Abstract
Purpose
It is unclear what kind of modifiable lifestyle factors are associated with long-time weight gain in adulthood. To clarify the lifestyle behavior related to body weight gain since the age of 20 years, we explored the lifestyle risk factor, independently associated with excessive weight gain after 20 years of age as compared to those in subjects with a stable weight, with matching of age, gender, and the current body mass index (BMI).
Patients and Methods
From baseline data of a general population-based cohort study, we designed a cross-sectional analysis collecting individual data of medical health check-ups and a questionnaire related to lifestyle, including amount of sleep, frequency of eating breakfast, average times per day engaged in walking and sitting in the prior year, and smoking habits. These data were compared between the subjects with weight gain ≥10kg (n=3601) and <10kg (n=3601) after age 20, matched by a propensity score model which included current BMI, age and gender. We used multivariable logistic regressions to assess the lifestyle factor’s association with high weight gain.
Results
Participants who gained ≥10 kg were significantly more likely to sleep <5 hours or ≥9 hours per night, skip breakfast, engage in walking <1 hour per day, and sit ≥5 hours per day than those who gained <10kg. Multivariable logistic regressions analyses showed that, with adjusting for potential confounder, the lifestyles with the positive association with high weight gain were skipping breakfast (OR 1.252; 95% CI 1.053–1.489, vs regularly), long sleeping duration (9 hours/day≤ OR 1.613; 95% CI 1.018–2.557 vs 5≤-<7 hours/day), and former smoker (OR 1.163; 95% CI 1.008–1.343 vs never smoker), while walking duration was negatively associated with high weight gain. Furthermore, despite similar current BMI, participants with weight gain ≥10kg had significantly higher values for waist circumference, blood pressure, HbA1c, LDL-C, triglycerides, and hepatic enzyme levels than those with weight gain <10kg. Similarly, the prevalence rates of hypertension, dyslipidemia, metabolic syndrome (MetS), and former smoker were higher in the participants with weight gain ≥10kg.
Conclusion
Major weight gain after 20 years of age was associated with unfavorable lifestyle factors and greater waist circumference, possibly leading to elevated risk for MetS and other non-communicable diseases. These findings highlight the importance of maintaining both weight at age 20 and a favorable lifestyle throughout adulthood.
Keywords: metabolic syndrome, physical activity, skipping breakfast, sleep duration, waist circumference, weight gain
Introduction
Rising rates of obesity have become a major public health concern worldwide,1 including in Japan,2 over the past 40 years. According to annual public reports by the National Nutrition Survey in Japan,3 the prevalence of obesity, which was defined as a body mass index (BMI) over 25, has consistently risen and remained elevated over the second decade of the 21st century in middle-aged and older individuals. Although the grade of obesity is obviously mild in East Asian populations as compared to that in Western countries, even modest body weight gain has an enormous impact on the development of metabolic disorders.4 In addition, Japanese subjects have a higher visceral fat area relative to abdominal subcutaneous fat area than Caucasians,5 which partially accounts for their greater predisposition to type 2 diabetes despite having only mildly elevated BMI.
Fat mass is thought to be determined by the number of adipocytes and fat storage capacity. Adipocytes are generated mainly during childhood when the total number of adipocytes is set and then remains constant after reaching maturity.6 Therefore, pediatric adiposity is a critical issue because it carries over into adult obesity, which is strongly associated with type 2 diabetes, hypertension, and atherosclerosis.
On the other hand, weight gain generally tends to accrue in middle age regardless of race or gender.7 Long-term weight gain during adulthood has been linked to increased risks of metabolic syndrome (MetS),8 chronic kidney disease,9 hypertension,10 non-alcoholic fatty liver disease (NAFLD),11 sleep disturbance,12 and dyslipidemia,13 independently of the peak BMI level reached. These widespread findings are in agreement with the results of studies conducted in Asian populations.14 Taking the reported observations together, body weight gain in both childhood and adulthood shows strong involvement in the development of obesity-related health problems.
There is a consensus that high weight gain in adulthood is related to higher risks for various forms of metabolic disease. Understanding underlying factors regarding weight development is needed to help control the obesity pandemic. However, the contribution of modifiable risk factors, such as physical activity, smoking status, and sleeping duration, to long-term weight gain during adulthood is less clear in an Asian country. Therefore, we aimed to investigate the association between lifestyle factors and high weight gain since age of 20 years. We thus compared, cross-sectionally, subjects who had gained a significant amount of weight after age 20 to a control group who had maintained relatively constant body weights, matching the two groups for age, gender, and current BMI, employing a Japanese general population-based cohort.15
Patients and Methods
Ethical Aspects
The present study was approved by the ethics committee of the Iwate Medical University School of Medicine (HG25-2), and written informed consent was obtained from all enrolled subjects. Moreover, all procedures were in accordance with the Declaration of Helsinki.
Study Population
The Tohoku Medical Megabank (TMM) was launched to promote creative solutions to medical problems encountered in the aftermath of The Great East Japan Earthquake. The TMM thus initiated a general population-based adult cohort study named the TMM Community-Based Cohort Study (TMM Comm Cohort Study).15 We asked participants to join our cohort in health check-up sites. After providing informed consent, the participants were handed a questionnaire for self-administration, inquiring comprehensive information regarding demographics, education, alcohol consumption, smoking, sleeping, exercise, medication. Then, the participants complete the questionnaire at home and send it back within 2 weeks. We asked the municipality to provide data of blood and urine tests measured during the health check-ups for participants. The inclusion criteria were persons aging 20 years or over. Individuals were excluded if they did not consent to participate in the study and/or if they were not able to complete the questionnaire. As the TMM Comm Cohort study, we recruited 87,865 participants from July 2013 to March 2016 from Miyagi and Iwate Prefecture.16 For the current study, we used the data of those living in Iwate prefecture (n = 32,675). We designed a cross-sectional study using baseline data of this cohort study, including medical health check-ups and a questionnaire related to lifestyle. Participants were excluded from this study if they had a self-reported past history of any malignant or primary wasting disorder, and they were under 40 years of age. Participants underwent physical measurements, biological specimens were collected, and they completed questionnaires.
Cohort Data Collection
At the health check-up site, the physical measurements for anthropometric data, including height, weight (dressed in light clothing), and waist circumference, were obtained by trained personnel. Waist circumference was measured once to the nearest 0.1 cm using a flexible metric measuring tape over bare skin with the participants in a standing position. Blood pressure was measured twice on the right arm with the participant in a seated position, using an electronic BP monitor. We have used the average of the two blood pressure measurements in this study. Blood specimens and random spot urine samples were collected for biochemical analyses. Blood glucose levels were determined by the hexokinase method. The HbA1c levels were measured using high-performance liquid chromatography (HPLC) method. Other laboratory parameters, such as triglyceride, were measured by standard enzymatic methods.
The individual medical data and anthropometric data at health check-ups and recent lifestyle and sociodemographic factors collected by questionnaire were obtained at the recruitment point and compared between subjects with weight gain ≥10kg and <10kg.
Hypertension was defined as systolic blood pressure (BP) ≥140mmHg and/or diastolic BP ≥90mmHg, and/or the use of antihypertensive medication. Diabetes was defined as glycated hemoglobin (HbA1c) value ≥ 6.5%, a fasting plasma glucose ≥126mg/dL or non-fasting glucose concentration ≥200mg/dL, and/or a prior diagnosis of diabetes, and/or undergoing treatment with antidiabetic drugs, including insulin. Type 2 diabetes could be diagnosed only after excluding self-reported type 1 diabetes. Dyslipidemia was defined as LDL-C ≥140mg/dL and/or triglyceride ≥150mg/dL and/or HDL-C <40mg/dL, a prior diagnosis of dyslipidemia, and/or the use of antihyperlipidemic medication. Hyperuricemia was defined as uric acid (UA) ≥7.0mg/dL, and/or a prior diagnosis of hyperuricemia, and/or undergoing treatment with antihyperuricemic agents. CKD was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and/or urinary albumin-to-creatinine ratio ≥30 mg/gCr. MetS as defined by the diagnostic criteria in Japan17 is a waist circumference of ≥85 cm in men and ≥90 cm in women, which is equivalent to a visceral fat area of ≥100 cm2, in addition to at least two of the following conditions: dyslipidemia (triglycerides ≥150 mg/dL or HDL-C < 40 mg/dL) or current lipid-lowering medications, high blood pressure (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg) or current antihypertensive medications, and hyperglycemia (fasting glucose ≥110 mg/dL) or current medications for diabetes mellitus.
Definition of Weight Change
The altered body shape is usually defined by either weight change in kilograms (kg) or BMI change. Since height gradually decreases with advancing age, BMI might be overestimated in the elderly population.18 Thus, in order to capture increases in fat mass more precisely, we measured weight change in kg from 20 years of age.19 We used self-reported body weight at 20 years of age and that at the time of the TMM medical check-up to calculate weight change in kg. Weight change was categorized into weight gain ≥10 kg and weight gain <10kg from 20 years of age to the timing of surveillance.
Questionnaire on Lifestyle and Sociodemographic Factor
The questionnaire included the following questions on daily lifestyle, including amount of sleep (categorized as <5 hours/day, 5–6 hours/day, 6–7 hours/day, 7–8 hours/day, 8–9 hours/day, >9 hours/day), frequency of eating breakfast (less than once/month, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, every day), and average times per day engaged in walking and sitting in the prior year (categorized as never, <30 minutes/day, 30–60 minutes/day, 1–3 hours/day, 3–5 hours/day, 5–7 hours/day, 7–9 hours/day, 9–11 hours/day, >11 hours/day). Smoking habits (never, former, current) were also collected by questionnaire. Subjects who reported eating breakfast every day, or almost daily, were defined as eating breakfast regularly, and those who reported eating a morning meal less than 5–6 times/week were categorized as skipping breakfast. The information on sociodemographic factors, such as marital status, formal education level, and employment status, was obtained by the questionnaire as well. Formal education level was classified as: completed elementary/middle school, completed high school, and completed university/college. Marital status was classified into four groups as married, single, divorced, and widowed. Employment status was categorized as having a paid employment or not.
Statistical Analysis
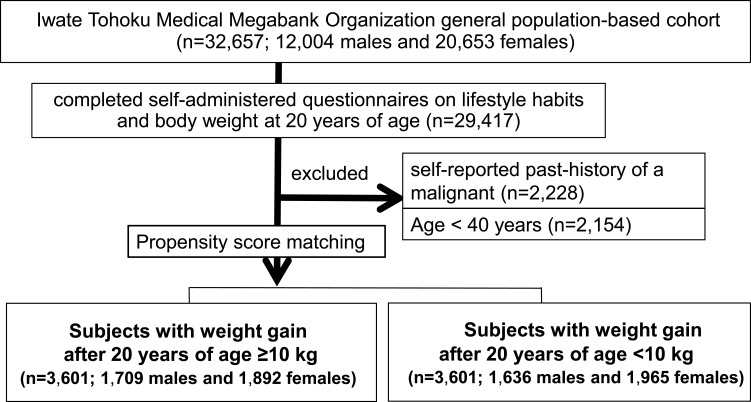
In order to minimize selection bias, a propensity score (PS) matching analysis was applied to those who gained ≥10kg and <10kg after 20 years of age. As current BMI was often higher in the “high weight gain” than in the “low weight gain” group, predictors matched in the PS model included BMI at the time of this study as well as age and gender, in order to precisely distinguish the effects of weight gain in adulthood. PS matching uses a nearest-neighbor algorithm to match weight gain ≥10kg with weight gain <10kg after 20 years of age, at 1:1, in the final dataset. For matching, the logit of the PS was used, with a caliper distance of less than 0.1 standard deviations. After the PS matching analysis, 3601 subjects (1709 males and 1892 females) who gained ≥10kg after 20 years of age were matched with 3601 subjects (1636 males and 1965 females) who gained <10kg, with a total of 7202 cohort subjects finally being included in this study (Figure 1).
Figure 1.
Study recruitment diagram. Patient selection for evaluating associations between lifestyle habits and adverse health outcomes in propensity score (PS)-matched subjects who gained ≥10 kg versus <10 kg after age 20 years is summarized in the study flowchart. The PS-matched analysis was conducted employing the following covariates: age, sex, and BMI at the time of the study. We used 0.1 times the pooled standard deviation of the logit of the PS as the caliper width for the PS matching.
Continuous variables were expressed as medians (25th–75th percentiles). Categorical variables were expressed as numbers and percentages. The characteristics and prevalence of major health outcomes of weight change were assessed using the chi-square test for categorical variables and the Mann–Whitney U-test for continuous variables. Adjusted standardized residuals were used to determine which groups presented significant differences in a cross table, enabling the significance of each criterion to be calculated independently. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the strength of associations between weight gain since age 20 and adverse health outcomes. The Mantel–Haenszel method was used to adjust for smoking status. Moreover, we used multivariable logistic regressions to assess the associations of lifestyle factors with marked weight gain. A P value <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS version 25 (SPSS Japan Inc., Tokyo, Japan).
Results
Table 1 shows the characteristics of study participants, categorized by weight gain ≥10kg and <10kg from 20 years of age to the timing of surveillance. As a result of PS matching, values of BMI at the time of this study, age, and gender were similar in the two groups. The median BMI at age 20 was 20.4 kg/m2 in the subjects with weight gain ≥10kg after 20 years of age, significantly smaller than the 23.8 kg/m2 in those with weight gain <10kg (p<0.001). In other words, those who gained <10kg were somewhat heavier in adolescence. Participants who gained ≥10kg had significantly higher values for waist circumference, systolic BP, diastolic BP, LDL-C, triglycerides, HbA1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and UA than those who gained <10kg. In contrast, the values for HDL-C, glycated albumin, and eGFR in the subjects with weight gain ≥10kg were significantly lower than in those who gained <10kg (Table 1, Supplemental Table 1). In addition, sociodemographic factors, including educational level, employment status, and marital status, are demonstrated in Table 1. Chi-square test and adjusted standardized residuals statistical tests revealed that the subjects with weight gain≥10 kg are more likely to complete high school, less likely to have a paid employment, and less likely to be single than the subjects with weight gain <10kg (p<0.05).
Table 1.
Characteristics of the Study Participants
| Weight Gain After 20 Years of Age | P value* | ||
|---|---|---|---|
| <10kg (n=3601) | ≥ 10kg (n=3601) | ||
| Gender (male/female) | 1709/1892 | 1636/1965 | |
| Age (years) | 64 (55–69) | 63 (55–67) | |
| BMI at the time of the study (kg/m2) | 25.9 (24.5–27.2) | 25.8 (24.5–27.3) | |
| BMI at 20 years of age (kg/m2) | 23.8 (22.4–25.6) | 20.4 (19.2–21.7) | <0.001 |
| Waist circumference (cm) | 87.3 (83.5–91.7) | 89.2 (85.4–93.5) | <0.001 |
| Systolic blood pressure (mmHg) | 129 (119–141) | 130 (120–143) | 0.007 |
| Diastolic blood pressure (mmHg) | 77 (70–84) | 78 (71–84) | 0.016 |
| Hypertension, n (%) | 1959 (54.4) | 2085 (57.9) | 0.003 |
| Dyslipidemia, n (%) | 2151 (59.7) | 2405 (66.8) | <0.001 |
| Type 2 Diabetes, n (%) | 653 (18.1) | 713 (19.8) | 0.071 |
| Chronic kidney disease, n (%) | 794 (22.0) | 830 (23.0) | 0.310 |
| Hyperuricemia, n (%) | 443 (12.3) | 527 (14.6) | 0.004 |
| Metabolic syndrome, n (%) | 755 (21.0) | 977 (27.1) | <0.001 |
| Stroke, n (%) | 79 (2.2) | 80 (2.2) | 0.936 |
| Coronary heart disease, n (%) | 97 (2.7) | 95 (2.6) | 0.884 |
| Aneurysm of aorta, n (%) | 22 (0.6) | 25 (0.7) | 0.661 |
| Chronic heart failure, n (%) | 13 (0.4) | 18 (0.5) | 0.368 |
| Marital status, n (%) | 0.007 | ||
| Single | 316 (8.9) | 263 (7.4)# | |
| Divorced | 137 (3.9) | 171 (4.8) | |
| Living with a spouse | 2835 (79.8) | 2827 (79.2) | |
| Widowed | 265 (7.5) | 310 (8.7) | |
| Education level, n (%) | <0.001 | ||
| Completed elementary/middle school | 1124 (31.6) | 931 (26.2)## | |
| Completed high school | 1514 (42.6) | 1652 (46.6)## | |
| Completes university/college | 916 (25.8) | 965 (27.2) | |
| Employment, n (%) | 2062 (58.2) | 1970 (55.5) | 0.022 |
Notes: *Mann–Whitney U-test or chi-square test. Median values are shown (25th percentile-75th percentiles). #Adjusted residual >|1.96|, ##adjusted residual>|2.58|.
Abbreviation: BMI, body mass index.
The non-communicable disease prevalence in each group is also presented in Table 1. Weight gain ≥10kg after 20 years of age was associated with a significantly increased OR for hypertension (OR 1.153; 95% CI 1.050–1.265; P<0.05, Mantel–Haenszel common OR estimate; 1.145; 95% CI; 1.043–1.257; p=0.005), dyslipidemia (OR 1.356; 95% CI 1.231–1.492; P<0.05, Mantel–Haenszel common OR estimate; 1.359; 95% CI; 1.234–1.496; P<0.001), hyperuricemia (OR 1.222; 95% CI 1.067–1.400; P<0.05, Mantel–Haenszel common OR estimate; 1.200; 95% CI 1045–1.379; P=0.011), and MetS (OR 1.404; 95% CI 1.259–1.565; P<0.05, Mantel–Haenszel common OR estimate; 1.396; 95% CI 1.251–1.558; P<0.001) as compared with weight gain <10kg. The prevalence of cardiovascular events was similar between two groups.
The number of factors comprising the MetS differed significantly, as analyzed employing the chi-square test, between the two groups (Table 2). The analyses conducted using the adjusted standardized residuals revealed that the participants who gained ≥10kg were more likely to have two or more and less likely to have one or fewer factors comprising the MetS than those who gained <10kg. These results showed major weight gain after 20 years of age to be closely associated with the high prevalence of metabolic disorders in middle age, in contrast to subjects with minor weight gain, even after matching their BMI at the time of this study.
Table 2.
The Comparison of the Number of Metabolic Syndrome Risk Factors by Weight Gain After 20 Years of Age
| Weight Gain After 20 Years of Age | P value* | ||
|---|---|---|---|
| <10kg (n=3601) | ≥ 10kg (n=3601) | ||
| 0, n (%) | 458 (54.4) | 307 (8.5)## | <0.001 |
| 1, n (%) | 1018 (50.0) | 802 (22.3)## | |
| 2, n (%) | 1154 (18.1) | 1237 (34.4)# | |
| 3, n (%) | 758 (9.6) | 962 (26.7)## | |
| 4, n (%) | 213 (12.3) | 293 (8.1)## | |
Notes: *Chi-square test or residual analysis. #Adjusted residual >|1.96|, ##adjusted residual>|2.58|. Factors comprising metabolic syndrome; (1) a waist circumference of ≥85 cm in men and ≥90 cm in women, (2) dyslipidemia (triglycerides ≥150 mg/dL or HDL-C < 40 mg/dL) or current lipid-lowering medications, (3) high blood pressure (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg) or current antihypertensive medications, and (4) hyperglycemia (fasting glucose ≥110 mg/dL) or current medications for diabetes mellitus.
Multivariate logistic analysis was employed to confirm the association between the prevalence of metabolic disorders and marked weight gain in adulthood adjusted for gender, age, smoking status, habitual drinking, skipping breakfast, walking time, sleep duration, marital status, educational level, and employment status. The adjusted odds ratio was 1.216 (95% CI; 1.095–1.350; p<0.001) for hypertension, 1.341 (95% CI; 1.209–1.487; p<0.001) for dyslipidemia, 1.307 (95% CI; 1.121–1.523; p=0.001) for hyperuricemia, and 1.460 (95% CI; 1.293–1.648; p<0.001) for MetS (Table 3).
Table 3.
Odds Ratios of Metabolic Disorder Prevalences from Multivariable Logistic Regression Model Associated with Weight Gain ≥10kg After 20 Years of Age vs Weight Gain <10kg
| Metabolic Disorders | OR (95% CI) |
|---|---|
| Hypertension | 1.26 (1.095–1.350) |
| Dyslipidemia | 1.341 (1.209–1.487) |
| Hyperuricemia | 1.307 (1.121–1.523) |
| MetS | 1.460 (1.293–1.648) |
| CKD | 1.087 (0.962–1.228) |
| Type 2 Diabetes | 1.100 (0.968–1.251) |
Notes: Independent variables: gender, age, smoking status, habitual drinking, eating breakfast, walking time, sleep duration, marital status, formal education level, employment status.
Abbreviations: MetS, metabolic syndrome, CKD, chronic kidney disease.
Next, the relationships between the weight gain in adulthood and recent lifestyle factors, as collected by questionnaire, were examined (Table 4). To determine which factors differed significantly between the two groups, post-hoc adjusted residuals analyses were performed after applying the chi-square test. Participants who gained ≥10 kg were significantly more likely to sleep <5 hours or ≥9 hours per night, to skip breakfast, to engage in walking <1 hour per day, to sit ≥5 hours per day, and to be former smokers than those who gained <10kg.
Table 4.
The Comparison of Lifestyle Factors by Weight Gain After 20 Years of Age
| Weight Gain After 20 Years of Age | P value* | ||
|---|---|---|---|
| <10kg (n=3601) | ≥ 10kg (n=3601) | ||
| Smoking status, n (%) | 0.049 | ||
| Never smokers | 2326 (64.6) | 2265 (62.9) | |
| Former smokers | 762 (21.2) | 848 (23.5)# | |
| Current smokers | 513 (14.2) | 488 (13.6) | |
| Habitual drinking, n (%) | 1830/3581 (51.1) | 1788/3594 (49.7) | 0.252 |
| Sleep, hours/day, n (%) | 0.010 | ||
| <5 | 153 (4.3) | 190 (5.3)# | |
| 5 - <7 | 2202 (61.1) | 2225 (61.9) | |
| 7 - <9 | 1205 (33.5) | 1119 (31.1)# | |
| ≥ 9 | 39 (1.1) | 59 (1.6)# | |
| Eating breakfast, n (%) | 0.001 | ||
| Skipping | 299 (8.5) | 381 (10.8) | |
| Regularly | 3217 (91.5) | 3142 (89.2) | |
| Walking time, hours/day, n (%) | <0.001 | ||
| <1 | 1453 (42.6) | 1623 (47.7)## | |
| 1 - <3 | 1203 (35.3) | 1120 (32.9)# | |
| 3 - <5 | 386 (11.3) | 354 (10.4) | |
| ≥ 5 | 365 (10.7) | 305 (9.0)# | |
| Sitting time, hours/day, n (%) | <0.001 | ||
| <1 | 454 (13.7) | 427 (12.8) | |
| 1 – <3 | 1012 (30.6) | 842 (25.3)## | |
| 3 – <5 | 980 (29.6) | 1022 (30.7) | |
| ≥ 5 | 865 (26.1) | 1037 (31.2)## | |
Notes: *Chi-square test or residual analysis. #Adjusted residual >|1.96|, ##adjusted residual>|2.58|.
Multivariable logistic regression analyses were performed to identify variables independently related to marked weight gain in adulthood (Table 5). Model 1, which was adjusted for gender, age, BMI, smoking status, habitual drinking, eating breakfast, walking time, and sleep duration, revealed former smoking to be positively (former vs never, OR 1.217; 95% CI 1.057–1.401), skipping breakfast positively (skipping vs regularly eating breakfast, OR 1.264; 95% CI 1.068–1.496) and walking time negatively (1–3 hours/day; OR 0.834; 95% CI 0.747–0.930, 3–5 hours/day; OR 0.821; 95% CI 0.697–0.967, >5 hours/day; OR 0.771; 95% CI 0.650–0.915 vs <1 hour/day) associated with major weight gain in adulthood, respectively. Model 2, with additionally controlling for sociodemographic factors (marital status, formal education level, and employment status) revealed former smoking (former vs never, OR 1.163; 95% CI 1.008–1.343), skipping breakfast (skipping vs regularly eating breakfast, OR 1.252; 95% CI 1.053–1.489), walking time (1–3 hours/day; OR 0.828; 95% CI 0.740–0.926, 3–5 hours/day; OR 0.818; 95% CI 0.691–0.968, >5 hours/day; OR 0.821; 95% CI 0.688–0.980 vs <1 hour/day) and long sleep duration (9 hours/day≤ OR 1.613; 95% CI 1.018–2.557 vs 5–7 hours/day) to be statistically significant predictors of marked weight gain, respectively.
Table 5.
Odds Ratios of Lifestyle Factors from Multivariable Logistic Regression Model Associated with Weight Gain ≥10kg After 20 Years of Age vs Weight Gain <10kg
| Model 1 | Model 2 | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Smoking status | ||
| Never smoker | 1.00 (reference) | 1.00 (reference) |
| Current smoker | 0.986 (0.846–1.159) | 0.974 (0.826–1.148) |
| Former smoker | 1.217 (1.057–1.401) | 1.163 (1.008–1.343) |
| Habitual drinking | 0.934 (0.837–1.042) | 0.914 (0.817–1.023) |
| Eating breakfast | ||
| Regularly | 1.00 (reference) | 1.00 (reference) |
| Skipping | 1.264 (1.068–1.496) | 1.252 (1.053–1.489) |
| Walking time, hours/day | ||
| <1 | 1.00 (reference) | 1.00 |
| 1 – <3 | 0.834 (0.747–0.930) | 0.828 (0.740–0.926) |
| 3 – <5 | 0.821 (0.697–0.967) | 0.818 (0.691–0.968) |
| ≥ 5 | 0.771 (0.650–0.915) | 0.821 (0.688–0.980) |
| Sleep, hours/day | ||
| <5 | 1.227 (0.972–1.549) | 1.264 (0.994–1.607) |
| 5 – <7 | 1.00 (reference) | 1.00 (reference) |
| 7 – <9 | 0.960 (0.863–1.069) | 0.978 (0.876–1.092) |
| ≥ 9 | 1.410 (0.914–2.172) | 1.613 (1.018–2.557) |
Notes: Model 1: independent variables: gender, age, BMI, smoking status, habitual drinking, eating breakfast, walking time, sleep duration. Model 2: independent variables: Model 1+ marital status, formal education level, employment status.
Discussion
This cross-sectional study obtained several important findings regarding major body weight gain in adulthood. First, gaining more than 10kg in adulthood was closely associated with unfavorable lifestyle factors at the time of surveillance, which presumably reflected prolonged maintenance of these poor habits. Second, even with matching for BMI at the time of the surveillance, major weight gain after maturity contributed significantly to the development of metabolic disorders.
Certain lifestyle factors, such as physical inactivity, high-fat and -protein diets, alcohol consumption, and certain health characteristics, are reportedly related to long-term weight gain.20 However, the associations between lifestyle factors and weight change in adulthood have not been extensively documented in large-scale studies. To the best of our knowledge, this is the first large population-based study examining these associations in an Asian country.
Our present study revealed lifestyle factors, identified by surveillance in middle-aged subjects, to be associated with weight gain in adulthood. Skipping breakfast has been suggested to be associated with overweight and obesity,21 because individuals who usually skip breakfast tend to have poorer diet quality22 and higher total daily energy intakes23 than those who consume breakfast regularly. In addition, skipping breakfast might be a marker of certain lifestyle characteristics, such as lower levels of physical activity.24 Nevertheless, the relationship between skipping breakfast and obesity remains controversial. Several studies have indicated not only the absence of a significant correlation between skipping breakfast and obesity,25 but also showed weight loss to be associated with skipping breakfast.26 Our current BMI, age, and gender-matched study suggests a relationship between routinely skipping breakfast by middle-aged individuals and weight gain in adulthood.
The results of this study indicated long sleep duration, such as ≥9 hours/day, to be closely associated with weight gain in adulthood. The relationship between an inadequate amount of sleep and MetS has been analyzed epidemiologically27 and in human studies focusing on metabolites.28 In contrast, little attention has been paid to the effects of excessively long sleep times on health outcomes. Although a recent meta-analysis revealed excessive amounts of sleep also to be associated with a significantly increased risk for negative health outcomes, including obesity,29 they have not investigated the association between body weight change for long periods, such as after 20 years of age, and health outcomes. Only one study explored the relationship of weight gain in adulthood with the amount of sleep and demonstrated an association of BMI at the time of the study, but not BMI gain between ages 20–40 years, and short sleep duration.30 According to our literature search, this was the first study to indicate a relationship between long sleep duration and weight gain from 20 years of age to middle age.
Smoking cessation is a well-established determinant of weight gain.31 Our results also showed former smoking, but not current smoking, to be significantly associated with weight gain ≥10kg after 20 years of age. Although mechanisms underlying smoking cessation-induced weight gain remain poorly understood, our results suggest that post-cessation weight gain might result from 1) smoking cessation being associated with increased energy intake and decreased energy expenditure,32 2) giving up cigarette smoking might return the body weight to a set-point, which is lowered by nicotine (and possibly other smoking products).33 Admittedly, smoking cessation might lead to substantial weight gain, resulting in an elevated risk of type 2 diabetes, but this would not diminish the benefits of quitting smoking for reducing cardiovascular and all-cause mortality.34
Weight gain ≥10kg in adulthood was found to be associated with higher levels of liver enzymes and high prevalence of hypertension, dyslipidemia, hyperuricemia, and MetS as compared to weight gain <10kg. The most important finding underlying these metabolic disorders was large waist circumference even in subjects with similar BMI, indicating visceral fat accumulation. Fat-free mass peaked in men at 35 to 44 years of age and in women at 45 to 54 years, declining thereafter, and fat mass and proportion of fat mass increased progressively in both genders with aging.35 Furthermore, weight gain during adulthood was closely associated with a larger amount of visceral fat and higher hepatic triglyceride contents in middle age, with no major change in subcutaneous fat, regardless of total body fat.36 Interestingly, men who were relatively slim as young adults, ie, had low BMI at age 20, often exhibited more weight gain-associated visceral fat accumulation,37 an observation compatible with our results of a large waist circumference at the time of the present surveillance in the subjects who had gained ≥10kg. From another perspective, we can also speculate that the participants who gained <10kg after 20 years of age, whose median BMI was 23.8 kg/m2 at age 20, might have had a large fat free mass, including muscle and/or subcutaneous fat mass in adolescence.
The molecular mechanism linking weight gain in adulthood and visceral fat accumulation might involve subjects with low BMI when young having a low fat-storage capacity in their subcutaneous adipose tissues because the total number of adipocytes remains constant in adulthood, rising only in childhood and adolescence,38 Even slight weight gain might easily overwhelm the lipid storage capacity of subcutaneous adipose tissue, and excess lipids may accumulate in visceral adipose tissue, especially in East Asian populations,39 In addition, excess lipids were also thought to spill over into the liver, muscle, epicardium, and pancreas, resulting in ectopic fat accumulation, which has been recognized as an emerging risk factor for metabolic morbidities and atherosclerosis,40 In this study, the subjects with marked weight gain in adulthood showed higher hepatic enzyme levels, possibly reflecting hepatic steatosis including NAFLD. The results of our study raise the possibility of major weight gain in adulthood being highly involved in ectopic fat accumulation, such as that in the liver and viscera.
Strengths of our study include the large population-based analysis through PS matching with age, gender, and BMI at the time of the surveillance. Most previous studies performed comparative analyses without matching for current BMI, causing critical bias in evaluating health conditions because the current degree of obesity has a major impact on metabolic phenotype. Our PS-matched model enabled us to distinguish the effects of weight gain from those of current body weight, leading to an unbiased investigation. In addition, a wide-ranging questionnaire concerning lifestyle factors enabled us to elucidate the relationships of unfavorable lifestyle factors with weight gain in adulthood. In order to carry out effective public health actions to control the obesity epidemic, recognition of the modifiable lifestyle factors related to weight gain is urgently required.
This study has limitations that must be considered. First, the dataset was cross-sectional, and causality thus cannot be inferred. The bidirectional associations between unfavorable lifestyle behavior and weight gain need to be taken into consideration. The bidirectional association of low physical activity with both body weight gain and mobility disability41 has been demonstrated. Furthermore, regarding the relationship between long sleep duration and weight gain, bidirectional effects have also been proposed, by which obesity-related metabolic disorders, such as depression, low-grade inflammation, and sleep-disordered breathing, might cause positive feedback for excessive sleep.42 As the second limitation, we calculated weight gain from the age of 20 years using a self-reported weight at age 20, which would be subject to recall errors. However, as a previous study showed the accuracy of long-term recall of past body weight,43 the self-reported past weights were thought to be relatively reliable. Moreover, large-scale epidemiological studies, as a series of The Japan Public Health Center-based Prospective Study (JPHC)44 or the Japan Multi-institutional Collaborative Cohort (J-MICC) study,45 have been conducted focusing on weight change after 20 years of age, using the same questionnaire as the current study. Therefore, misclassification of weight gain after 20 years of age ≥10kg or <10kg might have less impact on the results. Third, the current study has not evaluated the validity and reliability of the questionnaire. However, we have adopted the lifestyle questionnaire, which was established in J-MICC study.46 Besides the current study, The Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT),47 and Yamagata Molecular Epidemiological Cohort Study48 use the same or similar questionnaire. The J-MICC and JPHC-NEXT have conducted a validity study by examining all questionnaires for integrated analysis.46 Therefore, we believe that the validity and reliability of the questionnaire used in the current study have been assured by many series of epidemiologic studies. Fourth, weight change time courses were not examined. It is noteworthy that weight gain in adulthood occurred gradually in some cases but quite dramatically at certain ages in others. Moreover, the subjects with weight loss in adulthood, who possibly had severe health concerns, were categorized as a weight gain <10kg group. Finally, we have yet to collect, by questionnaire or interview, lifestyle habits at age 20. Thus, we cannot speculate as to whether changes in lifestyle or lifestyle habits that were present at baseline impacted the results. This cohort mainly includes the disaster area residents whose lifestyle habits might have been markedly altered in response to the Great East Japan Earthquake and resulting tsunami. However, we believe that it is important to investigate both the risk factors for and the protective factors against obesity, especially in this area, because numbers of patients suffering from obesity-related comorbidities have increased in this area since the disaster.
Conclusion
In conclusion, unfavorable lifestyle factors, such as low physical activity, skipping breakfast, inappropriate sleep duration, and smoking, were closely associated with weight gain in adulthood. Moreover, major weight gain after 20 years of age was associated with larger waist circumference, possibly leading to an elevated risk for MetS and other non-communicable diseases. These findings, obtained from a general population-based cohort, highlight the importance of maintaining both normal weight and favorable lifestyle habits throughout adulthood.
Acknowledgments
This work was supported by AMED under Grant Numbers JP17km0105003, JP17km0105004, and Grants-in-Aid for Scientific Research (18K08523) to Y.I., from the Japan Society for the Promotion of Science. The authors would like to thank all of the participants of The Tohoku Medical Megabank Community-Based Cohort Study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London). 2017;390(10113):2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funatogawa I, Funatogawa T, Nakao M, et al. Changes in body mass index by birth cohort in Japanese adults: results from the National Nutrition Survey of Japan 1956–2005. Int J Epidemiol. 2009;38(1):83–92. doi: 10.1093/ije/dyn182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Division of Health Promotion and Nutrition MoH, Labour and Welfare. The 2016 National Health and Nutrition Survey; 2016. Available from: https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2016.pdf. Accessed March25, 2021.
- 4.Odegaard AO, Koh WP, Vazquez G, et al. BMI and diabetes risk in Singaporean Chinese. Diabetes Care. 2009;32(6):1104–1106. doi: 10.2337/dc08-1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40(Suppl 1):S302–304. doi: 10.1007/s00592-003-0093-z [DOI] [PubMed] [Google Scholar]
- 6.Spalding KL, Bernard S, Näslund E, et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat Commun. 2017;8(1):15253. doi: 10.1038/ncomms15253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the multi-ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168(9):928–935. doi: 10.1001/archinte.168.9.928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Tamakoshi K, Yatsuya H, et al. Long-term body weight fluctuation is associated with metabolic syndrome independent of current body mass index among Japanese men. Circ J. 2005;69(1):13–18. doi: 10.1253/circj.69.13 [DOI] [PubMed] [Google Scholar]
- 9.Ochiai H, Shirasawa T, Yoshimoto T, et al. Association of the combination of weight gain after 20 years of age and current obesity with chronic kidney disease in Japan: a cross-sectional study. BMJ Open. 2019;9(6):e027752. doi: 10.1136/bmjopen-2018-027752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie YJ, Ho SC, Su X, et al. Changes in body weight from young adulthood to middle age and its association With blood pressure and hypertension: a Cross-Sectional Study in Hong Kong Chinese Women. J Am Heart Assoc. 2016;5(1):e002361. doi: 10.1161/JAHA.115.002361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WJ, Chen LL, Zheng J, et al. Association of adult weight gain and nonalcoholic fatty liver in a cross-sectional study in Wan Song Community, China. Braz J Med Biol Res. 2014;47(2):151–156. doi: 10.1590/1414-431X20133058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai GH, Janson C, Haglöw J, et al. both weight at age 20 and weight gain have an impact on sleep disturbances later in life: results of the EpiHealth Study. Sleep. 2018;41(1). doi: 10.1093/sleep/zsx176 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Xuan YJ, Yang LS, et al. Weight changes since age 20 and cardiovascular risk factors in a middle-aged Chinese population. J Public Health (Oxf). 2018;40(2):253–261. doi: 10.1093/pubmed/fdx057 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa-Takata K, Ohta T, Moritaki K, et al. Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. Eur J Clin Nutr. 2002;56(7):601–607. doi: 10.1038/sj.ejcn.1601364 [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama S, Yaegashi N, Nagami F, et al. The Tohoku Medical Megabank Project: design and mission. J Epidemiol. 2016;26(9):493–511. doi: 10.2188/jea.JE20150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hozawa A, Tanno K, Nakaya N, et al. Study profile of The Tohoku Medical Megabank Community-Based Cohort Study. J Epidemiol. 2020. doi: 10.2188/jea.JE20190271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzawa Y. Metabolic syndrome–definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12(6):301. doi: 10.5551/jat.12.301 [DOI] [PubMed] [Google Scholar]
- 18.Malhotra R, Ostbye T, Riley CM, et al. Young adult weight trajectories through midlife by body mass category. Obesity. 2013;21(9):1923–1934. doi: 10.1002/oby.20318 [DOI] [PubMed] [Google Scholar]
- 19.Hu FB. Obesity Epidemiology. Oxford: Oxford University Press; 2008:xiii, 498. [Google Scholar]
- 20.MacInnis RJ, Hodge AM, Dixon HG, et al. Predictors of increased body weight and waist circumference for middle-aged adults. Public Health Nutr. 2014;17(5):1087–1097. doi: 10.1017/S1368980013001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X, Chen Q, Pu Y, et al. Skipping breakfast is associated with overweight and obesity: a systematic review and meta-analysis. Obes Res Clin Pract. 2020;14(1):1–8. doi: 10.1016/j.orcp.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Smith KJ, McNaughton SA, Cleland VJ, et al. Health, behavioral, cognitive, and social correlates of breakfast skipping among women living in socioeconomically disadvantaged neighborhoods. J Nutr. 2013;143(11):1774–1784. doi: 10.3945/jn.113.181396 [DOI] [PubMed] [Google Scholar]
- 23.Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev. 2007;65(6 Pt 1):268–281. doi: 10.1111/j.1753-4887.2007.tb00304.x [DOI] [PubMed] [Google Scholar]
- 24.Smith KJ, Gall SL, McNaughton SA, et al. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the childhood determinants of Adult Health Study. Am J Clin Nutr. 2010;92(6):1316–1325. doi: 10.3945/ajcn.2010.30101 [DOI] [PubMed] [Google Scholar]
- 25.Dhurandhar EJ. True, true, unrelated? A review of recent evidence for a causal influence of breakfast on obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):384–388. doi: 10.1097/MED.0000000000000281 [DOI] [PubMed] [Google Scholar]
- 26.Geliebter A, Astbury NM, Aviram-Friedman R, et al. Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: a randomised controlled trial. J Nutr Sci. 2014;3:e56. doi: 10.1017/jns.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaput JP, McNeil J, Després JP, et al. Short sleep duration as a risk factor for the development of the metabolic syndrome in adults. Prev Med. 2013;57(6):872–877. doi: 10.1016/j.ypmed.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet (London). 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8 [DOI] [PubMed] [Google Scholar]
- 29.Jike M, Itani O, Watanabe N, et al. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. doi: 10.1016/j.smrv.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 30.Japas C, Knutsen S, Dehom S, et al. Body mass index gain between ages 20 and 40 years and lifestyle characteristics of men at ages 40–60 years: the Adventist Health Study-2. Obes Res Clin Pract. 2014;8(6):e549–557. doi: 10.1016/j.orcp.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiolero A, Faeh D, Paccaud F, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi: 10.1093/ajcn/87.4.801 [DOI] [PubMed] [Google Scholar]
- 32.Hofstetter A, Schutz Y, Jéquier E, et al. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med. 1986;314(2):79–82. doi: 10.1056/NEJM198601093140204 [DOI] [PubMed] [Google Scholar]
- 33.Perkins KA. Weight gain following smoking cessation. J Consult Clin Psychol. 1993;61(5):768–777. doi: 10.1037/0022-006X.61.5.768 [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Zong G, Liu G, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379(7):623–632. doi: 10.1056/NEJMoa1803626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyle UG, Genton L, Slosman DO, et al. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17(7–8):534–541. doi: 10.1016/S0899-9007(01)00555-X [DOI] [PubMed] [Google Scholar]
- 36.Verkouter I, Noordam R, de Roos A, et al. Adult weight change in relation to visceral fat and liver fat at middle age: the Netherlands epidemiology of obesity study. Int J Obes. 2019;43(4):790–799. doi: 10.1038/s41366-018-0163-5 [DOI] [PubMed] [Google Scholar]
- 37.Koda M, Kitamura I, Okura T, et al. Men who were thin during early adulthood exhibited greater weight gain-associated visceral fat accumulation in a study of middle-aged Japanese men. Obes Sci Pract. 2018;4(3):289–295. doi: 10.1002/osp4.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 39.Azuma K, Kadowaki T, Cetinel C, et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 2009;58(8):1200–1207. doi: 10.1016/j.metabol.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1 [DOI] [PubMed] [Google Scholar]
- 41.de Munter JS, Tynelius P, Ahlström G, et al. The bidirectional association between body weight and mobility disability: a population-based cohort. Disabil Health J. 2016;9(4):632–637. doi: 10.1016/j.dhjo.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 42.Grandner MA, Drummond PA. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–360. doi: 10.1016/j.smrv.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamakoshi K, Yatsuya H, Kondo T, et al. The accuracy of long-term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord. 2003;27(2):247–252. doi: 10.1038/sj.ijo.802195 [DOI] [PubMed] [Google Scholar]
- 44.Chei CL, Iso H, Yamagishi K, et al. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based Study. Int J Obes. 2008;32(1):144–151. doi: 10.1038/sj.ijo.0803686 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki W, Wuren KK. Associations between family factors and body weight gain from 20 years old. BMC Womens Health. 2019;19(1):33. doi: 10.1186/s12905-019-0719-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi K, Naito M, Kawai S, et al. Study profile of the Japan Multi-institutional Collaborative Cohort (J-MICC) Study. J Epidemiol. 2020. doi: 10.2188/jea.JE20200147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawada N, Iwasaki M, Yamaji T, et al. The Japan Public Health Center-based Prospective Study for the Next Generation (JPHCNEXT): study design and participants. J Epidemiol. 2020;30(1):46–54. doi: 10.2188/jea.JE20180182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narimatsu H; Yamagata University Genomic Cohort Consortium. Constructing a contemporary gene-environmental cohort: study design of the Yamagata Molecular Epidemiological Cohort Study. J Hum Genet. 2013;58(1):54–56. doi: 10.1038/jhg.2012.128 [DOI] [PubMed] [Google Scholar]