Katalin Karikó rose to world fame as the principal creator of the vaccine against the Covid-19 pandemic, 2020–2021. She emigrated from a hopeless situation to further hopeless situations. However, she had a sound scientific idea and dedication. Her perseverance and stamina, combined with her researcher’s acumen, paid off. It brought rescue to the world and appreciation to her, once again demonstrating the benefit of science for humankind.
Katalin Karikó (1955, Fig. 1) was born into the family of a butcher in Kisújszállás, a town some 140 kilometers (about 85 miles) to the east from Budapest. Kisújszállás is adjacent to Karcag, a similarly small town, the birth place of the chemistry Nobel laureate Avram Hershko. Karikó went to the local schools and has praised her teachers both in the general school and the Móricz Zsigmond Gimnázium. They inculcated in her the love of mathematics and the sciences. Upon graduation from high school, she enrolled at the University of Szeged. She majored in biochemistry and completed her equivalent of a Master’s degree in 1978. She started at the Szeged Biological Research Center (BRC) of the Hungarian Academy of Sciences whose scholarship allowed her to embark on her doctoral studies. Jenő Tomasz was her mentor, a caring organic chemist of broad vision who’s impact further strengthened Karikó’s dedication to biochemistry. She has remembered the sizzling scientific atmosphere at the laboratory. Tomasz spent much of his time in the United States in subsequent years, and, at the conclusion of his research career, retired in Hungary.
Fig. 1.

Katalin Karikó, 1980 and 2015. Courtesy of Katalin Karikó
Karikó completed her doctorate in 1982 and stayed on at the BRC. Alas, the Center was experiencing hard time in the 1980s, and could not offer permanent employment to some of the young people, however promising they might have been. This was unusual under the socialist system, which boasted full employment, so the economic situation was precarious indeed. In 1985, Karikó received the news that it will not be possible for her to continue at the BRC, and this happened on the day of her 30th birthday, when she and her young family were about to move into a new apartment. There seemed no other venue in Hungary at that point that could have absorbed the researchers who were looking for jobs. The state-run economy was undergoing a crisis, and then there was no European Union yet for Hungary to bail it out or provide employment for surplus scientists.
The United States was thought to have ample opportunities and, following persistent applications, she landed a postdoctoral position at the Department of Biochemistry at Temple University in Philadelphia. This position lasted for three years. It was followed by another, for two years, at the Department of Pathology of the Uniformed Armed Services University of the Health Sciences (USU), a federal medical school in Bethesda, Maryland, near Washington, DC. Her project revolved about the signal protein interferons. When a cell is infected by viruses, it sends these interferons to neighboring cells to prompt their anti-viral defense. This project provided a good opportunity for her to learn a great deal about the modern techniques of molecular biology.
In 1989, Karikó began a long-lasting, at times bumpy, relationship with the University of Pennsylvania (UPenn) in Philadelphia. It is a most prestigious private university, one of the eight Ivy League schools. She hoped for a tenure-track position, which did not work out, but she rather continued in a demoted position than leave UPenn. Long before Karikó’s recent Covid-19 vaccine fame, Harvard University invited her to talk about her career. She was asked to speak especially about her failures. She remarked that this was easy, because she had plenty. Actually, failures may indicate daring research ideas and pioneering research attempts, not just unsuccessful trials. There have been examples of brilliant research careers whose roads were paved by initial failures. Paul Nurse comes to mind who won the Nobel Prize for his discoveries of proteins that regulate the life cycle of cells [1]. When Karikó was on a so-called soft-money position at UPenn, she needed outside support to continue her research; alas, such support was not forthcoming. She always found enthusiastic colleagues who shared her vision, at least for a while.
In 1995, there was a break, still at UPenn. It came from a chance meeting with a recently appointed professor, Drew Weissman of the UPenn Perelman School of Medicine (Fig. 2). The encounter injected new hopes for Karikó to continue her efforts. Weismann was an MD/PhD immunologist with his degrees from Brandeis University. He did postdoctoral work with Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases of NIH. Anthony Fauci is currently (2021) the Chief Medical Advisor to President Joe Biden. At UPenn, Weissman had ambitious plans for finding remedies for diseases. When Karikó told Weissman about her ideas concerning the use of mRNA, he recognized their unique potentials. Hence, a most fruitful interaction developed between them.
Fig. 2.
Drew Weissman and Katalin Karikó in their UPenn laboratory, 2015. Courtesy of Katalin Karikó
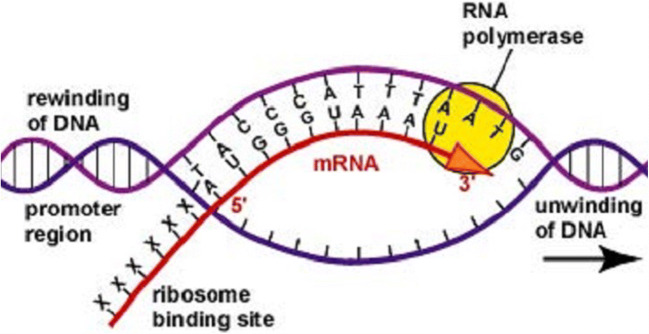
Messenger RNA, mRNA, was discovered some thirty years before and quite a number of scientists, among them some future Nobel laureates, acted as midwifes at its discovery [2]. The molecule mRNA is a single-strand nucleic acid with the complementary sequence of nucleotides as the double-strand deoxynucleic acid (DNA) from which the mRNA carries the genetic information between the cell nucleus and the ribosome (Fig. 3).
Fig. 3.
The mRNA among the other components. Courtesy of Katalin Karikó
The function of mRNA explains its name, “messenger.” The genetic information from the DNA is transcribed by means of an enzyme (a protein) called RNA polymerase. According to the information the ribosome receives from the mRNA, it produces proteins. The ribosome is called the protein factory and the mRNA the software of protein production of the cell. The entire process is called translation and it constitutes a part of the “central dogma,” as labeled by Francis Crick. According to it, the information flow has a one-way direction,
and never in the reverse direction. These basic principles, however, were far from suggesting any pharmaceutical applications. Incidentally, the “central dogma” is no longer rigorously so as the enzyme called reverse transcriptase is capable of generating DNA from RNA.
One of Weissman’s projects was using DNA for developing a vaccine against HIV (the human immunodeficiency virus). He used mice for testing. When it did not work, Karikó suggested to try mRNA. The advantage of RNA over DNA was manifold. For DNA, to bring about change, it has to penetrate the cell nucleus; for RNA, it suffices to enter the cytoplasm, that is, the cell material outside the nucleus. The RNA acts quickly in producing the necessary protecting proteins. It does not affect the genetic content of the target cells whereas DNA may cause unwanted mutations. Once the DNA is brought into the cell nucleus, it stays, regardless of its efficacy. In contrast, RNA decomposes, so it is no longer there if it is not needed. In case its continued presence is needed because it has the desired efficacy, it can be augmented by providing additional amounts. This is just like it happens in any treatment with other medications, which can be taken as long as is needed. Weissman followed up on Karikó’s suggestion and injected the mice with mRNA. The first results were discouraging; many of the mice suffered inflammation and some died. Weissman and Karikó together solved the problem with “clever biochemistry.” In order to make the mRNA non-inflammatory, they had to remove the double-stranded RNA contaminants in addition to the nucleoside modification [3].
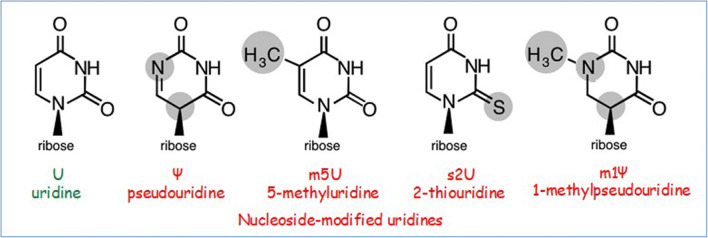
Indeed, the solution originated from Karikó’s deep knowledge of RNA chemistry. They replaced one of the four nucleosides of the RNA molecule by a similar building block (Fig. 4) whose participation did not alter the efficacy of the treatment, but the inflammation no longer occurred [4]. In principle, they had now a reliable tool of using mRNA for creating vaccines. However, as of now (Spring 2021), except for the emergency approval for Covid-19 vaccine, none has so far been approved. The work continues. For example, there is an mRNA treatment being developed in Weissman’s lab for sickle-cell anemia, a pathological blood condition. The name sickle cell refers to the sickle shape of the red blood cells rather than the donut shape of the healthy red blood cells. Sickle-cell anemia was the first pathological condition for which Linus Pauling used the expression “a molecular disease” for the first time [5].
Fig. 4.
Nucleoside-modified uridines. Courtesy of Katalin Karikó
The road toward the general application of mRNA has opened. Putting it in one sentence, it is the use of mRNA to induce the organism itself to produce the proteins for protection to preempt infection or for cure, once there is already a pathological condition. Karikó and Weissman jointly started a biotech company as a spinoff, but Karikó left it and assumed a leading position at a biotech company in Mainz, Germany. It is the BioNTech Company and her official title is Executive Vice President. She does now supervision and follows the literature, and no longer conducts hands-on experiments although she used to love doing them. She has become the great statesperson of mRNA science for medical applications. It appears that the possibilities of mRNA-based therapeutics are limitless [6]. From the interviews she is giving, a most interesting route to success emerges. Few had thought to seek her out before her world fame, but at least one science writer did [7].
Karikó left Hungary under unpleasant circumstances. Had she stayed, as she put it in an interview, “I would have become a mediocre, disgruntled researcher” [8]. Her emigration led her, eventually, and finally, to world-wide international success and saving lives, not just a few, but improbably many. However, immigration in the United States did not put her on the right track instantly. She could have become “a mediocre and disgruntled researcher” in America, just as well. However, rather than succumbing to the temptation of some secure job in which she would have had to work on other peoples’ ideas, she insisted on working on her own ideas. She stuck to her idée fixe that the potential of mRNA in medicine is there and only waiting to be uncovered and utilized. As of lately, supports of millions and millions of dollars assist the research and development of the field. This is now. However, it was only as recently as 2006 that her research had finally won a grant for the first time; it was a one-hundred-thousand dollar grant from the NIH.
Some failures occur necessarily in any truly original and successful career; otherwise, the scientist may have not probed the frontiers of science to its fullest. But she had a far larger share of failures than what should be considered a healthy amount. She had a good start and good teachers, an inspiring mentor, and dedicated colleagues with whom she has maintained pleasant and most fruitful interaction. In 2020, Katalin Karikó and Drew Weissman received the prestigious Rosenstiel Award of Brandeis University, Weissman’s alma mater. It is for distinguished work in basic medical research and the prize has been given since 1971, annually. Close to thirty future Nobel laureates have received this distinction. As we contemplate in spring 2021 about what the future may bring for Karikó, her anticipated Nobel Prize may not even express fully and sufficiently the significance of her achievement.
She had a bumpy road to the summit of science, which began when she earned her doctorate in Szeged, and soon after, emigrated to the United States. So it was a great event that she was asked to address the annual general assembly of the Hungarian Academy of Sciences in Budapest, on May 3, 2021. She told the story of how mRNA has become a therapeutic agent and a vaccine. She voiced no complaints, only praises to all who, in one way or another, helped her to acquire knowledge and research experience. She made a subtle, bitter-sweet remark though at the end of the presentation. She realized that she would not be here had the pandemic not happened and she wished rather not to be here if in that case the pandemic had not happened. She was obviously happy, and moved by the honor, throughout the well-structured and superbly delivered lecture.
Acknowledgments
We thank Dr. Katalin Karikó for the art reproduced in this communication and Dr. Michele Hargittai for consultation.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Istvan Hargittai, Email: istvan.hargittai@gmail.com.
Magdolna Hargittai, Email: hargittaim@gmail.com.
References
- 1.Hargittai I (2020) Mosaic of a Scientific Life (Springer Nature), Chapter 33, “Paul Nurse,” p 120
- 2.Cobb M. Who discovered messenger RNA? Curr Biol. 2015;25:R526–R532. doi: 10.1016/j.cub.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Weissman D (2021), private communication by e-mail of February 15.
- 4.Karikó K, Weissman D. Naturally-occurring nucleoside modifications suppress the immunostimulatory activity of RNA: implication for therapeutic RNA development. Curr Opin Drug Discov Dev. 2007;10:523–532. [PubMed] [Google Scholar]
- 5.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a Molecular Disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 6.Sahin U, Karikó K, Türeci O. mRNA-based therapeutics – developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 7.Silberer V. “Jó hírt hoz a messenger RNA” (an interview, in Hungarian, Messenger RNA brings good news) Magyar Kémikusok Lapja. 2019;74(4):118–121. [Google Scholar]
- 8.Ha Magyarországon maradok, panaszkodó, középszerű kutató lettem volna | G7 - Gazdasági sztorik érthetően