Abstract
Survivors from COVID-19 pneumonia can present with persisting multisystem involvement (lung, pulmonary vessels, heart, muscle, red blood cells) that may negatively affect exercise capacity. We sought to determine the extent and the determinants of exercise limitation in patients with COVID-19 at the time of hospital discharge. Eighteen consecutive patients with COVID-19 and 1:1 age-, sex-, and body mass index-matched controls underwent: spirometry, echocardiography, cardiopulmonary exercise test and exercise echocardiography for the study of pulmonary circulation. Arterial blood was sampled at rest and during exercise in patients with COVID-19. Patients with COVID-19 lie roughly on the same oxygen consumption isophlets than controls both at rest and during submaximal exercise, thanks to supernormal cardiac output (P < 0.05). Oxygen consumption at peak exercise was reduced by 30% in COVID-19 (P < 0.001), due to a peripheral extraction limit. In addition, within COVID-19 patients, hemoglobin content was associated with peak oxygen consumption (R2 = 0.46, P = 0.002). Respiratory reserve was not exhausted (median [IRQ], 0.59 [0.15]) in spite of moderate reduction of forced vital capacity (79 ± 40%). Pulmonary artery pressure increase during exercise was not different between patients and controls. Ventilatory equivalents for carbon dioxide were higher in patients with COVID-19 than in controls (39.5 [8.5] vs. 29.5 [8.8], P < 0.001), and such an increase was mainly explained by increased chemosensitivity. When recovering from COVID-19, patients present with reduced exercise capacity and augmented exercise hyperventilation. Peripheral factors, including anemia and reduced oxygen extraction by peripheral muscles were the major determinants of deranged exercise physiology. Pulmonary vascular function seemed unaffected, despite restrictive lung changes.
NEW & NOTEWORTHY At the time of hospital discharge, patients with COVID-19 present with reduced functional capacity and exercise hyperventilation. Peripheral factors, namely reduced oxygen extraction (myopathy) and anemia, which are not fully compensated by a supernormal cardiac output response, account for exercise limitation before exhaustion of the respiratory reserve. Enhanced chemoreflex sensitivity, rather increased dead space, mainly accounts for exercise hyperventilation. The pulmonary vascular response to exercise circulation of survived patients with COVID-19 does not present major pathological changes.
Keywords: cardiopulmonary exercise test, COVID-19, echocardiography, hemodynamics
BACKGROUND
Novel coronavirus-19 disease (COVID-19) pandemic has affected more than 100 million people all around the world up to February 2nd (1). Despite obvious concerns on mortality rates, 98% of subjects survived (1), but survival was often associated with residual fibrotic lung abnormalities at discharge from hospitalization, as witnessed by preliminary studies with chest CT-scans (2) and pulmonary function tests (3). Given the often severe involvement of multiple organs body functions during COVID-19, other abnormalities might persist at the resolution of the acute phase, potentially affecting subjects’ wellbeing. Various forms of pulmonary vascular involvement [pulmonary macro- or micro-embolism (4), as well as inflammatory endothelitis with neo-angiogenesis (5) and blunted hypoxic pulmonary vascoconstriction (6)] have been reported, especially in the most severe cases. Cardiac involvement has been described in a small but not negligible proportion of patients during the acute phase (7). In addition, the huge systemic inflammatory response during COVID-19 could lead to anemia, which is a common finding in these patients and reduces blood oxygen carrying capacity (8). Furthermore, hospitalization for COVID-19 generally lasts for several weeks, is characterized by prolonged bed rest and administration of myotoxic medications, that can promote profound deconditioning and muscle atrophy, especially in patients admitted to the intensive care unit. In addition, concern has been raised on the potential of COVID-19 to be directly or indirectly associated with myopathic changes (9, 10). However, the impact of all these multisystem alterations on patients’ functional capacity at the time of hospital discharge after COVID-19 is still unknown. Based on the above considerations, we hypothesized that the oxygen flow from the mouth to mithocondria could be impaired at several steps (ventilatory or cardiac pump, pulmonary circulation, blood oxygen carrying capacity, muscular oxygen extraction) immediately after clinical resolution of COVID-19 pneumonia. Thus, we sought to quantify and describe the extent and the main mechanisms of exercise limitation in these patients at the time of hospital discharge, combining cardiopulmonary exercise test with exercise echocardiography and comparing patients healed from COVID-19 with matched controls. Based on analogy with previous reports on survivors from Severe Acute Respiratory Syndrome, we anticipated that COVID-19 could be associated with a ∼20% reduction of exercise capacity (11–13).
METHODS
Patients with COVID-19
Between 22nd of April and 5th of May 2020, we consecutively assessed patients just before their hospital discharge from San Luca Hospital, Istituto Auxologico Italiano, Milan, where they had been admitted for PCR-positive COVID-19 pneumonia.
We included patients that, at the time of evaluation, were become PCR-negative, were judged clinically healed and weaned from oxygen, could perform an exercise test and did not present relevant preexisting cardiac, respiratory, or musculoskeletal comorbidities. In particular, we excluded patients with preexisting reduced left ventricular (LV) ejection fraction (<50%) or valvular heart disease, pulmonary embolism, chronic obstructive lung disease (COPD) GOLD class > II, muscular myopathy, active malignancy, or cognitive decline.
On the day before hospital discharge, enrolled patients underwent a full cardiorespiratory assessment, including pulmonary function test, echocardiography at rest, and cardiopulmonary exercise test combined with exercise echocardiography. In all patients, a radial artery catheter was inserted into the right radial artery, using sterile techniques and local anesthetic.
Control Subjects
We queried the database of patients who underwent a full cardiorespiratory assessment for unexplained dyspnea at Istituto Auxologico Italiano, Milan, between September 2016 and May 2018. This cardiorespiratory assessment consisted of lung spirometry, cardiopulmonary exercise test combined with exercise stress echocardiography, and dynamic assessment of operating lung volumes. We excluded patients with a cardiac or respiratory limitation to exercise (14), patients with reduced LV ejection fraction (<50%) or valvular heart disease, those with echocardiographic estimate of high LV filling pressure, pulmonary embolism, COPD GOLD > grade II, muscular myopathy, active malignancy, or cognitive decline.
Control subjects were matched 1:1 with patients with COVID-19 for age, sex, and body mass index (BMI). In case that more than one control subject matched with a COVID-19 patient, we chose the subject who underwent exercise cardiorespiratory study more recently.
The Ethics Committees of the Istituto Auxologico Italiano approved the study on 21st of April 2020 (Procotol No. 2020_04_21_04). All patients with COVID-19 signed a written informed consent at the time of enrollment before undergoing a full cardiorespiratory assessment. All controls had signed a written informed consent for the use of their data for research purposes.
Pulmonary Function Tests
Lung spirometry was performed using automated equipment (Vmax SensorMedics 2200, Yorba Linda, CA).
Echocardiography at Rest and During Exercise
Echocardiography at rest was performed according to current recommendations of the European/American Society of Echocardiography (15, 16). Left and right ventricular strain analysis was possible in patients with COVID-19 only.
Exercise stress echocardiography was performed at the same time of cardiopulmonary exercise test (see Cardiopulmonary Exercise Test). At rest, during the last minute of the warm-up phase and at peak exercise, consecutive pairs of tricuspid regurgitant jet velocity and velocity time integral of the left ventricular outflow tract were collected. These two parameters allow the estimation of pulmonary artery pressure (PAP) and cardiac output (CO), respectively, as previously described (17–19). We have already demonstrated the accuracy of exercise echocardiography for the assessment of pulmonary hemodynamics in our laboratory (18). Results reflect the agreement of two independent and experienced cardiologists. Multipoint PAP-CO slopes were calculated from linear regressions. Average slopes were also calculated from pooled mPAP-CO relationships of each study group using an adjustment for individual variability as previously described (19). At the same time, also tissue Doppler imaging of the tricuspid annulus was recorded, to assess the S′ wave, as a measure of right ventricular longitudinal systolic function. The ratio between S′ wave and systolic PAP was computed as an estimate of right ventricular to pulmonary artery coupling, albeit it has been less validated than other echocardiographic parameters (20). A single operator performed all echocardiographic examinations with a GE Logiq E9 ultrasound machine (General Electric Company, Boston, MA). Stored images were blindly reviewed and analyzed by two readers.
Cardiopulmonary Exercise Test
Patients wore a nonrebreathing Hans-Rudolph mask connected to the V-MAX metabolic cart (Vmax SensorMedics 2200, Yorba Linda, CA).
A symptom-limited exercise test was performed on a semi-recumbent cycle ergometer. Exercise started with a 20–30 W warm-up phase (depending on subject’s fitness) lasting 3 min, followed by a personalized ramp increment in workload, in order to achieve exhaustion in 6–12 min (14). Subjects were encouraged to exercise up to their maximal volitional effort, with the aim of obtaining a peak respiratory quotient > 1.1. Key measurements included standard breath-by-breath cardiorespiratory and breathing pattern parameters, and dynamic operating lung volumes calculated from inspiratory capacity maneuvers. At rest, during the last minute of the warm-up phase and at peak exercise, a flow-volume loop maneuver was performed (21) roughly at the same time of echocardiographic acquisition, and, only in patients with COVID-19, 2 mL of blood were sampled from the radial artery catheter. An average of the last 30-s period of exercise was taken as peak value for the variables of interest. The V̇e/V̇co2 slope was calculated over the linear component of V̇e versus V̇co2 (22). Results reflect the agreement of two independent and experienced operators.
Derived Cardiorespiratory Parameters
The content of oxygen in arterial blood (CaO2) was calculated using the following formula: CaO2 = 1.39 × SaO2× Hb + 0.0031 × PaO2 (16), where SaO2 is oxygen saturation in arterial blood, Hb is hemoglobin, and PaO2 is arterial oxygen partial pressure. In controls, the minor role of PaO2 in determining CaO2 was neglected, given that a radial artery catheter was not routinely placed.
By resolving Fick equation to obtain the arterial-venous oxygen difference from the direct measure of oxygen consumption (V̇o2) and from the echocardiographic estimate of cardiac output, we derived the content of oxygen in venous blood (23).
Physiological dead space was calculated in patients with COVID-19 by using V̇e, V̇co2, as well as PaCO2 directly measured from arterial blood (24, 25).
Statistics
In the absence of previous data on exercise limitation after COVID-19 at the time in which the study was conducted, but considering reports of ∼20% reduction of exercise capacity after Severe Acute Respiratory Syndrome (11–13), we tested the hypothesis that V̇o2 at peak exercise would be 20% lower in patients with COVID-19 as compared with controls. Considering a power of 80% and a significance level (alpha) of 0.05, and a standard deviation of 20%, we needed to include 18 patients with COVID-19 and 18 controls.
All continuous variables were reported as median and interquartile range [IQ] for homogeneity of data representation. Categorical data were reported as absolute numbers and proportions. Distribution of variables in terms of proximity to the normal curve and the homogeneity of variances were detected by the Shapiro–Wilk test and Bartlett test, respectively. Numerical variables were analyzed with t test or Wilcoxon rank sum, according to their distributions. Categorical variables were analyzed with Chi-squared test or Fisher exact test in case of small cell sizes. Correlation analysis was performed with the Pearson product-moment or with Kendall’s tau, where appropriate. Multiple linear regressions were performed to assess factors associated with V̇o2 and the ratio between minute ventilation (V̇e) and carbon dioxide (V̇co2) at peak. The variable selection for each model was performed by a stepwise method using Akaike's information criterion (AIC). All regression analysis met the assumptions for linearity, homoscedasticity, multicollinearity, and normality of residuals. An α level of 0.05 was used for all hypothesis tests. All data analyses were performed using R Core Team (2020), Vienna, Austria.
RESULTS
General and Echocardiographic Characteristics
Out of 25 patients evaluated at the time hospital discharge after resolution of laboratory-confirmed COVID-19 pneumonia between 22nd of April and 5th of May 2020, 20 satisfied inclusion criteria for the study and 18 accepted to participate. They underwent a full cardiorespiratory assessment at the time of hospital discharge. Eighteen age-, sex-, and BMI-matched controls were chosen among 115 outpatients who underwent cardiopulmonary exercise test combined with exercise-stress echocardiography, pulmonary function test, and dynamic assessment of operating lung volumes.
As shown in Table 1, it was a middle-aged, overweight population with a high prevalence of male sex. The main general characteristics of patients with COVID-19 did not differ from control group (Table 1), including the burden of mild comorbidities. Obesity was slightly but nonsignificantly more prevalent in controls, in spite of a nondifferent BMI between the two groups.
Table 1.
General characteristics of the study population
| Control Patients n = 18 | COVID-19 Patients n = 18 | P Value | |
|---|---|---|---|
| Demographics and anthropometrics | |||
| Age, yr | 65 (20) | 66 (21) | 0.911 |
| Male sex, N (%) | 13 (72) | 13 (72) | 1.000 |
| BMI, kg/m2 | 26 (5) | 26 (4) | 0.298 |
| Comorbidities | |||
| Arterial hypertension, N (%) | 11 (61) | 11 (61) | 1.000 |
| Diabetes mellitus, N (%) | 1 (6) | 2 (11) | 0.547 |
| Obesity, N (%) | 5 (28) | 1 (6) | 0.074 |
| Smoke, N (%) | 3 (17) | 3 (17) | 1.000 |
| COPD, N (%) | 3 (17) | 3 (17) | 1.000 |
| CAD, N (%) | 0 (0) | 1 (6) | 0.347 |
| Atrial fibrillation | 1.000 | ||
| Paroxismal, N (%) | 1 (6) | 1 (6) | |
| Permanent, N (%) | 1 (6) | 1 (6) | |
| Pulmonary function test | |||
| FVC, L | 4.02 (2.33) | 2.66 (1.47) | 0.002 |
| FVC, % predicted | 101 (10) | 79 (47) | 0.009 |
| FEV1, L/min | 3.06 (1.47) | 2.01 (1.13) | 0.016 |
| FEV1, % predicted | 105 (22) | 79 (40) | 0.059 |
| FEV1/FVC | 0.78 (0.09) | 0.83 (0.11) | 0.045 |
| Echocardiography at rest | |||
| LV EDV, mL | 106 (21) | 109 (39) | 0.735 |
| LV EF | 0.61 (0.07) | 0.58 (0.08) | 0.801 |
| LV mass index, g/m2 | 67 (16) | 85 (21) | 0.027 |
| LA volume, mL | 53 (20) | 57 (26) | 0.373 |
| E/E′ average | 8 (3) | 9 (5) | 0.450 |
| RV diameter, mm | 35 (6) | 40 (7) | 0.029 |
| S′ RV, cm/s | 11 (4) | 14 (3) | 0.155 |
| Hemodynamics, oxygen delivery and extraction at rest | |||
| SBP, mmHg | 134 (30) | 130 (20) | 0.343 |
| SPAP, mmHg | 25 (10) | 27 (11) | 0.034 |
| S′ RV /SPAP, cm/s/mmHg | 0.54 (0.23) | 0.49 (0.11) | 0.817 |
| HR, bpm | 75 (15) | 82 (20) | 0.760 |
| CO, L/min | 4.3 (0.9) | 5.3 (1.5) | <0.001 |
| SaO2, % | 99.5 (2.5) | 98.0 (2.0) | 0.007 |
| CaO2, mL/dL | 19.6 (2.9) | 17.1 (4.8) | <0.001 |
| CvO2, mL/dL | 12.3 (3.1) | 10.5 (5.4) | 0.318 |
| C(a−v)O2 mL/dL | 7.3 (2.3) | 5.7 (2.5) | <0.001 |
| C(a−v)O2/CaO2 | 0.37 (0.10) | 0.36 (0.17) | 0.219 |
BMI, body mass index; CAD, coronary artery disease; CaO2, content of oxygen in arterial blood; C(a-v)O2, arteriovenous oxygen difference; CO, cardiac output; COPD, chronic obstructive lung disease; COVID-19, novel coronavirus-19 disease; EDV, end-diastolic volume; EF, ejection fraction; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; HR, heart rate; LA, left atrium; LV, left ventricle; RV, right ventricle; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure. Data are expressed as median (IQR).
Patients with COVID-19 spent in median 30 [23–37] days in the hospital, where they had been treated with antiretroviral agents, steroids, antibiotics, hydroxychloroquine, and low-molecular weight heparin, in all cases at anticoagulant dosage as per local recommendations. Five patients (28%) underwent mechanical ventilation, 9 patients (50%) received noninvasive ventilation, and 4 patients (22%) necessitated only oxygen support.
Blood tests were within normal limits, except for mild anemia: patients with COVID-19 presented with lower hemoglobin values than controls (11.3 [2.3] vs. 14.5 [2.0] g/dL, P < 0.001).
Cardiorespiratory Function at Rest
Lung volumes of patients recovering from COVID-19 were smaller than controls (Table 1), with a relative reduction of both forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) of 22% and 26% in median, respectively (P < 0.05). Forty-five percent of patients with COVID-19 presented with at least moderate restrictive lung disease, whereas 67% of controls had normal lung function tests (P < 0.01).
At the same basal ventilation of controls, respiratory dynamics of COVID-19 were characterized by higher respiratory rate (24.0 [6.0] vs. 18.5 [9.3]/min, P = 0.021) with high dead space ventilation (VD/VT 0.63 [0.05]), higher end-inspiratory lung volume (1.97 [1.25] vs. 1.06 [1.00] L, P = 0.002; Supplemental Table S1; all Supplemental material is available at https://doi.org/10.5281/zenodo.4549376), higher ventilatory equivalents for carbon dioxide (V̇e/V̇co2 61 [7] vs. 42 [16], P = 0.003) and lower end-tidal carbon dioxide pressure (PetCO2 29 [2] vs. 32 [7], P = 0.023). SaO2 was in the normal range albeit slightly lower in COVID-19 as compared with controls (P < 0.01).
CaO2 of a patient with COVID-19 was lower than controls (P < 0.01), and associated with hemoglobin levels (tau 0.62, P < 0.001). Arteriovenous oxygen difference was lower in COVID-19 (P < 0.01) but peripheral oxygen extraction at rest was not different between COVID-19 and controls.
Standard echocardiography did not show any relevant abnormality in patients with COVID-19, except for a mildly dilated right ventricle (Table 3). Global longitudinal strain of the left ventricle and right ventricular free wall strain in patients with COVID-19 were −18 [4]% and −22 [8]%, respectively. Accordingly, one third of patients with COVID-19 presented with an abnormal left or right ventricular strain, defined by cut-off values >−17% and −20%, respectively.
Both cardiac output and PAP were higher in COVID-19 than controls (P < 0.05), leading to similar total pulmonary resistance (TPR) in the two groups. The ratio between right ventricular S′ wave and systolic PAP was not different between the two groups.
Cardiorespiratory Function during Exercise
Cardiorespiratory function at peak exercise is shown in Table 2. All patients affirmed to have performed a maximal volitional effort up to their limit. At peak exercise, V̇o2 was lower in COVID-19 patients than in controls (P < 0.001). The same held true for peak workload (64 [40] vs. 128 [89] W, P < 0.001). Ninety-five percent of patients with COVID-19 had a reduced exercise capacity, with a peak V̇o2 less than 70% of predicted in 61% of cases (vs. 17% of controls, P = 0.006). Also the V̇o2/work slope was lower in COVID-19 than in controls (P < 0.001).
Table 2.
Ventilatory and hemodynamic parameters of study population at peak exercise
| Control Patients n = 18 | COVID-19 Patients n = 18 | P Value | |
|---|---|---|---|
| Oxygen flow | |||
| V̇o2, mL/kg/min | 22.8 (9.3) | 14.8 (6.1) | <0.001 |
| V̇o2, % predicted | 90 (19) | 59 (32) | <0.001 |
| V̇o2/work slope | 10.9 (1.9) | 8.1 (1.2) | <0.001 |
| O2 pulse, mL/bpm | 12.3 (3.6) | 9.1 (2.0) | 0.002 |
| SaO2, % | 97.0 (2.8) | 96.8 (4.5) | 0.042 |
| CaO2, mL/dL | 19.5 (2.5) | 16.9 (5.0) | 0.001 |
| CvO2, mL/dL | 3.3 (6.2) | 6.3 (3.1) | 0.081 |
| C(a−v)O2, mL/dL | 15.3 (5.6) | 11.7 (2.5) | <0.001 |
| C(a−v)O2/CaO2 | 0.81 (0.31) | 0.66 (0.19) | 0.006 |
| RQ | 1.18 (0.15) | 1.08 (0.28) | 0.263 |
| Ventilatory adaptation | |||
| V̇e, L/min | 54.5 (36.1) | 41.9 (20.6) | 0.038 |
| V̇e/MVV | 0.45 (0.19) | 0.41 (0.15) | 0.369 |
| RR, per min | 32.5 (9.3) | 34.5 (12.0) | 0.035 |
| V̇e/V̇o2 | 32 (10) | 40 (10) | 0.023 |
| V̇e/V̇co2 | 30 (9) | 40 (9) | <0.001 |
| PetO2, mmHg | 112 (9) | 117 (8) | 0.037 |
| PetCO2, mmHg | 39 (10) | 34 (5) | 0.001 |
| Dynamic hyperinflation, N(%) | 10 (56) | 7 (39) | 0.317 |
| Expiratory flow limitation, N (%) | 5 (29) | 3 (17) | 0.370 |
| V̇e/V̇co2 slope | 28 (8) | 32 (7) | 0.007 |
| Cardiovascular adaptation | |||
| HR, beats/min | 142 (36) | 120 (30) | 0.023 |
| HR, % predicted | 89 (13) | 78 (17) | 0.009 |
| CO, L/min | 10.3 (2.8) | 10.6 (2.7) | 0.872 |
| CO, % of predicted | 86 (20) | 105 (28) | 0.004 |
| SBP, mmHg | 195 (31) | 165 (38) | 0.020 |
| SPAP, mmHg | 54 (28) | 52 (10) | 0.835 |
| TPR, WU | 3.6 (2.1) | 3.4 (1.7) | 0.646 |
| S′ RV, m/s | 16 (3) | 19 (5) | 0.560 |
| S′ RV /SPAP, m/s/mmHg | 0.41 (0.26) | 0.36 (0.18) | 0.831 |
CaO2, content of oxygen in arterial blood; C(a-v)O2, arteriovenous oxygen difference; CO, cardiac output; HR, heart rate; MVV, maximal voluntary ventilation; PetO2, end-tidal pressure for oxygen; PetCO2, end-tidal pressure for carbon dioxide; RQ, respiratory quotient; RR, respiratory rate; RV, right ventricle; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TPR, total pulmonary vascular resistance; V̇e, minute ventilation; V̇co2, carbon dioxide production; V̇o2, oxygen consumption. Data are expressed as median (IQR).
Oxygen Delivery and Extraction During Exercise
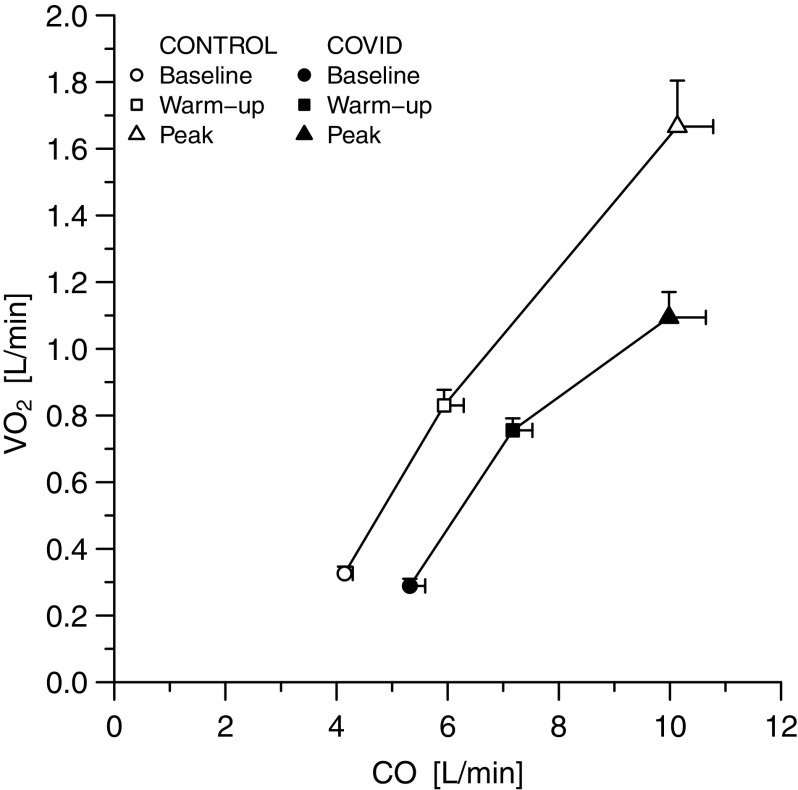
Figure 1 show cardiac output behavior as a function of arteriovenous oxygen difference and of peripheral oxygen extraction: patients with COVID-19 lie roughly on the same isophlets than controls at rest and during submaximal exercise, at higher cardiac output and lower arteriovenous oxygen difference and peripheral extraction. Accordingly, the relationship between V̇o2 and cardiac output was rightward-shifted in patients with COVID-19: at any V̇o2, cardiac output was higher in patients with COVID-19 than in controls (Fig. 2). At peak exercise, CaO2, arteriovenous difference and peripheral oxygen extraction were lower in COVID-19 than in the control group (P < 0.05). Absolute cardiac output value was not different between groups at peak exercise, but it was supernormal in COVID-19 when expressed as percentage of predicted values (P = 0.004). This occurred in spite of a mild chronotropic incompetence and a reduced systemic arterial pressure response in COVID-19 as compared with controls (P < 0.05). Finally, when analyzing patients with COVID-19 alone, V̇o2 at peak was associated both with CaO2 (tau = 0.58, P = 0.012) and with hemoglobin (R2 = 0.46, P = 0.01; Supplemental Fig. S1.
Figure 1.
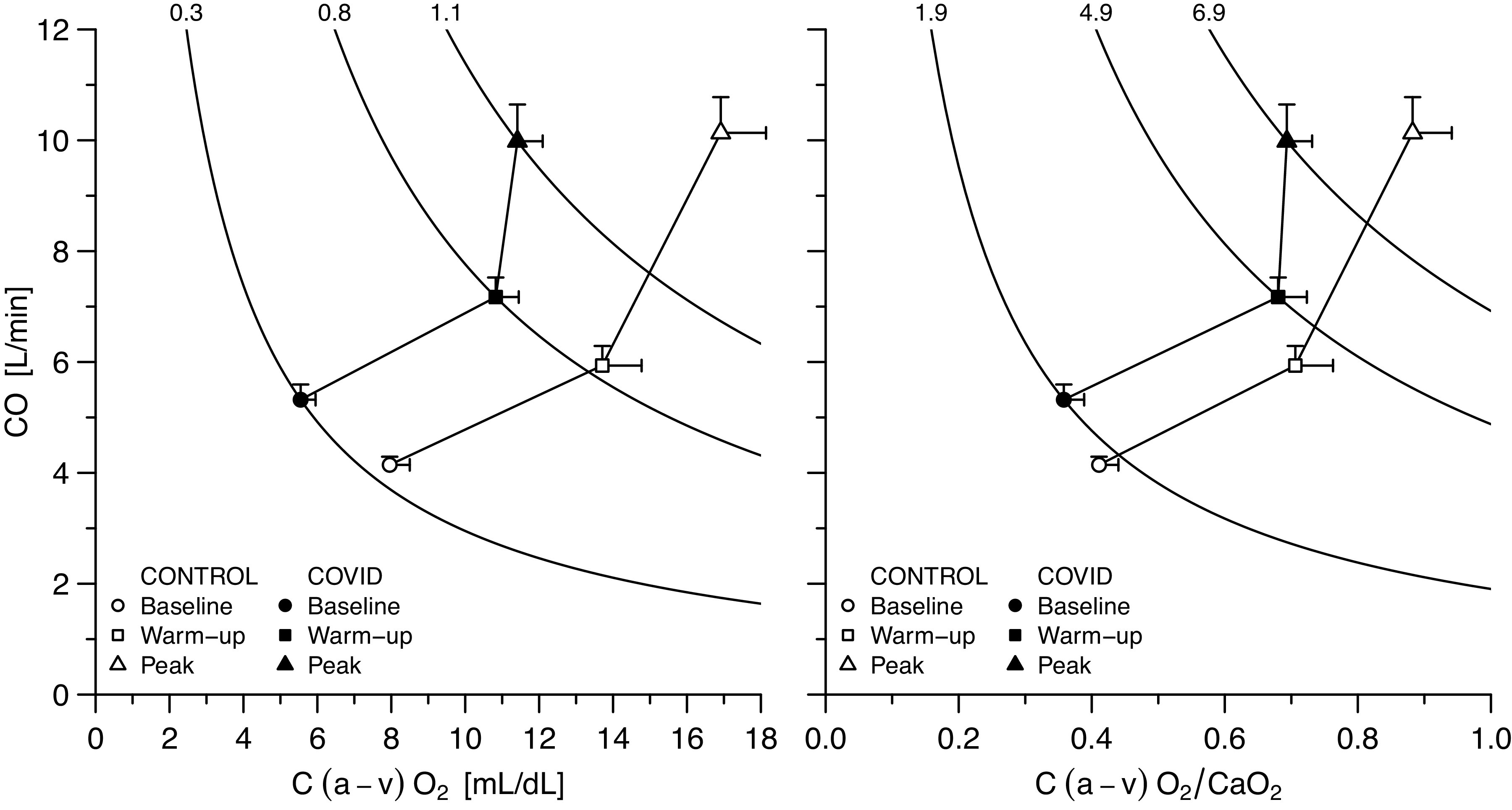
Cardiac output as a function of arteriovenous oxygen difference (left) and of peripheral oxygen extraction (right) at rest and during exercise. Data are represented as means ± standard error of the mean. CaO2, arterial oxygen content; C(a−v)O2, arteriovenous oxygen difference; CO, cardiac output.
Figure 2.
Oxygen consumption as a function of cardiac output. This relationship was rightward-shifted in patients with COVID-19 as compared with controls at rest and during submaximal exercise. Data are represented as means ± standard error of the mean. CO, cardiac output; COVID-19, novel coronavirus-19 disease; V̇o2, oxygen consumption.
Ventilation During Exercise
Overall, respiratory reserve was not exhausted in patients with COVID-19 and similar to that of controls (Table 2). In addition, the occurrence of expiratory or inspiratory flow limitation, as well as of dynamic hyperinflation during exercise did not differ between patients with COVID-19 and controls (Table 2 and Supplemental Table S1). Only two patients had a pattern consistent with respiratory limitation to exercise, presenting reduced lung volumes at rest and a respiratory reserve of 16%, but only one had mild oxygen desaturation at peak (from 98 to 93%).
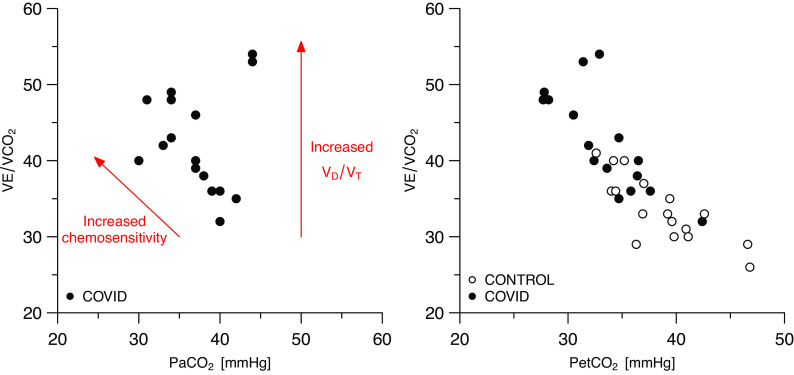
Ventilation during exercise was lower in patients with COVID-19 than in control subjects, with lower tidal volume and higher respiratory rate (P < 0.05). Nevertheless, ventilatory equivalents for O2 and CO2, PetCO2, and V̇e/V̇co2 slope were higher in patients with COVID-19 than in controls, whereas PetCO2 was lower (P < 0.05). Accordingly, exercise hyperventilation as expressed by the V̇e/V̇co2 and PetCO2 was higher in patients with COVID-19 also during submaximal exercise (P < 0.001), and was mainly linked with a low PaO2 set-point (Fig. 3). As a notable exception, only the two patients presenting with respiratory limitation to exercise configured as outliers from this latter plot, and both of them had high VD/VT (0.63 and 0.64, respectively). Complete blood gas analysis at rest and at peak exercise in patients with COVID-19 is shown in Table 3.
Figure 3.
Exercise hyperventilation in patients with COVID-19 and in controls. Each dot represents an individual. COVID-19, novel coronavirus-19 disease; PaCO2, arterial partial pressure for carbon dioxide; PETCO2, end-tidal partial pressure for carbon dioxide; V̇co2, carbon dioxide production; VD, dead space; VE, minute ventilation; VT, tidal volume.
Table 3.
Blood gas-analysis in patients with COVID-19 at rest and at peak exercise
| Rest | Peak Exercise | P Value | |
|---|---|---|---|
| PH | 7.44 (0.02) | 7.40 (0.05) | <0.001 |
| PaO2, mmHg | 81 (14.5) | 79 (25) | 0.271 |
| PaO2, mmHg | 38 (5) | 37 (5) | 0.328 |
| (ET-a)O2, mmHg | 32 (19) | 39 (26) | 0.106 |
| (a-ET)CO2, mmHg | 9 (6) | 4 (6) | <0.001 |
| Lac, mmol/L | 1.0 (0.4) | 3.5 (2.8) | <0.001 |
| VD/VT | 0.63 (0.05) | 0.38 (0.16) | <0.001 |
(a-ET)CO2, delta arterial/end-tidal partial pressure for carbon dioxide; COVID-19, novel coronavirus-19 disease; (Et-a)O2, delta end-tidal/arterial partial pressure for oxygen; Lac, lactate; PaCO2, arterial partial pressure for carbon dioxide; PaO2, arterial partial pressure for oxygen; VD/VT, dead space ventilation. Data are expressed as median (IQR).
Pulmonary Circulation during Exercise
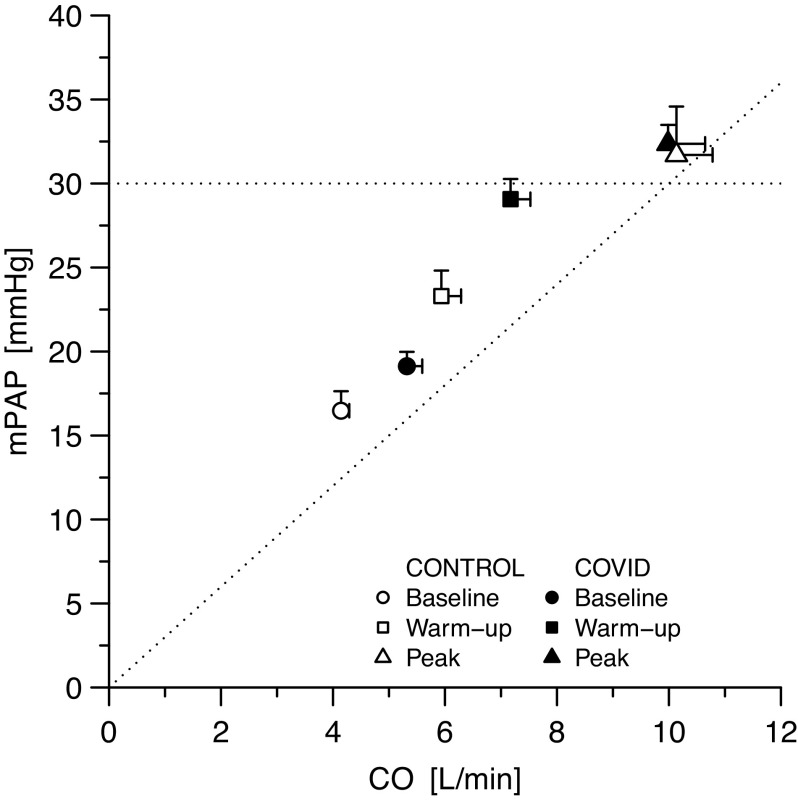
Three patients with COVID-19 and seven controls did not have a tricuspid regurgitant jet at peak exercise (P = 0.137). Pressure-flow pairs of patients with COVID-19 at rest and during submaximal exercise were upward and rightward shifted as compared with controls, but lay on the same regression slope (Fig. 4), with nondifferent peak values (TPR of the whole population: 3.5 [1.7] WU). Individual pressure-flow pairs as well as averaged slopes for both patients’ groups are shown in Supplemental Figs. S2 and S3. In patients with COVID-19, SPAP and TPR at peak resulted unrelated to SaO2, PaO2, and CaO2. Moreover, right ventricular function, even when normalized for right ventricular afterload, was similar in COVID-19 and controls.
Figure 4.
Pressure-flow relationship of the pulmonary circulation in patients with COVID-19 and controls. The horizontal dotted line marks the value of 30 mmHg; the oblique dotted line corresponds to a slope of the pressure/flow relationship of 3 mmHg/L/min. Data are represented as means ± standard error of the mean. CO, cardiac output; COVID-19, novel coronavirus-19 disease; mPAP, mean pulmonary artery pressure.
Cardiorespiratory Adaptation to Exercise according to COVID-19 Severity
We performed an exploratory analysis to highlight between-groups differences according to the type of ventilatory support undertaken during hospitalization (mechanical ventilation, noninvasive ventilation, oxygen mask), as shown in Supplemental Tables S2 and S3. As expected, both the length of hospitalization, as well as that of ventilatory support, decreased from mechanical ventilation to noninvasive ventilation to oxygen (P < 0.05). Patients treated with mechanical ventilation tended to have lower lung volumes as well as lower V̇o2 at peak, in spite of a not-exhausted ventilatory reserve. Patients treated with noninvasive ventilation were older, had nonsignificantly higher E/E′ and higher PAP at rest, as well as higher PAP at peak in spite of nondifferent TPR. There were no major differences between the three groups in blood tests, echocardiographic parameters at rest and at peak exercise, as well as in oxygen delivery and extraction.
DISCUSSION
With this paper, we provide the first description of the cardiorespiratory adaptation to exercise in the recovery phase from COVID-19 pneumonia, at the time of hospital discharge. In particular, our results show that: 1) recovering COVID-19 patients presented a significantly reduced exercise capacity; 2) impairment of functional capacity was mainly related to peripheral factors (anemia and oxygen extraction) rather than a respiratory or cardiac limitation; 3) in spite of parenchymal lung disruption, pulmonary vascular function was not impaired after COVID-19; and 4) exercise hyperventilation after COVID-19 is frequent and principally due to enhanced chemoreflex sensitivity rather than increased VD/VT. This information could be relevant not only for a better understanding of COVID-19 consequences, but also to give clearer indications to survivors on how they should behave once discharged from the hospital, especially in previously healthy subjects, who would expect to resume their usual lifestyle. In particular, our findings might help reassuring survivors from COVID-19 about the benignity of residual symptoms in most cases.
V̇o2 at peak at the time of hospital discharge was reduced by 30% in patients with COVID-19. The extent of such functional impairment was larger than previous reports in survivors from severe acute respiratory syndrome caused by coronavirus, investigated either by 6-min walking test (two studies) or standard cardiopulmonary exercise test (one study) later on during follow-up, i.e., 3–12 mo after hospital discharge (11–13). Nonetheless, the extent of such limitation is similar to a recent report describing cardiorespiratory fitness in patients with COVID-19, 2–3 mo after disease onset [19, 26]. Coherently with such previous reports (11, 26), and extending those evidences providing a deeper insight in exercise pathophysiology, the great majority of our patients did not present respiratory limitation to exercise (normal respiratory reserve, absence of relevant expiratory or inspiratory flow limitation, or dynamic hyperinflation) despite restrictive pulmonary changes accounting for a reduction of 34% of lung volumes.
Instead, functional limitation seems to be mainly caused by reduced oxygen content secondary to anemia as well as by impaired peripheral extraction after COVID-19. In particular, patients with COVID-19 lie roughly on the same V̇o2 and V̇o2/CaO2 isophlets of control subjects both at rest and during low-workload exercise, in spite of lower arteriovenous difference and oxygen extraction, thanks to higher cardiac output. However, at peak exercise the further increase of V̇o2 in COVID-19 appeared to be mainly driven by cardiac output increase, while reaching an apparent limit of oxygen extraction. This finding might reflect myopathic changes occurring as a consequence of medications administered during the hospital stay (e.g., steroids, which were used in all our patients) as well as of the potential direct or indirect myopathic damage from COVID-19 (9, 10), rather than muscle disuse. Indeed, it has been previously shown that prolonged bed rest should impair more stroke volume reserve rather than peripheral extraction in healthy subjects (27). At variance, cardiac output reserve was supernormal in our COVID-19 patients, despite prolonged bed rest. We may try to explain this finding based on the peculiar hyperinflammatory setting of COVID-19, which can be associated with a longstanding high-output hemodynamic state (6). Interestingly, such supernormal cardiac output reserve occurred in spite of subtle echocardiographic abnormalities (mild right ventricular dilation and abnormal myocardial deformation indexes in one third of patients at rest, which is coherent with previous reports of cardiac involvement after COVID-19 (7) and of blunted heart rate and systemic blood pressure responses to exercise, potentially indicating a persisting autonomic imbalance after COVID-19 (28). Based on this latter, we may also speculate that such autonomic derangement might be associated with suboptimal distribution of cardiac output to exercising muscles, thus contributing to low peripheral oxygen extraction.
Furthermore, the cytokine storm and systemic inflammatory response that characterize COVID-19 can be responsible for iron-restricted erythropoiesis with acute phase anemia (8), which could persist for a quite long time after resolution of pneumonia. Since each gram of hemoglobin can bind ∼100 mL of oxygen (29), also anemia was obviously associated with reduced V̇o2. Thus, in patients with COVID-19 that uniformly presented with a limit in oxygen extraction as compared with control subjects, lower hemoglobin values were associated with lower peak V̇o2. The combination of these two peripheral factors (anemia and suboptimal extraction) was only partially compensated by a supernormal cardiac output, thus limiting exercise capacity well before exhaustion of the respiratory reserve. This result is encouraging since anemia and myopathic changes are expected to spontaneously recover during extended follow-up (12).
In addition, it is interesting to note that, despite significant parenchymal disruption, pulmonary vascular function of COVID-19 was similar to that of control subjects. The pressure-flow relationship of the pulmonary circulation was upward and rightward shifted in patients with COVID-19 as compared with controls, with a similar ratio between pulmonary artery pressure and cardiac output, which is consistent with invasive hemodynamic findings obtained in mechanically-ventilated COVID-19 patients (6). Nonetheless, TPR resulted in both groups at the upper limit of normal at peak exercise. Indeed, both population were overall at increased risk for having heart failure with preserved ejection fraction, based on age, clinical history, and comorbidities (30). At variance from a recent report in severe COVID-19 evaluated in the acute phase of disease, where right ventricular to pulmonary artery coupling was independently associated with survival, in our cohort S′/systolic PAP was not different between survived patients with COVID-19 and controls, neither at rest nor during exercise (31).
Another peculiar aspect of COVID-19 exercise pathophysiology is hyperventilation. V̇e/V̇co2 was high at rest and during exercise, due to enhanced chemoreflex sensitivity. Indeed, only two patients with respiratory limitation to exercise presented with a high V̇e/V̇co2 due to high VD/VT, whereas in all other patients, the ventilatory response was leftward shifted probably due to reflex, peripheral factors (24, 25).
Study Limitations
Despite enrolling a small sample of patients with COVID-19, our study was adequately powered to detect meaningful differences in exercise pathophysiology between COVID-19 and control subjects. Although the small sample size of our study may limit generalizability of our results, it did not prevent us from conducting an in-depth pathophysiological exploration of a representative population healed from severe COVID-19 pneumonia that was evaluated with cardiopulmonary exercise test combined with blood gas analysis and echocardiography at the time of their hospital discharge. Furthermore, the characteristics of our population are in line with those of larger studies (32), with a quite fair distribution of the kind of ventilatory support required during hospitalization. However, the small representation of each group of patients corresponding to a given ventilatory support, prevented us from finding meaningful between-groups differences, an aim that goes beyond the scope of the present work. Furthermore, despite heterogeneous ventilatory support, in median our patients had a severe lung restriction after COVID-19.
Controls were not asymptomatic matched individuals, but outpatients who underwent a full cardiorespiratory assessment to investigate the etiology of shortness of breath, including 17% of patients with mild COPD (GOLD I or II). In addition, given the unresolved issues related to the noninvasive diagnosis of heart failure with preserved ejection fraction, we cannot exclude that some of these subjects (as well as of patients with COVID-19) may have had an early phase of such disease (33, 34), explaining TPR at the upper limits of normal. However, control subjects ended up even after this test without any sign of cardiac or respiratory limitation to exercise, and presented a burden of mild comorbidities comparable with that of our representative COVID-19 population. Since a radial artery catheter was routinely placed only in COVID-19 survivors but not in controls, we could not have a comparison of blood gas analysis between controls and patients with COVID-19. However, having excluded control subjects with severe respiratory disease, we believe that this does not importantly affect the interpretation of our results. Finally, we could not measure lung diffusion properties in our patients. Nonetheless, in survivors from Severe Acute Respiratory Syndrome, the functional disability has been suggested to be out of proportion to the degree of lung function impairment (13).
Interpretation
Exercise limitation is a frequent finding at the time of hospital discharge after COVID-19 pneumonia. Reduced oxygen content and extraction, secondary to anemia and myopathic changes, rather than respiratory, pulmonary vascular, or cardiac impairment, were the main contributors to reduced exercise capacity. In addition, enhanced chemoreflex sensitivity triggers exercise hyperventilation after resolution of COVID-19 pneumonia. These findings might help reassuring survivors from COVID-19 on the benignity of residual symptoms in most cases.
SUPPLEMENTAL DATA
Supplemental Tables S1–S3 and Figs. S1–S3: https://doi.org/10.5281/zenodo.4549376
GRANTS
This study was funded by the Italian Ministry of Health (Progetti di Ricerca Corrente, IRCCS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B. and S.C. conceived and designed research; C.B. and S.C. performed experiments; C.B., S.C., and A.F. analyzed data; C.B., S.C., and A.F. interpreted results of experiments; A.F. prepared figures; C.B. and S.C. drafted manuscript; A.F., G.B.P., M.S., L.P.B., and G.P. edited and revised manuscript; C.B., S.C., A.F., G.B.P., M.S., L.P.B., and G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Laboratori Guidotti S.p.A. for providing free-of-charge software for speckle-tracking analysis.
REFERENCES
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic (Online). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [20 February 2021].
- 2.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology 296: E55–E64, 2020. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 55: 2001217, 2020. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, Huisman MV, Klok FA. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res 193: 86–89, 2020. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caravita S, Baratto C, Di Marco F, Calabrese A, Balestrieri G, Russo F, Faini A, Soranna D, Perego GB, Badano LP, Grazioli L, Lorini FL, Parati G, Senni M. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail 22: 2228–2237, 2020. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Oz AG, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 142: 342–353, 2020. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol 99: 1421–1428, 2020. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985) 129: 864–867, 2020. doi: 10.1152/japplphysiol.00321.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrandi PJ, Alway SE, Mohamed JS. Last word on viewpoint: the interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985) 129: 872–872, 2020. doi: 10.1152/japplphysiol.00785.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, Earnest A. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J 24: 436–442, 2004. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 12.Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, Sung JJY. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 128: 2247–2261, 2005. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, Ko FW, Chan MC, Chan DP, Tony MW, Rainer TH, Ahuja AT, Cockram CS, Sung JJY. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60: 401–409, 2005. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman K, Hansen JE, Sue DY, Whipp BJ, Froelicher VF. Principles of exercise testing and interpretation, 3rd Edition. Philadelphia, PA: Lippincott, Williams, and Wilkins, 1999. [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39.e14, 2015. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Rudski LG, Gargani L, Armstrong WF, Lancellotti P, Lester SJ, Grünig E, D’Alto M, Aneq AM, Ferrara F, Saggar R, Saggar R, Naeije R, Picano E, Schiller NB, Bossone E. Stressing the cardiopulmonary vascular system: the role of echocardiography. J Am Soc Echocardiogr 31: 527–550.e11, 2018. doi: 10.1016/j.echo.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Caravita S, Yerly P, Baratto C, Dewachter C, Faini A, Rimouche A, Branzi G, Perego GB, Bondue A, Parati G, Vachiéry J-L. Noninvasive versus invasive pressure-flow relationship of the pulmonary circulation: bias and error. Eur Respir J 54: 1900881, 2019. doi: 10.1183/13993003.00881-2019. [DOI] [PubMed] [Google Scholar]
- 19.Simaga B, Vicenzi M, Faoro V, Caravita S, Di Marco G, Forton K, Deboeck G, Lalande S, Naeije R. Pulmonary vascular function and exercise capacity in black sub-Saharan Africans. J Appl Physiol (1985) 119: 502–507, 2015. doi: 10.1152/japplphysiol.00466.2015. [DOI] [PubMed] [Google Scholar]
- 20.Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, Sommer N, Gall H, Richter MJ. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 12: e009047, 2019. doi: 10.1161/CIRCIMAGING.119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 116: 488–503, 1999. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 22.Caravita S, Faini A, Deboeck G, Bondue A, Naeije R, Parati G, Vachiéry JL. Pulmonary hypertension and ventilation during exercise: Role of the pre-capillary component. J Heart Lung Transplant 36: 754–762, 2017. doi: 10.1016/j.healun.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 8: 286–294, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weatherald J, Sattler C, Garcia G, Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J 51: 1700860, 2018. doi: 10.1183/13993003.00860-2017. [DOI] [PubMed] [Google Scholar]
- 25.Naeije R, Faoro V. The great breathlessness of cardiopulmonary diseases. Eur Respir J 51: 1702517, 2018. doi: 10.1183/13993003.02517-2017. [DOI] [PubMed] [Google Scholar]
- 26.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-AlmagroF , et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 31: 100683, 2021. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltin B, Blomqvist G, Mitchell JH, JohnsonRL , Jr., Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 37: VII1–VII78, 1968. [PubMed] [Google Scholar]
- 28.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174: 156–161, 2010. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agostoni P, Salvioni E, Debenedetti C, Vignati C, Cattadori G, Contini M, Magri D, Palermo P, Gondoni E, Brusoni D, Fiorentini C, Apostolo A. Relationship of resting hemoglobin concentration to peak oxygen uptake in heart failure patients. Am J Hematol 85: 414–417, 2010. doi: 10.1002/ajh.21698. [DOI] [PubMed] [Google Scholar]
- 30.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery J-L, Lewis GD. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 187: 576–583, 2013. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Alto M, Marra AM, Severino S, Salzano A, Romeo E, De Rosa R, Stagnaro FM, Pagnano G, Verde R, Murino P, Farro A, Ciccarelli G, Vargas M, Fiorentino G, Servillo G, Gentile I, Corcione A, Cittadini A, Naeije R, Golino P. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care 24: 670, 2020. doi: 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naeije R, Vonk Noordegraaf A, Kovacs G. Exercise-induced pulmonary hypertension: at last!. Eur Respir J 46: 583–586, 2015. doi: 10.1183/09031936.00061015. [DOI] [PubMed] [Google Scholar]
- 34.Senni M, Caravita S, Paulus WJ. Do existing definitions identify subgroup phenotypes or reflect the natural history of heart failure with preserved ejection fraction? Circulation 140: 366–369, 2019. doi: 10.1161/CIRCULATIONAHA.119.041657. [DOI] [PubMed] [Google Scholar]