The coronavirus disease 2019 (COVID-19) pandemic has confronted us with the fast devastating spread of a new highly contagious respiratory airborne pathogen, against which no single person on this planet had any protective immunity. Collapse or near-collapse of healthcare systems occurred in highly developed as well as low- and middle-income countries; critical care capacity turned out to be the bottleneck [1, 2]. In the absence of herd immunity, rigorous community measures and lockdowns had and have to be imposed, often recurrently, with dramatic impact on economies and mental health.
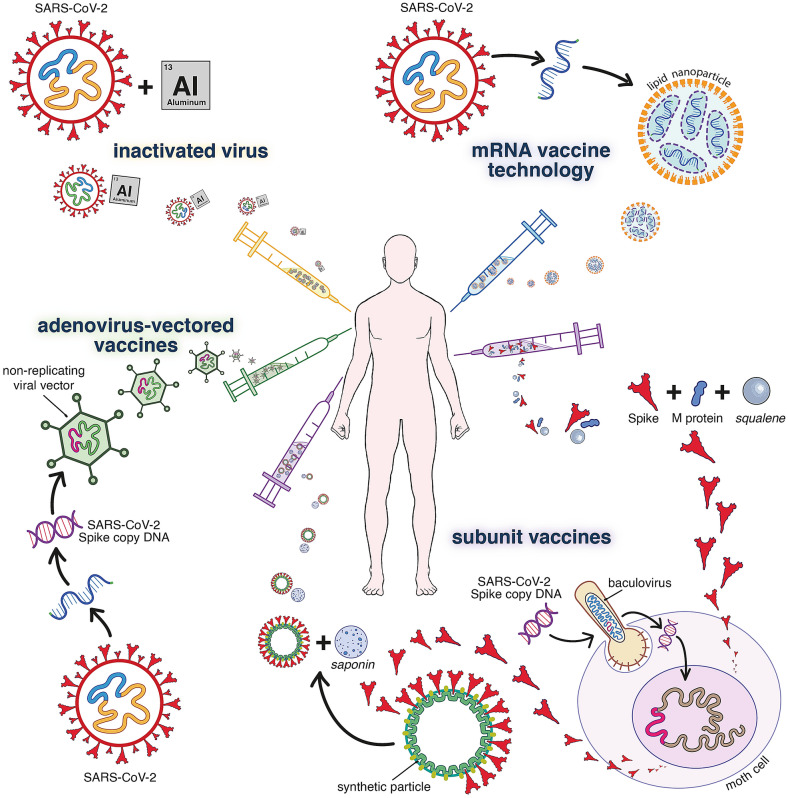
Vaccines are the only valuable option towards herd immunity without excess mortality. Within 11 days of the first official reports of viral pneumonia clusters in Wuhan, China, the genetic sequence of SARS-CoV-2 was published (January 11th 2020). Based on knowledge on other coronaviruses, the viral Spike (S) protein was selected early on as potential antigen for vaccine development. One year later, 14 COVID-19 vaccines have undergone full development [3]. Globally, at least nine of them already received approval from competent national and international regulatory agencies (such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency), mostly under emergency or conditional terms, where the prospected benefit of population-wide immunization is balanced against the potential risks of deploying novel vaccines in the absence of long-term safety records. The approved vaccines provide good to excellent protection. Overall, COVID-19 vaccines belong to two main classes: (i) whole inactivated virus vaccines, and (ii) vaccines that present the SARS-CoV-2 S protein, the main target of neutralizing antibodies, to the immune system (Fig. 1).
Fig. 1.
Technologies on which the main current COVID-19 vaccines or vaccine candidates are based. Inactivated vaccines such as Sinovac (CoronaVac®), Sinopharm (BBIBP-CorV®) or Bharat Biotech (BBV152/Covaxin®) are produced with traditional methods (similar to rabies or hepatitis A vaccines), where the SARS-CoV-2 virus is grown in cell culture, inactivated by β-propiolactone and adsorbed to alum (and other excipients). Current S-based vaccines consist of either (i) recombinant S protein (like hepatitis B vaccines), (ii) replication-deficient viral vectors expressing S, or (iii) mRNA encoding S. Because of an optimized design, these vaccines induce rapid and vigorous immune responses. For the subunit vaccines, recombinant S is produced in insect cells and formulated with saponin or other emulsions as adjuvant. In the case of NVX-CoV2373 by Novavax, S subunits are mounted on a synthetic protein-scaffold to further enhance their immunogenicity. The vector-based vaccines by AstraZeneca (AZD1222/Covishield®), Gamalenya (Gam-COVID-Vac/Sputnik V®) or Johnson & Johnson (Ad26.COV2.S/JNJ-78436735) use modified replication-deficient adenoviruses (respectively chimpanzee AdV; a combination of AdV 26 and AdV5; Adv26) to deliver an expression cassette for the copy DNA encoding for S (alike established e.g. for gene therapy). For the novel mRNA approach, synthetic mRNA encoding for S is encapsulated in lipid nanoparticles. Representative mRNA vaccines are BNT162b2 (Comirnaty®) by Pfizer/BioNtech, mRNA-1273 by Moderna and CVnCoV by Curevax. All current COVID-19 vaccines are administered by intramuscular injection; following a two-dose regimen (with as exception Ad26.COV2.S as single dose). For further information, see Kyriakidis et al. NPJ Vaccines. (2021) https://doi.org/10.1038/s41541-021-00292-w
So far, > 600 million doses have been administered (updates via [4]). In countries with significant coverage (two doses: Israel > 54%, UAE > 23%, USA > 16%; first dose: UK > 60%), a clear reduction in the number of severe COVID-19 cases and admissions in the intensive care unit (ICU) is noted. The impact of vaccination on hospitalizations and thus also ICU occupation is remarkable with a drop in cases up to 85–95% within weeks to months after population-wide vaccine rollout [5, 6]. This sparks hope that COVID-19 may be pushed back from a highly endemic to a more readily to manage epidemic or seasonal situation [7].
Frontline health care workers (HCW) accounted for up to 25% of COVID-19 patients in China in early January 2020, and for approximately 10–20% of all cases during first waves in Italy and Spain. Since HCW have been prioritized for vaccination, their risk of SARS-CoV-2 infection and hospitalization dropped by a factor 10 [8, 9]. Vaccination reduces as well the number of HCW that need to quarantine, as a consequence of high-risk exposure, by ~ 90% [10].
The new waves now occuring, with an additional impact on healthcare systems, are mostly linked to the emergence of Variants of Concern (VoC). In particular, variants that emerged from the UK (B.1.1.7), South Africa (B.1.351) and Brazil (P1) are more infectious and may appear as well to have an increased virulence [11]. The South African and Brazilian VoC are less efficiently neutralized by the serum of subjects previously infected with the original variants [12] or who had been vaccinated. Some vaccines showed reduced efficacy in clinical studies running in regions with widespread transmission of variant B.1.351 [13]. Despite the fear that VoC may escape naturally acquired or vaccine induced immunity [12, 13], fast rollout of current vaccines has a clear impact on the number of patients needing care; thus also stress on intensive care [5–7]; in particular also in countries such as Israel or the UK with an high incidence of circulating VoC B.1.1.7.
The emergence of VoCs raises the question as to whether vaccines will need to be regularly updated. Interestingly, convalescent sera from patients with past B.1.351 infection elicits cross-reactive neutralizing antibodies against viruses from the first wave and P1 [14], providing a rationale for the design of vaccines covering most circulating strains. So far, a convergent evolution (on different continents) of VoC is observed, offering hope that yet very different escape mutants may not readily emerge. Coronaviruses mutate typically at a slower pace than influenza viruses. Hence, in contrast to influenza, annual updates may not be required. A related question is how long protective immunity will last, only time will tell whether booster/recurrent vaccination will (regularly) be needed. Some evidence suggests that the vaccines may reduce virus shedding from the upper respiratory tract and thus lower the chance of transmission, which is highly relevant in the context of the prevention of nosocomial and occupational transmission.
This pandemic will not be the last one. One day, viruses may even emerge that can be as contagious as measles (R0 of SARS-CoV-2 = 1.5–3.5 versus R0 of measles = 12–18), as lethal as MERS-CoV (mortality of COVID-19 ~ 2% versus mortality of MERS ~ 34%), or, also like measles, with a preference for children instead of the elderly. The rapid development of vaccines against SARS-CoV-2 was unprecedented but is certainly not a given. In the interval between the emergence of a new virus and the rollout of a vaccination campaign, the world is not ready to deal with the impact of a large next pandemic. Therefore, in addition to vaccines, it will be of utmost importance to develop safe, ultrapotent oral antiviral drugs that cover entire families of viruses. Such drugs could help to curb local outbreaks such as during the first weeks of an emergence [15] and will be an essential tool to control further spread. In addition, such drugs can be administered prophylactically and protect health care workers.
In summary, the available vaccines will be our way out of this pandemic. Unfortunately, in many regions, including most African countries, vaccination has not, or almost not been initiated. SARS-CoV-2 can only be beaten if the whole world population gets equal access to safe and potent vaccines.
Funding
GM is funded by the Research Foundation, Flanders (FWO), as senior clinical investigator (1843118 N) and receives project research funding from the KU Leuven (C24/17/072), and the Belgian Federal Health Care Knowledge Center (KCE) (COV201003: Donated antibodies working against nCoV (DAWN-Plasma)). The original work by JN is funded by the COVID-19 call of FWO (G0G4820N), the European Union’s Horizon 2020 research and innovation program under grant agreements No 101003627 (SCORE project) and Bill & Melinda Gates Foundation (BGMF) under grant agreement INV-00636.
Declarations
Conflicts of interest
The authors declare that they no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richards-Belle A, Orzechowska I, Gould DW, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed 1 April 2021
- 4.https://coronavirus.jhu.edu/vaccines/international. Accessed 1 April 2021. Accessed 1 April 2021
- 5.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torjesen I. COVID-19: First doses of vaccines in Scotland led to a substantial fall in hospital admissions. BMJ. 2021;372:n523. doi: 10.1136/bmj.n523. [DOI] [PubMed] [Google Scholar]
- 7.Shilo S, Rossman H, Segal E. Signals of hope: gauging the impact of a rapid national vaccination campaign. Nat Rev Immunol. 2021 doi: 10.1038/s41577-021-00531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benenson S, Oster Y, Cohen MJ, Nir-Paz R. BNT162b2 mRNA COVID-19 vaccine effectiveness among health care workers. N Engl J Med. 2021 doi: 10.1056/NEJMc2101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021 doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021 doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabino EC, Buss LF, Carvalho MPS, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahase E. COVID-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021;372:n296. doi: 10.1136/bmj.n296. [DOI] [PubMed] [Google Scholar]
- 14.Moyo-Gwete T, Madzivhandila M, Makhado Z, et al. SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies. bioRxiv. 2021 doi: 10.1101/2021.03.06.434193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torneri A, Libin P, Vanderlocht J, Vandamme AM, Neyts J, Hens N. A prospect on the use of antiviral drugs to control local outbreaks of COVID-19. BMC Med. 2020;18(1):191. doi: 10.1186/s12916-020-01636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]