Highlights
-
•
Retrospective clinical review of 167 patients with breast cancer bone metastases.
-
•
Comprehensive study of clinical characteristics, prognostic factors, and survival.
-
•
First study to assess outcomes associated with operative vs. nonoperative treatment.
-
•
Utilizing surgical treatment involves a complex, personalized care decision.
Keywords: Breast cancer, Bone metastasis, Prognostic factor, Cancer management
Abstract
Introduction
Bone is the most common distant site of breast cancer metastasis. Skeletal lesions can cause significant morbidity due to pain, pathologic fracture, and electrolyte abnormalities. Current treatment for patients with bone metastases (BoM) from breast cancer is highly personalized and often involves a multidisciplinary approach with chemotherapy, hormone therapy, bone-targeted antiresorptive agents, radiation therapy, and surgery. We have retrospectively collected clinical data from a series of patients with bone metastases to evaluate the clinical characteristics, prognostic factors, and survival patterns of patients with breast cancer BoM receiving standard multimodal therapy.
Methods
A consecutive series of 167 patients with breast cancer BoM treated at a single institution between August 2013 and March 2020 were identified. Clinical information was obtained from the medical record and survival analyses were performed to evaluate patient outcomes and identify prognostic factors.
Results
Thirty-seven patients (22%) presented with de novo BoM – bone metastases at the time of breast cancer diagnosis – and were 2.6 times more likely to die within the study period than those with asynchronous BoM (HR = 2.62, p = <0.0001). Patients who received bone-targeted medical therapy were 61% less likely to die after BoM diagnosis than those who did not (HR = 0.39, p = 0.001). Operative stabilization of BoM was more frequently employed in patients with lytic (p = 0.02) or mixed (p = 0.02) tumors than it was for those with blastic lesions. Patients treated with surgery had a lower overall bone metastasis survival than those treated without (p < 0.03).
Discussion
These findings reflect the current patterns in metastatic breast cancer treatment and associated outcomes. In a series of 167 consecutive patients, we demonstrate the natural history of breast cancer with BoM being treated with modern multimodal therapy. Understanding these treatment patterns and prognostic factors enhances the provider’s ability to counsel patients and direct appropriate treatments.
1. Introduction
Breast cancer is the most common cancer diagnosis in women globally with an estimated two million new cases each year [1]. In the U.S., breast cancer currently affects over 3.5 million men and women. Metastatic disease is the primary cause of cancer-related mortality and affects approximately 30% of patients with breast cancer [2], [3], [4], [5]. While early diagnosis and treatment for primary disease has seen significant improvements over the last three decades [6], the overall 5-year survival rate for patients with metastatic disease has remained unchanged at around 20–30% [7]. Bone is the most common site of distant breast cancer metastasis and is involved in 65–80% of patients with metastatic disease [8]. BoM can be lytic, blastic, or mixed [9], [10] and are associated with pathologic fracture, endocrine dysregulation, damage to local structures [10], [11], [12], [13], and increased mortality [11], [14]. Living with BoM has a dramatic impact on the patients’ quality of life and causes significant pain, physical disability, and potential loss of employment.
There have been significant improvements in breast cancer therapy in recent years and more patients are living longer with BoM with the use of adjuvants such as chemotherapy, radiation therapy, and hormonal therapy to reduce the incidence and morbidity of metastasis. Better strategies have also been developed specifically for loco-regional and systemic control of BoM, including advances in orthopaedic surgery techniques, radiotherapy, and bone-targeting agents [10]. Results from recent trials consistently support the efficacy of bone-targeting agents (i.e. bisphosphonates, denosumab) in reducing the incidence of BoM and the rate of BoM-related morbidity. As such, these have become a mainstay of treatment [15]. These agents decrease the lytic action of cancer in bone through osteoclast inhibition. This inhibition also interrupts physiologic bone remodeling, which predisposes this population to atypical fractures, osteonecrosis of the jaw, systemic electrolyte abnormalities, and renal failure [9], [10]. If these side effects are experienced, treatment with bone-targeting therapy generally needs to be discontinued.
There is currently no universally accepted paradigm for the treatment of BoM in breast cancer. At our institution, patients with BoM that have estrogen receptor positive (ER+) also known as hormone receptor positive breast tumors are treated with sequential endocrine therapy with aromatase inhibitors and fulvestrant in combination with agents such as CDK 4/6 inhibitors (palbociclib, ribociclib, or abemaciclib), or the mTOR inhibitor, everolimus. Additional chemotherapy may be given if the patient has visceral metastasis or if there has been progression despite multiple lines of endocrine therapy. In patients with triple-negative breast cancer, chemotherapy is generally employed starting with a taxane or capecitabine with transition to other agents as needed if the disease progresses. For patients with HER2+ breast tumors, initial treatment generally includes a taxane with trastuzumab or pertuzumab followed by trastuzumab emtansine as a second line agent. After these initial lines of therapy, many patients continue to receive individualized treatment as deemed appropriate by their medical oncologist. The decision to pursue surgical intervention for bone lesions in patients with metastatic breast cancer is multifactorial and made based on a thorough, personalized assessment with the patient that involves discussion with the medical and radiation oncologists. The surgical treatment of metastatic disease is often preceded by systemic therapy of the patient’s primary disease as directed by medical oncology.
The absence of clear treatment guidelines is a challenge to both patients and physicians. The surgical treatment of BoM involves stabilizing or replacing the compromised bone. This is a palliative solution that does not aim to “cure” the disease or address the formation or growth of new metastases. There remains significant variability in the treatment indicated in each case of BoM based on breast cancer type, prior treatments, patient symptoms and characteristics, including the degree of pain at rest and with ambulation, overall performance and health status, the location of skeletal disease, the predominance of lytic or blastic disease, and other considerations related to the individual patient’s circumstances.
If surgical intervention is indicated, native bone is preserved with intramedullary or extramedullary fixation with or without a structural adjuvant such as polymethyl methacrylate bone cement whenever feasible. Replacement of bone or joints with endoprostheses is performed if the volume of healthy bone is determined to be inadequate to support bone/joint preservation. The goal of any surgical intervention is to maximize the patient’s immediate mobility and quality of life [16], [17].
A major obstacle in the care of patients with metastatic breast cancer is the poor understanding of BoM biology and lack of clear guidelines driving the decision to pursue surgical treatment. In this study, we aim to identify the clinical characteristics, prognostic factors, and survival of a cohort of patients with breast cancer BoM to provide an updated understanding of the clinical patterns of this disease and to assist in the multidisciplinary decisions surrounding surgical intervention. We have retrospectively reviewed the clinical data of all patients seen in our orthopedic oncology practice between August 2013 and March 2020 to identify prognostic factors in the context of modern treatment strategies employed by a multidisciplinary musculoskeletal oncology team.
2. Methods
2.1. Patient inclusion criteria and chart review
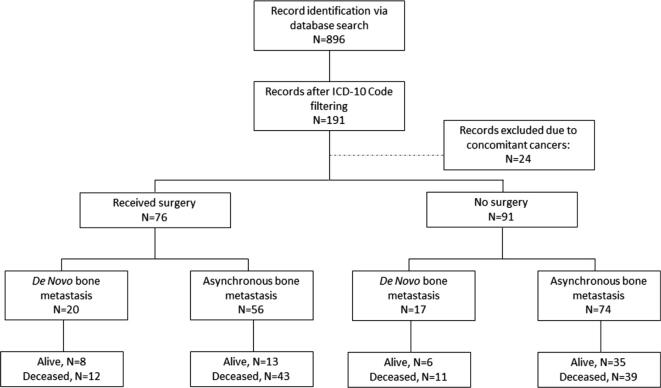
This study was approved by the University of Pittsburgh Institutional Review Board committee (STUDY20010034). We performed a retrospective chart review using the ICD-10 codes C79.51 (secondary malignant neoplasm of bone), C79.9 (secondary malignant neoplasm of unspecified site), C79.81 (secondary malignant neoplasm of breast), and C50 (malignant neoplasm of breast) to identify patients with suspected breast cancer BoM seen in our practice by four board-certified, fellowship-trained orthopaedic oncologists between August 2013 and March 2020. This search yielded 896 subjects. After preliminary chart review, 191 patients were confirmed to have a final pathologic diagnosis of metastatic breast cancer and underwent further review. In surgically treated patients, a board-certified pathologist confirmed the diagnosis of breast cancer BoM as dictated by our treatment paradigm. Twenty-four patients were excluded from complete review as they were found to have concomitant primary carcinomas of different histological subtypes other than breast cancer.
Demographic information collected on the remaining 167 patients included sex, race, and age at breast cancer and BoM diagnosis; tumor histology; primary tumor and BoM receptor status including the quantitative estrogen receptor (ER) and/or progesterone receptor (PR) H-scores (a measure of nuclear immunoreactivity applicable to steroid receptors); types of treatment received (chemotherapy, radiation therapy, hormonal therapy, bone-targeted therapy, and surgery); type and location of bone lesions; and date of last follow up or death.
2.2. Statistical analyses
Overall survival (OS) was defined as the time from primary breast cancer diagnosis until death or last follow-up. BoM survival (BMS) was defined as the time from BoM diagnosis until death or last follow-up. Survival functions were plotted using the Kaplan-Meier method. Cox proportional hazards models were used to assess relationships between each of the two survival outcomes and prognostic factors (de novo status, diagnosis age, received surgery, ER primary, endocrine therapy, radiotherapy, chemotherapy, bone-targeted therapy, CDK inhibitor treatment, race, and histological subtype). Multivariable Cox proportional hazards models for each of the two survival outcomes were created using the factors that were significant in the univariate models with backwards selection to develop a final model. Chi-square tests with post-hoc probing adjusted with the Holm-Bonferroni method were employed to compare the rate of surgical intervention across types and sites of bone lesions. Age was analyzed as a continuous variable and the remainder of variables were considered categorical. An alpha level of 0.05 was used for all statistical tests. Analysis was performed in SAS version 9.4.
3. Results
3.1. Patient characteristics
Patients with histologically confirmed breast cancer BoM (n = 191) underwent full review and demographics were obtained (Fig. 1). Twenty-four subjects were excluded from further analyses due to concomitant or historical diagnoses of another carcinoma. Patient demographics and disease characteristics are illustrated in Table 1.
Fig. 1.
Flow-chart for retrospective study design.
Table 1.
Patient demographics and disease characteristics.
| Category | Subcategory | N | % |
|---|---|---|---|
| Life Status | Alive | 62 | 37.1 |
| Deceased | 105 | 62.9 | |
| Race | Caucasian | 152 | 91.3 |
| African-American | 13 | 7.6 | |
| Asian | 2 | 1.2 | |
| de novo BM | Yes | 37 | 22.2 |
| No | 130 | 77.8 | |
| Metastases | Bone only | 24 | 14.4 |
| Other sites | 143 | 85.6 | |
| Surgery | Yes | 76 | 45.5 |
| No | 91 | 54.5 | |
| Pathology Subtype | Ductal | 134 | 80.2 |
| Lobular | 14 | 8.4 | |
| Ductal and Lobular (Mixed) | 9 | 5.4 | |
| Unknown | 10 | 6.0 |
The majority of patients were Caucasian (n = 152), while patients identifying as African American (n = 13) and Asian (n = 2) were present in smaller numbers. All patients were female. Of the 167 patients, 37 (22.2%) presented with BoM at the time of breast cancer diagnosis (de novo BoM).
4. Breast cancer histologic subtypes:
The histologic subtype was ductal in 80.2% of patients (Table 1) and the majority of primary breast tumors were ER+ and HER2− (71.6%). The majority of patients received endocrine therapy, chemotherapy, and radiation therapy (Table 2). The majority of BoM were also ER+ and HER2− (71.3%) (Table 3). Receptor discordance was highest among ER− BoM; the corresponding primary tumors from these patients were often ER+ (45.5%). Mean patient age and average interval of time from cancer diagnosis to diagnosis of BoM are shown in Table 4.
Table 2.
Treatments received based on receptor status of breast primary.
| Breast Primary Hormonal Status | N (%)* | Received Endocrine Therapy | Received HER2 Therapy | Received Chemotherapy | Received Radiation | Received Palbociclib |
|---|---|---|---|---|---|---|
| ER+/HER2− | 106/148(71.6%) | 105/106 | 8/106 | 100/106 | 98/106 | 62/106 |
| ER+/HER2+ | 26/148 (17.6%) | 26/26 | 22/26 | 26/26 | 24/26 | 10/26 |
| ER-/HER2+ | 3/148 (2.0%) | 2/3 | 3/3 | 3/3 | 1/3 | 0/3 |
| ER-/HER2− | 13/148 (8.8%) | 4/13 | 0/13 | 13/13 | 10/13 | 2/13 |
*Total number of patients (n = 148) that receptor status and all treatment information were documented in clinical records for ER, PR, and HER2 for primary tumors.
Table 3.
Characteristics of patient bone metastases.
| Bone Metastasis Hormonal Status | N (%)* | Receptor Discordanceǂ | De Novo | Other Metastatic Sites | Received Bone-Targeted Therapy | Received Surgery |
|---|---|---|---|---|---|---|
| ER+/HER2− | 57/80 (71.3%) | 12/57 (21%) | 15/57 (26.3%) | 50/57 (87.7%) | 53/57 (98.1%) | 35/57 (61.4%) |
| ER+/HER2+ | 10/80 (12.5%) | 2/10 (20%) | 1/10 (10%) | 9/10 (90%) | 9/10 (90%) | 4/10 (40%) |
| ER-/HER2+ | 2/80 (2.5%) | 2/2 (100%) | 1/2 (50%) | 1/2 (50%) | 2/2 (100%) | 0/2 (0%) |
| ER-/HER2− | 11/80 (13.7%) | 5/11 (45.5%) | 0/11 (0%) | 9/11 (81.8%) | 7/11 (63.6%) | 2/11 (18.2%) |
*Total number of patients (n=80) that receptor status was documented in clinical records for ER, PR, and HER2 for both primary tumors and bone metastases.
ǂRecorded if any of the receptors (ER, PR, HER2) switched from what was reported for the primary tumor.
Table 4.
Mean patient age at time of primary breast cancer diagnosis and diagnosis of bone metastasis, and interval between diagnoses.
| Mean (years) | Range (years) | |
|---|---|---|
| Age at primary breast cancer diagnosis | 54 | 31–91 |
| Age at onset of metastatic bone disease | 59 | 31–93 |
| Time from primary dx to first bone met (including de novo disease) | 5 | 0–35 |
4.1. Bone metastasis subtypes
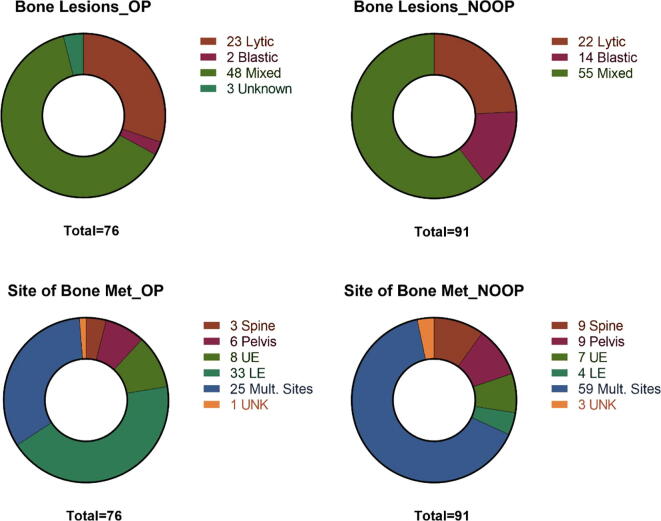
BoM were characterized by lesion type (lytic, blastic, or mixed) and site (spine, pelvis, upper extremity, lower extremity, or multiple sites) (Fig. 2). Patients with lytic or mixed lesions had higher rates of surgical intervention compared with patients who had blastic lesions (p = 0.02). Patients with lower extremity BoM were more likely to be treated surgically than patients with BoM in the pelvis (p = 0.005) or the spine (p = 0.001).
Fig. 2.
Characteristics of BoM categorized into those that received surgery (OP) and those that did not (NOOP) for A.) lesion type (lytic, blastic, or mixed) and B.) site of lesion (spine, pelvis, upper extremity = UE, lower extremity = LE, and multiple sites). UNK = unknown in cases where this information was not recorded.
4.2. Overall survival and bone metastasis survival
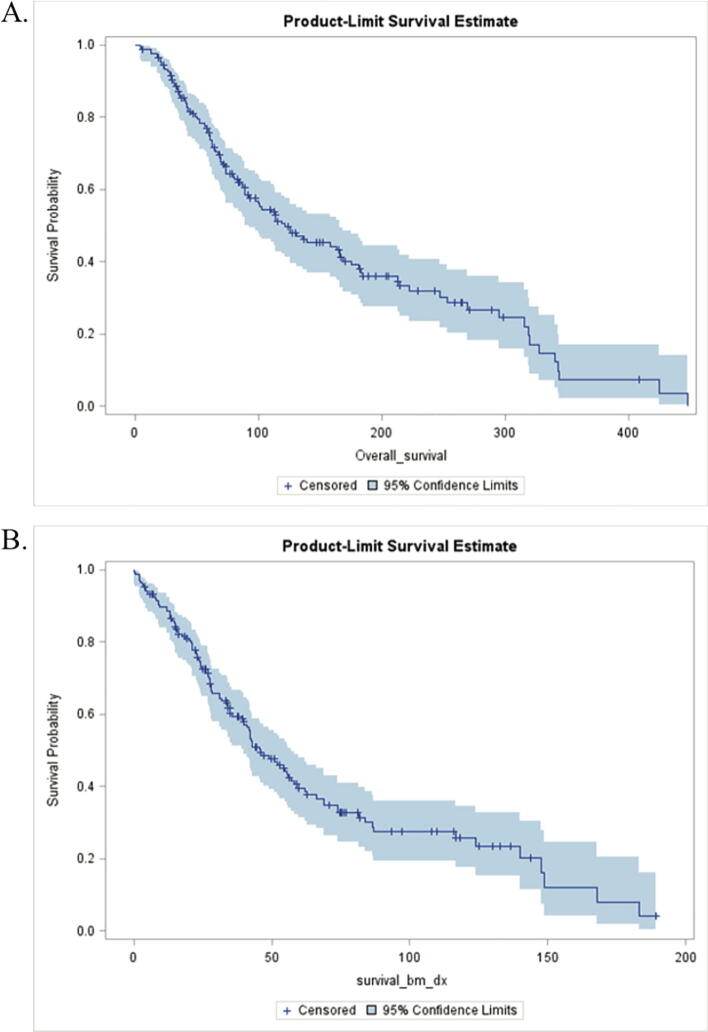
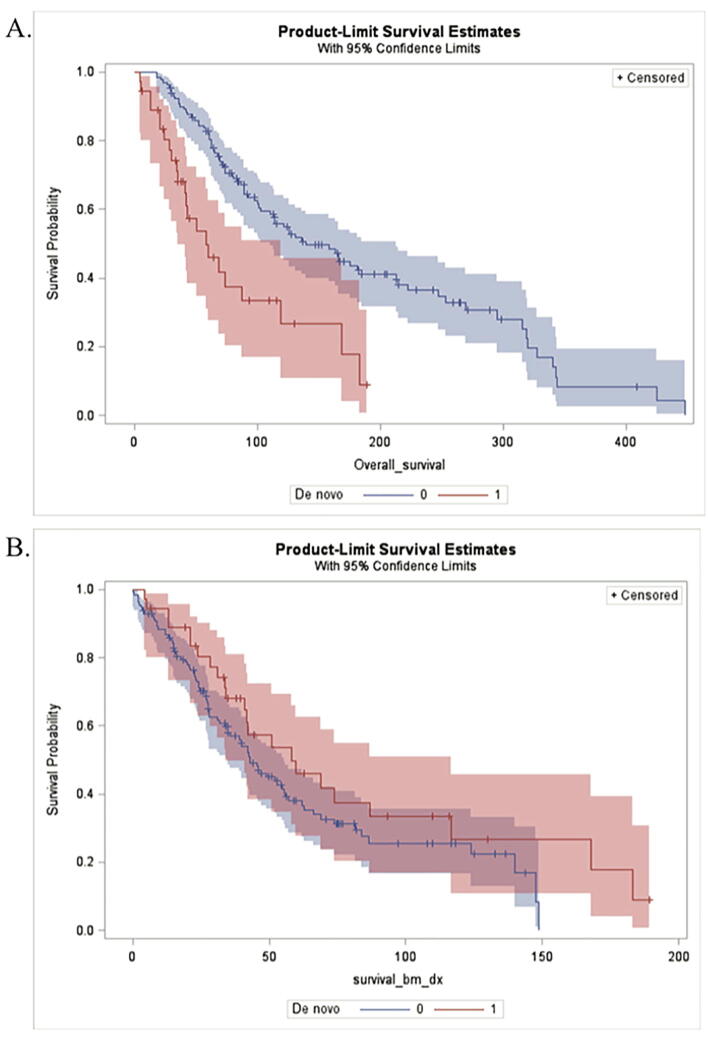
Median OS was 121.2 months (95% CI: 92.7, 165.7) (Fig. 3A), and median BMS was 45.9 months (95% CI: 39.9, 56.9) (Fig. 3B). Median OS for patients with de novo BoM was 58.3 months (95% CI: 34.9, 118.8) compared with 139.7 months (95% CI: 110.1, 212.6) in patients with asynchronous BoM (Fig. 4A). Median BMS was higher in patients with de novo disease compared to patients with asynchronous metastases although this difference was not statistically significant (HR = 0.69, p-value = 0.14) (Fig. 4B, Table 5). Overall mortality was much higher in patients with de novo BoM; this group was almost three times more likely to die within the study period than those with asynchronous BoM while controlling for age and primary ER status (HR = 2.62, p = <0.0001). Our multivariable model revealed that de novo status remained associated with lower OS after controlling for age at diagnosis and ER status (Table 6).
Fig. 3.
Kaplan-Meier survival estimates of survival for all patients. A.) Median survival after primary breast cancer diagnosis was 121.2 months (95% CI: 92.7, 165.7). B.) Median survival after diagnosis of BoM was 45.9 months (95% CI: 39.9, 56.9).
Fig. 4.
Kaplan-Meier survival estimates to compare patients with de novo BoM versus those with asynchronous metastases. A.) Median overall survival for patients with de novo BoM was 58.3 months (95% CI: 34.9, 118.8), while median survival for patients with asynchronous BoM was 139.7 months (95% CI: 110.1, 212.6). B.) Median BoM survival for de novo patients was 58.3 months (95% CI: 33.9, 116.7), and 42.9 months (95% CI: 35, 55.6) for those with asynchronous metastases.
Table 5.
Results from univariate Cox proportional hazards model assessing influence of de novo status, age at diagnosis (dx age), receiving surgery, ER status of primary tumor, race, treatment endocrine therapy, chemotherapy, radiation, bone-targeted therapy, and CDK inhibitors on overall survival (OS) and bone metastasis survival (BMS). *Mixed- refers to patients with tumors displaying both ILC and IDC subtypes.
| Parameter | OS |
BMS |
|||
|---|---|---|---|---|---|
| Hazard Ratio | P-value | Hazard Ratio | P-value | ||
| De novo Status | <0.0001 | 2.62 | 0.14 | 0.69 | |
| Age at Dx | 0.0003 | 1.03 | 0.11 | 1.01 | |
| Received Surgery (ref = yes) | 0.2 | 1.29 | 0.03 | 1.52 | |
| ER primary, n = 160 (ref = negative) | 0.03 | 0.5 | 0.001 | 0.32 | |
| Race | African American vs. Caucasian | 0.01 | 2.5 | 0.12 | 1.72 |
| Asian vs. Caucasian | 0.79 | 1.3 | 0.63 | 0.62 | |
| Endocrine Therapy (ref = no) | 0.13 | 0.59 | 0.0001 | 0.27 | |
| Chemotherapy (ref = no) | 0.1 | 3.32 | 0.16 | 2.8 | |
| Radiotherapy, n = 163 (ref = no) | 0.48 | 0.79 | 0.76 | 0.9 | |
| Bone-targeted therapy, n = 165 (ref = no) | 0.25 | 0.72 | 0.001 | 0.39 | |
| CDK inhibitor therapy (ref = no) | 0.08 | 0.7 | 0.003 | 0.56 | |
| Histological Subtype, n = 157 | ILC vs IDC | 0.32 | 1.4 | 0.81 | 1.1 |
| Mixed* vs IDC | 0.02 | 2.4 | 0.01 | 2.7 | |
Table 6.
Results from multivariable Cox proportional hazards model reveals significant parameters assessing influence of de novo status, age at diagnosis, and ER status of primary tumor on overall survival (OS).
| Parameter | OS |
||
|---|---|---|---|
| P-value | 95% CI | Hazard Ratio | |
| De novo Status | 0.002 | 1.36, 3.81 | 2.27 |
| Age at Dx | 0.001 | 1.01, 1.05 | 1.03 |
| ER primary, n = 160 (ref = negative) | 0.005 | 0.21, 0.75 | 0.39 |
4.3. Age, receptor status, and treatment as prognostic factors
Multivariable modeling revealed that increased age at diagnosis was associated with decreased OS (HR = 1.03, 95% CI: 1.01, 1.05, p = 0.001) while controlling for de novo status and primary ER status. ER-positivity in primary tumors was associated with increased OS (HR = 0.5, p = 0.03) and BMS (HR = 0.32, p = 0.001). Patients with an ER+ primary tumor were 61% less likely to die within the study period than patients with ER− primary tumors (HR = 0.39, 95% CI: 0.21, 0.75, p = 0.005) (Table 6). African American patients exhibited decreased OS (HR = 2.5, p = 0.01) compared with Caucasian patients. Patients receiving endocrine (HR = 0.27, p = 0.0001), bone-targeted (HR = 0.39, p = 0.001), and CDK-inhibitor (HR = 0.56, p = 0.003) therapies were more likely to survive after BoM diagnosis (Table 5).
Patients treated with endocrine therapy demonstrated increased BMS and were 67% less likely to die after BoM diagnosis than those who did not receive endocrine therapy, while controlling for use of surgery and bone-targeted therapy (HR = 0.33, 95% CI: 0.14, 0.82, p = 0.02). Patients who received bone-targeted therapy were 54% less likely to die after BoM diagnosis than those who did not while controlling for use of endocrine therapy and surgery (HR = 0.46, 95% CI: 0.23, 0.93, p = 0.03). Patients with mixed invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) histological subtypes were over 3 times more likely to die after BoM diagnosis than patients with IDC or ILC alone while controlling for use of endocrine therapy, bone-targeted therapy, and surgery (HR = 3.49, 95% CI: 1.65, 7.41, p = 0.001) (Table 7).
Table 7.
Results from multivariable Cox proportional hazards model reveals significant parameters assessing influence of receiving surgery, endocrine therapy, bone-targeted therapy, and histological subtype on bone metastasis survival (BMS). *Mixed- refers to patients with tumors displaying both ILC and IDC subtypes.
| Parameter | BMS |
|||
|---|---|---|---|---|
| P-value | 95% CI | Hazard Ratio | ||
| Received Surgery (ref = yes) | 0.003 | 1.24, 2.92 | 1.9 | |
| Endocrine Therapy (ref = no) | 0.02 | 0.14, 0.82 | 0.33 | |
| Bone-targeted therapy, n = 165 (ref = no) |
0.03 | 0.23, 0.93 | 0.46 | |
| Histological Subtype, n = 157 | ILC vs IDC | 0.23 | 0.76, 3.20 | 1.56 |
| Mixed* vs IDC | 0.001 | 1.65, 7.41 | 3.49 | |
4.4. Surgical treatment as a prognostic factor
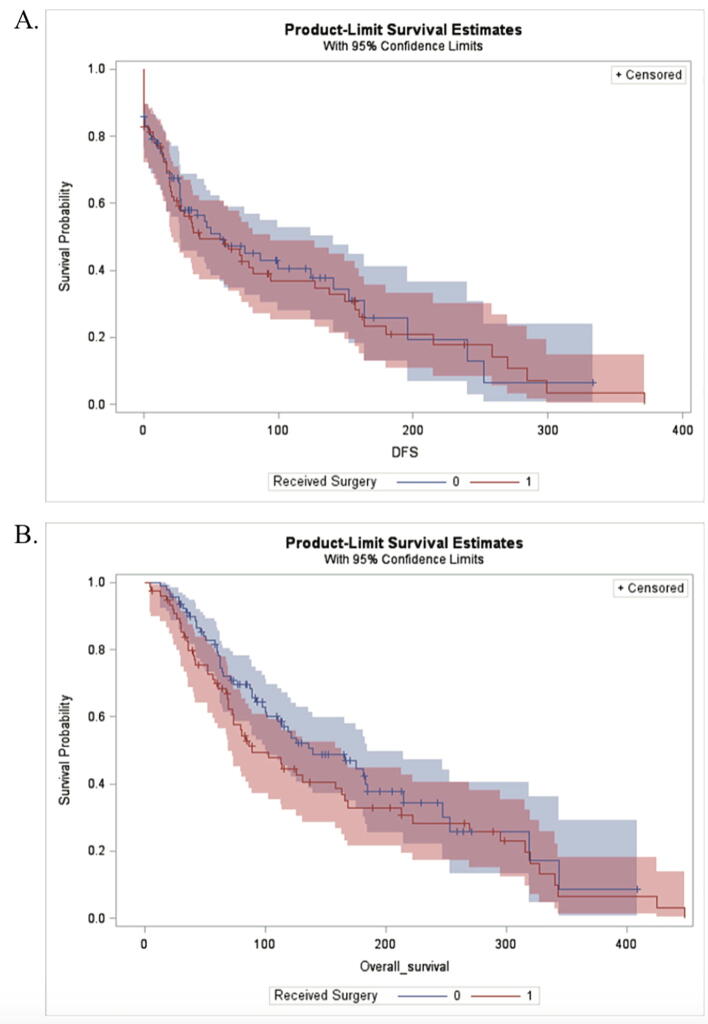
Kaplan-Meier (KM) survival estimates were used to compare patients treated with and without surgery. Median OS for operative patients was 88.9 months (95% CI: 69.6, 163.7) and for non-operative was 139.7 months (95% CI: 99.8, 184.3). Median BMS for operative was 39.9 months (95% CI: 27.9, 45.9), and 59.5 months (95% CI: 42.3, 86.4) for non-operative. Overall, patients that received surgery had decreased OS and BMS (Fig. 5).
Fig. 5.
Kaplan-Meier survival estimates to compare patients that received surgery versus those that did not have this in their treatment plan. A.) Median overall survival for operative was 88.9 months (95% CI: 69.6, 163.7), while median survival for non-operative was 139.7 months (95% CI:99.8, 184.3). B.) Median BoM survival for operative was 39.9 months (95% CI: 27.9, 45.9), and 59.9 months (95% CI: 42.3, 86.4) for non-operative.
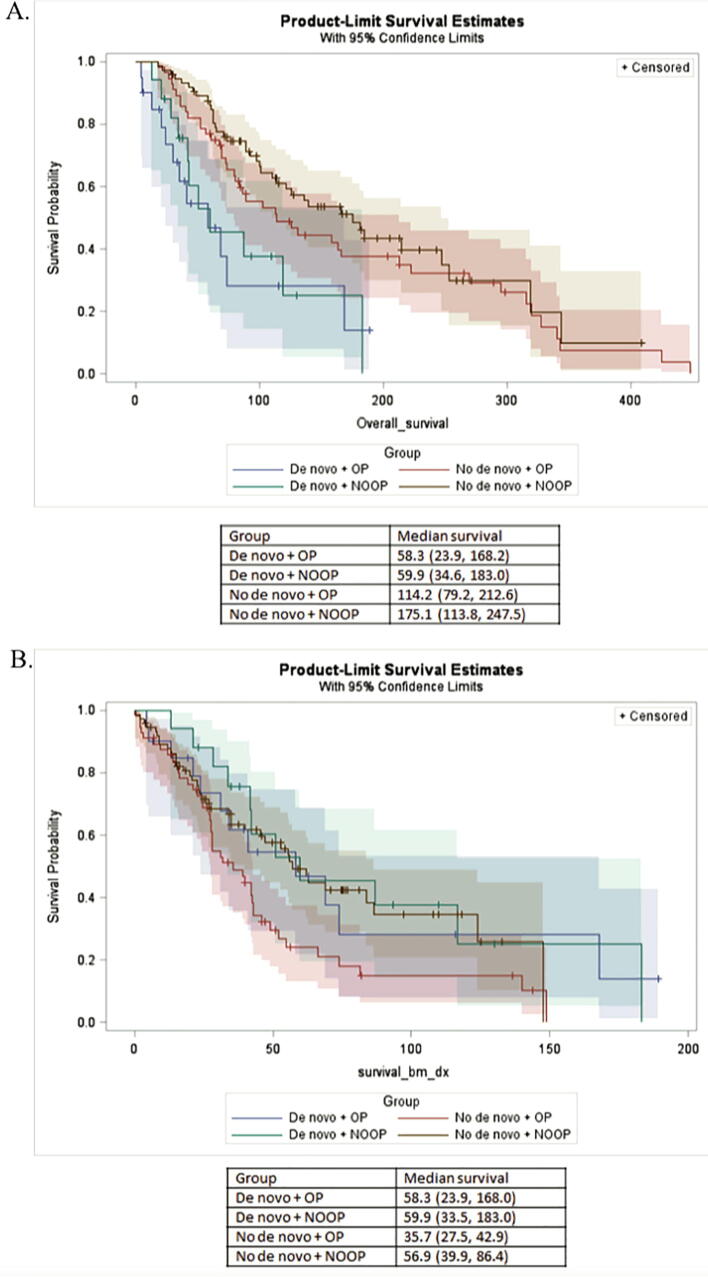
KM plots were also utilized to compare survival between patients with de novo disease and those with asynchronous BoM who were treated with and without surgery (Fig. 6). Median OS for patients with de novo disease treated operatively was 58.3 months (95% CI: 23.9, 168.2) compared with 59.9 months (95% CI: 34.6, 183) in patients with de novo disease treated nonoperatively. In patients with asynchronous metastases, those treated operatively had an OS of 114.2 months (95% CI: 79.2, 212.6) compared with 175.1 months (95% CI: 113.8, 247.5) for those treated nonoperatively. Patients with de novo metastases treated operatively were roughly 3 times more likely to die within the study period than those without de novo metastases who were treated nonoperatively (p = 0.0005). Furthermore, operative patients with de novo metastases were 2.5 more likely to die within the study period than operative patients without de novo disease (p = 0.01). Patients with asynchronous metastases treated operatively were 73% more likely to die within the study period than those treated nonoperatively (p = 0.01).
Fig. 6.
Kaplan-Meier survival estimates to compare patients with de novo or asynchronous metastases that received surgery versus those that did not have this in their treatment plan. A.) Median overall survival for de novo, operative was 58.3 months, while median survival for de novo, non-operative was 59.9 months. Asynchronous metastases, operative was 114.2 months, while non-operative was 175.1 months. B.) Median BoM survival for de novo, operative was 58.3 months, while median survival for de novo, non-operative was 59.9 months. Asynchronous metastases, operative was 35.7 months, while non-operative was 56.9 months. OP = operative, NOOP = non-operative.
5. Discussion
Review of the basic clinical characteristics of this series of patients corroborates the findings reported previously in the literature. The mean age of breast cancer diagnosis in our study was 54 years. In similar cohorts of patients with breast cancer BoM, median age has been found to be between 46 and 67 years [18], [19], [20], [21], [22], [23]. Our analysis demonstrated that older age at diagnosis is a poor prognostic factor for overall survival, an association previously found in some series [24] but not others [21].
In our cohort, median OS was 121.2 months (10.1 years). Previous studies have reported survival of 7–9 years in patients with breast cancer BoM [21], [22]. Median survival after BoM diagnosis has been reported to be 2.7 years [19], which was lower than the 45.9 months (3.8 years) BMS we observed in our patient population. This may be due to the evolving treatments and modern multimodal therapeutic approaches for metastatic disease.
We observed a high percentage of ER+ cases in our sample, reflecting our cohort’s consistency with the well-established tendency for ER+ breast cancer to metastasize to bone [24], [25], [26], [27]. BoM occurs predominantly in ER+ breast cancers, and generally maintains ER+ receptor status [12], [20], [28], [29], [30]. Our assessment of receptor discordance, which was established if the ER or HER2 status of the primary tumor was not mirrored in the BoM histology, demonstrated that it was more common for ER+ tumors to switch to ER− metastases than the converse. This is consistent with other reported literature [31], [32] and may be associated with acquired endocrine therapy resistance [33], [34], [35]. Previous research has found a strong association between ER status and survival in patients with BoM [24], which is recapitulated by our findings: ER-positivity was associated with longer OS (HR = 0.5, p = 0.03) and BMS (HR = 0.32, p = 0.001).
Patients with de novo skeletal metastases represented 22.2% of patients in our group, which is similar to what has been found by others: in a study of 1445 patients with bone-only metastases, Parkes et al. found 31% of patients to have de novo BoM [22]. In a series of patients with Stage IV breast cancer, Shen et al. found that 35% of patients had de novo metastatic disease, and about half of these were de novo bone lesions [36]. The Cox proportional hazards model demonstrated that patients diagnosed with BoM at the time of breast cancer diagnosis were almost three times more likely to die than women with asynchronous metastases, which is consistent with previous results [20], [24].
Patients treated nonoperatively had better BMS (p = 0.03). Interestingly, we reported that patients who received bone-targeted therapy were 54% less likely to die after BoM diagnosis than those who did not while controlling for use of endocrine therapy and surgery. This finding was unexpected, and its explanation is likely multifactorial. At our institution, bone-targeting therapy (BTT) in the form of bisphosphonates or denosumab is uniformly employed in patients with bone metastases. Exceptions are made in rare cases of allergy, patient refusal for concerns of side effects, or history of poor dentition or other serious dental issues. Patients treated operatively probably had more aggressive lesions (i.e. lytic, larger in size, completed fracture, weight-bearing bone, more painful), which would have prompted the decision to proceed with surgical intervention. We investigated this in more detail and found that patients treated operatively were more likely to have lytic or mixed lesions compared to blastic lesions and they occurred more frequently in their lower extremities than other areas. Surgical intervention was not associated with a difference in OS or BMS in patients with de novo BoM. These findings reflect the complex, highly personalized care decision to involve surgical treatment; timing of metastasis has not been shown to be a useful factor in directing the decision to proceed with surgical intervention. Rather, the lesion’s characteristics, location, size, and associated symptoms along with the patient’s performance status directs operative versus nonoperative treatment. At our Institution, fellowship-trained musculoskeletal oncologists employ a system similar to that described by Mirels, et al. [37], [38] who espoused a multifaceted approach to surgical decision-making in patients with osseous metastases. Lesion characteristics refer to the lesion’s radiographic appearance as lytic, blastic, or mixed. Purely lytic lesions present a greater threat to skeletal integrity and typically warrant a more aggressive approach than mixed or lytic disease. Tumor location is in itself multifaceted as both the location of the affected bone and the location of disease within that bone are important in surgical decision-making. For example, lower extremity long bones (femur, tibia) are typically approached much more urgently than upper extremity bones (humerus, ulna, radius) for the simple reason that ambulation is crucial for nearly all activities of daily life. A lower extremity pathological fracture is therefore a devastating event that should be prevented as it would likely render the patient immobile, bed-bound, and in considerable pain. Additionally, the location within the bone is important as cortical bone accounts for a greater proportion of the bone’s structural integrity than intermedullary bone. Cortically destructive lesions are therefore more worrisome from a biomechanical standpoint. The most important associated symptom is pain. Patients who experience pain with every step and/or pain at rest are closer to an adverse skeletal event than patients who only have occasional or activity-associated pain. All of these factors, along with the patient’s goals and wishes are weighed, and evaluated for a personalized approach to each patient’s particular disease.
This study has several limitations. It is a retrospective analysis with a relatively small sample size, and certain variables, such as treatment, were relatively homogenous among the group based on current treatment standards. The majority of our patients had ER+/HER2− breast tumors, with many of them receiving similar systemic therapy, thus limiting our ability to detect differences in outcome based on specific treatment regimens. However, the relatively uniform combination of therapies received by most patients, including surgical intervention by four board-certified, fellowship-trained orthopaedic oncology surgeons with consistent medical and radiation oncology collaboration from a single institution may have assisted in limiting confounding factors in the survival analysis.
The majority of our patients were Caucasian, which reflects the demographic of our geographical catchment area of southwestern Pennsylvania. Even so, barriers related to socioeconomic factors for underrepresented minorities [39] as well as a racial survival disparity for African American women compared to their Caucasian counterparts has been previously described [40]. We observed that African American women had decreased OS (p = 0.01) compared with Caucasian women. These data must be interpreted with caution by virtue of the retrospective nature and data source, missing values for key prognostic factors, and the aforementioned demographic factors inherent to our study population. Additional research is needed in the form of larger, prospective studies that are powered to explore the possible contributions of race to differences in metastatic outcomes.
To our knowledge, this is the most comprehensive examination of clinical characteristics and prognostic factors in patients with breast cancer BoM treated with and without orthopaedic surgery, and is the first study to look specifically at outcomes associated with operative versus nonoperative treatment in a population of patients with breast cancer BoM. Even though surgery is a commonly rendered treatment for patients with breast cancer bone metastases, we were not aware of any studies in the literature that compared outcomes between surgical and non-surgical patients within the same clinical practice. It seems that our curiosity was warranted, as we would not have predicted that non-operative patients would have better survival. Indeed, the surgery versus non-surgery groups (76 versus 91 patients, respectively) were an almost even split which made comparison between these groups attractive to us.
To date, we have no reliable means of preventing or curing BoM [30], [41] and metastatic disease continues to cause the majority of morbidity and mortality associated with breast cancer. Further investigation of the disease characteristics in these patients and analysis of their outcomes is needed to develop a better understanding of prognostic factors. This will allow providers to better educate patients and direct goals of care.
Future directions include identifying and validating novel genetic therapeutic targets in breast cancer BoM. We have established a prospectively maintained tissue repository to house BoM samples from breast cancer patients. In conjunction with our clinical information, this is a singularly unique resource which provides the potential to identify and validate genomic profiles that could be utilized to uncover novel therapeutic interventions.
6. Ethical Review Committee Statement
This study was approved by the University of Pittsburgh Institutional Review Board committee (STUDY20010034).
CRediT authorship contribution statement
Margaret L. Hankins: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing - original draft, Writing - review & editing. Clair N. Smith: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Beverly Hersh: Investigation, Writing - review & editing. Tanya Heim: Investigation, Writing - review & editing. Rebekah Belayneh: Data curation, Investigation, Visualization, Writing - review & editing. Sean Dooley: Investigation, Writing - review & editing. Adrian V. Lee: Conceptualization, Writing - review & editing. Steffi Oesterreich: Conceptualization, Writing - review & editing. Peter C. Lucas: Investigation, Methodology, Resources, Writing - review & editing. Shannon L. Puhalla: Conceptualization, Writing - review & editing, Writing - review & editing. Kurt R. Weiss: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing. Rebecca J. Watters: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Lucas reports other from Amgen, other from Bayer/Loxo, outside the submitted work. Dr. Puhalla reports personal fees from Abbvie, personal fees from Medimmune, personal fees from Puma, personal fees from Pfizer, grants and personal fees from Astra Zeneca, personal fees from Esai, personal fees from Nanostring, grants and non-financial support from Abbvie, grants from Pfizer, grants from Lilly, grants from Novartis, grants from Incyte, grants from Covance-Bayer, grants from Astra-zeneca, grants from Genentech, grants from Medivation, outside the submitted work. All other authors certify that he or she has no commercial associations that might pose a conflict of interest to this submitted article.
Acknowledgements
We thank Mark Goodman MD, Stella Joo Lee, MD, and Alma Heyl, LAS, RTR, CCRC for assistance with the collection of samples and clinical information, and the patients that consented and donated their tissues for this study. We thank Richard McGough, MD for careful review of our manuscript. We also thank Mr. Jeffrey Martin for his assistance with the ICD-10 codes needed to identify our patient populations of interest for this study. The work was funded in part by the METAvivor Research and Support Inc. Young Investigator Award and the Susan G. Komen Career Catalyst Award (CCR18548418).
Contributor Information
Margaret L. Hankins, Email: hankinsml@upmc.edu.
Clair N. Smith, Email: cns45@pitt.edu.
Beverly Hersh, Email: bev_hersh@mac.com.
Tanya Heim, Email: heimte@upmc.edu.
Rebekah Belayneh, Email: belaynehr2@upmc.edu.
Sean Dooley, Email: Dooley.Sean@medstudent.pitt.edu.
Adrian V. Lee, Email: leeav@upmc.edu.
Steffi Oesterreich, Email: oesterreichs@upmc.edu.
Peter C. Lucas, Email: lucaspc@upmc.edu.
Shannon L. Puhalla, Email: puhallasl@upmc.edu.
Kurt R. Weiss, Email: weiskr@upmc.edu.
Rebecca J. Watters, Email: rjw63@pitt.edu.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herrinton L.J., Barlow W.E., Yu O., Geiger A.M., Elmore J.G., Barton M.B. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J. Clin. Oncol. 2005 Jul 1;23(19):4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 3.Berman A.T., Thukral A.D., Hwang W.-T., Solin L.J., Vapiwala N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin. Breast Cancer. 2013;13(2):88–94. doi: 10.1016/j.clbc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Brockton N.T., Gill S.J., Laborge S.L., Paterson A.H.G., Cook L.S., Vogel H.J. The Breast Cancer to Bone (B2B) Metastases Research Program: a multi-disciplinary investigation of bone metastases from breast cancer. BMC Cancer. 2015;10(15):512. doi: 10.1186/s12885-015-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 6.Tevaarwerk A.J., Gray R.J., Schneider B.P., Smith M.L., Wagner L.I., Fetting J.H. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119(6):1140–1148. doi: 10.1002/cncr.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steeg P.S. Targeting metastasis. Nat. Rev. Cancer. 2016;16(4):201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Body J.-J., Quinn G., Talbot S., Booth E., Demonty G., Taylor A. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit. Rev. Oncol. Hematol. 2017;115:67–80. doi: 10.1016/j.critrevonc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Macedo F., Ladeira K., Pinho F., Saraiva N., Bonito N., Pinto L. Bone metastases: an overview. Oncol. Rev. 2017;11(1):321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Oronzo S., Coleman R., Brown J., Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019;15 doi: 10.1016/j.jbo.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 12.Suva L.J., Griffin R.J., Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr. Relat. Cancer. 2009;16(3):703–713. doi: 10.1677/ERC-09-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casimiro S., Guise T.A., Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell. Endocrinol. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 14.R. Coleman, J.J. Body, M. Aapro, P. Hadji, J. Herrstedt, ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 Suppl 3:iii124–37. [DOI] [PubMed]
- 15.Coleman R., Cameron D., Dodwell D., Bell R., Wilson C., Rathbone E. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15(9):997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 16.Goodman M.A., Weiss K.R. Surgical approach to metastatic bone disease. Oper. Tech. Orthop. 2014;24(2):85–90. [Google Scholar]
- 17.McGough R.L., Goodman M.A., Weiss K.R. Unusual acetabular and proximal femur reconstructions: technical considerations from the orthopaedic oncology perspective. Oper. Tech. Orthop. 2017;27(3):198–206. [Google Scholar]
- 18.Jensen A.Ø., Jacobsen J.B., Nørgaard M., Yong M., Fryzek J.P., Sørensen H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;24(11):29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yavas O., Hayran M., Ozisik Y. Factors affecting survival in breast cancer patients following bone metastasis. Tumori. 2007;93(6):580–586. doi: 10.1177/030089160709300611. [DOI] [PubMed] [Google Scholar]
- 20.Parkes A., Warneke C.L., Clifton K., Al-Awadhi A., Oke O., Pestana R.C. Prognostic factors in patients with metastatic breast cancer with bone-only metastases. Oncologist. 2018;23(11):1282–1288. doi: 10.1634/theoncologist.2018-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson J.D., McNair M., Cribb G.L., Cool W.P. Prognostic factors for patients with skeletal metastases from carcinoma of the breast. Bone Joint J. 2016;98-B(2):266–270. doi: 10.1302/0301-620X.98B2.36185. [DOI] [PubMed] [Google Scholar]
- 22.Parkes A., Clifton K., Al-Awadhi A., Oke O., Warneke C.L., Litton J.K. Characterization of bone only metastasis patients with respect to tumor subtypes. NPJ Breast Cancer. 2018;25(4):2. doi: 10.1038/s41523-018-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diessner J., Wischnewsky M., Stüber T., Stein R., Krockenberger M., Häusler S. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer. 2016;12(16):307. doi: 10.1186/s12885-016-2345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman R.E., Smith P., Rubens R.D. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer. 1998;77(2):336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross-Innes C.S., Stark R., Teschendorff A.E., Holmes K.A., Ali H.R., Dunning M.J. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481(7381):389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James J.J., Evans A.J., Pinder S.E., Gutteridge E., Cheung K.L., Chan S. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br. J. Cancer. 2003;89(4):660–665. doi: 10.1038/sj.bjc.6601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomayer E.F., Diel I.J., Meyberg G.C., Gollan C., Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res. Treat. 2000;59(3):271–278. doi: 10.1023/a:1006308619659. [DOI] [PubMed] [Google Scholar]
- 28.Piggott R.P., Waters P.S., Kerin M.J. The influence of breast cancer subtype on bone metastases development and survival in women with metastatic breast cancer. Ir. J. Med. Sci. 2017;186(1):97–102. doi: 10.1007/s11845-016-1512-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson R.W., Suva L.J. Hallmarks of bone metastasis. Calcif. Tissue Int. 2017;102(2):1–11. doi: 10.1007/s00223-017-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y.-F., Liao Y.-Y., Yang M., Peng N.-F., Xie S.-R., Xie Y.-F. Discordances in ER, PR and HER2 receptors between primary and recurrent/metastatic lesions and their impact on survival in breast cancer patients. Med. Oncol. 2014;31(10):214. doi: 10.1007/s12032-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 32.Meng X., Song S., Jiang Z., Sun B., Wang T., Zhang S. Receptor conversion in metastatic breast cancer: a prognosticator of survival. Oncotarget. 2016;7(44):71887–71903. doi: 10.18632/oncotarget.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angus L., Beije N., Jager A., Martens J.W.M., Sleijfer S. ESR1 mutations: Moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat. Rev. 2017;52:33–40. doi: 10.1016/j.ctrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Toy W., Shen Y., Won H., Green B., Sakr R.A., Will M. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45(12):1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeselsohn R., Yelensky R., Buchwalter G., Frampton G., Meric-Bernstam F., Gonzalez-Angulo A.M. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 2014;20(7):1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen T., Gao C., Zhang K., Siegal G.P., Wei S. Prognostic outcomes in advanced breast cancer: the metastasis-free interval is important. Hum. Pathol. 2017;13(70):70–76. doi: 10.1016/j.humpath.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 37.H. Mirels, Metastatic disease in long bones: A proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin. Orthop. Relat. Res. 2003;(415 Suppl):S4–13. [DOI] [PubMed]
- 38.H. Mirels, Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. Relat. Res. 1989;(249):256–264. [PubMed]
- 39.Haley S.J., Southwick L.E., Parikh N.S., Rivera J., Farrar-Edwards D., Boden-Albala B. Barriers and strategies for recruitment of racial and ethnic minorities: perspectives from neurological clinical research coordinators. J. Racial Ethn. Health Disparit. 2017;4(6):1225–1236. doi: 10.1007/s40615-016-0332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yee M.K., Sereika S.M., Bender C.M., Brufsky A.M., Connolly M.C., Rosenzweig M.Q. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123(11):2061–2069. doi: 10.1002/cncr.30575. [DOI] [PubMed] [Google Scholar]
- 41.Esposito M., Guise T., Kang Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Med. 2018;8(6) doi: 10.1101/cshperspect.a031252. [DOI] [PMC free article] [PubMed] [Google Scholar]