Αbstract
Remdesivir (GS-5734), a drug initially developed to treat hepatitis C and Ebola virus disease, was the first approved treatment for severe coronavirus disease 2019 (COVID-19). However, apart from remdesivir, there is a paucity of other specific anti-viral agents against SARS-CoV-2 infection. In 2017, researchers had documented the anti-coronavirus potential of remdesivir in animal models. At the same time, trials performed during two Ebola outbreaks in Africa showed that the drug was safe. Although vaccines against SARS-CoV-2 infection have emerged at an enormously high speed, equivalent results from efforts towards the development of anti-viral drugs, which could have played a truly life-saving role in the current stage of the pandemic, have been stagnating. In this review, we will focus on the current treatment options for COVID-19 which mainly consist of repurposed agents or treatments conferring passive immunity (convalescent plasma or monoclonal antibodies). Additionally, potential specific anti-viral therapies under development will be reviewed, such as the decoy miniprotein CTC-445.2d, protease inhibitors, mainly against the Main protein Mpro, nucleoside analogs, such as molnupiravir and compounds blocking the replication transcription complex proteins, such as zotatifin and plitidepsin. These anti-viral agents seem to be very promising but still require meticulous clinical trial testing in order to establish their efficacy and safety. The continuous emergence of viral variants may pose a real challenge to the scientific community towards that end. In this context, the advent of nanobodies together with the potential administration of a combination of anti-viral drugs could serve as useful tools in the armamentarium against COVID-19.
Keywords: Antiviral treatment, Baricitinib, boceprevir, COVID-19, Favipiravir, Molnupiravir, Monoclonal antibodies, Nanobodies, Plitidepsin, Protease inhibitors, Ribavirin, SARS-CoV-2, Zotatifin
List of abbreviations
- AAK1
AP2-associated protein kinase 1
- ACE2
Angiotensin Converting Enzyme 2
- ACTT-2
Adaptive Covid-19 Treatment Trial 2
- ALT
Alanine aminotransferase
- BMI
Body Mass Index
- CI
Confidence Interval
- COVID-19
Coronavirus disease 19
- CRP
C-Reactive Protein
- ECMO
Extracorporeal Membrane Oxygenation
- ED
Emergency Department
- eGFR
estimated Glomerular Filtration Rate
- EMA
European Medicinal Agencies
- EUA
Emergency Use Authorization
- FDA
Food and Drug Administration
- ICU
Intensive Care Unit
- NIH
National Institute of Health
- NSPs
Non Structural Proteins
- RCT
Randomized Controlled Trial
- RTC
Replication Transcription Complex
- RECOVERY
Randomized Evaluation of Covid-19 Therapy
- REMAP-CAP
Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia
- RNA
Ribonucleic Acid
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- VTE
Venous Thromboembolism
- WHO
World Health Organization
1. Introduction
The novel coronavirus disease (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been declared a global pandemic by the WHO since March 11, 2020. As of May 15, 2021, there have been 161,513,458 confirmed cases of COVID-19 with 3,352,109 deaths, and 1,264,164,553 vaccine doses administered/reported from 223 countries/areas all over the world [1]. Currently, there is no specific treatment; only the use of repurposed drugs [[2], [3], [4], [5], [6], [7]]. Up until now, anti-viral agents have played a crucial role in fighting infections from RNA viruses, such as HCV infection and HIV infection. Regarding SARS-CoV-2, anti-viral drugs, such as remdesivir, which was initially developed for the treatment of Ebola virus disease, are being used for the time being, while other anti-viral drugs are much anticipated [8]. In this review, we present the current treatment options for COVID-19 and we discuss current advances in the development of potential anti-viral therapies.
2. What do we have so far in our armamentarium?
2.1. Remdesivir
Remdesivir is a nucleotide analog that has shown activity against SARS-CoV-2 in vitro [8]. In the United States, the Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for remdesivir for hospitalized patients with severe COVID-19. The suggested adult dose is 200 mg intravenously on day 1, followed by 100 mg daily for 5 days total (with extension to 10 days if there is no clinical improvement and in patients on mechanical ventilation). Remdesivir is not recommended in patients with alanine aminotransferase (ALT) ≥5 times the upper limit of normal and should be discontinued if serum ALT rises above this level during treatment. Remdesivir is not recommended in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min per 1.73 m2 [8]. Data from randomized trials are rapidly emerging. Overall, available data suggests that there are some clinical benefits of remdesivir [9,10]. Results from one large trial have indicated that remdesivir reduced the recovery time from severe COVID-19 [10]. In particular, patients who received remdesivir had a median recovery time of 10 days (95% CI, 9 to 11), as compared with 15 days (95% CI, 13 to 18), with those who received placebo [10]. However, whether remdesivir reduces mortality remains uncertain. It is noteworthy that remdesivir is not recommended for the treatment of mild or moderate COVID-19 [8].
2.2. Immunomodulators
Glucocorticoids, e.g. dexamethasone, exert anti-inflammatory and immunosuppressive effects on the immune cells, thus decreasing the production of pro-inflammatory cytokines, such as interleukin (IL)-2, IL-6, and tumor necrosis factor (TNF)-α, and suppressing the activation of T cells, monocytes, and macrophages. The RECOVERY trial is evaluating a wide range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal. In this Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial, 2,104 hospitalized patients receiving dexamethasone at a dose of 6 mg daily for up to 10 days were compared with 4,321 controls. The incidence of death was lower in the dexamethasone group than in the usual-care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81). Evidence from this trial that has been published by the RECOVERY Collaborative Group in the New England Journal of Medicine on 25 February 2021, advocates the use of dexamethasone in the management of severe COVID-19, as dexamethasone was demonstrated to reduce mortality in severely ill patients, i.e., patients who have SpO2 ≤ 94% on room air, requiring supplemental oxygen or mechanical ventilation. Dexamethasone is administered at a dose of 6 mg daily for 10 days or until discharge. On the contrary, there is currently no recommendation for dexamethasone in patients with mild to moderate COVID-19 [11].
Baricitinib is a selective Janus kinase 1 and 2 inhibitor used for the treatment of rheumatoid arthritis. Besides its immunomodulatory effects, which include inhibition of IL-2, IL-6, IL-10, interferon-γ, and granulocyte–macrophage colony stimulating factor, baricitinib may possess antiviral effects through interfering and potentially hampering viral entry. In particular, AP2-associated protein kinase 1 (AAK1) is suggested to be a regulator of endocytosis; therefore, the disruption of AAK1 might block the entrance of the SARS-CoV-2 into cells [12]. Indeed, baricitinib has been documented to possess a high affinity for AAK1, thus hampering the entry of SARS-C0V-2 into the host cells. In the USA, the FDA issued an EUA for baricitinib administered at 4 mg orally once daily for up to 14 days, to be used in combination with remdesivir in patients with COVID-19, who require oxygen or ventilatory support [13]. This EUA was based upon the results of the Adaptive Covid-19 Treatment Trial (ACTT-2), which was published in the NEJM on March 4, 2021. The ACTT-2 enrolled 1,033 patients with severe COVID-19, with 515 receiving the combination treatment and 518 receiving remdesivir plus placebo. Patients on baricitinib plus remdesivir had a 30% increase in odds of improving their clinical status at day 15 (odds ratio/OR 1.3, 95% CI: 1 to 1.6). Further studies are mandatory to investigate the efficacy of the combination of dexamethasone and baricitinib for patients with severe COVID-19. For the rare occurrence of a patient who has contraindications to glucocorticoids and is being administered remdesivir, adding baricitinib is a quite reasonable approach. Notably, baricitinib should not be used without remdesivir, as only their combination has been documented to reduce recovery time and accelerate improvement. No increase in the rate of adverse effects, including infection rates and venous thromboembolism with baricitinib was reported [14].
Markedly elevated inflammatory markers (e.g. D-dimer, ferritin) and elevated pro-inflammatory cytokines, such as IL-6, have been related to critical COVID-19 [7]. Therefore, blocking this inflammatory pathway may prevent disease progression [12]. Various agents targeting the IL-6 pathway have been evaluated in several studies for the treatment of COVID-19; these comprise the IL-6 receptor blockers tocilizumab and sarilumab, and the direct IL-6 inhibitor siltuximab. Tocilizumab at 8 mg/kg as a single dose, is recommended for individuals who require high-flow oxygen or more intensive respiratory support within 24–48 h of admission to an ICU or who receive care of ICU-level [15]. Tocilizumab may also be used for selected patients on low-flow oxygen supplementation, if they are clinically progressing towards high-flow oxygen therapy despite administration of dexamethasone and have significantly elevated inflammatory markers, such as a C-reactive protein (CRP) level of ≥75 mg/L. Administration of tocilizumab may be used only in conjunction with dexamethasone (or another glucocorticoid) and is generally limited to a single dose. It should not be administered among patients with hypersensitivity to tocilizumab, uncontrolled severe infections apart from COVID-19, absolute neutrophil count <500 cells/μL, platelet count <50.000 cells/μL, ALT >5 times the upper limit of normal range, and patients at increased risk of gastrointestinal perforation. Tocilizumab should be used with caution in immunocompromised individuals as very few of them have been included in clinical trials. The National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel recommends adding tocilizumab to dexamethasone in recently hospitalized patients who are at least on high-flow oxygen and have either been admitted to the ICU within the prior 24 h or have greatly increased inflammatory markers; some members have suggested adding tocilizumab to patients on conventional oxygen supplementation if they exhibit rapidly increasing oxygen needs and a CRP level ≥75 mg/L [15]. The Infectious Diseases Society of America (IDSA) suggests adding tocilizumab to standard of care (i.e., glucocorticoids) for hospitalized adults who have progressively severe or critical COVID-19 and elevated inflammatory markers [15]. The latest suggestion for the use of tocilizumab stemmed from the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Study. In this trial, 353 patients had been administered tocilizumab, 48 sarilumab, and 402 were controls. An analysis of 90-day survival confirmed improved survival in the IL-R6 antagonist groups, with a hazard ratio for the comparison with the control group of 1.61 (95% CI, 1.25 to 2.08) and a probability of superiority greater than 99.9%. Based on the results of the REMAP-CAP study, in critically ill patients receiving organ support in ICUs, treatment with the IL-R6 antagonists tocilizumab and sarilumab improved outcomes, including mortality [16].
According to preliminary unpublished results of an open-label trial in the United Kingdom that included 4,116 patients with suspected or confirmed COVID-19, hypoxia (oxygen saturation < 92% on room air or oxygen supplementation of any kind), and a CRP level ≥75 mg/L, adding one to two doses of weight-based tocilizumab to usual care reduced the 28-day mortality rate compared with usual care alone (29% versus 33%, relative risk 0.86, 95% CI 0.77 to 0.96) [8,9]. Among those who were not on mechanical ventilation at baseline, tocilizumab similarly reduced the combined endpoint of progression to mechanical ventilation or death. There did not appear to be a statistically significant difference in mortality risk reduction after adjusting for baseline respiratory support. Most of the trial participants (82%) were also under glucocorticoid treatment, and subgroup analysis suggested that they were more likely to benefit from tocilizumab than were individuals who did not receive glucocorticoids. In particular, the RECOVERY Study among the 4,116 adults, who were included, 562 (14%) patients were receiving invasive mechanical ventilation, 1,686 (41%) receiving non-invasive respiratory support, and 1,868 (45%) receiving no respiratory support other than oxygen. Overall, 596 (29%) of the 2,022 patients on tocilizumab and 694 (33%) of the 2094 patients on usual care died within 28 days (rate ratio 0.86; 95% CI 0.77 to 0.96; p = 0.0028). A clear mortality benefit was seen in those receiving systemic corticosteroids. Patients allocated to tocilizumab were more likely to be discharged from hospital alive within 28 days (57% vs. 50%; rate ratio 1.22; 95% CI 1.12 to 1.33; p < 0.0001). Among those not receiving invasive mechanical ventilation at baseline, patients administered tocilizumab were less likely to reach the composite endpoint of invasive mechanical ventilation or death (35% vs. 42%; risk ratio 0.84; 95% CI 0.77 to 0.92; p < 0.0001) [16,17].
However, several other trials, such as COVACTA, a phase 3, randomized controlled trial (RCT) to estimate the efficacy and safety of tocilizumab in hospitalized patients with severe Covid-19 pneumonia, have not established any mortality benefit or other clear clinical benefit with the use of these agents [18,19].
2.3. Anti-thrombotic prophylaxis and treatment
Venous thromboembolism (VTE) was frequently observed in acutely ill patients with COVID-19 during the early stages of the pandemic, approaching a proportion of one-third of patients in the ICU, even when prophylactic anticoagulation was used [8]. In a recent study published in JAMA, Sadeghipour et al. have reported the results of a multicenter RCT of intermediate-dose versus standard-dose heparin-based thromboprophylaxis in critically ill patients with COVID-19 [20]. Among 562 inpatients enrolled, no significant difference in the primary efficacy outcome was ascertained [a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation (ECMO)], or mortality within 30 days of enrollment) or in the main safety outcomes, such as major bleeding and severe thrombocytopenia between the two groups. Primary efficacy outcome occurred in 126 patients (45.7%) in the intermediate-dose group and 126 patients (44.1%) in the standard-dose prophylaxis group, death occurred in 43.1% vs 40.9%, while VTE occurred in 3.3% vs 3.5%, and major bleeding occurred in 2.5% vs 1.4%, respectively. However, there are several issues worth mentioning mainly regarding the low thrombotic event rate reported in both study groups, raising the possibility of a considerable number of potentially “missed” cases. VTE rates reported in clinical studies regarding COVID-19 have substantially varied, with higher rates noted in early investigations and in studies that used VTE screening and lower rates reported in more recent investigations and in studies that did not screen for VTE [20]. Sadeghipour et al. did not use universal screening for VTE. Therefore, currently available data support the use of standard rather than escalated thromboprophylaxis dosing in critically ill patients with COVID-19 [20]. However, pending the publication of final results from the ATTACC, REMAP-CAP, and ACTIV-4a multi-platform trial confirming the interim report, escalated thromboprophylaxis could be appropriate in moderately ill hospitalized patients with COVID-19, while bearing in mind the need for balancing between known comorbidities and bleeding risk. Recent thromboprophylaxis guidelines (from CHEST and the American Society of Hematology) recommend a standard thromboprophylactic dose of LMWH over an intermediate dose in critically ill patients with COVID-19 [21,22].
Additional important questions regarding thromboprophylaxis in COVID-19 remain under investigation, including the utility of post-discharge thromboprophylaxis and the effect of outpatient thromboprophylaxis for patients with mild COVID-19, who do not require hospital admission [23,24].
2.4. Other main repurposed drugs
The RECOVERY trial was designed with the condition that drugs could be added or withdrawn, as evidence from various sources would come to light. Since November 2020, the trial had included an arm comparing colchicine to usual care alone. As part of a routine meeting on 4 March 2021, the Data Monitoring Committee of the trial reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone. The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine when compared to usual care alone among hospitalized patients with COVID-19, and therefore the recruitment for the colchicine arm has been stopped (20% vs 19%; RR, 1.02; 95% CI, 0.94 to 1.11; p = 0.63) [12].
Prior to stopping colchicine, WHO on 4 July 2020, accepted the recommendation from the Solidarity Trial's International Steering Committee to stop hydroxychloroquine and lopinavir/ritonavir arms. The Solidarity Trial was established by the WHO in an attempt to find an effective COVID-19 treatment for hospitalized patients. The International Steering Committee jumped to this recommendation based upon the evidence for hydroxychloroquine versus standard-of-care and for lopinavir/ritonavir versus standard-of-care from the Solidarity trial interim results, and from reviewing the evidence from all trials presented at the 1–2 July WHO Summit on COVID-19. The use of hydroxychloroquine and lopinavir/ritonavir resulted in little to no reduction in the mortality of hospitalized COVID-19 patients, when compared to patients receiving standard of care [12].
In the RECOVERY Trial, 7,763 patients were randomly assigned to either usual standard of care alone or usual standard of care plus azithromycin 500 mg once per day orally or intravenously for 10 days or until discharge. The primary outcome was 28-day all-cause mortality, assessed in the intention-to-treat population. No significant difference was noted in duration of hospital stay (median 10 days [IQR 5 to >28] vs 11 days [5 to >28]) or in the proportion of patients discharged from hospital alive within 28 days (rate ratio 1.04, 95% CI 0.98 to 1.10; p = 0.19). In addition, no significant difference was reported in the proportion meeting the composite endpoint of invasive mechanical ventilation or death (risk ratio 0.95, 95% CI 0.87 to 1.03; p = 0.24). Therefore, among patients admitted with COVID-19, azithromycin did not improve survival or other clinical outcomes and its use should be restricted to inpatients who have a clear antimicrobial indication [24]. Moreover, the PRINCIPLE Study, enrolled 2,120 outpatients in the UK, 500 patients in the azithromycin plus usual care group, 823 in the usual care alone group, and 797 in other intervention groups. The results of this study do not support the routine use of azithromycin for reducing time to recovery or risk of hospitalization among people with COVID-19 in the community [25]. Therefore, based on the RECOVERY and the PRINCIPLE Studies, which were both published in the Lancet, the routine use of azithromycin to outpatients as well as inpatients with COVID-19 is not recommended [24,25].
Early in the course of the pandemic, the potential efficacy of agents traditionally used as anthelminthics was examined for the treatment of SARS-Cov-2 infection. Ivermectin, an inexpensive agent used since the 1980s for the treatment of parasitic infections is known to exert a strong antiviral effect against RNA viruses in vitro. It was also shown to potently inhibit SARS-CoV-2 replication in Vero-hSLAM cell cultures [26]. In a subsequent small scale, randomized pilot study, early (within 72h of symptom onset) initiation of ivermectin among patients without risk factors for severe disease demonstrated no significant effects on viral loads and PCR positivity after 7 days, although active treatment receivers showed an earlier recovery from hyposmia/anosmia [27]. In a recently published single-center placebo-controlled randomized trial conducted among individuals with mild disease, a 5-day ivermectin course showed no substantial benefits on symptom duration compared with placebo [28]. In another randomized, single blind study in a population of severely ill COVID-19 patients, the addition of ivermectin for 5 days on a 3-agent treatment protocol consisting of hydroxychloroquine, favipiravir and azithromycin had a diminishing effect on CRP, D-dimer and ferritin levels after an additional 5 day period without significantly affecting mortality, the rate of clinical improvement or oxygenation indices [29]. Citing a current lack of concrete evidence to point towards a clear benefit of ivermectin for SARS-CoV-2 infection, both the EMA and the WHO have advised against its use outside the context of clinical trials for the time being. Niclosamide is another anthelminthic agent used against tapeworm infections with a proven in vitro antiviral activity against SARS coronavirus [30]. The effectiveness of niclosamide for various grades of COVID-19 infection severity is currently tested in phase 2 clinical trials (NCT04436458, NCT04753619, NCT04603924). Nitazoxanide is an FDA approved antiprotozoal drug with demonstrated in vitro and clinical activity against a broad viral spectrum, including multimodal actions against SARS-CoV-2 [31]. In a multicenter, randomized, double-blind, placebo-controlled trial among individuals with mild COVID-19, a 5-day course of nitazoxanide resulted in significantly lower viral loads at the end of treatment vs. placebo without a significant effect on symptom resolution, which was the primary endpoint [32]. This finding has been also ascertained in a still unpublished interim analysis of an ongoing study (NCT04463264) conducted in a similar population of patients with mild SARS-CoV-2 infection. Moreover, a number of phase 2 trials to examine the efficacy of nitazoxanide in various combinations and clinical settings (including post-exposure prophylaxis) are either ongoing or planned (NCT04459286, NCT04435314, NCT04360356).
3. Polyclonal and monoclonal antibody therapy: A bridge between vaccines and specific anti-viral treatment
3.1. Convalescent plasma
Convalescent plasma obtained from individuals who have recovered from COVID-19 can provide antibody-based passive immunity. Neutralizing antibodies are suggested to be the main active component, while other immune mediators in plasma could also play a role. Convalescent plasma containing high neutralizing antibody titers is thought to have clinical benefit when given early in the course of disease, and it may be of particular interest for individuals with deficits in antibody production, such as those receiving anti-CD20 therapies [33]. However, the available evidence does not support a clear role for convalescent plasma in patients with severe disease. In the USA, an EUA has been granted for high-titer convalescent plasma among hospitalized patients with COVID-19 early in the course of the disease or for those who have impaired humoral immunity [34]; nevertheless, because of the lack of significant clinical benefits until today, it should not be used outside the context of clinical trials. It is also being assessed among outpatients with non-severe COVID-19 and as post-exposure prophylaxis. The results of these trials are eagerly anticipated.
3.2. Monoclonal antibodies
Monoclonal antibodies, which target the S (Spike) protein of SARS-CoV-2 are currently being evaluated among outpatients with mild to moderate disease with very encouraging results [[35], [36], [37], [38]]. In the USA, the combinations of bamlanivimab-etesevimab and carisivimab-imdevimab are available with EUA by the FDA, while in Europe the EMA has also approved the abovementioned monoclonal antibodies combinations for selected outpatients at risk for severe disease. These risk factors for patients ≥18 years are any of the following:
-
•
Body mass index (BMI) ≥35 kg/m2
-
•
Chronic kidney disease
-
•
Diabetes mellitus
-
•
Immunosuppression
-
•
≥65 years
-
•
≥55 years presenting underlying cardiovascular disease, and/or hypertension, and/or chronic respiratory disease.
These combinations of monoclonal antibodies must be administered as a single intravenous dose as soon as possible after a positive SARS-CoV-2 test, preferably within 3–7 days, but no longer than 10 days after the onset of symptoms [[35], [36], [37], [38]].
In preliminary results of a Phase 3 study of BLAZE-1, 1,035 non-hospitalized adults with mild to moderate COVID-19 with risk factors for severe disease received a single dose of bamlanivimab-etesevimab, 2800 mg of each or placebo [35]. At one month, rates of hospitalization or death were lower among those who received the combination of bamlanivimab-etesevimab, when compared with placebo (2% vs 5% and 0% vs 2%, respectively). All 10 deaths occurred among patients in the placebo group. Nausea and infusion-related adverse effects, such as pruritus, fever and rash were reported with the administration of bamlanivimab-etesevimab but were uncommon and mild.
This phase 3 trial results were based upon the administration of 2800 mg dose of each antibody, although the EUA dose for combination treatment is 700 mg of bamlanivimab and 1400 mg of etesevimab. The reduced dosage is based upon virologic and clinical data in conjunction with pharmacokinetic models, suggesting that the lower dose is expected to have a similar clinical efficacy to the 2800 mg combined dosage [35].
SARS-CoV-2 variants, in particular those with mutations involving the S protein, may affect the clinical efficacy of monoclonal antibody treatment [[35], [36], [37], [38]]. In fact, based on the increasing prevalence of SARS-CoV-2 variants resistant to bamlanivimab, the FDA has banned monotherapy with bamlanivimab, which should only be administered in combination with etesevimab [[35], [36], [37], [38]]. Further clinical studies among patients affected by SARS-CoV-2 variants are needed in order to determine the efficacy of these therapies in the context of the evolution of SARS-CoV-2 variants.
Regarding casirivimab-imdevimab combination, in an unpublished study including 799 non-hospitalized adults with mild to moderate COVID-19, the combination of the two monoclonal antibodies, at two different doses, i.e. 2400 and 8000 mg total doses, was compared to placebo [38]. In preliminary results, both doses reduced viral loads, when compared to placebo. At 28 days, the rate of visits to the Emergency Department (ED) or hospitalization was lower with casirivimab-imdevimab (3% in the combination group vs 9% in the placebo group). Among those receiving casirivimab-imdevimab, infusion-related reactions of mild to moderate severity were infrequently reported, such as fever, urticaria, pruritus, abdominal pain, and flushing. A single episode of anaphylaxis was also noted. The combination of casirivimab-imdevimab may also be administered subcutaneously and has been demonstrated to reduce the risk of symptomatic SARS-CoV-2 infection by 81%. This phase 3 prevention trial enrolled 1,505 individuals, who lived in the same household with individuals infected with SARS-CoV-2 and who received the combination at a dose of 1200 mg subcutaneously [39]. These results are very encouraging and suggestive of the potential of the combination of monoclonal antibodies, which could serve as a bridge between the vaccination and the specific anti-viral treatment of SARS-CoV-2 infection. Notably, supplies of monoclonal antibodies are still limited, expensive and their administration requires supporting infrastructure, such as infusion centers, which is a barrier in many countries, for the time being. Nevertheless, the subcutaneous administration of casirivimab-imdevimab seems to facilitate this process.
4. Specific anti-viral treatment
4.1. Life cycle of SARS-CoV-2
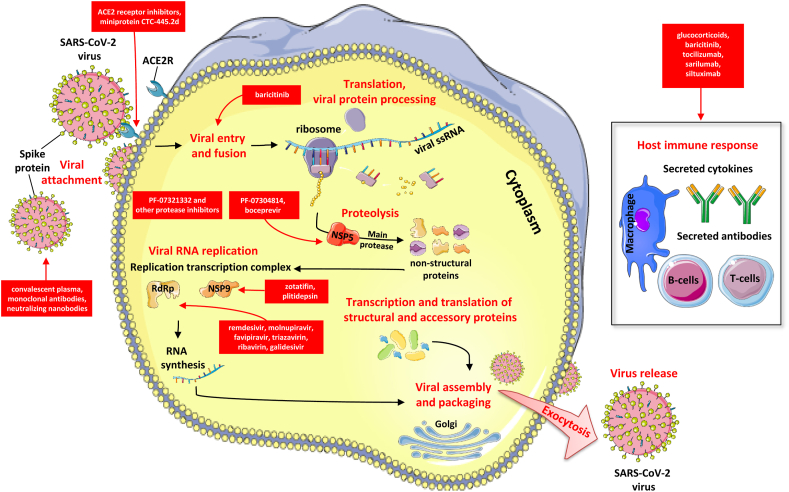
SARS-CoV-2 is a betacoronavirus with a viral genome encoding for 29 viral proteins, categorized in three main classes: structural proteins that comprise the outer coat; non-structural proteins (NSPs), which help the virus to replicate; and several accessory proteins, which appear to interfere with the host's immune response. Thus far, drug hunters have aimed mainly at the structural and replication proteins, concentrating on molecules similar to those that have been already effective in fighting other viruses. SARS-CoV-2 has just four structural proteins. The envelope and membrane proteins make up the viral spherical shell, and the nucleocapsid protein shields its genome. The fourth protein, spike or S protein, protrudes from the shell, creating the crown of thorns that gives the virus its name (coronavirus) and enables it to bind to the angiotensin-converting enzyme 2 (ACE2) receptors, its main entry point necessary for membrane fusion and introduction of SARS-CoV-2 into the host cell [40,41]. Fig. 1 depicts the life cycle of SARS-CoV-2 in the host cell as well as potential anti-viral agents and immunomodulators.
Figure 1.
Life cycle of SARS-CoV-2 and potential anti-viral agents.
SARS-CoV-2 infects host cells, reproduces itself and spreads depending on a variety of viral and host proteins. Key steps of the SARS-CoV-2 life cycle inhibited by current treatment options as well as promising antiviral agents that are currently being examined, represent attractive antiviral targets and are highlighted in red. These include ACE2 receptor inhibitors, ACE2 mimic compounds, fusion inhibitors, several protease inhibitors, RNA polymerase inhibitors, passive immunization with convalescent plasma, monoclonal antibodies or nanobodies. Furthermore, immunomodulators such as corticosteroids, a selective Janus kinase 1 and 2 inhibitor (baricitinib), and various agents targeting the interleukin-6 pathway are presented.
4.2. Potential drugs based on the SARS-CoV-2 life cycle
As ACE2 is necessary for the entry of the virus into the host cell, scientists are designing ACE2 structural analogs to serve as decoys, thus, drawing SARS-CoV-2 away from the host cell. For example, researchers at Neoleukin Therapeutics and their partners, have reported the development of a miniprotein, CTC-445.2d, which mimics ACE2, binding firmly to S protein. The compound protected human cells from infection in vitro. When administered to Syrian hamsters as a nasal spray, this decoy also prevented severe disease after having received a normally lethal load of SARS-CoV-2 [42]. Although these results are really intriguing, there is a long way ahead for these decoys to be used as anti-viral drugs in humans.
Protease inhibitors bind to a viral enzyme, which is known as a protease, thus, preventing the virus from replicating in the host cell. Protease inhibitors have already been effectively used for the treatment of infections caused by other viral pathogens, such as HIV and hepatitis C virus. Currently marketed therapeutics that target viral proteases are not generally associated with toxicity and therefore, these substances may represent well-tolerated treatment options against COVID-19 [40].
Pfizer scientists had developed a drug to target NSP5, a protease specific to SARS-CoV-2 as well as other coronaviruses. The drug was developed in 2003 to block the NSP5, also known as Main protease (Mpro), in patients with severe acute respiratory syndrome (SARS), the coronavirus infection that emerged in 2002. That work was set aside when the SARS pandemic subsided. A newfound interest in this compound revealed that it may hinder the reproduction of SARS-CoV-2 inside human cells. Pfizer researchers have slightly changed its structure to make a more soluble version, known as PF-07304814. This compound has significantly reduced viral load in mice, while in other animals, increased concentrations of the drug could reach several tissues. In September 2020, Pfizer launched a clinical trial to test the safety of PF-07304814, delivered intravenously, which is currently in a Phase 1b multi-dose trial in hospitalized patients with COVID-19 [40].
On 23 March 2021, Pfizer announced that it is progressing to multiple ascending doses after completing the dosing of single ascending dose in a Phase 1 study among healthy adults in order to evaluate the safety and tolerability of an investigational, novel oral antiviral drug for SARS-CoV-2, which is being conducted in the USA. The oral antiviral clinical candidate PF-07321332, which is a SARS-CoV-2-3CL protease inhibitor, has demonstrated in vitro anti-viral activity against SARS-CoV-2. The results of studies regarding PF-07321332 are eagerly anticipated in the near future [40].
Apart from remdesivir, there has been growing optimism about other nucleoside analogs. Molnupiravir, a nucleoside analog that can be taken orally, was originally developed to combat influenza. It has been documented that molnupiravir integrates into RNA strands in place of the nucleoside cytidine, thus, initiating errors in the copying process and causing a lethal series of mutations to the virus. That mechanism has raised doubts about the drug causing similar mutations in host cells. Nevertheless, such phenomena have not been reported so far in animal studies. Molnupiravir has been demonstrated to reduce replication of multiple coronaviruses, including SARS-CoV-2, when used in mice, while it led to significantly reduced SARSCoV-2 replication in human airway epithelial cells [42]. Wahl et al. only recently have shown that this compound decreased viral replication 100,000-fold in mice engineered to have human lung tissue [43]. In the meantime, Cox et al. reported that molnupiravir might do more than just prevent symptoms. More specifically, after the drug was administered to ferrets, which readily spread the coronavirus, transmission levels diminished within 24 h. They concluded that this was the first demonstration of an orally available drug to block SARS-CoV-2 transmission, which could play a key role in slowing the spread of SARS-CoV-2 [44]. Overall, molnupiravir may be administered per os and early in the disease course, when SARS-CoV-2 replication typically peaks, in contrast to injectable drugs and seems to be well tolerated, with no serious adverse effects in healthy volunteers. Molnupiravir is now in Phase 2/3 clinical trial run by Merck and Ridgeback Biotherapeutics, with the results being anticipated in September or October 2021. A trial of molnupiravir for post-exposure prophylaxis is also scheduled for the second half of 2021, according to the manufacturers [45].
Many researchers have been working on Mpro inhibitors, worldwide. In September 2020, researchers in China reported on two drugs, boceprevir, a hepatitis C drug, and GC376, which was designed to target a feline coronavirus. Both substances have been demonstrated to slow SARS-CoV-2 replication in cells. Up to date, 807 molecules have been designed to specifically inhibit SARS-CoV-2's Mpro but remain at a much earlier research stage [40].
Researchers are optimistic about other re-purposed nucleoside and nucleotide analogs, which work by “misleading” the coronavirus’ RNA-dependent RNA-polymerase (RdRp). The most known candidates are favipiravir and triazavirin, which were originally designed against flu viruses; ribavirin, a drug for respiratory syncytial virus and hepatitis C; and galidesivir, which may be active against Ebola, Zika, and yellow fever viruses [40].
Additionally, there have been attempts to block other replication transcription complex (RTC) proteins. Two compounds, called zotatifin and plitidepsin, appear to halt viral replication by interfering with NSP9, the RNA-grabbing enzyme. Plitidepsin is currently in a phase 2/3 trial by the Spanish pharma company PharmaMar [40].
Overall, there is growing interest in anti-viral drugs against SARS-CoV-2 with mounting evidence suggesting a promising efficacy for certain agents.
5. What is next? Nanobodies
New therapies effective against SARS-CoV-2 variants also offering an alternative to intravenously administered monoclonal antibody drugs are much anticipated [46]. In this context, the results of a very recent study by Koenig et al. on camelid-derived, single-domain antibodies or nanobodies are really intriguing. The researchers immunized alpacas and llamas with SARS-CoV-2 S protein and identified nanobodies that specifically bind to the receptor-binding domain of the virus. They characterized four neutralizing nanobodies (namely E, U, V, and W) based upon their structure and functions. The nanobodies neutralize the virus by inducing a premature structural transition from a pre-fusion conformation to an irreversible post-fusion conformation, the latter of which cannot bind to ACE2 and thus, makes SARS-CoV-2 incapable of inducing membrane fusion and entering the host cell. Koenig et al. then progressed to make biparatopic nanobodies, i.e., nanobodies which possess two antigen-binding sites in one molecule, by fusing nanobodies targeting distinct epitope regions (e.g., E + V, V + E, E + W, and W + E) [47].
Due to their relatively small size, nanobodies possess favorable biophysical potential and are cheaper and easier to produce, when compared to the standard monoclonal antibodies. Their small size and their long, heavy-chain regions make them capable of targeting specific epitopes, such as the receptor-binding site of the S protein. Nanobodies can be manufactured with the use of prokaryotic organisms (e.g., from bacteria or yeast) because they lack the glycan-harboring Fc domain, making them easier to make than the standard monoclonal antibodies. The absence of the Fc region diminishes the risk of antibody-dependent enhancement of infection, but it also shortens the half-life of these molecules, a disadvantage that could be overcome by attaching them to polyethylene glycol or human albumin. In addition, nanobodies are capable of being nebulized and delivered directly to the lung tissue of COVID-19 patients via inhalation, thus presenting an attractive alternative to the intravenously administered monoclonal antibodies. Aerosol formulation of nanobodies has already shown some promising non-clinical results [47].
6. Conclusion
Despite the fact that there is currently no specific treatment for SARS-CoV-2, there are meticulous efforts towards developing new and promising compounds to combat SARS-CoV-2. Although there is a true and seemingly efficacious rally for the development of vaccines, additional focus should be given to the development and testing of anti-viral drugs, which could play a life-saving role during this pandemic. Apart from the efficacy and safety of candidate agents across the spectrum of COVID-19 severity, additional questions to be addressed include their potential utility as post-exposure or even pre-exposure prophylaxis (e.g. in areas with a high prevalence of viral variants, whereby the efficacy of available vaccines is still uncertain). Another issue to be examined would be their potential value in treatment schemes with multiple drugs of complementary modes of action, especially in the severely ill. The COVID R&D Alliance, a consortium of more than twenty life-science companies worldwide is planning to create an organization that will accelerate the development of drugs against coronaviruses. The Alliance is aiming to prepare twenty-five candidate drugs for trials in humans. Together with another global project, known as the Rapidly Emerging Antiviral Drug Development Initiative, they are in the process of raising funds from industry and governments. The US National Institutes of Health is also planning to invest in developing drugs to fight SARS-CoV-2. It is high time for the development of anti-viral drugs, following the rapid and successful paradigm of vaccine emergence against SARS-CoV-2.
Declaration of competing interest
No conflict of interest to disclose.
Contributor Information
Natalia G. Vallianou, Email: natalia.vallianou@hotmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
Abbreviations
- ACE2R
Angiotensin Converting Enzyme 2 Receptor
- RdRp
RNA-dependent RNA-polymerase
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- ssRNA
single-stranded Ribonucleic Acid
(All images are derived from the free medical site http://smart.servier.com/by Servier licensed under a Creative Commons Attribution 3.0 Unported License).
Financial support
None.
References
- 1.reportWHO Report. Coronavirus disease (COVID-19) pandemic. (Assessed on May 15, 2021).
- 2.Dalamaga M., Karampela I., Mantzoros C. Commentary: could iron chelators prove to be useful as an adjunct to COVID-19 Treatment Regimens? Metabolism. 2020;108:154260. doi: 10.1016/j.metabol.2020.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalamaga M., Karampela I., Mantzoros C. Commentary: phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism. 2020;109 doi: 10.1016/j.metabol.2020.154282. 154282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karampela I., Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res. 2020;51(7) doi: 10.1016/j.arcmed.2020.06.004. 741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kritis P., Karampela I., Kokoris S., Dalamaga M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabolism Open. 2020;8:100066. doi: 10.1016/j.metop.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallianou N., Evangelopoulos A., Kounatidis D., Stratigou T., Christodoulatos G.S., Karampela I., Dalamaga M. Diabetes mellitus and SARS-CoV-2 infection: pathophysiologic mechanisms and implications in management. Curr Diabetes Rev. 2020 doi: 10.2174/1573399817666210101110253. [DOI] [PubMed] [Google Scholar]
- 7.Dalamaga M., Christodoulatos G.S., Karampela I., Vallianou N., Apovian C.M. Understanding the Co-epidemic of obesity and COVID-19: current evidence, comparison with previous epidemics, mechanisms, and preventive and therapeutic perspectives. Curr Obes Rep. 2021:1–30. doi: 10.1007/s13679-021-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus disease 2019 (COVID-2019) management in hospitalized adults. 2021. www.uptodate.com [Google Scholar]
- 9.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A., Zingman B., Kalil A. ACTT-1 study group members. Remdesivir for the treatment of covid-19-final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. 10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nissen C.B., Sciascia S., Andrade D., Atsumi T., Bruce I.N., Cron R.Q. The role of antirheumatics in patients with COVID-19. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00062-X. Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalil A., Patterson T., Mehta A., Tomaschek K., Wolfe C., Ghazarian V. Baricitinib plus remdesivir for hospitalized patients with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stebbing J., Sánchez Nievas G., Falcone M. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1) doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosas I., Brau N., Waters M., Go R., Hunter B., Bhagani S. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone J.H., Frigault M.J., Serling-Boyd N.J. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383:2333. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermine O., Mariette X., Tharaux P.L. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the Inspiration randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Samkari H. Finding the optimal thromboprophylaxis dose in patients with COVID-19. JAMA. 2021;325(16):1613–1615. doi: 10.1001/jama.2021.4295. [DOI] [PubMed] [Google Scholar]
- 22.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert Panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–878. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY). A randomized, controlled, open-label platform trial. Lancet. 2021;397:605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The PRINCIPLE Trial Collaboration Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomized, controlled, open-label adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;78:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaccour C., Casellas A., Blanco-Di Matteo A., Pineda I., Fernandez-Montero A., Ruiz-Castillo P., Mary-Ann Richardson M.A. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32:100720. doi: 10.1016/j.eclinm.2020.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eduardo López-Medina E., Pío López P., Isabel C., Hurtado I.C., Diana M., Dávalos D.M., Oscar Ramirez O., Ernesto Martíne E. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okumuş N., Demirtürk N., Çetinkaya R.A., Güner R., Avcı I.Y., Orhan S. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 2004;48(7):2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lokhande A.S., Devarajan P.V. A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19. Eur J Pharmacol. 2021;891:173748. doi: 10.1016/j.ejphar.2020.173748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocco P.R.M., Silva P.L., Cruz F.F., Antonio Marco, Tierno P., Moura M.A. Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021:2003725. doi: 10.1183/13993003.03725-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. BioRxiv. 2020 doi: 10.1101/2020.12.28.424451. 12.28.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark E., Guilpain P., Filip I.L. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol. 2020;190:e154. doi: 10.1111/bjh.16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19?
- 36.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-uthorizes-monoclonal-antibodies-treatment-covid-19
- 37.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-uthorizes-monoclonal-antibodies-treatment-covid-19-0?
- 38.The COVID-19 treatment guidelines panel's statement on the emergency use authorization of anti-SARS-CoV-2 monoclonal antibodies for the treatment of COVID-19. https://www.covid19treatmentguidelines.nih.gov/statement-on-anti-sars-cov-2-monoclonal-antibodies-eua
- 39.https://investor.regeneron.com/news-releases/news-release-details/phase-3-prevention-trial-showed-81-reduced-risk-symptomatic-sars
- 40.Service R. A call to arms. Science. 2021;371(6534):1092–1095. doi: 10.1126/science.371.6534.1092. [DOI] [PubMed] [Google Scholar]
- 41.Collins S. Covid-19 lessons for research. Science. 2021;371(6534):1081. doi: 10.1126/science.abh3996. [DOI] [PubMed] [Google Scholar]
- 42.Linsky T.W., Vergara R., Codina N., Nelson J.W., Walker M.J., Su W. De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2. Science. 2020;370:1208–1214. doi: 10.1126/science.abe0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.E. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;571(7850):451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6(1):11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.https://merckcovidresearch.com
- 46.Sasisekharan R. Preparing for the future-Nanobodies for COVID-19? N Engl J Med. 2021;84(16):1568–1571. doi: 10.1056/NEJMcibr2101205. [DOI] [PubMed] [Google Scholar]
- 47.Koenig P.A., Das H., Liu H., Kummerer B.M., Gohg F.N., Jenster L.M. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371(6530) doi: 10.1126/science.abe6230. [DOI] [PMC free article] [PubMed] [Google Scholar]