efficacy in decreasing viral titers and virus-induced cytokine production, including IL-6, IL-8, and IFN-β, in coronavirus 229E (HCoV-229E)-infected primary human nasal and tracheal epithelial cell cultures (85). In fact, the combination of these 3 drugs exerted additive inhibitory effects. A poster presented at the American Academy of Allergy, Asthma & Immunology 2021 Annual Meeting, however, reported bronchodilator usage, either alone or in combination with steroids to be associated with increased COVID-19 hospitalization risk (99). The use of oral SABA was also an independent contributor toward the increased total medical cost burden in patients with COVID-19 with underlying asthma (100). Despite these mixed reports, patients with asthma are advised to continue their prescribed asthma medications as per the recent 2020 Global Initiative for Asthma report to maintain optimal asthma control which may help reduce the risk of adverse outcomes in COVID‐19 (101, 102).
Monoclonal Antibodies
Anti-IgE monoclonal antibody (mAb), Omalizumab, is another therapeutic approved for the treatment of severe, persistent asthma. Since the crosslinking of IgE with its high affinity receptor, FcεRI, impairs the antiviral response in patients with asthma (31), it is logical to think that blocking IgE using omalizumab in patients with asthma could reduce susceptibility to COVID-19 infection by boosting IFN signaling, as seen in the case of RV infections (86, 103). Furthermore, in patients with allergic asthma, omalizumab treatment also decreased circulating IL-33 levels (89), that is known to induce the release of proinflammatory mediators (IL-6, IL-1β, TNF-α, MCP-1, and prostaglandin D2) by mast cells (88). Omalizumab has shown clinical efficacy in decreasing susceptibility of patients with asthma to viral infections such as RV by reducing viral disease duration, viral shedding, and viral disease risk (86), as well as reducing lower respiratory tract symptoms and improving lung function (90). In a case report, the absence of asthma exacerbation, loss of asthma control or pneumonia in a patient with severe, early‐onset allergic asthma on omalizumab treatment during SARS-CoV-2 infection led the authors to attribute this protective effect to the underlying disease (allergic asthma) or the antibody used for treatment (Omalizumab) (104).
Benralizumab treatment and other asthma maintenance medications in two patients with severe eosinophilic asthma who had been affected by COVID-19 was accompanied by good response to infection (105). Another observational study on a cohort of 676 severe asthma patients indicated a low incidence (2.1%) of COVID-19 infection and that severe asthma patients using biologics (omalizumab, mepolizumab, benralizumab, and reslizumab) may not be at increased risk of severe COVID-19 compared with those not on biologics (106). Interestingly, none of the patients with COVID-19 experienced severe asthma exacerbations, ICU admissions, noninvasive/mechanical ventilation or extracorporeal membrane oxygenation, and no deaths. In a similar study, the incidence of COVID-19 in patients with severe asthma who received biological therapy (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab) was 1.4% (107). In this study however, severe asthma patients on biological therapy exhibited severe course and poor outcome of COVID-19. Since eosinopenia has been suggested as an early diagnostic marker of COVID-19 (108), the use of eosinophil-depleting treatments such as monoclonal antibodies targeting IL-5 (mepolizumab and reslizumab) or its receptor (benralizumab) is a matter of concern among patients with asthma and their treating physicians in the wake of COVID-19. The limited studies available provide a reassuring picture regarding the use of omalizumab (104), and benralizumab (105) during COVID-19 infection. However, there is a need for large-scale studies to assess the treatment mechanistics in asthma patients with COVID-19.
There are also several mAbs targeting the IL-17 pathway (anti-IL-17A and anti-IL-17RA) that are currently approved for the treatment of psoriasis and psoriatic arthritis. The three commercially available biological agents include secukinumab (anti-IL-17), ixekizumab (anti-IL-17), and brodalumab (anti-IL-17R). Although these biological agents have demonstrated efficacy in the treatment of IL-17-mediated diseases such as psoriasis, psoriatic arthritis, and rheumatoid arthritis, their efficacy is yet to be proven in severe uncontrolled asthma. The disappointing results from clinical trials investigating these mAbs in asthma (109) may be attributed to inappropriate study design and patient stratification where neither the asthma endotype nor the activity of the Th17 pathway was taken into consideration while recruiting patients with asthma. IL-17 inhibition may thus be a prospective therapeutic option for patients with IL-17-driven chronic inflammatory disorders.
Repurposing these currently marketed drugs is an attractive therapeutic option for COVID-19 as emerging studies have suggested the potential of IL-17 to serve as a biomarker of disease severity as well as a plausible therapeutic target to prevent ARDS in COVID-19 (110). IL-17 being important for host defense, the use of these drugs could entail an increased risk of infections. However, clinical trials indicate unchanged or low risk of serious infections over the short term (110), recommending their use in the acute setting of COVID-19. In addition, Wu et al. (93) tested the effects of an FDA-approved JAK2 inhibitor, Fedratinib, on Th17 cytokine production. In addition to suppressing the expression of IL-17, Fedratinib also reduced the expression of IL-22 by murine Th17 cells. Fedratinib is an attractive treatment option for COVID-19 as it is specific to JAK2 with no inhibitory effect on JAK1, JAK3, and TYK2, that are key for type I IFN signaling in antiviral response. Furthermore, GM-CSF signaling also involves JAK2, and thus, a JAK2 inhibitor can also prospectively impede GM-CSF function. Fedratinib thus, has the potential to hinder several Th17-associated cytokines and thereby prevent the deteriorating outcomes of cytokine storm in patients with COVID-19 (93).
Azithromyzin
Azithromyzin is usually administered as an add-on therapy in persistent asthma as it has been shown to contribute to fewer asthma exacerbations and improved quality of life in patients with uncontrolled persistent asthma (111). Since azithromyzin augments IFN response and ISG expression, while suppressing RV replication and release in airway epithelium (91, 92), azithromyzin-mediated innate antiviral immunity is likely to reduce the risk of severe outcomes in COVID-19-infected patients with asthma (112).
LACUNAE IN KNOWLEDGE AND FUTURE DIRECTIONS
There are key COVID-19-related questions concerning asthma that are in need of answers to guide patient care and management. 1) Are the various severities, phenotypes and endotypes of asthma associated with different levels of risk and altered course of COVID-19? 2) Does asthma affect the COVID-19-associated lung pathology? 3) Are Th2-high and Th2-low/Th-17 asthma associated with unique COVID-19 features? 4) Do asthma therapeutics alter COVID-19 susceptibility and/or disease course?
In a large population-based cohort study, patients with nonallergic asthma were found to be at a higher risk of severe COVID-19 when compared with allergic asthma (113). One possible explanation for this could be the reduced ACE2 expression in allergic asthma, potentially rendering their airways less vulnerable to viral intrusion. In addition to the disease mechanisms in play, the role of asthma medications on the severity of COVID-19 is lacking clarity. The staggering figures associated with COVID-19 and the absence of specific treatment has led to the repurposing of currently available FDA-approved drugs, including dexamethasone, azithromycin, remdesivir, hydroxychloroquine, and tocilizumab, in the pursuit for improved outcomes in patients with COVID-19. It is therefore, a logical reasoning to screen all severe patients with COVID-19 for hyperinflammation to be able to segregate them for treatment with existing, approved therapies with proven safety profiles, such as selective cytokine blockade, glucocorticosteroids, JAK inhibition, and intravenous immunoglobulin, to curb the rising mortality rates (114). For instance, targeting IL-17 in severe COVID-19 patients with ARDS is an attractive option considering its role in the induction of IL-1β, IL-6, TNF-α, GM-CSF, and neutrophil recruitment, that are known to drive the pathogenesis of ARDS.
CONCLUSIONS
Consistent with the epidemiological findings made during the 2009 influenza pandemic (38), asthmatic population appear to be no more likely to acquire COVID-19 and suffer severe morbidity and mortality than the general public during the COVID-19 pandemic. SARS-CoV-2 infection during heightened asthmatic inflammation could in turn play a beneficial role in certain asthma subtypes. However, asthma being a multi-factorial disease, their susceptibility to SARS-CoV-2 infection, COVID-19 severity and mortality is likely to depend on multiple factors. Demographics (age and sex), exacerbation frequency, asthma phenotype, asthma severity, asthma treatment, level of asthma control, lung function, and underlying comorbidities such as obesity are potential risk factors (115). Given the known phenotypic heterogeneity in asthma, we speculate that the different endotypes of asthma may contribute to the differential regulation of ACE2 and TMPRSS2 expression and be a critical factor responsible for the differential susceptibility of patients with asthma to COVID-19 across the globe. In addition to ACE2 expression, a combination of factors such as Th2/Th17 inflammation, eosinophilic/neutrophilic influx, smoking status, and presence of comorbidities, could influence susceptibility to SARS-CoV-2 and COVID-19 outcomes in patients with asthma (Fig. 1). There is a need for large-scale epidemiologic studies, mechanistic studies, and clinical trials that should include appropriate patient stratification to shed some clarity on the contribution of each of these factors in modulating COVID-19 disease outcome and severity in patients with asthma.
Figure 1.
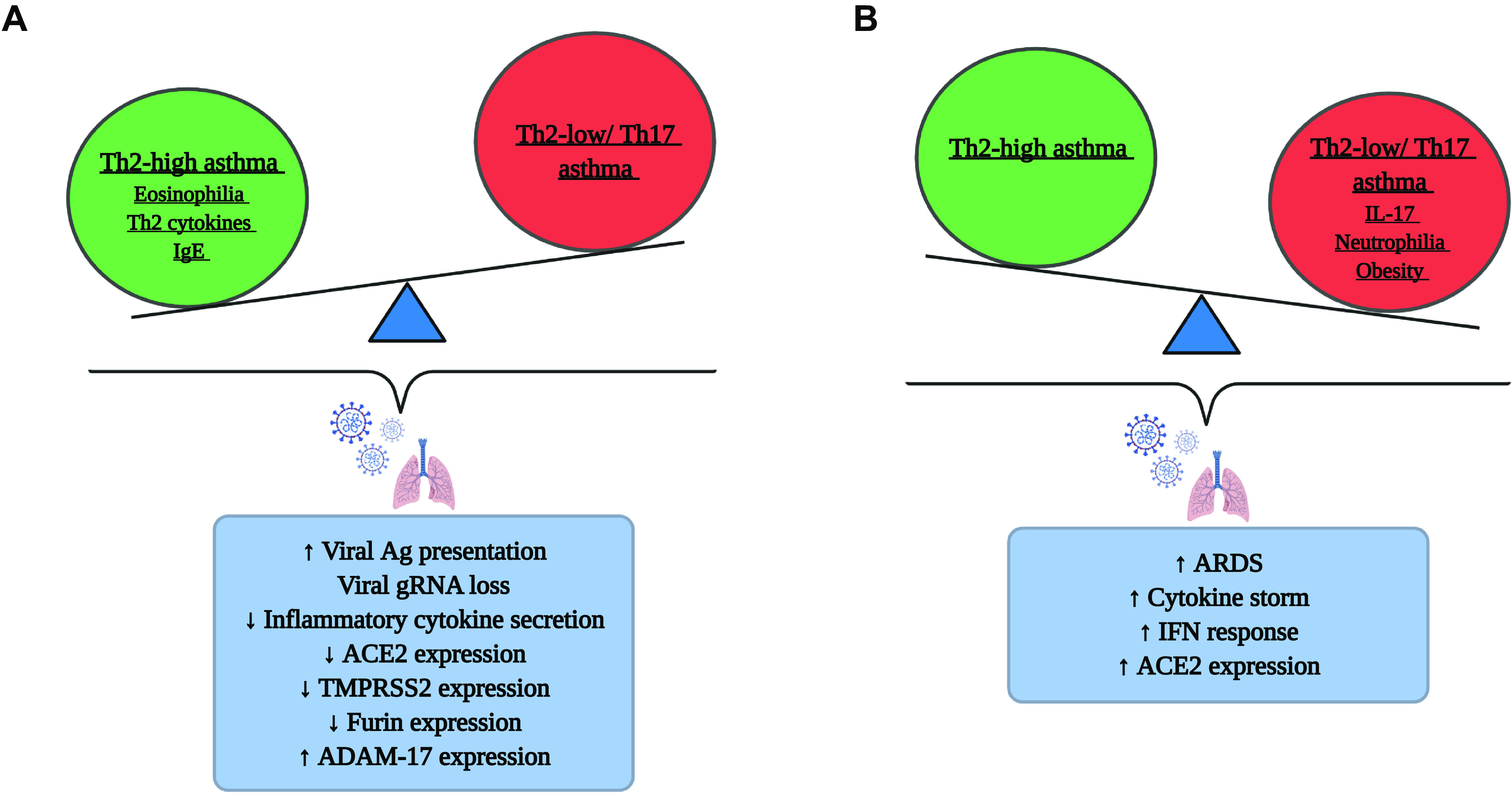
The balance between Th2-high and Th2-low/Th17 asthma in COVID-19. A schematic representation of the possible mechanisms by which Th2-high (A) and Th2-low/Th17 (B) endotypes of asthma contribute to the risk of COVID-19 infection. Created with BioRender.com. ACE, angiotensin-converting enzyme; Ag, antigen; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; gRNA, genomic RNA; Th2, T helper type 2; TMPRSS2, transmembrane protease serine 2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.K.R. and S.A.H. prepared figure; R.K.R. drafted manuscript; R.K.R., S.A.H., and Q.H. edited and revised manuscript; R.K.R., S.A.H., and Q.H. approved final version of manuscript.
REFERENCES
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard [https://covid19.who.int/] [9 March 2021].
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 173: 362–367, 2020. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020. [Erratum in Lancet Respir 8: e26, 2020]. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. In press. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71: 762–768, 2020. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, Stroberg E, Duval EJ, Pradhan D, Tzankov A, Parwani AV. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) - anatomic pathology perspective on current knowledge. Diagn Pathol 15: 103, 2020. doi: 10.1186/s13000-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caminati M, Lombardi C, Micheletto C, Roca E, Bigni B, Furci F, Girelli D, Senna G, Crisafulli E. Asthmatic patients in COVID-19 outbreak: Few cases despite many cases. J Allergy Clin Immunol 146: 541–542, 2020. doi: 10.1016/j.jaci.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 146: 110–118, 2020. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75: 1730–1741, 2020. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 12.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, , et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55: 2000547, 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurnier A, Jutant EM, Jevnikar M, Boucly A, Pichon J, Preda M, Frank M, Laurent J, Richard C, Monnet X, Duranteau J, Harrois A, Chaumais MC, Bellin MF, Noël N, Bulifon S, Jaïs X, Parent F, Seferian A, Savale L, Sitbon O, Montani D, Humbert M. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J 56: 2001875, 2020. doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pignatti P, Visca D, Cherubino F, Zampogna E, Spanevello A. Impact of COVID-19 on patients with asthma. Int J Tuberc Lung Dise 24: 1217–1219, 2020. doi: 10.5588/ijtld.20.0608. [DOI] [PubMed] [Google Scholar]
- 15.Avdeev S, Moiseev S, Brovko M, Yavorovskiy A, Umbetova K, Akulkina L, Tsareva N, Merzhoeva Z, Gainitdinova V, Fomin V. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy 75: 2703–2704, 2020. doi: 10.1111/all.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asthma Australia. Impact of COVID-19 on asthma: Rapid review for Asthma Australia [https://asthma.org.au/wp-content/uploads/2020/10/COVID-rapid-review_Summarised-report-for-technical-audience-VF-clean_v2.pdf] [15 February 2021].
- 17.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. The journals of gerontology Series A. J Gerontol A Biol Sci Med Sci 75: 2224–2230, 2020. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, , et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 69: 458–464, 2020. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 173: 268–277, 2020. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): people with certain medical conditions. [https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html#asthma] [22 October 2020].
- 21.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med 382: 2012–2022, 2020. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York city. N Engl J Med 382: 2372–2374, 2020. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovinsky-Desir S, Deshpande DR, De A, Murray L, Stingone JA, Chan A, Patel N, Rai N, DiMango E, Milner J, Kattan M. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol 146: 1027–1034, 2020. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, Kudlaty E, Mai Q, Yeh C, Muhammad LN, Harris KE, Bochner BS, Grammer LC, Greenberger PA, Kalhan R, Kuang FL, Saltoun CA, Schleimer RP, Stevens WW, Peters AT. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol 146: 307–314, 2020. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369: m1985, 2020. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler MW, O'Reilly A, Dunican EM, Mallon P, Feeney ER, Keane MP, McCarthy C. Prevalence of comorbid asthma in COVID-19 patients. J Allergy Clin Immunol 146: 334–335, 2020. doi: 10.1016/j.jaci.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MMA, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: a systematic review and meta-analysis. J Glob Health 10: 020503, 2020. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev 21: e13128, 2020. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussein MH, Toraih EA, Attia AS, Burley N, Zhang AD, Roos J, Houghton A, Aniemeka N, Omar M, Aboueisha M, Shama MA, Duchesne J, Kandil E. Asthma in COVID-19 patients: an extra chain fitting around the neck? Respir Med 175: 106205, 2020. doi: 10.1016/j.rmed.2020.106205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzales-van Horn SR, Farrar JD. Interferon at the crossroads of allergy and viral infections. J Leukoc Biol 98: 185–194, 2015. doi: 10.1189/jlb.3RU0315-099R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol ) 184: 5999–6006, 2010. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedke N, Sammut D, Green B, Kehagia V, Dennison P, Jenkins G, Tatler A, Howarth PH, Holgate ST, Davies DE. Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PloS one 7: e44580, 2012. doi: 10.1371/journal.pone.0044580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, Stanciu LA, Gnesini G, Pastore A, Spanevello A, Morelli P, Johnston SL, Caramori G, Papi A. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70: 910–920, 2015. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 34.Van Bever HP, Chng SY, Goh DY. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr Allergy Immunol 15: 206–209, 2004. doi: 10.1111/j.1399-3038.2004.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol 146: 1–7, 2020. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177: 1458–1464, 1998. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 37.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110: 1578–1586, 2007. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 38.Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, Lee JJ, Hurwitz JL, Thomas PG, McCullers JA. Eosinophils promote antiviral immunity in mice infected with Influenza A virus. J Immunol ) 198: 3214–3226, 2017. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang SM, Na BJ, Jung Y, Lim HS, Seo JE, Park SA, Cho YS, Song EH, Seo JY, Kim SR, Lee GY, Kim SJ, Park YS, Seo H. Clinical and laboratory findings of middle east respiratory syndrome coronavirus infection. Jpn J Infect Dis 72: 160–167, 2019. doi: 10.7883/yoken.JJID.2018.187. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, Yu W, Zhang J. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis 95: 183–191, 2020. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, Rosenstreich D, Ramesh M. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract 9: 1152–1162.e3, 2021. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Zhi Y, Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol 59: 78–88, 2020. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson DJ, Busse WW, Bacharier LB, Kattan M, O'Connor GT, Wood RA, Visness CM, Durham SR, Larson D, Esnault S, Ober C, Gergen PJ, Becker P, Togias A, Gern JE, Altman MC. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol 146: 203–206, 2020. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wark PAB, Pathinayake PS, Kaiko G, Nichol K, Ali A, Chen L, Sutanto EN, Garratt LW, Sohal SS, Lu W, Eapen MS, Oldmeadow C, Bartlett N, Reid A, Veerati P, Hsu AC, Looi K, Iosifidis T, Stick SM, Hansbro PM, Kicic A. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology. In press. doi: 10.1111/resp.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song J, Zeng M, Wang H, Qin C, Hou HY, Sun ZY, Xu SP, Wang GP, Guo CL, Deng YK, Wang ZC, Ma J, Pan L, Liao B, Du ZH, Feng QM, Liu Y, Xie JG, Liu Z. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy 76: 483–496, 2021. doi: 10.1111/all.14517. [DOI] [PubMed] [Google Scholar]
- 46.Saheb Sharif-Askari F, Saheb Sharif-Askari N, Goel S, Fakhri S, Al-Muhsen S, Hamid Q, Halwani R. Are patients with chronic rhinosinusitis with nasal polyps at a decreased risk of COVID-19 infection? Int Forum Allergy Rhinol 10: 1182–1185, 2020. doi: 10.1002/alr.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, Billheimer D, Kraft M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol 146: 80–88.e88, 2020. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, HCA Lung Biological Network, , et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang EH, Willis AL, Romanoski CE, Cusanovich DA, Pouladi N, Li J, Lussier YA, Martinez FD. Rhinovirus infections in individuals with asthma increase ACE2 expression and cytokine pathways implicated in COVID-19. Am J Respir Crit Care Med 202: 753–755, 2020. doi: 10.1164/rccm.202004-1343LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradding P, Richardson M, Hinks TSC, Howarth PH, Choy DF, Arron JR, Wenzel SE, Siddiqui S. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma-implications for COVID-19. J Allergy Clin Immunol 146: 208–211, 2020. doi: 10.1016/j.jaci.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kermani NZ, Song WJ, Badi Y, Versi A, Guo Y, Sun K, Bhavsar P, Howarth P, Dahlen SE, Sterk PJ, Djukanovic R, Adcock IM, Chung KF; U-BIOPRED Consortium . Sputum ACE2, TMPRSS2 and FURIN gene expression in severe neutrophilic asthma. Respir Res 22: 10, 2021. [Erratum in Respir Res 22: 47, 2021]. doi: 10.1186/s12931-020-01605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 56: 219–233, 2019. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilbert TW, Bacharier LB, Fitzpatrick AM. Severe asthma in children. J Allergy Clin Immunol Pract 2: 489–500, 2014. doi: 10.1016/j.jaip.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhopal S, Bagaria J, Bhopal R. Children's mortality from COVID-19 compared with all-deaths and other relevant causes of death: epidemiological information for decision-making by parents, teachers, clinicians and policymakers. Public Health 185: 19–20, 2020. doi: 10.1016/j.puhe.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 69:: 422–426, 2020. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milani GP, Bottino I, Rocchi A, Marchisio P, Elli S, Agostoni C, Costantino G. Frequency of children vs adults carrying severe acute respiratory syndrome coronavirus 2 asymptomatically. JAMA Pediatr 175: 193–194, 2021. doi: 10.1001/jamapediatrics.2020.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, Temsah MH, Al Heialy S, Hamid Q, Halwani R. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev 18: 1–6, 2020. doi: 10.1016/j.omtm.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, Rios CL, Pruesse E, Nolin JD, Plender EG, Wechsler ME, Mak ACY, Eng C, Salazar S, Medina V, Wohlford EM, Huntsman S, Nickerson DA, Germer S, Zody MC, Abecasis G, Kang HM, Rice KM, Kumar R, Oh S, Rodriguez-Santana J, Burchard EG, Seibold MA. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun 11: 5139, 2020. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams EM, McGill G, Bhopal SS, Sinha I, Fernandes RM. COVID-19, asthma, and return to school. Lancet Respir Med 8: 847–849, 2020. doi: 10.1016/S2213-2600(20)30353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 123: 1185–1187, 2009. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108: 430–438, 2001. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 62.Ramakrishnan RK, Al Heialy S, Hamid Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev Respir Med 13: 1057–1068, 2019. doi: 10.1080/17476348.2019.1666002. [DOI] [PubMed] [Google Scholar]
- 63.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620–2629, 2020. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, O'Kane CM, McAllister K, Fitzgerald DC, Kissenpfennig A, McAuley DF, Ingram RJ. Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med 193: 407–416, 2016. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 66.Xie M, Cheng B, Ding Y, Wang C, Chen J. Correlations of IL-17 and NF-κB gene polymorphisms with susceptibility and prognosis in acute respiratory distress syndrome in a chinese population. Biosci Rep 39: BSR20181987, 2019. doi: 10.1042/BSR20181987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. [Erratum in Lancet Respir Med8: 420–422, 2020]. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faure E, Poissy J, Goffard A, Fournier C, Kipnis E, Titecat M, Bortolotti P, Martinez L, Dubucquoi S, Dessein R, Gosset P, Mathieu D, Guery B. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PloS one 9: e88716, 2014. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Josset L, Menachery VD, Gralinski LE, Agnihothram S, Sova P, Carter VS, Yount BL, Graham RL, Baric RS, Katze MG. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio 4: e00165–e00213, 2013. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. [https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports] [22 October 2020].
- 71.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep 15: 28, 2015. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leynaert B, Sunyer J, Garcia-Esteban R, Svanes C, Jarvis D, Cerveri I, Dratva J, Gislason T, Heinrich J, Janson C, Kuenzli N, de Marco R, Omenaas E, Raherison C, Gómez Real F, Wjst M, Zemp E, Zureik M, Burney PG, Anto JM, Neukirch F. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax 67: 625–631, 2012. doi: 10.1136/thoraxjnl-2011-201249. [DOI] [PubMed] [Google Scholar]
- 73.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 377: 965–976, 2017. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 74.Fernando M, Agusti A, Dharmage S, Lodge C. Are women with asthma at increased risk for severe COVID-19? Lancet Respir Med 9: 125–126, 2021. doi: 10.1016/S2213-2600(21)00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ignacio RM, Kim CS, Kim SK. Immunological profiling of obesity. J Lifestyle Med 4: 1–7, 2014. doi: 10.15280/jlm.2014.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leiria LO, Martins MA, Saad MJ. Obesity and asthma: beyond T(H)2 inflammation. Metabolism 64: 172–181, 2015. doi: 10.1016/j.metabol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Camiolo M, Gauthier M, Kaminski N, Ray A, Wenzel SE. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol 146: 315–324, 2020. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 7: 301ra129, 2015. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 79.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 134: 1175–1186, 2014. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staton TL, Peng K, Owen R, Choy DF, Cabanski CR, Fong A, Brunstein F, Alatsis KR, Chen H. A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers. BMC Pulm Med 19: 5, 2019. doi: 10.1186/s12890-018-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baraket M, Oliver BG, Burgess JK, Lim S, King GG, Black JL. Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study. Respir Res 13: 11, 2012. doi: 10.1186/1465-9921-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pelaia G, Vatrella A, Busceti MT, Fabiano F, Terracciano R, Matera MG, Maselli R. Molecular and cellular mechanisms underlying the therapeutic effects of budesonide in asthma. Pulm Pharmacol Ther 40: 15–21, 2016. doi: 10.1016/j.pupt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, Shimojima M, Fukushi S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol 95: e01648–e01720, 2020. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, Woodruff PG, Mauger DT, Erzurum SC, Johansson MW, Denlinger LC, Jarjour NN, Castro M, Hastie AT, Moore W, Ortega VE, Bleecker ER, Wenzel SE, Israel E, Levy BD, Seibold MA, Fahy JV. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med 202: 83–90, 2020. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, Shimotai Y, Momma H, Ichinose M, Kawase T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig 58: 155–168, 2020. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, Gruchalla RS, Kattan M, Kercsmar CM, Khurana Hershey G, Kim H, Lebeau P, Liu AH, Szefler SJ, Teach SJ, West JB, Wildfire J, Pongracic JA, Gern JE. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med 196: 985–992, 2017. doi: 10.1164/rccm.201701-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, Wildfire JJ, Gergen PJ, Cohen RT, Pongracic JA, Kercsmar CM, Khurana Hershey GK, Gruchalla RS, Liu AH, Zoratti EM, Kattan M, Grindle KA, Gern JE, Busse WW, Szefler SJ. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 136: 1476–1485, 2015. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parveen S, Saravanan DB, Saluja R, Elden BT. IL-33 mediated amplification of allergic response in human mast cells. J Recept Signal Transduct Res 39: 359–367, 2019. doi: 10.1080/10799893.2019.1690515. [DOI] [PubMed] [Google Scholar]
- 89.Yalcin AD, Uzun R. Anti-IgE significantly changes circulating interleukin-25, vitamin-D and interleukin-33 levels in patients with allergic asthma. Curr Pharm Des 25: 3784–3795, 2019. doi: 10.2174/1381612825666190930095725. [DOI] [PubMed] [Google Scholar]
- 90.Heymann PW, Platts-Mills TAE, Woodfolk JA, Borish L, Murphy DD, Carper HT, Conaway MR, Steinke JW, Muehling L, Gerald TW, Kennedy JL, Irani AM, McGraw MD, Early SV, Wheatley LM, Adams AP, Turner RB. Understanding the asthmatic response to an experimental rhinovirus infection: exploring the effects of blocking IgE. J Allergy Clin IImmunol 146: 545–554, 2020. doi: 10.1016/j.jaci.2020.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J 36: 646–654, 2010. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 92.Porter JD, Watson J, Roberts LR, Gill SK, Groves H, Dhariwal J, Almond MH, Wong E, Walton RP, Jones LH, Tregoning J, Kilty I, Johnston SL, Edwards MR. Identification of novel macrolides with antibacterial, anti-inflammatory and type I and III IFN-augmenting activity in airway epithelium. J Antimicrob Chemother 71: 2767–2781, 2016. doi: 10.1093/jac/dkw222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53: 368–370, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 26: 625–632, 2020. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, , et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA 324: 1–13, 2020. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP; OpenSAFELY Collaborative, , et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med 8: 1106–1120, 2020. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solinas C, Perra L, Aiello M, Migliori E, Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev 54: 8–23, 2020. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 214: 108393, 2020. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang BZ, Chen Z, Sidell MA, Eckel SP, Martinez MP, Lurmann F, Thomas DC, Gilliland FD, Xiang AH. Associations of asthma, COPD and medication history with the risk of severe COVID-19. In: American Academy of Allergy, Asthma & Immunology 2021 Annual Meeting 2021, 2021. [Google Scholar]
- 100.Choi YJ, Park JY, Lee HS, Suh J, Song JY, Byun MK, Cho JH, Kim HJ, Lee JH, Park JW, Park HJ. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J 57: 2002226, 2021. doi: 10.1183/13993003.02226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Global Initiative for Asthma. Pocket guide for asthma management and prevention. [https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/] [15 February 2021].
- 102.Johnston SL. Asthma and COVID-19: is asthma a risk factor for severe outcomes? Allergy 75: 1543–1545, 2020. doi: 10.1111/all.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, Gern JE, Togias A, Busse WW. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol 141: 1735–1743, 2018. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lommatzsch M, Stoll P, Virchow JC. COVID-19 in a patient with severe asthma treated with Omalizumab. Allergy 75: 2705–2708, 2020. doi: 10.1111/all.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.García-Moguel I, Díaz Campos R, Alonso Charterina S, Fernández Rodríguez C, Fernández Crespo J. COVID-19, severe asthma, and biologics. Ann Allergy Asthma Immunol 125: 357–359, 2020. doi: 10.1016/j.anai.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanon S, Brusselle G, Deschampheleire M, Louis R, Michils A, Peché R, Pilette C, Rummens P, Schuermans D, Simonis H, Vandenplas O, Schleich F. COVID-19 and biologics in severe asthma: data from the Belgian Severe Asthma Registry. Eur Respir J 56: 2002857, 2020. doi: 10.1183/13993003.02857-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eger K, Hashimoto S, Braunstahl GJ, Brinke AT, Patberg KW, Beukert A, Smeenk F, van der Sar-van der Brugge S, Weersink EJM, Bel EH. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med 177: 106287, 2020. doi: 10.1016/j.rmed.2020.106287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xia Z. Eosinopenia as an early diagnostic marker of COVID-19 at the time of the epidemic. EClinicalMedicine 23: 100398, 2020. doi: 10.1016/j.eclinm.2020.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188: 1294–1302, 2013. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 110.Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol 20: 345–346, 2020. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Marks GB, Baraket M, Powell H, Taylor SL, Leong LEX, Rogers GB, Simpson JL. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 390: 659–668, 2017. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 112.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56: 105949, 2020. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol 146: 327–329, 2020. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maes T, Bracke K, Brusselle GG. Reply to Lipworth et al.: inhaled corticosteroids and COVID-19. Am J Respir Crit Care Med 202: 900–902, 2020. doi: 10.1164/rccm.202006-2129LE. [DOI] [PMC free article] [PubMed] [Google Scholar]


