Abstract
A generally accepted framework derived predominately from animal models asserts that repeated cycles of chronic intermittent ethanol (EtOH; CIE) exposure cause progressive brain adaptations associated with anxiety and stress that promote voluntary drinking, alcohol dependence, and further brain changes that contribute to the pathogenesis of alcoholism. The current study used CIE exposure via vapor chambers to test the hypothesis that repeated episodes of withdrawals from chronic EtOH would be associated with accrual of brain damage as quantified using in vivo magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), and MR spectroscopy (MRS). The initial study group included 16 male (~325g) and 16 female (~215g) wild-type Wistar rats exposed to 3 cycles of 1-month in vapor chambers + 1 week of abstinence. Half of each group (n = 8) was given vaporized EtOH to blood alcohol levels approaching 250 mg/dL. Blood and behavior markers were also quantified. There was no evidence for dependence (i.e., increased voluntary EtOH consumption), increased anxiety, or an accumulation of pathology. Neuroimaging brain responses to exposure included increased cerebrospinal fluid (CSF) and decreased gray matter volumes, increased Choline/Creatine, and reduced fimbria-fornix fractional anisotropy (FA) with recovery seen after one or more cycles and effects in female more prominent than in male rats. These results show transient brain integrity changes in response to CIE sufficient to induce acute withdrawal but without evidence for cumulative or escalating damage. Together, the current study suggests that nutrition, age, and sex should be considered when modeling human alcoholism.
Keywords: repeated withdrawal, White matter, Magnetic resonance spectroscopy, Diffusion tensor imaging, Liver, behavior
1. Introduction
Alcohol Use Disorder (AUD) is marked by repeated periods of abstinence and relapse. Clinical signs of withdrawal – including altered physiological (e.g., heart rate) and psychological (e.g., anxiety) homeostasis – are thought to contribute to relapse risk (Kushner et al., 2000; Lee et al., 2005; O’Connor et al., 1991). Relative to those without prior detoxification experiences, individuals with a history of multiple detoxifications have more severe withdrawal symptoms (Malcolm et al., 2000), including frequency and intensity of delirium tremens (Cushman, 1987), seizures (Ballenger and Post, 1978; Booth and Blow, 1993; Brown et al., 1988; Lechtenberg and Worner, 1992), and anxiety (Breese et al., 2005a; Castaneda et al., 1996; Faingold et al., 2004; Malcolm et al., 2002). In addition, patients with multiple detoxifications compared with those experiencing their first detoxification show greater severity of cognitive impairment (Duka et al., 2002, 2011, 2003; Glenn et al., 1988; Loeber et al., 2009, 2010; Trick et al., 2014; Wollenweber et al., 2014), lower brain activity (George et al., 1999), and greater deficits in regional brain volumes, notably frontal gray matter (Duka et al., 2011; Le Berre et al., 2014; Trick et al., 2014) and cerebellum (Le Berre et al., 2014). Human studies frequently report compromised white matter in alcoholism (e.g., Alhassoon et al., 2012; Cardenas et al., 2011; Demirakca et al., 2011; Pfefferbaum et al., 2001; Segobin et al., 2014) with evidence of recovery of white matter integrity with abstinence (Pfefferbaum et al., 2014). Together, results from human studies suggest that recurring rehabilitation attempts and attendant abstinence periods are associated with changes in the brain that may contribute to anxiety and dependence severity with concomitant brain changes.
In animal models, a large body of data supports the hypothesis that repeated withdrawals from cycles of chronic alcohol exposure contribute to a progressive development of persisting adaptive brain changes that promote stress and anxiety during abstinence and enhance the likelihood of relapse (i.e., voluntary alcohol drinking) (cf., Breese et al., 2005b). Animal experiments using protocols such as chronic intermittent ethanol (CIE) treatment have thus been undertaken to determine whether a history of previous ethanol (EtOH) withdrawal episodes results in brain adaptations contributing to the development and maintenance of a dependent phenotype. In rodent studies, repeated withdrawals from EtOH are associated with exacerbation of seizure activity (in mice: Becker et al., 1997; Becker et al., 1998; Becker and Lopez, 2004; but see Cox et al., 2013); temporary increases in drinking as quantified using both simple consummatory measures and operant procedures (in mice: Becker and Lopez, 2004; Cowen et al., 2003; Cox et al., 2013; Khisti et al., 2006; Melendez et al., 2006; Sanchis-Segura et al., 2006; Sparta et al., 2009; Zghoul et al., 2007; in rats: Backstrom et al., 2004; Bell et al., 2004; Colombo et al., 2003; Dayas et al., 2004; Fullgrabe et al., 2007; Funk et al., 2004; Hauser et al., 2019; Heyser et al., 1997; Holter et al., 2000; Oster et al., 2006; Spanagel and Holter, 1999; Sparta et al., 2009; but see: Stephens et al., 2001; Lundqvist et al., 1995); deficits in fear conditioning (Duka et al., 2004; Stephens et al., 2001) and spatial learning (Swartzwelder et al., 2014); and increased anxiety (Fernandes et al., 2018; Mejia-Toiber et al., 2014; Overstreet et al., 2002; Somkuwar et al., 2017; Varlinskaya et al., 2017, 2020).
Whether brain damage accrues with repeated withdrawal episodes in animal models is unclear (e.g., Madeira et al., 1997). Repeated intraperitoneal injections of EtOH caused long-lasting (> 21 days) compromise of motor coordination and persistent loss of cerebellar Purkinje cells in male and female Sprague-Dawley rats (Forbes et al., 2013). Over a 1-month exposure period, intermittent relative to continuous EtOH exposure (in drinking water) was associated with greater loss of hippocampal pyramidal neurons (Lundqvist et al., 1995). Following withdrawal from 5-cycles of 1-week vapor + 1-week recovery, c-Fos expression showed various patterns (i.e., up, down): critically, persistent reductions in c-Fos expression were observed at a prolonged withdrawal time point (i.e., 7 days) in several brain regions implicated in relapse drinking (Smith et al., 2019). With respect to white matter specifically, male Wistar rats (Kim et al., 2015) and mice (Samantaray et al., 2015) exposed to CIE show reduced levels of myelin basic protein, which is critically involved in myelination (Deber and Reynolds, 1991).
In a previous magnetic resonance imaging (MRI) study, animals were exposed to 5 cycles of 4 days of intragastric (“binge”) EtOH treatment and 10 days of recovery (Zahr et al., 2016). Blood alcohol levels (BALs) at each of the 5 binge episodes was ~300 mg/dL. Cerebrospinal fluid (CSF) volumes of the lateral ventricles and cisterns showed enlargement with each binge EtOH exposure but recovery with each abstinence period. Similarly, changes in MR Spectroscopy (MRS) metabolite levels were transient: levels of N-acetylaspartate (NAA) and total creatine (tCr) decreased, while those of choline-containing compounds (Cho) increased with each binge EtOH exposure cycle, and then recovered during each abstinence period. Despite repeated exposure and withdrawal cycles, this EtOH binge protocol provided limited evidence for enduring brain compromise. A failure to observe an increase in voluntary drinking as a measure of dependence may have been due to the inordinately high BALs achieved [i.e., escalated drinking is typically observed in animals with BALs < 250 mg/dL (Bell et al., 2004; Holter et al., 2000; Spanagel and Holter, 1999)] or because of an aversion derived from the exposure to EtOH via intragastric gavage (e.g., Ciccocioppo et al., 1999; Nachman et al., 1970).
The current study was thus conducted to determine whether greater withdrawal frequency from CIE using vaporized EtOH would be associated with exacerbated anxiety, greater voluntary EtOH consumption, and more enduring MRI-detectable brain structural and MRS-measurable neurochemical changes than previously achieved. To test these hypotheses, male and female wild-type Wistar rats were exposed to 3 cycles of 1 month in vapor chambers (e.g, Becker et al., 1997; Seo et al., 2004) and 1 week of recovery. This EtOH exposure protocol – with 14hr ON EtOH to BALs < 250 mg/dL and daily “withdrawals” to BALs of zero in the 10hr OFF period – is reported to elicit dependence (i.e., increases in voluntary drinking, e.g., Gilpin et al., 2008). Further, this paradigm enabled testing the alternative hypothesis that EtOH-related exposure effects on brain structure, biochemistry, behavior, and blood biomarkers would lessen at later cycles, consistent with neuroadaptation as previously observed with a repeated binge model (Zahr et al., 2016). With respect to MRS metabolite patterns, if the alcohol vapor chamber exposure produced the same MRS results as were obtained with the binge-EtOH exposure protocol, it would be expected that Cho would increase and NAA and tCr would decrease; an increase the ratio of Cho/tCr would be the most sensitive measure. Inclusion of male and female rats enabled examination of sex differences in these measures and investigation of the effects of EtOH exposure on brain growth over the 5 months of MRI data acquisition.
2. Methods
2.1. Animals
Animals used in these experiments were maintained in facilities fully accredited by the Association of the Assessment and Accreditation of Laboratory Animal Care (AAALAC). The Institutional Animal Care and Use Committees (IACUC) at SRI International and Stanford University approved all research protocols in accordance with the guidelines of the IACUC of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
The study group initially comprised 32 wild-type Wistar rats (Charles River Laboratories) aged ~8 weeks (corresponding to mid-to-late human adolescence)(Sengupta, 2013), including 16 male (324.6 ± 32.5) and 16 female (212.6 ± 30.5) animals singly housed with free access to food and water and accustomed to a reverse 12hr light/12hr dark cycle with lights ON from 20:00 to 8:00. Red lights were available for visibility when personnel needed to be in the room. In the airtight vapor chambers, male and female animals were segregated to separate sides of the room. For all other activities, male and female animals were segregated by time (experiments conducted on separate days) or space (moved to separate rooms).
Animals were monitored daily. Once per week, animals were weighed and had blood draws for determination of blood alcohol levels (BALs). Weights were additionally recorded at each of the scan time points. Two EtOH-exposed rats [one female after scan 2 had severe withdrawal signs observed as tremors (Majchrowicz and Hunt, 1976); one male after scan 3 had severe intoxication observed as heavily impaired motor coordination (Majchrowicz, 1975)] died during the course of the experiment because of excessive sensitivity to EtOH.
2.2. Ethanol exposure
Following the baseline scan, animals were triaged to control (n = 8 female; n = 8 male) or EtOH (n = 8 female; n = 8 male) groups based on weight and baseline scan structural brain characteristics, then acclimated to the regulated airflow of the specialized airtight chambers (La Jolla Alcohol Research Inc., La Jolla, CA.) for 10 days before EtOH exposure. Once initiated, vaporized EtOH was administered 7 days per week on a 14hr ON/10hr OFF schedule starting at 16:00 each day.
EtOH vapor was created by dripping 95% alcohol into a 4000 mL Erlenmeyer vacuum flask kept at 50 °C on a warming tray. Air was blown over the bottom of the flask at 11L/min to vaporize EtOH. Vapor concentrations were adjusted by varying the rate at which EtOH was pumped into the flask and ranged from 15 to 30 mg/L.
Saphenous vein blood (~0.5 mL) collected for determination of BALs once per week (8:00, within 2hr of EtOH OFF) in non-heparinized microcuvettes was assayed for alcohol content based on direct reaction with the enzyme alcohol oxidase (Analox Instruments, Ltd., U.K.). Air and EtOH flow rate were optimized to limit BALs to approximately 250 mg/dL.
Animals underwent 3 cycles of 4–5 weeks of vaporized EtOH exposure followed by 1 week of recovery: cycles 1 and 2 each included 5 weeks (38 days) of vaporized EtOH exposure; cycle 3 was only 4 weeks (29 days). EtOH exposure was designed to model cyclical intoxication and withdrawal periods to mimic cycles of human drinking behavior: exposure for 4–5 weeks models human drinking for 24–30 months; recovery for 7 days models approximately 6 months of abstinence (Sengupta, 2013).
2.3. Behavioral tests
2-bottle choice (2BC).
The 2-bottle choice assay between 15% w/v EtOH or reverse-osmosis water was given in the dark (i.e., while rats were awake) for 24hr every other day (Q.O.D) for the first 6 baseline sessions. The next 3 baseline sessions and all consequent Q.O.D sessions were 2hr each. The 2 bottles were positioned on 2 separate scales: scales were zeroed before the experiment began. The position of EtOH and water bottles was alternated randomly to avoid side preferences. The amount of EtOH or water consumed (in grams) was recorded for each session. In total, there were 9 sessions prior to vaporized EtOH exposure. Two weeks into vaporized EtOH cycle I, 2BC data was collected for 8 sessions Q.O.D.; 1 session was collected 4 days into the recovery week. Three weeks into EtOH cycle II, 2BC data was collected for 6 sessions Q.O.D.; 1 session was collected 7 days into the recovery week. Three weeks into EtOH cycle III, 2BC data was collected for 1 session; 1 session was collected 7 days into the recovery week.
Elevated Plus Maze (EPM), von Frey, and Neurological testing occurred at 7 time points: baseline (1); during the last week of each EtOH exposure cycle at peak withdrawal (~7hr after vaporized EtOH; 2, 4, 6); and after each week of recovery (3, 5, 7).
Elevated Plus Maze (EPM).
A standard rat apparatus 100 cm in length and 10 cm in width with two opposing arms (50 cm each) enclosed within high walls and the two remaining arms open and exposed was used for the EPM assay. Animals were placed in center of the maze and allowed to explore for 5min. Trials were recorded, and entries + time spent in proximal or distal open or closed arms was analyzed using BehaviorCloud (BehaviorCloud LLC, San Diego CA). Ethological variables such as head dips, stretch-attend postures, and rearing into either open or closed arms were manually evaluated and scored (Wilson et al., 1998). As a control for EPM performance, animals were also recorded for 10min using BehaviorCloud while in a custommade open field apparatus constructed from opaque black Plexiglas measuring 40 × 40 cm, surrounded by 40 cm-high walls. Variables quantified included average motor velocity, time spent in periphery, entries + time in large arena (25×25), entries + time in small center (5×5).
Von Frey Test for Allodynia.
Filaments were presented to the hind (right) paw of each animal using a “simplified up-down method” (e.g., Bonin et al., 2014; Dixon, 1965) to determine paw-withdrawal threshold. Filament size was converted to force in grams using: force (g) = 1.043*log(filament size)+3.997.
Neurological Testing.
Central, autonomic, sensory, and motor functions were evaluated and scored as normal = 0 or abnormal = 1 (e.g., Becker, 2000; Roberts et al., 1996; Yaksh et al., 1977). As examples, an abnormal autonomic response was piloerection; an abnormal sensory response as an accentuated startle to a clap; an abnormal posture a hunched back; and abnormal motor function ataxia. Abnormal responses were tallied and summed (Zahr et al., 2016).
Bottle Brush Irritability Test (BBIT).
The BBIT was conducted at 4 time points: 2 weeks into EtOH cycle II; at 1 and 3 weeks into EtOH cycle III; and after recovery III. Behavioral responses were recorded. Scoring by 2 different observers summed the aggressive or defensive postures in response to motions of a bottle brush in the animal’s home cage (Riittinen et al., 1986; Somkuwar et al., 2017).
2.4. Blood assays
Large volume (3.0 cc) samples were drawn at 4 time points (baseline and within 7hr of each of the 3 vaporized-EtOH exposure cycles) from the jugular vein under anesthesia. Complete blood count and liver panel assays were performed at the Stanford University Department of Comparative Medicine. Individual measures that were 4 or more standard deviations (SD) from the mean of the groups were identified with an iterative procedure and excluded from analysis.
2.5. MR scanning procedures and data analysis
Schedule.
Animals were scanned at baseline which occurred ~1 month following arrival (scan 1); at the end of each EtOH exposure cycle, within 7hr of vaporized EtOH (scans 2, 3, 6); and after 1 week of abstinence (scans 3, 5, 7).
Anesthesia and Monitoring.
Isoflurane anesthesia was initiated at 2–3% followed by a single subcutaneous injection of dexmedetomidine hydrochloride (0.05 mg/kg). At scan commencement, isoflurane was reduced to 0.5–1.5%. Rats were placed on an animal cradle equipped with built-in water circulation for body temperature control. Eye lubricant was used to anoint eyes for protection from dehydration. Silicon earplugs were affixed to protect ears from scanner gradient volume. A rat-brain surface coil was secured over each animal. Temperature and respiration were monitored throughout the ~2hr experiment. Animals received subcutaneous saline (10 cc) for hydration at the end of the scan.
MRI Acquisition.
MR data were collected on a Bruker 70/16 US AVANCE III 7.0T system (Karlsruhe, Germany) with 380 mT/m gradient strength on each (X, Y, and Z) axis, slew rate of 3420 T/m/s, 16 cm bore size using a Bruker surface coil and ParaVision 6.1 software. A gradient-recalled echo (GRE) localizer scan was used to position the animals in the scanner and for graphical prescription of subsequent scans. The T2-weighted, high-resolution, TurboRare acquisition for structural analysis had the following parameters: repetition time (TR) = 6000 ms; echo time (TE) = 33 ms; 0.2 mm isotropic voxels; matrix = 160 × 160; 2 averages; echo train length = 8; slice thickness = 0.5 mm; 32 slices.
MRI Processing.
Preprocessing of each image included removal of noise (Coupe et al., 2008) and inhomogeneity correction via ANTS 2.1.0 (Tustison et al., 2011). Each image was skull stripped by aligning a template to the scan via symmetric diffeomorphic registration (Tustison et al., 2010) and the resulting deformation map was applied to the brain mask of the template. Image inhomogeneity correction was repeated on skull-stripped images. Bias-corrected, skull-stripped images were rigidly aligned to a template via ANTS 2.1.0 and used as input for further analysis. Structural images were segmented into CSF, gray matter, and white matter with finite mixture modeling (FMM) segmentation (ANTS atropos) producing a probability for each of the three tissue types for each voxel in the brain. The final unit of measure for each tissue type was the integrated probability over the entire brain, yielding whole brain gray matter, white matter, and CSF volumes.
MRS Acquisition.
Proton metabolite data were collected from a large voxel (9×7×8 mm) subtending the anterior half of the brain with a Point-Resolved Spin Echo Sequence (PRESS): TR = 2500 ms; TE = 135 ms; 2048 acquisition data points; spectral width = 4006 Hz; with VAPOR (variable power and optimized relaxation delays) water suppression (bandwidth 200 Hz). Eight repetitions were collected without water suppression followed by 50 with water suppression.
MRS Metabolite Quantification.
MRS data were analyzed using LCModel (Version 6.3–1J) (Provencher, 1993, 2001). The analysis was run with the “water reference” option that provided reasonably meaningful absolute metabolite concentrations based on the unsuppressed water content of the voxel. The analysis window was 0.2–4.0 ppm. Data were pre-processed with zero-order phasing, referencing, and residual water line removal. Data were fitted to a linear combination of a number of metabolites in a simulated basis set designed for Bruker 7T acquisition at TE = 135 ms provided by Stephen Provencher [sp@lcmodel.CA] containing alanine, creatine + phosphocreatine (tCr), glutamine (Gln), glutamate (Glu), glycerophosphorylcholine + phosphorylcholine (Cho), glutathione (GSH), inositol, lactate, N-acetylaspartate + N-acetylaspartylglutamate (NAA), scyllo-inositol, taurine (Tau), and several lipids and macromolecules. Only metabolite concentrations derived from fitted spectra consistently within average Cramér-Rao bounds < 15% were considered and comprised NAA, tCr, Cho, Glu, and Tau.
DTI Acquisition.
DTI echoplanar data acquisitions used TR = 8000 ms; TE = 22.57 ms; 0.5 mm isotropic voxels, field of view (FOV) = 32 mm; matrix = 64 × 64; slice thickness = 1.0 mm; 24 slices. One data set include six 3-D frames of b = 0, 32 directions of b = 500, and 32 directions of b = 1500. A second data set with six b = 0 and 6 directions of b = 1500 with reversed readout polarity was used for echoplanar spatial distortion correction.
DTI Metric Quantification.
In subject space, FSL “topup” (Jenkinson et al., 2012; Smith et al., 2004) was used to minimize echoplanar spatial distortion. FSL “dtifit” was used to create the tensors and extract fractional anisotropy (FA) and mean diffusivity (MD) measures. Native FA images were upsampled into laboratory atlas space with concatenated, non-rigid registration of b = 0 to T2 structural to atlas with 0.2 mm 3D isotropic resolution. DTI metrics were measured in genu, splenium, and left and right hippocampal fimbria-fornix drawn on the atlas and transferred to each animal’s FA and MD images.
2.6. Statistical analysis
For each blood and behavioral variable of interest, a repeated-measures (time), full-factorial (group, sex, group-by-sex) multivariate analysis of variance (MANOVA) was conducted in JMP® Pro version 14.1.0 (SAS Institute Inc., Cary N.C., 1989–2019). Group (that is, treatment comparing EtOH-exposed vs. control) and interaction effects are reported. When relevant, a follow-up MANOVA considered group effects separately for male and female rats; this was followed by t-tests.
MRI, MRS, and DTI measures were analyzed first with linear models (lm) using R 3.5.1 (R Core Team, 2019), which examined general growth trends. To capture the potential cyclical effect of EtOH exposure followed by withdrawal, difference scores were calculated between pairs of measures, that is, baseline (scan 1) to exposure (scan 2), exposure (scan 2) to recovery (scan 3) and so on. Each difference score was tested with directional t-tests with the predictions that exposure minus baseline or recovery differences would be greater in the EtOH than control groups for tissue measures, whereas recovery minus exposure differences would be less in the EtOH than controls groups. The opposite pattern was predicted for CSF volumes. Familywise Bonferroni correction for 6 cycles with α = 0.05 required p = .017.
3. Results
3.1. Weight and blood alcohol levels (BALS)
Statistics on weight included 18 measures. Over the course of the experiment, weight showed effects of sex [F(1,26) = 79.2, p < .0001)] and time [F(17,442) = 55.7, p < .0001]. Female rats were smaller than male rats throughout the experiment (Fig. 1a). Male rats gained 31% and female rats gained 25% of their baseline weight over the course of the experiment. Growth and sex differences were present despite EtOH exposure.
Fig. 1.
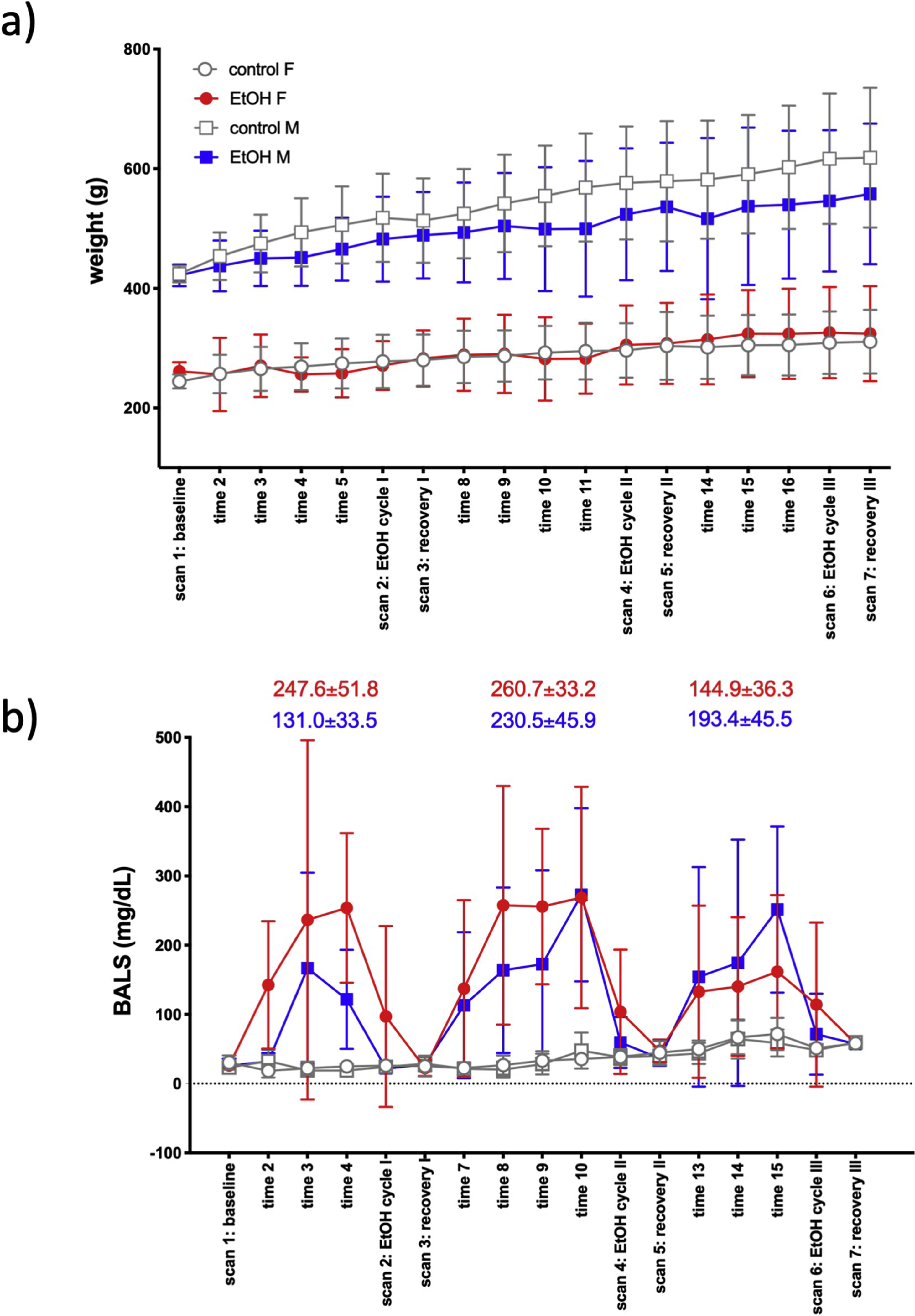
a) Weight (mean ± SD) of animals across the course of the experiment. b) Blood alcohol levels (BALs) over the course of the experiment. BALs (mean ± SD) for each EtOH cycle are presented separately for female (red) and male (blue) EtOH-exposed rats. [EtOH cycle I: average of BALs at times 3, 4; EtOH cycle II: average of times 8, 9, 10; EtOH cycle II average of times 13, 14, 15.].
An in-house pilot study found an alcohol elimination rate of about 25 mg/dL/hour for BALs under 200 mg/dL; a lower elimination rate was observed for higher levels. As blood samples were collected 2hr into the OFF period of vaporized EtOH, our estimates indicate that BALs presented herein are slightly, but insignificantly underestimated. BALs showed effects of treatment (that is, group comparison of control vs. EtOH exposed) [F(1,26) = 106.5, p < .0001], time [F(17,442) = 8.2, p < .0001], and group-by-time interactions [F(17,442) = 8.6, p < .0001. Fig. 1b shows average BALs in each cycle at 2hrs after removal from vapor exposure. Male EtOH-exposed rats reached average peak BALs of 147.9 ± 138.7 mg/dL in cycle 1 (time 3), 301.7 ± 141.8 mg/dL in cycle 2 (time 10), and 251.5 ± 120.1 mg/dL in cycle 3 (time 15). Female EtOH-exposed rats reached peak BALs of 251.1 ± 243.6 mg/dL in cycle 1 (time 2), 268.8 ± 159.9 mg/dL in cycle 2 (time 10), and 161.8 ± 110.6 mg/dL in cycle 3 (time 15). EtOH levels were ramped up during each cycle, were marginal during EtOH scans, and measured zero during the recovery scans.
3.2. Behavior
2-bottle choice (2BC).
Because of length differences (24hr vs. 2hr), the first 6 time points (24hr) were analyzed separately from the 21 subsequent time points (2hr). For EtOH consumption, the first 6 time points showed a treatment-by-sex-by-time interaction [F(5,140) = 3.3, p = .008] (Fig. 2a). In female rats, a treatment-by-time interaction [F (5,70) = 3.4, p = .009] showed\ that female controls began by consuming more EtOH than the EtOH-treated group at time 1 (that is, before EtOH vapor exposure) (p = .04) and time 2 (p = .04). For the next 21 time points, there were a number of interactions including treatment-by-time [F(20,480) = 1.9, p = .01] and treatment-by-sex-by-time [F(20,480) = 1.8, p = .02]. Follow-up tests significant in female rats only showed a treatment-by-time interaction [F (20,240) = 2.5, p = .0005]: 2-bottle choice EtOH consumption during EtOH cycle II was significantly greater in the EtOH-exposed female rats at time 22 (p = .03) (Fig. 2a). Water consumption was not related to treatment group (Fig. 2b).
Fig. 2.
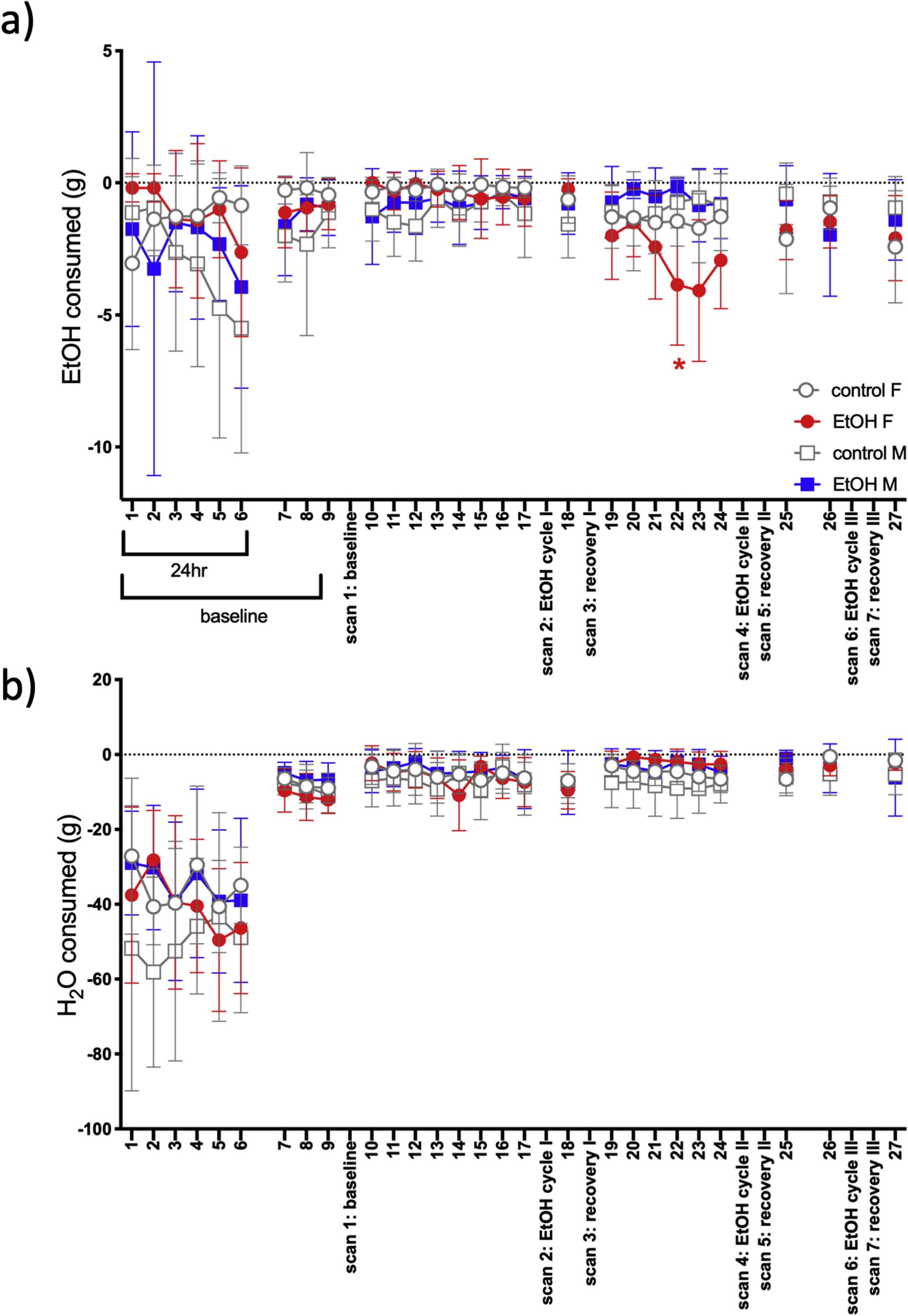
Two-bottle choice of a) EtOH or b) water consumption over the course of the experiment. Consumption is in the negative direction because data presented is total grams consumed starting from 0 (i.e., consumption decreased weight of the bottle). A red asterisk indicates t-test significance (p ≤ .05) between female EtOH and control groups.
Elevated Plus Maze (EPM).
In the EPM, neither entries nor time in distal open arms distinguished treatment groups. Further, time spent in proximal open arms did not distinguish treatment groups, and male EtOH and control animals did not differ in proximal entries into open arms. In the female animals, however, a repeated measures MANOVA for proximal entries was significant for diagnosis (p = .02): EtOH-exposed rats had fewer entries at all time points except baseline and the last evaluation (after the 3rd recovery period).
Open field test (OFT):
Neither entries nor duration in the center distinguished the male treatment groups in the OFT. Within female animals, duration did not distinguish groups, but center entries were significant: treatment groups were not different at baseline (p = .99), but EtOH-exposed animals had fewer entries following EtOH-exposure cycle I (p = .01) that persisted after the first week of recovery (p = .03) but then resolved for all remaining time points (p = .34-.90). Further, average velocity was significant for a treatment-by-time interaction [F (6,114) = 3.2, p = .006] (Fig. 3a). Follow-up analyses significant in the female rats only showed a treatment effect [F(1,13) = 9.9, p = .008) and a group-by-time interaction [F(6,78) = 4.9, p = .0003]: EtOH-exposed female rats had a slower average motor velocity following EtOH-exposure cycle I (p = .0001), EtOH-exposure cycle II (p = .0004), recovery II (p = .006), and EtOH-exposure cycle III (p = .03).
Fig. 3.
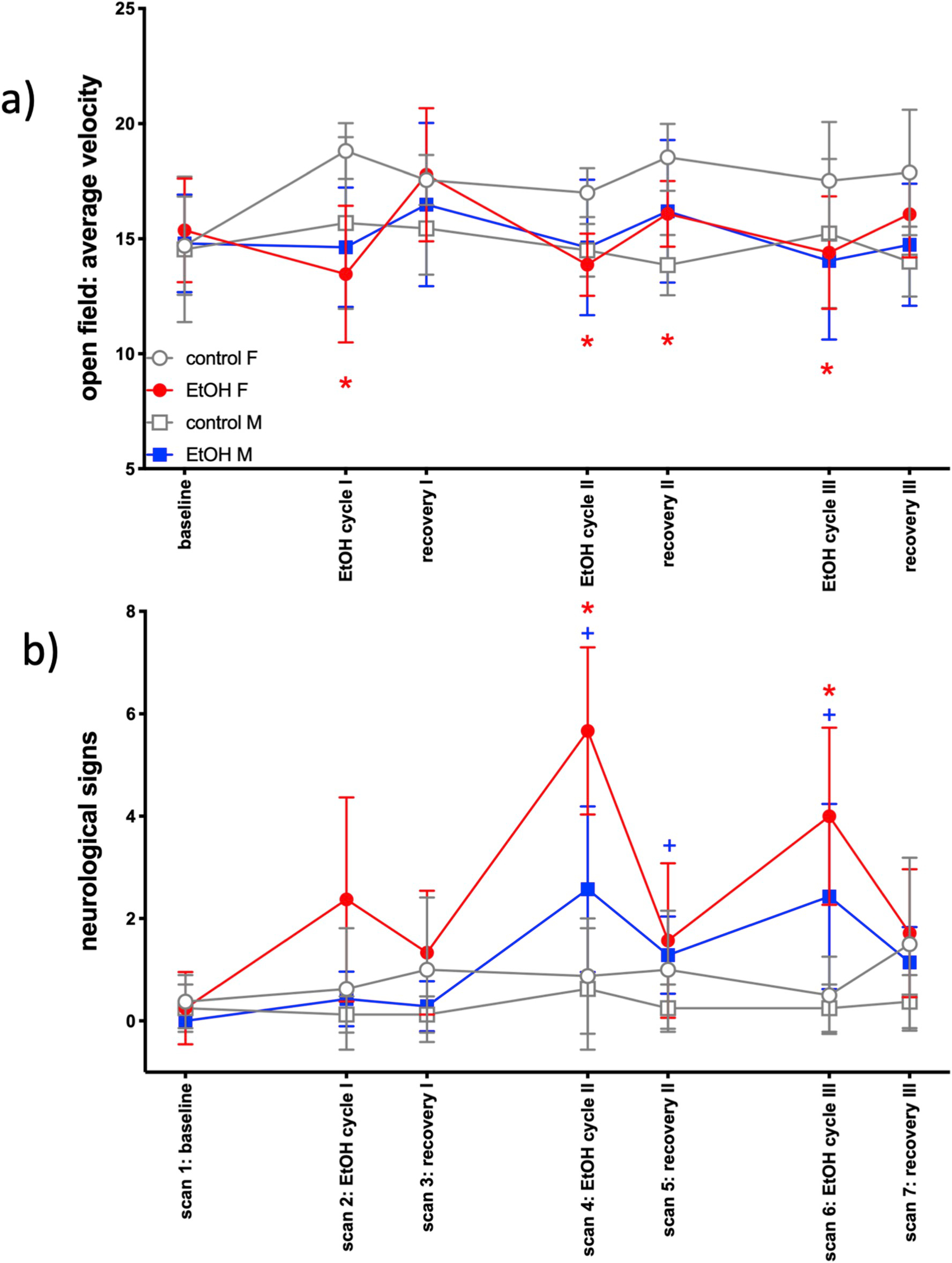
Behavioral measures: a) open field average velocity; b) neurological signs. Red asterisk indicates t-test significance (p ≤ .05) between female EtOH and control groups; blue cross indicates t-test significance (p ≤ .05) between male EtOH and control groups.
Von Frey Test for Allodynia.
Analysis of the von Frey threshold showed treatment effects [F(1,26) = 5.8, p = .02] that were not significant with the Bonferroni correction. Among female but not male rats, a MANOVA was significant for treatment [F(1,13) = 5.4, p = .04]: allodynia was observed in the female EtOH-exposed rats at EtOH cycle II (p = .002) and recovery II (p = .01).
Neurological Testing.
A MANOVA on the sum score was significant for treatment [F(1,19) = 29.2, p < .0001] and the interactions treatment-by-time [F(6,114) = 17.5, p < .0001] and treatment-by-sex-by-time [F(6,114) = 3.2, p = .007]. Among male rats, there were effects of treatment [F(1,10) = 34.1, p = .0002] and a treatment-by-time interaction [F(6,60) = 9.0, p < .0001]. EtOH-exposed male rats had more signs of impairment at scan 4 (EtOH cycle II, p = .002), scan 5 (recovery II, p = .02), and scan 6 (EtOH cycle III, p = .02). Female rats demonstrated the same significance pattern [treatment F (1,9) = 11.7, p = .008, treatment-by-time interaction F(6,54) = 10.6, p < .0001] with EtOH-exposed female rats showing more signs of neurological impairment at scan 4 (p = .004) and scan 6 (p = .01) relative to control rats (Fig. 3b).
Bottle Brush Irritability Test (BBIT).
Defensive behaviors showed effects of treatment [F(1,26) = 5.6, p = .03] and treatment-by-time interactions [F(3,78) = 3.5, p = .02] that did not meet significance with Bonferroni correction. Only the female rats showed follow-up effects of treatment [F(1,13) = 5.6, p = .04] due to a greater presence of defensive postures (p = .01) in EtOH-exposed rats at the 2nd BBIT time point (i.e., 1 week into EtOH cycle III). Escape behaviors showed effects of treatment [F(1,26) = 7.1, p = .01] and treatment-by-time interactions [F(3,78) = 3.1, p = .03]. In female rats only, follow-up showed treatment effects [F(1,13) = 5.2, p = .04], evidenced by a greater presence of escape postures in the EtOH-exposed rats at 2nd BBIT time point (p = .04).
3.3. Blood
Hematological [red blood count (RBC), red cell distribution width (RDW), hemaglobin (Hgb), hematocrit (HCT), mean corpuscular volume (MCV), MCH)] and hepatic enzyme [(aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase] indices were measured at baseline and 7hrs after each vapor cycle. Analyses were preceded by outlier elimination using an iterative procedure identifying and excluding values that were 4 or more SD from the mean; this procedure resulted in one or two exclusions for 5 of the 9 measures. For each measure, the groups were compared independently after each cycle with a linear model (lm), followed by the model with the addition of the baseline value as a second independent variable to determine whether any difference survived control for baseline differences.
Significant group differences emerged only after cycle 1 and only for female rats (Fig. 4). Specifically, relative to the female control group, the female EtOH group had lower Hgb and higher AST and ALT and trend for higher RDW; only AST (t = 3.205, p = .0069) and ALT (t = 2.566, p = .0235) survived adjustment for baseline value. No significant treatment group differences emerged for cycles 2 or 3 in either sex.
Fig. 4.
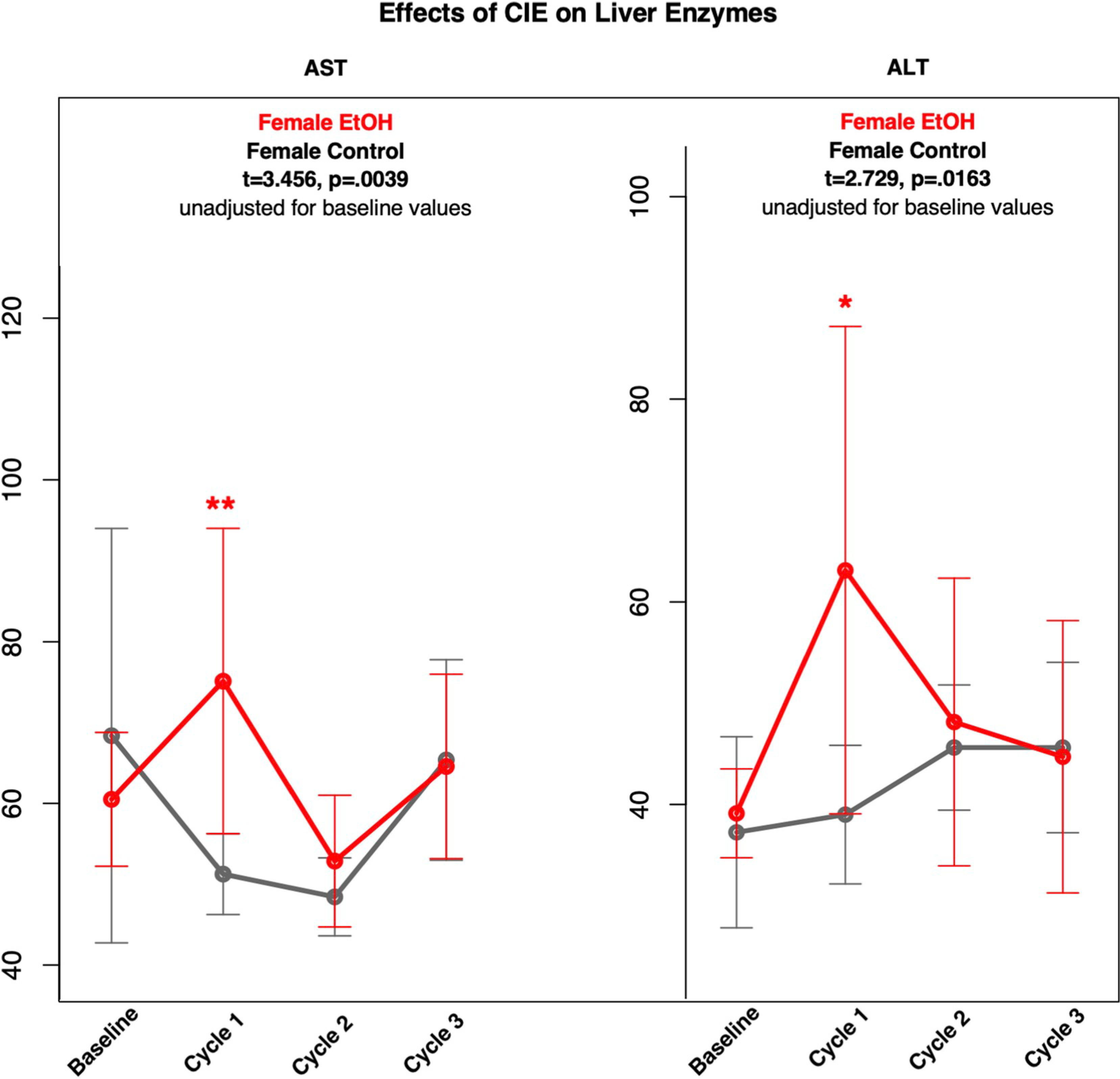
Means and standard deviations (unadjusted for baseline values) of control (gray) and females (red) animals across 3 cycles for AST and ALT.
3.4. Brain
Statistical analysis results for the MRI, MRS, and DTI by treatment group and sex comparisons are presented in Figs. 5–7 and Supplementary Tables 1, 2, and 3.
Fig. 5.
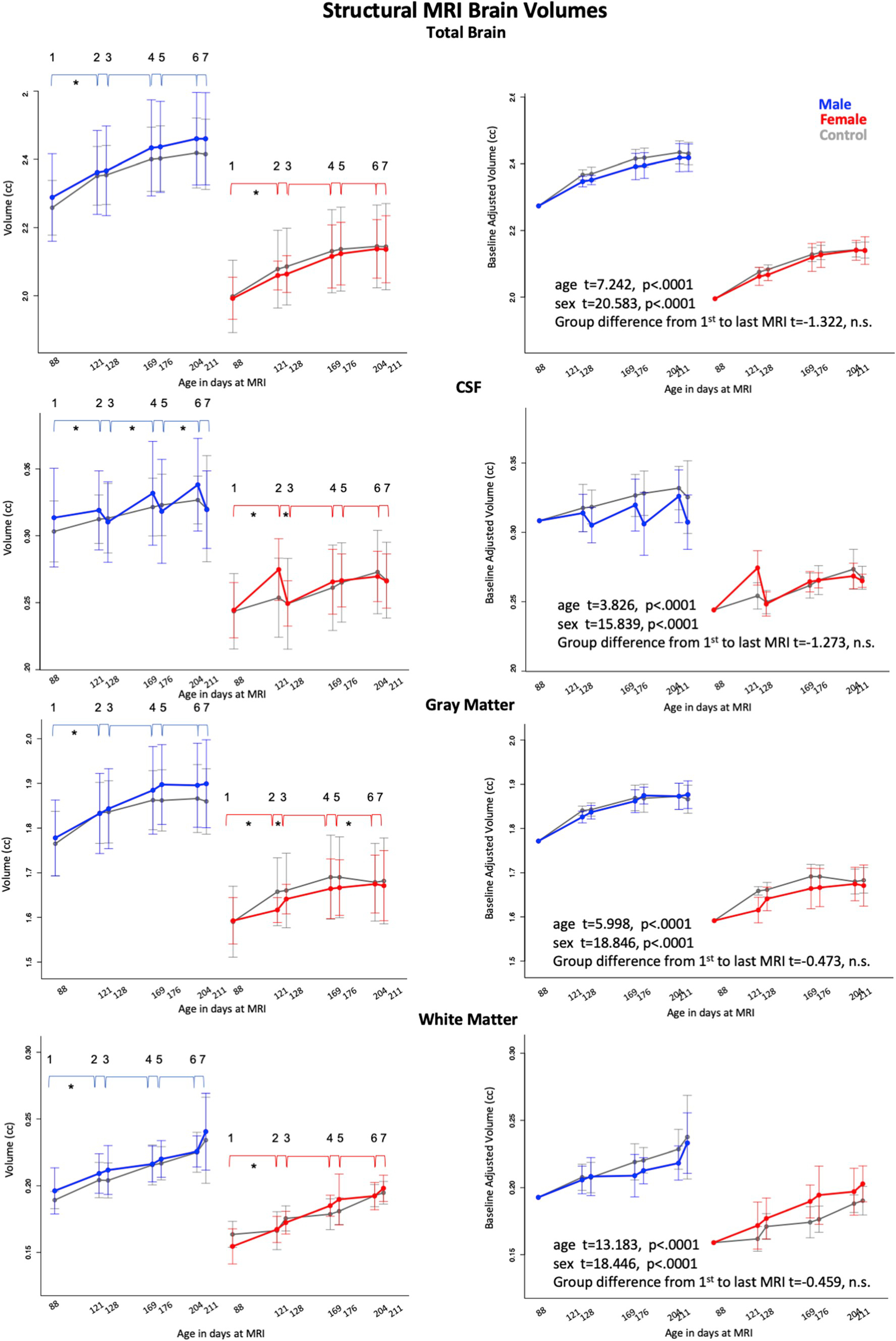
Means and standard deviations of control (gray) and exposed animals by sex (males = blue, females = red) for 7 scan sessions across 3 cycles for total brain volume (i.e, growth), and volumes of CSF, gray matter and white matter. Left column is unadjusted values; right column are data adjusted for baseline volume. * indicate statistically significant difference between exposed and control animals. Also see Supplementary Table 1.
Fig. 7.
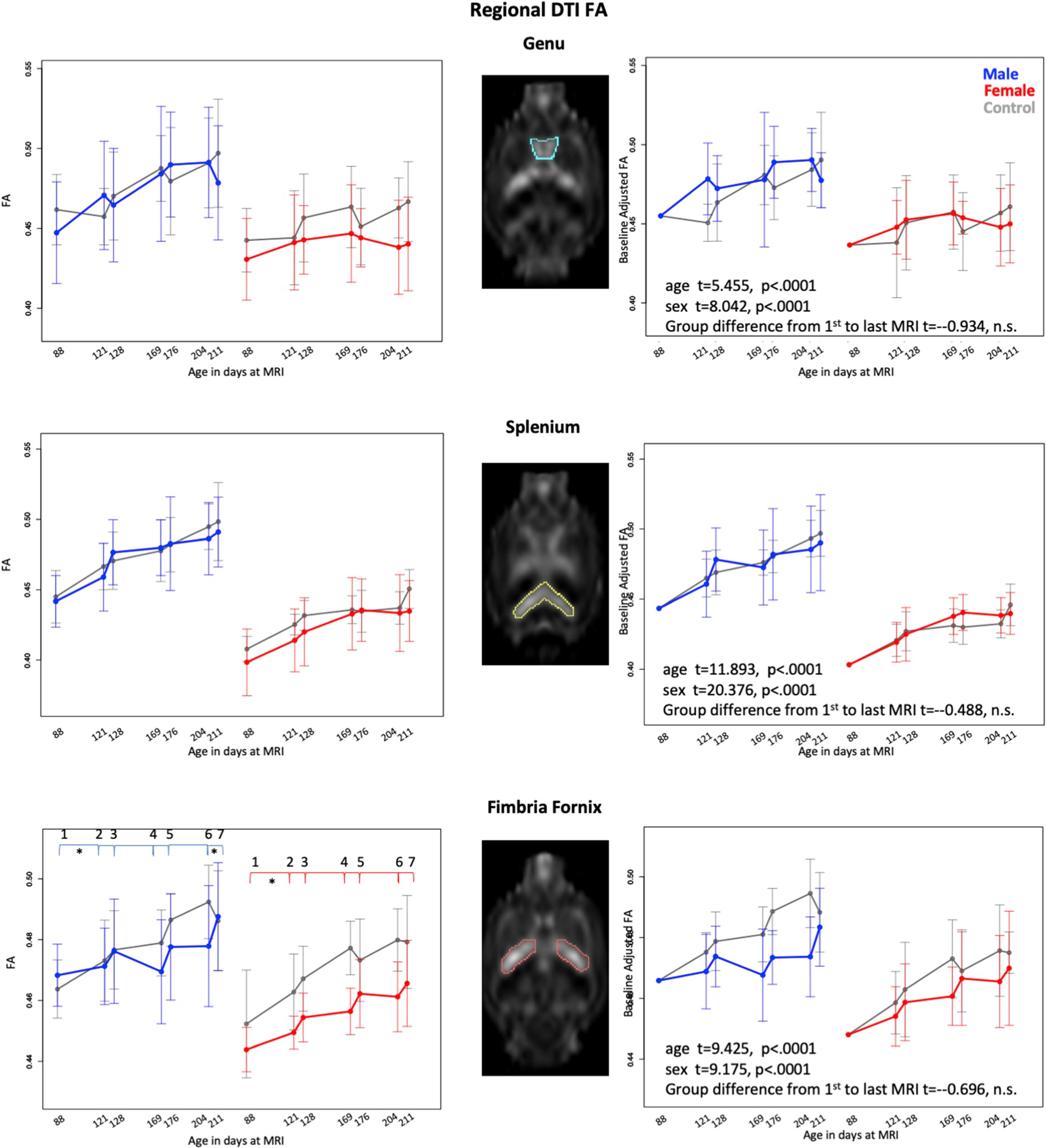
Means and standard deviations of control (gray) and exposed animals by sex (males = blue, females = red) for 7 scan sessions across 3 cycles for DTI FA in genu, splenium, and fimbria-fornix. Left column is unadjusted values; right column are data adjusted for baseline volume. * indicates statistically significant difference between exposed and control animals. Also see Supplementary Table 3.
3.4.1. MRI structural volumes
The lm analysis modeling growth measured by total brain volume revealed significant time (that is, age) and sex effects for both groups (all p’s < 0.0001) with no EtOH-exposure effect. This pattern indicated that although the female groups were smaller than the male groups, the control and EtOH groups showed similar growth trajectories for total brain volume and for each segmented brain volume (CSF, gray matter, and white matter) despite EtOH exposure. This effect was confirmed when analyzing volumes adjusted for baseline differences (Fig. 5, right panel).
Analyses of the tissue types by exposure cycle detected predicted changes in several contrast pairs. The figures and tables present the statistics and visual depiction of observed changes (Fig. 5, Supplementary Table 1). For the EtOH-treated female rats, for the first exposure cycle, CSF volume significantly increased from baseline (pre-exposure) to exposure, followed by a significant decrease from exposure to recovery. Subsequent exposure cycles 2 and 3 did not reach statistical significance for EtOH-treated relative to control animals. Conversely, gray matter volume growth for the EtOH-treated female rats was significantly less from pre-exposure to first exposure, followed by significantly greater expansion from exposure to recovery than in the control rats. The third cycle pre-to post-exposure revealed modestly greater expansion for the EtOH-treated than control animals, the latter group showing essentially no growth from scans 5 to 6.
For the EtOH-treated male rats, CSF volume decreased significantly from the second cycle of exposure to recovery and then increased modestly from recovery to exposure in the third exposure cycle. As with the female rats, gray matter volume growth of the male EtOH-treated animals was less from pre-exposure to exposure for the first exposure cycle.
None of the white matter volume or EtOH-exposure differences was significant.
3.4.2. MRS metabolite values
With the exception of Cho, which was higher between pre-exposure and the first exposure cycle in the EtOH-treated female rats than the female controls, predicted treatment vs. control effects for metabolites were not forthcoming. The Cho/tCr pattern was more highlighted differences with an increase from pre-exposure to exposure followed by a decrease from exposure to recovery (Fig. 6, Supplementary Table 2) and met adjusted significance level for the first cycle in the female animals. Although the repeated exposure for cycles 2 and 3 showed increases, neither met statistical significance compared with controls. By contrast, the exposure to recovery decrease in Cho/tCr occurred at the p < .05 (uncorrected) significance level for all three cycles.
Fig. 6.
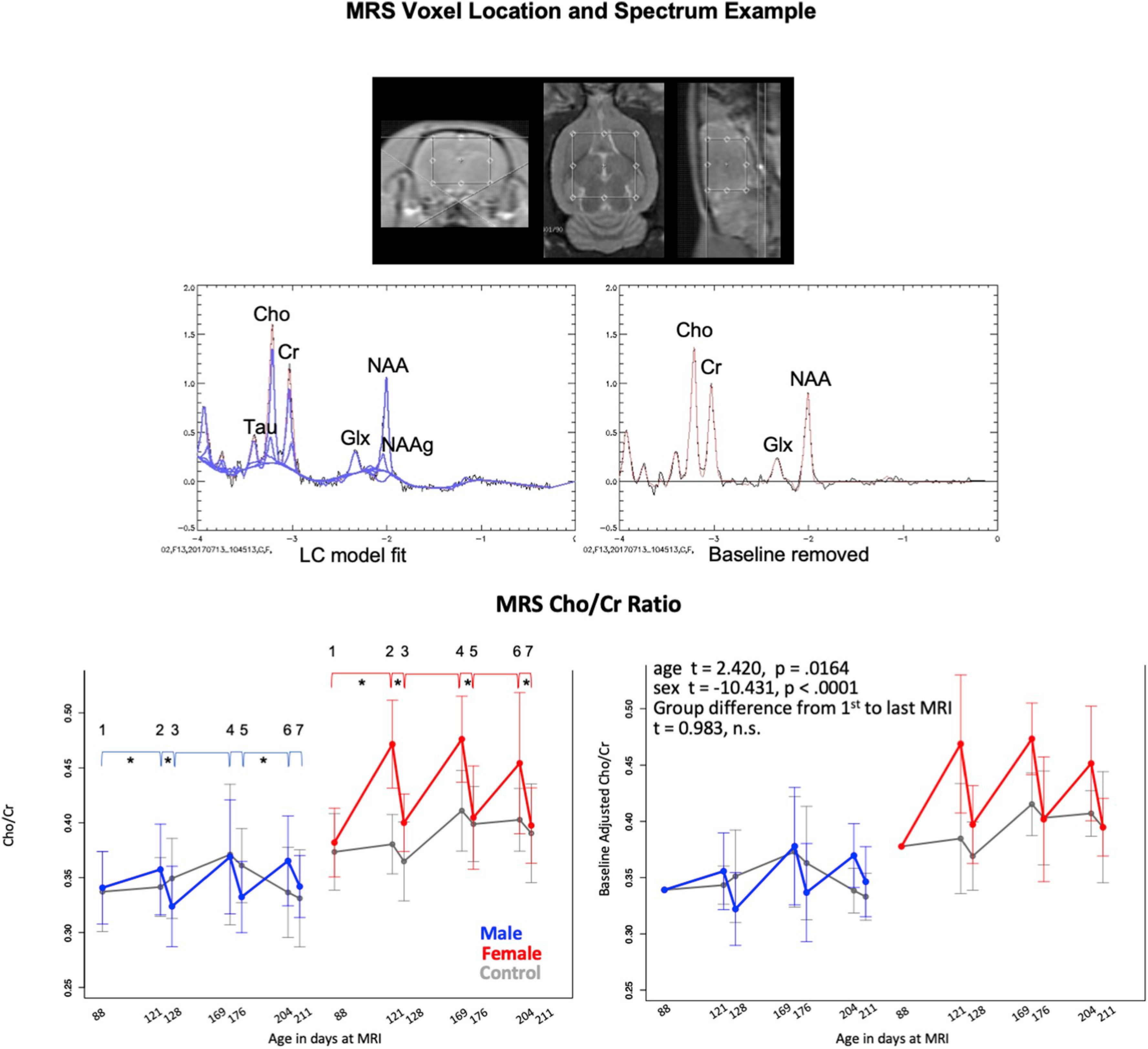
Top: MRS voxel placement. Middle: representative spectrum before (left) and after (right) spectral baseline removal. Bottom: Means and standard deviations of control (gray) and exposed animals by sex (males = blue, females = red) for 7 scan sessions across 3 cycles for MRS measured Choline/Creatine. Left column is unadjusted values; right column are data adjusted for baseline volume. * indicate statistically significant difference between exposed and control animals. Also see Supplementary Table 2.
For the male rats, the pattern for Cho/tCr was similar showing only trends for cycle 1 exposure to recovery and cycle 3 recovery to exposure. Overall, the pattern was an exposure effect with return to baseline for the first cycle without evidence of cumulative alcohol effect that did not recover. Analysis of the baseline adjusted ratios indicated significant age and sex effects but no treatment group effect between the first and last MRS measurement (Fig. 6, right panel).
3.4.3. White matter microstructure
This set of analyses removed one male, EtOH-exposed rat with outlying data. The lm analyses revealed growth (p < .0001) and sex (p < .0001) effects for FA of the genu, splenium, and bilateral hippocampal fimbria-fornix (Fig. 7, Supplementary Table 3). Only the fimbria-fornix showed a treatment group (that is, EtOH) effect (p ≤ .0001). In addition, fimbria-fornix FA exhibited treatment-by-age (p = .034) and treatment-by-sex (p = .013) interactions, indicating that age-related increases in FA were attenuated in the EtOH-exposed groups relative to the control groups and that the effect was prominent in the female EtOH-exposed rats. Treatment effects were not detected in MD.
Although none of the cycle pairings yielded significant treatment differences in the expected direction for any measured region, review of Fig. 7 suggests differential response to EtOH exposure and recovery, notable in the male rats. To explore whether EtOH-related responses were dampened by baseline difference seen in Fig. 7, baseline values were reset to the within-sex means, which served to adjust the levels of remaining values - for each subject baseline level was subtracted from all subsequent scans and the mean for all males added to the males and the mean of all females added to the females for realistic graphical presentation. The resulting adjusted FA data were subjected to the cycle pairings analysis and revealed significant recovery at the last cycle in the male rats (p = .0052). Analysis of baseline adjusted FA indicated significant age and sex effects of the genu, splenium, and bilateral fimbria-fornix but no treatment effect between the first and last DTI measurement (Fig. 7, right panel).
4. Discussion
It has been argued that alcohol dependence is more difficult to produce with protocols using high, acute EtOH doses (upwards of 350–400 mg/dL) by gavage than with protocols using a less aversive EtOH exposure protocol with vapor chambers and targeting sustained, lower BALs (typically below 250 mg/dL)(Griffin et al., 2009). Despite use of vapor delivery of relatively low doses of EtOH, this study did not show clear evidence of dependence as determined by an escalation of voluntary EtOH consumption. Further, no evidence emerged for accumulated neuropathology as quantified by gross measures of brain macrostructure, microstructure, or MRS-detectable metabolites despite evidence for non-accruing, transient effects, indicative of neuroadaptation. These findings are consistent with our previously-noted observations (i.e., Zahr et al., 2010) that the rate or pattern of achieving BALs is critical in determining the extent of neuropathology (cf., Bonthius and West, 1990; West et al., 1990). Specifically, our work has shown that ventricular enlargement is far more pronounced in rats achieving average BALs of 300 mg/dL in just 4 days of intragastric EtOH (Zahr et al., 2010) than in rats achieving average BALs of 250 mg/dL over 24 weeks via vaporized EtOH where only modest ventricular enlargement was observed (Pfefferbaum et al., 2008). Further support for this concept is provided by the minimal responsiveness of male rats to EtOH in this study possibly due to the relatively lower BALs achieved in cycle I relative to the female EtOH-exposed rats.
4.1. Behavioral markers of chronic EtOH exposure
Behaviors affected by EtOH exposure were generally transient, specific to female rats, and did not accumulate in response to repeated withdrawals. The female EtOH-exposed rats showed reduced proximal open arm entries in the EPM, fewer center entries and a reduced average velocity in the OFT. Together, these results support a transient increase in anxiety in the female, EtOH-exposed animals following the first EtOH-exposure cycle and correspond with neuroimaging findings in indicating a higher sensitivity of the exposure paradigm in the female animals. This is in contrast to published evidence suggesting male relative to female rats are more sensitive to withdrawal-induced anxiety as quantified on the elevated plus maze following one 5 day EtOH-exposure cycle (Overstreet et al., 2004), on the social interaction test following a single dose of EtOH (Varlinskaya and Spear, 2004), and greater acoustic startle response following 2 weeks of an EtOH liquid diet (Reilly et al., 2009).
The female rats also showed a reduced von Frey threshold and a greater presence of neurological signs. A decreased paw-withdrawal threshold (mechanical allodynia) has been reported as a result of EtOH exposure (Bergeson et al., 2016; Tiwari et al., 2009) and comports with reports of peripheral neuropathy in AUD (Zahr et al., 2019). Our own work in animal models (Zahr et al., 2009, 2010, 2016) has demonstrated the reliability of the neurological examination (Becker, 2000; Pitkin and Savage, 2001; Yaksh et al., 1977) to discern differences between EtOH-exposed and control animals, but these were mild and also transient after repeated EtOH exposures.
4.2. Blood markers of chronic EtOH exposure
Elevated levels of AST and ALT after cycle I with recovery in the female animals observed herein suggests a hepatic effect of EtOH exposure. Changes in hepatic indices, however, did not significantly correlate with brain alterations in the EtOH-exposed rats. Evidence for a brain-liver relation may have been below detection with our measures; alternatively, the animals which died may have been the most physically fragile and as such their data would have increased detection of a relation.
4.3. Brain structural and metabolite markers of chronic EtOH exposure
Throughout the experiment, male rats had larger brains than female rats, and all animals, including the EtOH-exposed, showed similar rates of brain growth. Evidence of structural response to vaporized EtOH exposure with elevated whole brain CSF and reduced gray matter volume with recovery at some cycles in both male and female animals comports with ventricular enlargement seen with a repeated gavage model (Zahr et al., 2016).
We have consistently seen in response to gavage-produced higher BALs (i.e., > 300 mg/dL) (Pfefferbaum et al., 2008; Zahr et al., 2009, 2010, 2013, 2014, 2016) reductions in NAA and tCr and elevations in Cho. While the current work did not replicate the binge model completely, it did show that bouts of vaporized EtOH exposure were associated with an increase in the Cho/tCr ratio with exposure in the female animals and recovery especially after cycle I.
Following each of the vaporized EtOH exposure cycles, FA fimbria-fornix was lower in EtOH-exposed relative to control female rats, but these effects were transient: FA values did not distinguish groups following each week of recovery. Integrity measures of the other white matter regions (i.e., callosal genu and splenium) were not significantly affected by EtOH exposure. This in vivo finding is consistent with postmortem evidence for reductions in myelin-related proteins in male Wistar rats following CIE (Kim et al., 2015; Samantaray et al., 2015) that are at least partially reversible with abstinence (Navarro and Mandyam, 2015; Yalcin et al., 2017). Postmortem tissue from individuals with AUD relative to healthy controls also shows alterations in the expression of myelin and oligodendrocyte-related mRNAs and proteins in various regions (Alexander-Kaufman et al., 2007; Liu et al., 2006; Mayfield et al., 2002; Miguel-Hidalgo et al., 2017). Further, in human alcoholics, both structural (Pfefferbaum et al., 1992, 1995, 2002; Sullivan et al., 1996; Zhao et al., 2019) and DTI studies have demonstrated the sensitivity of white matter microstructure to alcoholism (Pfefferbaum et al., 2006a, b; Pfefferbaum et al., 2009; Pfefferbaum et al., 2010; Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2000) and the potential for white matter recovery with abstinence (Alhassoon et al., 2012; Cardenas et al., 2007; Estilaei et al., 2001; Gazdzinski et al., 2010; Monnig et al., 2013; Pfefferbaum et al., 2014). Thus, available animal and human evidence indicates significant effects of alcohol exposure on the plasticity of white matter constituents and the potential for recovery as a consequence of remyelination during abstinence (e.g., Mi et al., 2009).
4.4. Limitations
Neuroimaging results were more forthcoming in the female than male animals. Although both sexes achieved high BALs during some cycles, that female rats had higher BALS than their male counterparts during cycle I may explain why their results reached statistical significance. A further limitation involves the age of the rats, which were in the range of young adult humans, and study exposure length, which was about half a year. While long for most animal model protocols, the age and alcohol exposure time of the rats were not comparable to those of human adults with long-standing AUD, who typically exhibit an age-alcoholism interaction, where regional brain volume deficits are more likely to be observed in alcohol-dependent individuals who are 45 years of age and older (cf., Pfefferbaum et al., 1997; Sullivan et al., 2018). The rodent/human age mismatch could account for the modest, reversible effects of EtOH vapor exposure, even with a CIE paradigm on the young adult rat brain.
5. Conclusions
In conclusion, this study demonstrates that repeated cycles of 1-month vaporized EtOH (to BALs approaching but not generally exceeding 250 mg/dL) + 1-week abstinence resulted in consistent, but reversible decreases in fimbria-fornix FA in female EtOH-exposed rats indicating transient disruption of white matter integrity. Increases followed by recovery in the Cho/Cr MRS metabolite marker also did not escalate over exposure cycles nor did the early acceleration of CSF volume expansion and shrinkage of gray matter volume accrue or endure in later cycles. This exposure protocol did not lead to reliable escalations in voluntary drinking, increased anxiety, or evidence for accumulation of neuropathology as determined using multi-modal in vivo imaging. Similarly, evidence of hepatic enzyme elevations in female animals for the first cycle only suggests some hepatic adaptation to ethanol exposure.
The fact that almost all measures - behavioral, hepatic, and neuroimaging - that revealed a response to exposure were followed by recovery is consistent with evidence for recovery with abstinence in humans. However, lack of escalation over repeated cycles suggests that alcohol alone in this model is not sufficient to mimic the human condition wherein cumulative damage ensued with continued drinking. Further, studies of human alcoholism provide evidence for subclinical occult signs of Wernicke’s encephalopathy in contributing to the neurocognitive deficits of “uncomplicated alcoholism,” indicating a large role for nutritional deficiency (Fama et al., 2019; Pitel et al., 2011). By contrast, the animals in this experiment were well fed and were, in fact, overweight. Extension of the CIE model should consider manipulation of nutrition (e.g., sub-clinical thiamine deficiency) to discern the independent and synergistic contribution of nutritional factors and alcohol per se on the apparent effects of significant alcohol exposure on brain structure, biochemistry, and behavior.
Supplementary Material
HIGHLIGHTS.
Male and female wild-type Wistar rats were exposed to repeated cycles of vaporized ethanol (EtOH).
2-bottle choice, elevated plus maze, von Frey, and neurological testing evaluated behavior.
Neuroimaging included structural MRI, diffusion tensor imaging (DTI), and MR spectroscopy.
Greater vulnerability of female rats to deficits are likely due to higher initial BALs.
No evidence for accrual of behavioral or brain deficits with repeated cycles.
Acknowledgments
Grants R01 AA005965 and U01 AA013521 by the U.S. Department of Health & Human Services (DHHS) National Institute on Alcohol Abuse and Alcoholism (NIAAA) supported this work.
Footnotes
Declaration of Competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2020.108066.
References
- Alexander-Kaufman K, Cordwell S, Harper C, Matsumoto I, 2007. A proteome analysis of the dorsolateral prefrontal cortex in human alcoholic patients. Proteomics Clin Appl 1, 62–72. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I, 2012. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin. Exp. Res 36, 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R, 2004. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology : off publ Am Coll Neuropsychopharmacology 29, 921–928. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM, 1978. Kindling as a model for alcohol withdrawal syndromes. The British journal of psychiatry : J. Ment. Sci 133, 1–14. [DOI] [PubMed] [Google Scholar]
- Becker HC, 2000. Animal models of alcohol withdrawal. Alcohol Res. Health 24, 105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL, 1997. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacol. Biochem. Behav 57, 179–183. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, 2004. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin. Exp. Res 28, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL, 1998. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology (Berl) 139, 145–153. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ, 2004. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin. Exp. Res 28, 1867–1874. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Blanton H, Martinez JM, Curtis DC, Sherfey C, Seegmiller B, Marquardt PC, Groot JA, Allison CL, Bezboruah C, Guindon J, 2016. Binge ethanol consumption increases inflammatory pain responses and mechanical and cold sensitivity: tigecycline treatment efficacy shows sex differences. Alcohol Clin. Exp. Res 40, 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y, 2014. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR, 1990. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin. Exp. Res 14, 107–118. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC, 1993. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcohol 28, 593–598. [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F, 2005a. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin. Exp. Res 29, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, 2005b. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 178, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC, 1988. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry 23, 507–514. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ, 2011. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry 70, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ, 2007. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda R, Sussman N, Westreich L, Levy R, O’Malley M, 1996. A review of the effects of moderate alcohol intake on the treatment of anxiety and mood disorders. J Clin Psychiatry 57, 207–212. [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Chhada M, Perfumi M, Froldi R, Massi M, 1999. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: influence of the method of ethanol administration. Pharmacol. Biochem. Behav 64, 563–566. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL, 2003. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend 70, 105–108. [DOI] [PubMed] [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C, 2008. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 27, 425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Schumann G, Yagi T, Spanagel R, 2003. Role of Fyn tyrosine kinase in ethanol consumption by mice. Alcohol Clin. Exp. Res 27, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE, 2013. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin. Exp. Res 37, 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman P Jr., 1987. Delirium tremens. Update on an old disorder. Postgrad. Med 82, 117–122. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Martin-Fardon R, Thorsell A, Weiss F, 2004. Chronic footshock, but not a physiological stressor, suppresses the alcohol deprivation effect in dependent rats. Alcohol Alcohol 39, 190–196. [DOI] [PubMed] [Google Scholar]
- Deber CM, Reynolds SJ, 1991. Central nervous system myelin: structure, function, and pathology. Clin. Biochem 24, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K, 2011. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin. Exp. Res 35, 1678–1685. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, 1965. The up-and-down method for small samples. J. Am. Stat. Assoc 60, 967–978. [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, Veatch LM, Becker HC, Crews FT, 2004. Consequences of multiple withdrawals from alcohol. Alcohol Clin. Exp. Res 28, 233–246. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN, 2002. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol Clin. Exp. Res 26, 785–795. [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN, 2003. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin. Exp. Res 27, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Williams SC, Critchley HD, Stephens DN, 2011. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry 70, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estilaei MR, Matson GB, Payne GS, Leach MO, Fein G, Meyerhoff DJ, 2001. Effects of abstinence from alcohol on the broad phospholipid signal in human brain: an in vivo 31P magnetic resonance spectroscopy study. Alcohol Clin. Exp. Res 25, 1213–1220. [PubMed] [Google Scholar]
- Faingold CL, Knapp DJ, Chester JA, Gonzalez LP, 2004. Integrative neurobiology of the alcohol withdrawal syndrome–from anxiety to seizures. Alcohol Clin. Exp. Res 28, 268–278. [DOI] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Hardcastle C, Sassoon SA, Pfefferbaum A, Sullivan EV, Zahr NM, 2019. Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict Biol 24, 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes LMP, Lopes KS, Santana LNS, Fontes-Junior EA, Ribeiro C, Silva MCF, de Oliveira Paraense RS, Crespo-Lopez ME, Gomes ARQ, Lima RR, Monteiro MC, Maia CSF, 2018. Repeated cycles of binge-like ethanol intake in adolescent female rats induce motor function impairment and oxidative damage in motor cortex and liver, but not in blood. Oxid Med Cell Longev 3467531 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Cooze J, Malone C, French V, Weber JT, 2013. Effects of intermittent binge alcohol exposure on long-term motor function in young rats. Alcohol 47, 95–102. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R, 2007. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol. Biochem. Behav. 86, 320–326. [DOI] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD, 2004. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 176, 82–87. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ, 2010. Cerebral white matter recovery in abstinent alcoholics–a multimodality magnetic resonance study. Brain 133, 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Teneback CC, Malcolm RJ, Moore J, Stallings LE, Spicer KM, Anton RF, Ballenger JC, 1999. Multiple previous alcohol detoxifications are associated with decreased medial temporal and paralimbic function in the post-withdrawal period. Alcohol Clin. Exp. Res 23, 1077–1084. [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF, 2008. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9 Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, Sinha R, Stevens L, 1988. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol Alcohol 23, 337–342. [DOI] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Becker HC, 2009. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin. Exp. Res 33, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA Jr., Knight CP, Waeiss RA, Truitt WA, Johnson PL, Bell RL, McBride WJ, Rodd ZA, 2019. Conditioned stimuli affect ethanol-seeking by female alcohol-preferring (P) rats: the role of repeated-deprivations, cue-pretreatment, and cue-temporal intervals. Psychopharmacology (Berl) 236, 2835–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF, 1997. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin. Exp. Res 21, 784–791. [PubMed] [Google Scholar]
- Holter SM, Linthorst AC, Reul JM, Spanagel R, 2000. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacol. Biochem. Behav 66, 143–151. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF, 2006. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 40, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD, 2015. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct. Funct 220, 1705–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Thuras P, Kaminski J, Anderson N, Neumeyer B, Mackenzie T, 2000. Expectancies for alcohol to affect tension and anxiety as a function of time. Addict. Behav 25, 93–98. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL, Beaunieux H, 2014. Impaired decision-making and brain shrinkage in alcoholism. Eur Psychiatry 29, 125–133. [DOI] [PubMed] [Google Scholar]
- Lechtenberg R, Worner TM, 1992. Seizure incidence enhancement with increasing alcohol intake. Ann. N. Y. Acad. Sci 654, 474–476. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jang MK, Lee JY, Kim SM, Kim KH, Park JY, Lee JH, Kim HY, Yoo JY, 2005. Clinical predictors for delirium tremens in alcohol dependence. J. Gastroenterol. Hepatol 20, 1833–1837. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD, 2006. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology 31, 1574–1582. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K, 2009. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol 44, 372–381. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, Flor H, 2010. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol 45, 541–547. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B, 1995. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcohol Alcohol 30, 737–748. [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM, 1997. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J. Neurosci 17, 1302–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E, 1975. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43, 245–254. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E, Hunt WA, 1976. Temporal relationship of the induction of tolerance and physical dependence after continuous intoxication with maximum tolerable doses of ethanol in rats. Psychopharmacology (Berl) 50, 107–112. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, 2002. The differential effects of medication on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am. J. Addict 11, 141–150. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF, 2000. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol 22, 159–164. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA, 2002. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J. Neurochem. 81, 802–813. [DOI] [PubMed] [Google Scholar]
- Mejia-Toiber J, Boutros N, Markou A, Semenova S, 2014. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav. Brain Res 266, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW, 2006. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin. Exp. Res 30, 2017–2025. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chedotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B, 2009. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol 65, 304–315. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Hall KO, Bonner H, Roller AM, Syed M, Park CJ, Ball JP, Rothenberg ME, Stockmeier CA, Romero DG, 2017. MicroRNA-21: expression in oligodendrocytes and correlation with low myelin mRNAs in depression and alcoholism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 79, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS, 2013. White matter volume in alcohol use disorders: a meta-analysis. Addict Biol 18, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman M, Lester D, Le Magnen J, 1970. Alcohol aversion in the rat: behavioral assessment of noxious drug effects. Science 168, 1244–1246. [DOI] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD, 2015. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience 293, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Gottlieb LD, Kraus ML, Segal SR, Horwitz RI, 1991. Social and clinical features as predictors of outcome in outpatient alcohol withdrawal. J. Gen. Intern. Med 6, 312–316. [DOI] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA, 2006. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol 38, 155–164. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR, 2002. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin. Exp. Res 26, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR, 2004. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol. Biochem. Behav 78, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV, 2006a. Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol. Aging 27, 994–1009. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV, 2006b. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry 59, 364–372. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV, 1992. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin. Exp. Res 16, 1078–1089. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E, 2001. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry 158, 188–197. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV, 2009. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV, 2002. Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin. Exp. Res 26, 400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV, 2014. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry 1, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV, 2010. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol Clin. Exp. Res 34, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, 2002. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 15, 708–718. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M, 2000. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin. Exp. Res 24, 1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO, 1997. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin. Exp. Res 21, 521–529. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO, 1995. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin. Exp. Res 19, 1177–1191. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Vinco S, Orduna J, Rohlfing T, Sullivan EV, 2008. Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcohol Clin. Exp. Res 32, 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, Sullivan EV, 2011. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without korsakoff’s syndrome. Neuropsychopharmacology 36, 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin SR, Savage LM, 2001. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behav. Brain Res 119, 167–177. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med 30, 672–679. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14, 260–264. [DOI] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL, 2009. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol 44, 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riittinen ML, Lindroos F, Kimanen A, Pieninkeroinen E, Pieninkeroinen I, Sippola J, Veilahti J, Bergstrom M, Johansson G, 1986. Impoverished rearing conditions increase stress-induced irritability in mice. Dev. Psychobiol 19, 105–111. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF, 1996. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin. Exp. Res 20, 1289–1298. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Patel KS, Mulholland PJ, Becker HC, Banik NL, 2015. Chronic intermittent ethanol induced axon and myelin degeneration is attenuated by calpain inhibition. Brain Res 1622, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R, 2006. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J. Neurosci. : the official journal of the Society for Neuroscience 26, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segobin SH, Chetelat G, Le Berre AP, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel AL, 2014. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin. Exp. Res 38, 739–748. [DOI] [PubMed] [Google Scholar]
- Sengupta P, 2013. The laboratory rat: relating its age with human’s. Int. J. Prev. Med 4, 624–630. [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Lee S, Rivier C, 2004. Prolonged exposure to intermittent alcohol vapors decreases the ACTH as well as hypothalamic nitric oxide and cytokine responses to endotoxemia. Alcohol Clin. Exp. Res 28, 848–854. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Anderson RI, Haun HL, Mulholland PJ, Griffin WC 3rd, Lopez MF, Becker HC, 2019. July 9. Dynamic c-Fos changes in mouse brain during acute and protracted withdrawal from chronic intermittent ethanol exposure and relapse drinking. Addict. Biol, e12804. 10.1111/adb.12804.e12804, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl. 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD, 2017. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 84, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, 1999. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol 34, 231–243. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE, 2009. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin. Exp. Res 33, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL, 2001. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur. J. Neurosci 14, 2023–2031. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A, 1996. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin. Exp. Res 20, 348–354. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, Pfefferbaum A, 2018 May 1. The role of aging, drug dependence, and hepatitis C comorbidity in alcoholism cortical compromise. JAMA Psychiatr 75 (5), 474–483. 10.1001/jamapsychiatry.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK, 2014. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol 48, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Chopra K, 2009. Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain 145, 129–135. [DOI] [PubMed] [Google Scholar]
- Trick L, Kempton MJ, Williams SC, Duka T, 2014 Nov. Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addict. Biol 19 (6), 1041–1054. 10.1111/adb.12175. Epub 2014 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC, 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Siqueira M, Gee JC, 2011. Topological well-composedness and glamorous glue: a digital gluing algorithm for topologically constrained front propagation. IEEE Trans. Image Process. 20, 1756–1761. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Hosova D, Towner T, Werner DF, Spear LP, 2020. Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res 378, 112292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Kim EU, Spear LP, 2017. Chronic intermittent ethanol exposure during adolescence: effects on stress-induced social alterations and social drinking in adulthood. Brain Res 1654, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2004. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin. Exp. Res 28, 40–50. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL, 1990. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin. Exp. Res 14, 813–818. [DOI] [PubMed] [Google Scholar]
- Wilson J, Watson WP, Little HJ, 1998. CCK(B) antagonists protect against anxiety-related behaviour produced by ethanol withdrawal, measured using the elevated plus maze. Psychopharmacology (Berl) 137, 120–131. [DOI] [PubMed] [Google Scholar]
- Wollenweber FA, Halfter S, Brugmann E, Weinberg C, Cieslik EC, Muller VI, Hardwick RM, Eickhoff SB, 2014. Subtle cognitive deficits in severe alcohol addicts–do they show a specific profile? J. Neuropsychol 8, 147–153. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Kohl RL, Rudy TA, 1977. Induction of tolerance and withdrawal in rats receiving morphine in the spinal subarachnoid space. Eur. J. Pharmacol 42, 275–284. [DOI] [PubMed] [Google Scholar]
- Yalcin EB, McLean T, Tong M, de la Monte SM, 2017. Progressive white matter atrophy with altered lipid profiles is partially reversed by short-term abstinence in an experimental model of alcohol-related neurodegeneration. Alcohol 65, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A, 2010. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry 67, 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, Pfefferbaum A, 2013. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology 38, 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A, 2009. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology 34, 1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pohl KM, Pfefferbaum A, Sullivan EV, 2019. Central nervous system correlates of “objective” neuropathy in alcohol use disorder. Alcohol Clin. Exp. Res 43, 2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Mayer D, Luong R, Sullivan EV, Pfefferbaum A, 2016. November. Transient CNS responses to repeated binge ethanol treatment. Addict. Biol 21 (6), 1199–1216. 10.1111/adb.12290. Epub 2015 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R, 2007. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology (Berl) 190, 13–19. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Pfefferbaum A, Podhajsky S, Pohl KM, Sullivan EV, 2019. April 1. Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder. Addict. Biol 10.1111/adb.12746. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


