Abstract
Understanding factors influencing conventional medical knowledge (CMK), general attitudes and risk perceptions of zoonotic diseases among rural residents who face risk of exposure to such diseases is important for human, livestock, and wildlife health. Focusing on Maasai from Makame, Kiteto District (Tanzania) who largely maintained a semi-nomadic lifestyle, we evaluated respondents’ CMK of causes, symptoms, treatments, and prevention methods of rabies, brucellosis, and anthrax. In addition, we identified socio-demographic correlates of CMK with respect to the target zoonoses. Finally, we assessed the relative frequency of practices that increase the risk of pathogen transmission, and compared the risk perception of the three diseases. We conducted structured interviews with Maasai respondents (n = 46) in six sub-villages of Makame and considered education, gender, age, and wealth (indicated by standardized number of livestock) as potential correlates of CMK. Respondents had greater CMK of rabies and anthrax, but feared anthrax the most. Receiving formal education increased rabies CMK (p ≤ 0.05). The CMK of anthrax and brucellosis was not associated with any of the tested variables (p > 0.05). Risk perceptions were correlated with knowledge scores for rabies and anthrax (p ≤ 0.05), and multiple interviewees reported engaging in practices that potentially enhance pathogen transmission. Specific socio-demographic attributes (i.e., formal education) may explain the observed variation in CMK of zoonotic diseases. This information can be used to develop and tailor health education programs for specific at-risk groups.
Keywords: Zoonotic disease knowledge, Risk perception, Rabies, Anthrax, Brucellosis
Zoonotic disease knowledge; risk perception; rabies; anthrax; brucellosis.
1. Introduction
Almost two-thirds of human infectious diseases are zoonotic and circulate across the wildlife-livestock-human interface [1]. High rates of spatio-temporal interactions between humans, their livestock and wildlife species enhance the risk of pathogen exposure and transmission [2]. Pathogens can be transmitted from wildlife to livestock and, ultimately, to humans as a result of habitat encroachment due to deforestation and expansion of livestock farming [3, 4]. Zoonoses can lead to decreased productivity or death which can negatively affect peoples' livelihoods and, subsequently, national economies [5]. Tanzania is one of the most biodiverse countries in the world, harboring c. 20% of Africa's large mammals [6]. Concomitantly Tanzania has the third largest population of livestock on the African continent. Because livestock and wildlife species often share the same pastures, livestock and pastoralists are potentially at high risk of infection with zoonotic pathogens. However, the central role of livestock herders in the wildlife-livestock interface also predestine them to be key actors for preventing zoonotic pathogen transmission [7, 8]. Thus, assessing the conventional knowledge, the general attitudes and risk perceptions of livestock herders towards zoonotic diseases is a first step in understanding and managing zoonotic diseases [2, 9, 10].
In northern Tanzania, parts of the Maasai ethnicity maintain a predominantly pastoralist lifestyle [11] and across most of their range (aided by their relatively high tolerance for most wildlife species), Maasai share their lands with species rich wildlife communities [12, 13, 14]. It is important to conduct studies on local knowledge and perceptions of zoonoses in such regions where individuals have limited access to professional health services, yet practice traditional lifestyles with nomadic or semi-nomadic pastoralism in close proximity to wildlife populations. People residing in such locations are potentially at greater risk of exposure to zoonoses and face increased challenges towards receiving professional health care.
Rabies, brucellosis, and anthrax are currently prioritized as the greatest national concern for Tanzania by the U.S. Centers for Disease Control and Prevention (CDC) in collaboration with Tanzanian officials representing the Ministry of Health, the Ministry of Livestock and Fisheries, and the Tanzanian Wildlife Research Institute, [6]. Prioritization was determined according to the diseases’ prevalence, potential social and economic impacts, available interventions, epidemic or pandemic causing potential, and the severity of the disease when acquired by humans.
Rabies is a fatal viral disease circulating in mammals, primarily dogs, which are the main source of exposure to humans in East Africa. Symptoms of rabies include fever, discomfort, headache, acute neurological symptoms, and death [15]. An estimated 90–400 deaths per year are attributed to rabies in Tanzania [6] where the population was approximately 58 million people as of 2019 [16]. Although rabies predominantly circulates in domestic dog populations in Tanzania, there are also frequent cases in wildlife populations [17]. Over 30% of unvaccinated dogs in northern Tanzania tested seropositive for rabies in one study [18]. The Maasai have frequent interactions with wildlife and as they use domestic dogs for herding, rabies is a potential threat to domestic dogs, livestock, and humans.
Brucellosis (Brucella spp.) is a bacterial zoonotic infection. Symptoms of brucellosis include fever, sweats, malaise, anorexia, headache, muscle and joint pain, swelling, fatigue, and depression [19]. From 2012 to 2014 in the southern highlands, northern zone, and eastern zones of Tanzania the incidence of brucellosis was 28% in humans [20]. In cattle and goats, the prevalence of brucellosis was 4–22% as of 2017 [6]. Humans can acquire brucellosis from direct contact with infected animals, via ingestion of infected food (e.g., consumption of raw milk or meat) and by aerosols [21]. Prior research has demonstrated that residents in pastoral communities in Tanzania reported that interactions between livestock and wildlife as well as the sharing of pastures and water supplies with wildlife have led to the transmission of brucellosis [10]. Although most human cases of brucellosis are contracted from livestock, transmission from wildlife cannot be dismissed in regions with high wildlife populations [22].
Anthrax (Bacillus anthracis) is another bacterial zoonotic infection that can be fatal. Symptoms include blisters, itchiness, ulcers, fever, chills, chest pain, cough, headache, dizziness, bloody coughs and vomit, diarrhea, and swelling [23]. In herbivores, anthrax typically presents as septicemia resulting in sudden death. Anthrax is contracted through contact with infected carcasses or through consumption of undercooked products from infected animals. Herbivores acquire anthrax through exposure to bacterial spores [24]. In parts of Tanzania, frequent outbreaks of anthrax have been reported in humans, livestock, and wildlife [25]. As of 2018, the prevalence of anthrax cases in humans in the Arusha region of northern Tanzania was about eight per 100,000 [25]. In northern Tanzania, there can be ten cases of human contracted anthrax per single infected animal carcass [6]. Anthrax can be indirectly transmitted to humans from wildlife. As anthrax spores can remain in the soil for long periods of time, the movement of livestock herds close to infected wildlife populations increases potential for infection [25]. To the best of our knowledge, data on the prevalence of rabies, brucellosis, and anthrax are not available for the Makame district.
In coupled social-ecological landscapes, such as Makame Wildlife Management Area (WMA1), understanding what factors contribute to conventional medical knowledge (CMK) of zoonotic diseases may help inform healthcare policies and programs geared at improving the prevention of rabies, brucellosis, and anthrax. Thus, the objectives of this study were (I) to determine the conventional knowledge of causes, symptoms, treatment, and prevention for each of the three diseases studied in Makame, Tanzania (II) to identify which factors (i.e. gender, age, education level, and wealth), lead to higher conventional knowledge scores for each disease (III) to assess the relative frequency of risky behaviors for pathogen transmission, and (IV) to determine the effect of knowledge on risk perceptions of the diseases.
2. Materials and methods
2.1. Study area
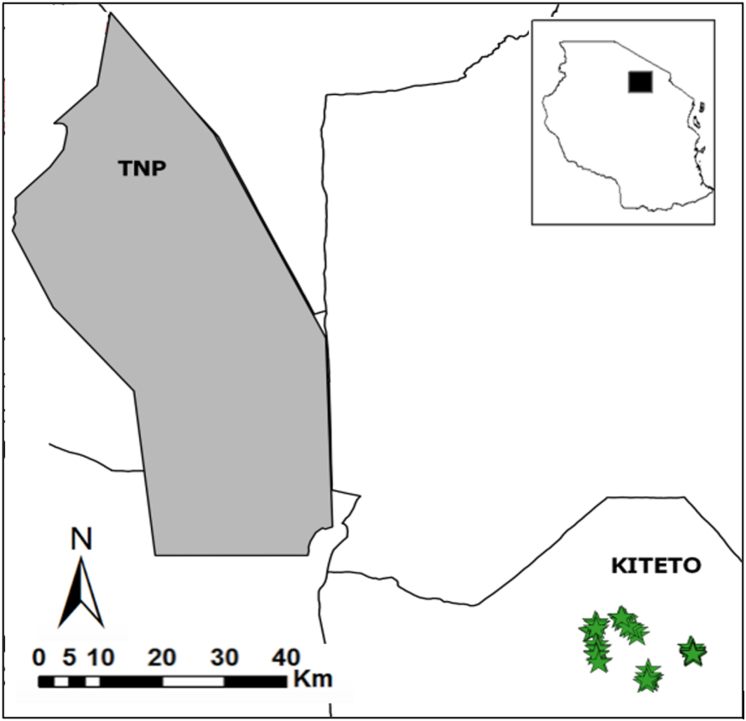
As our study site, we chose six sub-villages of Makame, a village in Kiteto District, mostly inhabited by members of the Maasai ethnicity who have retained many of their traditional practices and predominantly retain a semi-nomadic lifestyle. Makame village is part of the Makame WMA which lies southeast of Tarangire National Park (TNP) (Figure 1). The wildlife management area is characterized by different land-use zones (e.g., specific areas set aside for either human settlement, livestock grazing, or trophy hunting). The Maasai residents of this area typically rely on livestock production and they seasonally shift grazing areas of their livestock. Livestock is herded by humans and dogs, and no fences are used during daytime. At night, livestock is typically corralled in livestock enclosures made of thorn bushes (“boma(s)”). The area supports a mostly intact large mammal community with several wildlife species occurring at relatively high densities creating substantial potential for direct and indirect livestock-wildlife contacts. According to a recent camera trap study, wildlife occurs at relatively high densities [26]. Wildlife species include a substantial elephant population (Loxodonta africana), multiple ungulate species, such as zebras (Equus quagga), wildebeest (Connochaetes taurinus), Kirk's dik-dik (Madoqua kirkii), lesser kudu (Tragelaphus imberbis), and bush duiker (Sylvicapra grimmia). Among carnivores, the area supports a variety of species, including (and not limited to) aardwolves (Proteles cristata), leopards (Panthera pardus), spotted hyenas (Crocuta crocuta), striped hyenas (Hyaena hyaena), African lion (Panthera leo) and African wild dogs (Lycaon pictus) [26].
Figure 1.
Map of the study area showing surveyed households (n = 46), represented by stars, and Kiteto district in Tanzania. Some stars are overlapping due to the scale of the map and proximity of households. Kiteto is located southeast of Tarangire National Park (TNP). The black rectangle in the inset indicates the approximate location of the study area.
2.2. Disease selection
Of the 39 zoonotic diseases considered for prioritization Tanzania by the CDC, we chose rabies, brucellosis and anthrax as the focus of our study in Makame, Tanzania because the pastoralist lifestyle practiced in the region potentially exposes humans to pathogens that cause these three diseases [6]. Additionally, communications with a local physician, Dr. F. Artress (FAME Africa, a hospital located in Karatu, northern Tanzania), indicated that patients in northern Tanzania are occasionally diagnosed with rabies, anthrax or brucellosis.
2.3. Ethics statement
Research was conducted with permission from the Tanzania Commission for Science and Technology (COSTECH) and Tanzania Wildlife Research Institute (TAWIRI) (permit # 2019-92-NA-2013-191), with supporting letters from Kiteto district; the publication of this manuscript was approved by TAWIRI. All interviewees were informed that participation was voluntary, that information was anonymized and there would be no compensation. All interviewees expressed verbal consent prior to interviews being conducted. The right to end the interview at any point and anonymity, with no data shared that could individually identify participants, was assured. Interviewees were household members that were at least 18 years old.
2.4. Interview protocol
Surveys within Kiteto district were completed in April 2019. A total of 46 interviews were carried out with the help of local guides, who were employees of the Makame WMA, and translators. The local guides spoke Maa (the language of Maasai) and were familiar with the region. Their role was to provide the researchers local directions to sub-villages and joined researchers on interviews to translate from/to Maa directly from/to English. Sample size was determined by the limited availability of households in Makame village as the region is sparsely populated. To represent potential spatial differences in CMK, we conducted interviews in six sub-villages of Makame.
The GPS coordinates were recorded at each interview site. The questionnaire was pre-tested with local guides to ensure that the translators and researchers were aware of key words and the specific Maa terminology for each disease. Meanings of questions were agreed upon between translators and investigators before interviews began. For each day of data collection, researchers conducted interviews in different sub-villages of Makame. In each sub-village surveyed, households of at least 100-meter intervals were selected to participate in the interview and a member of the household was asked for voluntary participation and verbal consent. If consent was obtained, translators conducted the interviews in Swahili or Maa according to the respondents preferred language. Interviews were conducted outside of the household in the presence of the researcher, the local guide, the translator, and other household members or neighbors who wanted to listen. Responses were immediately translated into English and were recorded in English [2].
A predetermined questionnaire was used to structure and conduct interviews (Appendix A.1). The questionnaire (Appendix A.1) was adapted from Kiffner et al. (2019) [2], and utilized individual, quantitative, closed-ended questions and open-ended questions that were scored for inclusion of specific information to allow for quantitative analysis. First, basic demographic information (age, gender, religion, education, ethnicity, number in household, number of cattle/shoats/donkeys/dogs/bicycles/motorcycles/sofas/radios/TVs, agriculture acreage) was collected from the interviewee. Then, questions that assessed the respondents’ CMK regarding three zoonotic diseases [Maa name for diseases; rabies: Alaitirwa looldiaini; brucellosis: Orkibiroto (disease in humans: Emoyian e kule); and anthrax: Engirowaji (disease in humans: Emporoto)] were asked. Respondents were initially asked if they heard of the disease, and if so, if they knew if it affected humans, domestic and/or non-domestic animals, or both. Respondents were also asked to identify the causes and transmission routes, associated clinical symptoms, possible treatment methods, and prevention measures of the disease. Next, interviewees were asked if they took part in pre-determined actions or behaviors, including milk and meat consumption (raw/cooked/both) or dog ownership (Appendix A.1). To conclude the interview, respondents were asked to rank each disease according to fear for livestock and human health; the highest ranked disease (3) is the disease the respondent feared the most. After interviews were completed, the responses were scored to CMK points against a modification of the scoring system outlined in Kiffner et al. (2019) [2] (Appendix B.1). The adjustments involved rescaling to max. scores of seven for rabies, nine for anthrax, and ten for brucellosis to account for a point allocation per question (Appendix B.1).
2.5. Data analysis
All data were analyzed using RStudio [27]. The CMK scores were obtained using the scoring system in Appendix B.1. The CMK scoring comprised responses to the questions: “Is the disease observed in humans, animals, or both?; What are the symptoms of the disease?; What causes the disease?; What is the treatment for the disease?; Have you sought professional help for the disease?; What are prevention methods for the disease?” The proportion of CMK (points acquired/total points available) was used to represent CMK scores in graphs. Box plots were developed to visualize the overall CMK proportion for each disease for respondents and to assess the attitude/risk perception of each disease according the rank that respondents gave the disease. Data points were included in analyses if they were within two standard deviations of the mean when calculating medians for box plots.
We determined the influence of different variables on CMK2 using separate linear mixed models for each of the three diseases. The target variable for the model was CMK score for each disease. Sub-village was included as a random effect to account for similarities in households within a sub-village and thus to account for potential non-independence between households. Additionally, prior to selecting explanatory variables, pairwise correlation tests were run to assess collinearity. Because the hypothesized explanatory variables were not significantly correlated (Appendix C.1), all of them were considered for hypothesis testing. Livestock ownership was not included in the model because all of the respondents owned livestock. Explanatory (fixed) variables in the linear mixed model for CMK of rabies, brucellosis, and anthrax included education, age, gender, and tropical livestock unit (TLU)3.
Proportions of respondents participating in certain risk behaviors (i.e., dog ownership, milk consumption, meat consumption) were calculated. To determine the association strength between perception rank and the CMK scores, Kendall's rank correlation tests were used [27]. Risk behavior questions and rank were not included in CMK scores.
3. Results
3.1. Socio-demographic characteristics of respondents
Respondents from the 46 households interviewed were not evenly represented by gender (males, n = 32 and females, n = 14) (Table 1). All respondents belonged to the Maasai ethnicity (n = 46) and had received varying amounts of formal education. All respondents owned livestock, although there was a large range in herd sizes. Additional demographic information and details regarding respondents are presented in Table 1.
Table 1.
Demographic information of interviewees in Makame, Tanzania.
| Number of Respondents (n = 46) | Proportion of Respondents | Mean | Range | |
|---|---|---|---|---|
| Gender | ||||
| Male | 32 | 0.70 | -- | -- |
| Female | 14 | 0.30 | -- | -- |
| Age (years) | -- | -- | 46 | 18–90 |
| Education | ||||
| None | 24 (males n = 14, females n = 10) | 0.52 | -- | -- |
| Primary | 20 (males n = 16, females n = 4) | 0.43 | -- | -- |
| Secondary and above | 2 (males n = 2, females n = 0) | 0.04 | -- | -- |
| Livestock Ownership | ||||
| Number of cattle | -- | -- | 110 | 1–900 |
| Number of goats/sheep | -- | -- | 128 | 0–2000 |
| Number of donkeys | -- | -- | 7 | 0–50 |
3.2. Zoonotic disease CMK
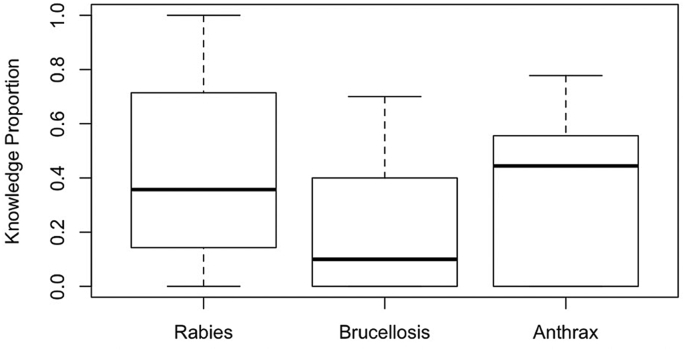
On average, respondents had the greatest median CMK of anthrax, followed by rabies and brucellosis. For all three diseases considered, the majority of respondents scored fewer than half of the CMK points. Among the interviewees, CMK was most variable for rabies, whereas the spread of CMK was narrower for anthrax and especially for brucellosis (Figure 2).
Figure 2.
Boxplot indicating the proportional conventional medical knowledge of rabies, brucellosis and anthrax among all respondents (n = 46) in Makame, Tanzania.
3.3. Demographic correlates of CMK
The two interviewees that had a secondary education were excluded from the linear mixed model analysis. With the remaining 44 respondents, none of the considered demographic explanatory variables yielded a significant (all p-values ≤ 0.05) signal for the anthrax or brucellosis models (Table 2). In the rabies model the education variable yielded a significant (p ≤ 0.05) correlation with CMK. Coefficient estimates of the models reflect the average changes in the number of CMK points earned according to each explanatory variable. For rabies, education compared to no education increased CMK score by about 24.2%. Gender, TLU, which is an indicator for wealth in the context of Maasai, were not significantly associated (p > 0.05) with CMK for any of the diseases (Table 2).
Table 2.
Coefficient estimates, standard errors, degrees of freedom, t-values, and p-values of socio-demographic variables associated with conventional medical knowledge of three diseases among Maasai in Makame, Tanzania, estimated with a general linear mixed effects model. The random effect was sub-village (n = 6). Significant values (p ≤ 0.05) are italicized. The linear mixed model was based on 44 respondents (i.e. excluding two interviewees with secondary education).
| Estimate | Std. Error | df | t-value | P-Value | |
|---|---|---|---|---|---|
| Rabies (Total points = 7) | |||||
| Education | 1.695 | 0.565 | 35.715 | 3.002 | 0.005 |
| Age | 0.007 | 0.018 | 38.490 | 0.394 | 0.696 |
| Gender (male vs. female) | 0.942 | 0.575 | 35.375 | 1.638 | 0.110 |
| TLU∗ |
-0.001 |
0.002 |
37.603 |
-0.343 |
0.733 |
| Brucellosis (Total points = 10) | |||||
| Education | 0.864 | 0.622 | 35.148 | 1.391 | 0.173 |
| Age | -0.010 | 0.019 | 38.400 | -0.543 | 0.590 |
| Gender (Male vs. female) | 0.605 | 0.633 | 34.755 | 0.957 | 0.345 |
| TLU |
0.001 |
0.002 |
37.353 |
0.639 |
0.527 |
| Anthrax (Total points = 9) | |||||
| Primary Education | 0.612 | 0.663 | 34.62 | 0.923 | 0.363 |
| Age | 0.028 | 0.021 | 37.39 | 1.392 | 0.181 |
| Gender (Male vs. female) | 0.602 | 0.674 | 34.34 | 0.893 | 0.378 |
| TLU | 0.001 | 0.002 | 36.30 | 0.059 | 0.954 |
Tropical Livestock Unit (TLU) is used as an indicator for wealth.
3.4. Risk practices and behaviors associated with zoonotic diseases
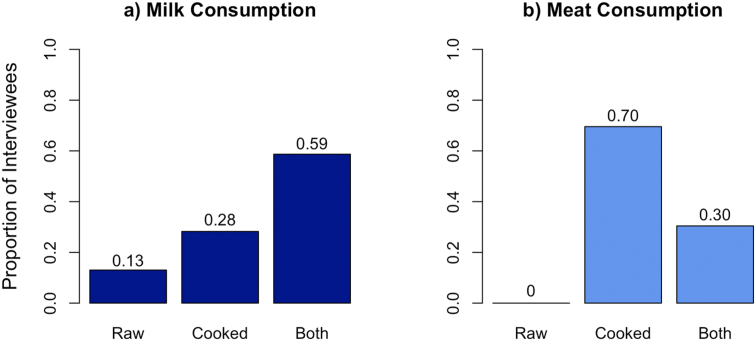
A majority of respondents reportedly consumed raw milk either as the only form of milk or in addition to cooked milk (72%) (Figure 3a). Thirty percent of respondents reported to eat both raw and cooked meat (Figure 3b). For households who reported owning dogs (n = 12), it was reported that mostly boys and men cared for the dogs (83%).
Figure 3.
Proportion of preparation of a) milk and b) meat before consumption as reported by Maasai respondents (n = 46) in Makame, Tanzania. Scores indicate the exact proportion of answers.
3.5. Risk perceptions of zoonotic diseases
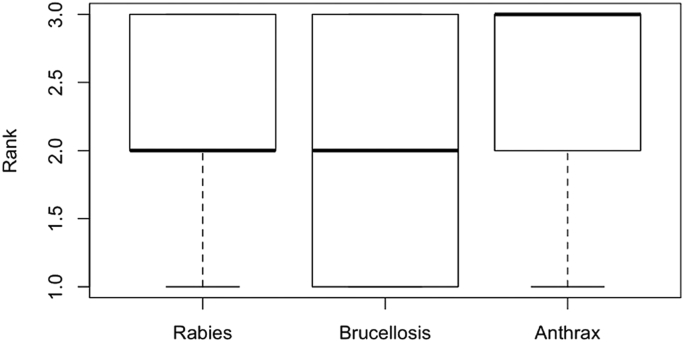
Respondents were asked to rank the zoonotic diseases considered from 1-3, with 3 as the most feared disease and 1 as the least feared disease. Anthrax was the most feared, while rabies and brucellosis had the same median rank (Figure 4). In some interviews, respondents were unfamiliar with a disease and could not rank them, leading to a smaller sample size than the total number of respondents interviewed (Figure 4).
Figure 4.
Boxplots indicating the risk perception rank for rabies (n = 36), brucellosis (n = 31), and anthrax (n = 35) among interviewed Maasai in Makame, Tanzania. A rank of 3 corresponds to the most feared and a rank of 1 corresponds to the least feared disease.
Perception ranks were compared to CMK scores using the Kendall's Rank Correlation; results indicated a significant negative association between CMK proportion and rank for rabies (tau = -0.279; p ≤ 0.05) and a significant positive association in anthrax (tau = 0.293; p ≤ 0.05). Data for brucellosis suggested no trends between CMK score and risk perception.
4. Discussion
This interview-based study in a traditional Maasai community in northern Tanzania revealed variable levels of CMK associated with rabies, brucellosis and anthrax. In addition, the strength of socio-demographic correlates with CMK differed across the three diseases. Practices associated with potentially contracting or transmitting rabies (e.g. dog ownership), brucellosis and anthrax (e.g. consumption of raw milk and meat) were frequently reported by the interviewees, and we observed mixed associations between risk perception of a disease and CMK of the disease.
4.1. Study location
Compared to a similar study on knowledge, perceptions and attitudes towards zoonotic diseases [2], Makame is more remote with several unique features. The study area provided valuable locations to complete this study as its residents are part of a traditional society, are semi-nomadic, and live in close proximity to wildlife. Also, the region is remote with poor access to conventional health services [28]. Practices of switching grazing and residential areas between rainy and dry season, based on unfenced livestock grazing regimes [11, 29, 30], differentiates survey participants from a more resident lifestyle in other parts of their range [2]. Such herding practices – in combination with high wildlife densities – bear potential for high contact rates between domestic livestock, wildlife, and humans [31, 32, 33].
4.2. CMK of zoonotic diseases
Interestingly, Maasai interviewed in Makame had the greatest median CMK about anthrax. Yet, there was wide variation in CMK scores among the interviewees for all three diseases (Figure 2). While Kiffner et al. (2019) also reported substantial variation in CMK scores [2], they reported that respondents had the most CMK regarding rabies, and the least regarding anthrax. This difference to the current study (where interviewees had the highest CMK scores of anthrax) can possibly be explained by differences in actual disease prevalence in the disparate study areas. However, as there are limited datasets available regarding anthrax outbreaks across mainland Tanzania [25], this remains speculative. However, prevalence of anthrax is also attributed to interactions between humans and animals, an essential part of Maasai culture and life [25].
Compared to respondents in the study conducted by Kiffner et al. (2019) in northern Tanzania [2], the Maasai in Makame scored lower on brucellosis and rabies CMK. Clinical manifestation of an illness could influence respondents’ CMK about pathogen transmission between animals and people. For example, anthrax has a short incubation period prior to symptomatic illness, so humans and animals may present symptoms at similar times. Furthermore, the symptoms of anthrax are similar in humans and animals. These factors potentially enable pastoralists to make connections that the same pathogens can be transmitted from animals to humans or that diseases may be present in both animals and humans [34]. This could in turn increase their CMK score.
For diseases with longer incubation periods and/or different symptoms in humans and animals, pastoralists may be less likely to associate the animal disease with human illness. For example, brucellosis has a longer incubation period, ranging from 1-3 weeks to months until clinical presentation [35]. Additionally, our finding of low brucellosis CMK is consistent with previous research that focused on pastoralists in Tanzania. Ntirandekura et al. (2018) observed that pastoral participants had limited knowledge of brucellosis and suggested future research to guide brucellosis’ control and prevention in Tanzania [10].
4.3. Socio-demographic factors and CMK
Regarding socio-demographic factors, studies in Tanzania and across the globe suggest that correlates of zoonotic disease knowledge vary in their importance and strength [2, 9, 36, 37, 38, 39]. Across all three diseases, gender was not a predictor of greater CMK scores when controlling for other variables (Table 2). This could be due to the traditional roles held by males and females in Maasai communities that encourage both genders to deal with livestock (though typically in different gender roles). While males in younger age-sets are responsible for herding and looking after livestock, females are responsible for taking care of the home, milking livestock and providing first aid. In this capacity, females and males would potentially be required to have knowledge about zoonotic diseases [40].
The current study only addressed CMK, whereas Maasai may also have more ethnoveterinary medicine (EVM) knowledge. Ethnoveterinary medicine includes traditional beliefs, knowledge, skills and practices regarding the healthcare of animals [41]. Because pastoral transhumance increases the risk of zoonotic pathogen transmission as a result of potentially more frequent interactions between livestock and wildlife [12], previous researchers have speculated that this persistent disease threat has made EVM knowledge important to be disseminated to all members of a group, including women [41]. Including and exploring EVM knowledge may thus increase knowledge scores for all respondents.
Not considering EVM knowledge may have contributed to results of the present study regarding age. In low income countries, household members are typically dependent on self-diagnosis and self-treatment of their livestock by drawing upon EVM knowledge in addition to contemporary veterinary biomedical knowledge [41]. According to Caudell et al. (2017), Maasai in northern Tanzania had greater EVM knowledge and this EVM knowledge was positively correlated with age [41]. Thus, the absence of age as significant correlate for knowledge towards zoonotic diseases could be due to the focus on conventional and not ethnoveterinary medicine knowledge.
Generally, variation in direction and strength of associations between socio-demographic variables and CMK were also evident regarding wealth, livestock, and education in previous studies [2, 9, 41]. In the current study, when excluding the two respondents with a secondary education, those with a primary education were indicative of greater CMK for rabies. As neither CMK of brucellosis nor rabies was correlated with education, our study mirrors the lack of agreement amongst previous studies as to whether education is correlated with knowledge of zoonotic diseases. While Sambo et al. (2014) concluded that education was a strong predictor of knowledge for rabies [9], Kiffner et al. (2019) concluded that there were variable effects between level of education and CMK for rabies and brucellosis [2]. Partly, mixed effects of education on CMK towards zoonotic diseases could be related to location and diversity throughout Tanzania. Tanzania's government requires children to attend primary school [42], yet school attendance in some rural communities is not always supported by parents. With education showing a significant association with CMK, this could be an area of focus when addressing measures towards preventing zoonoses.
4.4. Risk behaviors
Certain behaviors or practices potentially put people at risk for contracting the considered zoonotic diseases. For example, dog ownership could increase the risk of acquiring rabies because most infections follow a bite from an infected dog [6]. Previous research indicated that respondents across Tanzania who owned dogs were more likely to have greater knowledge of rabies [9]. In Makame, it is possible that lower rates of dog ownership (less than a third of households) resulted in lower median CMK scores compared to the findings in three other districts in northern Tanzania where each district had a higher median CMK score and a larger proportion of households owning dogs [2]. Although, to our knowledge, no literature exists regarding rabies outbreaks and risk factors present in Makame, previous studies in other regions of Tanzania have indicated that stray dogs are ubiquitous in certain locations, which may increase the rabies virus transmission risk at the human-dog-wildlife interface [18].
Brucellosis, as well as other pathogens, can be acquired through the consumption of raw milk [21, 43]. Yet, about three-quarters of respondents reported consuming raw milk as the sole source of milk or in addition to cooked milk (Figure 3). Milk plays an important cultural role in Maasai pastoral communities who heavily depend on it as a staple dietary element and as a symbol of prosperity. Although milk is commonly consumed boiled by elders with tea leaves and sugar, other members of Maasai communities may less frequently boil their milk [44]. Cultural considerations must be included when introducing alternatives to raw milk consumption to prevent pathogen transmission pathways. Cultural benefits and the local context of milk must be preserved in order to best implement milk pasteurization, boiling, or other alternatives among Maasai. Drastically changing the raw milk consumption may impede such campaigns due to its cultural importance [7].
4.5. Risk perception of zoonotic diseases
For all three diseases, associations between CMK and perceptions of the zoonoses studied varied. Anthrax was the most feared disease considered (Figure 4). Anthrax is a disease that causes illness and death quickly, and such shocking events may lead to fear among respondents in Makame. One prior study in northern and eastern Tanzania reported that anthrax was a feared illness among participants; however, most respondents were not afraid of eating meat from an animal that died of anthrax [7]. Brucellosis was the least feared disease in our study (Figure 4). Ntirandekura et al. (2018) studied pastoralists in Kagera, Tanzania, and also reported limited knowledge regarding brucellosis as well as a perception that it was less important than other diseases [10]. In contrast, Mangesho et al. (2017) reported that among pastoralists in Tanzania, brucellosis was ranked as the most problematic disease in an interior district [7]. Varied results in multiple studies could be the result of specific populations, locations, and experiences with a particular disease.
4.6. Limitations and future directions
The present study must be interpreted within the context of its limitations. We only addressed CMK, limiting conclusions regarding the full scope of the knowledge of zoonotic diseases in the area. To provide a more holistic view on the topic, future studies could include EVM knowledge, traditional medical knowledge, folk etiologies, and traditional remedies. Maasai culture is rich in EVM, as exemplified for example by studies on EVM on ticks and tick-borne diseases or the use of animal products as ailments for various diseases [45, 46]. The inclusion of such indigenous perspectives would allow for a more comprehensive understanding of Maasai knowledge and perceptions of zoonotic diseases as their dependence on livestock has resulted in various diagnostic methods, disease control practices, and treatment protocols of animal diseases prior to modern medicine [47]. Additionally, future research could include interviews with specialists in the region, such as local livestock officers, health workers, medical doctors, and/or veterinarians in order to provide a valuable comparison to rural residents. For diseases such as brucellosis, additional research could explore the prevalence of consuming soured milk in addition to boiled milk and raw milk to further elucidate risk behaviors for acquiring such diseases. Ideally, future research would aim to document disease occurrence and assess if CMK is correlated with actual disease prevalence. Furthermore, the role of extension services, non-government organizations, vaccination campaigns, and other informative programs could be explored. Documenting how and with whom traditional and conventional medical knowledge are acquired, and which social, cultural, and environmental or epidemiological parameters influence the perceptions and practices regarding zoonotic diseases will likely benefit future health initiatives in Tanzania.
4.7. Conclusions
While our study demonstrated variation in CMK among the interviewed population and occurrence of potentially risky behaviors, education is likely a key tool for preventing or reducing the transmission of zoonotic pathogens. We recognize the plurality of knowledge systems, including the local Maasai knowledge, and that education of CMK regarding zoonoses may only be a part of a necessary transdisciplinary approach to preventing zoonoses in a way that is most effective and embraced by Maasai communities. Education on zoonoses could, for example, start in primary school in order to reach most people. In addition, specific workshops or training sessions for specific at-risk groups would allow for zoonotic disease training among adults within livestock keeping communities as part of ongoing human health extension services or veterinary services and should be a high priority because preventing zoonotic diseases improves human, livestock, and wildlife health. This initiative is especially important in regions where wildlife live in close proximity to human activities, such as among the Maasai in Makame, Tanzania.
Declarations
Author contribution statement
ER Kriegel: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
DJR Cherney: Analyzed and interpreted the data; Wrote the paper.
C Kiffner: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was initially presented to the faculty of the Department of Animal Science of Cornell University in partial fulfillment of the requirements for a bachelor's degree with Distinction in Research by the senior author as an honors thesis. We thank translators and guides, S. Darabe and B. Victor and staff of the Makame WMA. We sincerely thank R. Becchina for assistance conducting interviews. J. Silaa developed the map. S. Parry of the Cornell Statistical Consulting Unit assisted with the statistical analyses.
Footnotes
Wildlife management areas (WMA) are community-based conservation models designed according to specific land-use plans. This form of conservation allocates user rights to local communities and allows villages to generate monetary income through wildlife-based tourism [11, 48].
CMK will be used interchangeably with CMK score or CMK proportion. This will refer to the points earned on the survey to assess CMK of each zoonotic disease studied.
Initially designed to summarize stocking rates and carrying capacity, tropical livestock unit (TLU) is a suitable index to assess relative wealth in Masai culture. In this method, one tropical livestock unit (TLU) is equivalent to 250 kg of live weight. According to this system, one cattle is 0.7 TLU, one sheep (Ovis aries) or one goat (Capra aegagrus hircus) is 0.1 TLU, and one donkey (Equus asinus) is 0.5 TLU [49].
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiffner C., Latzer M., Vise R., Benson H., Hammon E., Kioko J. Comparative knowledge, attitudes, and practices regarding anthrax, brucellosis, and rabies in three districts of northern Tanzania. BMC Publ. Health. 2019;19 doi: 10.1186/s12889-019-7900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomley F.M., Shirley M.W. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2637–2642. doi: 10.1098/rstb.2009.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caron A., Cappelle J., Cumming G.S., de Garine-Wichatitsky M., Gaidet N. Bridge hosts, a missing link for disease ecology in multi-host systems. Vet. Res. 2015;46:83. doi: 10.1186/s13567-015-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoonotic diseases guide launched. Bull. World Health Organ. 2019;97:249. [Google Scholar]
- 6.One Health Zoonotic Disease Prioritization for Multisectoral Engagement in Tanzania. 2017. https://www.cdc.gov/onehealth/pdfs/tanzania-report-508.pdf [Google Scholar]
- 7.Mangesho P.E., Neselle M.O., Karimuribo E.D., Mlangwa J.E., Queenan K., Mboera L.E.G., Rushton J., Kock R., Häsler B., Kiwara A., Rweyemamu M. Exploring local knowledge and perceptions on zoonoses among pastoralists in northern and eastern Tanzania. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klous G., Huss A., Heederik D.J.J., Coutinho R.A. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Heal. 2016;2:65–76. doi: 10.1016/j.onehlt.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambo M., Lembo T., Cleaveland S., Ferguson H.M., Sikana L., Simon C., Urassa H., Hampson K. Knowledge, attitudes and practices (KAP) about rabies prevention and control: a community survey in Tanzania. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntirandekura J.B., Matemba L.E., Ngowi H.A., Kimera S.I., Karimuribo E.D. Knowledge, perceptions and practices regarding brucellosis in pastoral communities of Kagera Region in Tanzania. J. Adv. Vet. Anim. Res. 2018;5:343–353. [Google Scholar]
- 11.Sulle E., Lekaita E., Nelson F. 2011. From Promise to Performance? Wildlife Management Areas in Northern Tanzania.https://www.ucl.ac.uk/pima/docs/reference/13_promise_to_performance.pdf [Google Scholar]
- 12.Bengis R.G., Kock R.A., Fischer J. Infectious animal diseases: the wildlife/livestock interface. OIE Rev. Sci. Tech. 2002;21:53–65. doi: 10.20506/rst.21.1.1322. [DOI] [PubMed] [Google Scholar]
- 13.Kiffner C., Kioko J., Baylis J., Beckwith C., Brunner C., Burns C., Chavez-Molina V., Cotton S., Glazik L., Loftis E., Moran M., O’Neill C., Theisinger O., Kissui B. Long-term persistence of wildlife populations in a pastoral area. Ecol. Evol. 2020;10:10000–10016. doi: 10.1002/ece3.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Western D., Tyrrell P., Brehony P., Russell S., Western G., Kamanga J. Conservation from the inside-out: winning space and a place for wildlife in working landscapes. People Nat. 2020;2:279–291. [Google Scholar]
- 15.Rabies signs and symptoms. Cent. Dis. Control Prev. 2019 https://www.cdc.gov/rabies/symptoms/index.html [Google Scholar]
- 16.The world factbook: Tanzania. https://www.cia.gov/library/publications/the-world-factbook/geos/print_tz.html (n.d.)
- 17.Fitzpatrick M.C., Hampson K., Cleaveland S., Meyers L.A., Townsend J.P., Galvani A.P. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mtui-Malamsha N., Sallu R., Mahiti G.R., Mohamed H., OleNeselle M., Rubegwa B., Swai E.S., Makungu S., Otieno E.G., Lupindu A.M., Komba E., Mdegela R., Assenga J.A., Bernard J., Marandu W., Warioba J., Makondo Z., Chang’a J., Mramba F., Nonga H., Killewo J., Kafeero F., Makonnen Y.J., Rivas A.L., Fasina F.O. Ecological and epidemiological findings associated with zoonotic rabies outbreaks and control in Moshi, Tanzania, 2017–2018. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16162816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brucellosis signs and symptoms. Cent. Dis. Control Prev. 2012 https://www.cdc.gov/brucellosis/symptoms/index.html [Google Scholar]
- 20.Chota A.C., Magwisha H.B., Stella B., Bunuma E.K., Shirima G.M., Mugambi J.M., Omwenga S.G., Wesonga H.O., Mbatha P., Gathogo S. Prevalence of brucellosis in livestock and incidences in humans in East Africa. Afr. Crop Sci. J. 2016;24:45–52. [Google Scholar]
- 21.Cash-Goldwasser S., Maze M.J., Rubach M.P., Biggs H.M., Stoddard R.A., Sharples K.J., Halliday J.E.B., Cleaveland S., Shand M.C., Mmbaga B.T., Muiruri C., Saganda W., Lwezaula B.F., Kazwala R.R., Maro V.P., Crump J.A. Risk factors for human brucellosis in Northern Tanzania. Am. J. Trop. Med. Hyg. 2018;98:598–606. doi: 10.4269/ajtmh.17-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodenham R.F., Lukambagire A.S., Ashford R.T., Buza J.J., Cash-Goldwasser S., Crump J.A., Kazwala R.R., Maro V.P., McGiven J., Mkenda N., Mmbaga B.T., Rubach M.P., Sakasaka P., Shirima G.M., Swai E.S., Thomas K.M., Whatmore A.M., Haydon D.T., Halliday J.E.B. Prevalence and speciation of brucellosis in febrile patients from a pastoralist community of Tanzania. Sci. Rep. 2020;10:7081. doi: 10.1038/s41598-020-62849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthrax symptoms. Cent. Dis. Control Prev. 2014 https://www.cdc.gov/anthrax/basics/symptoms.html [Google Scholar]
- 24.Hampson K., Lembo T., Bessell P., Auty H., Packer C., Halliday J., Beesley C.A., Fyumagwa R., Hoare R., Ernest E., Mentzel C., Metzger K.L., Mlengeya T., Stamey K., Roberts K., Wilkins P.P., Cleaveland S. Predictability of anthrax infection in the Serengeti, Tanzania. J. Appl. Ecol. 2011;48:1333–1344. doi: 10.1111/j.1365-2664.2011.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwakapeje E.R., Høgset S., Fyumagwa R., Nonga H.E., Mdegela R.H., Skjerve E. Anthrax outbreaks in the humans - livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006-2016. BMC Publ. Health. 2018;18 doi: 10.1186/s12889-017-5007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley C., Hassanali M., Baran P., Foley L., Lobora A., Durant S. 2018. An Assessment of Mammal Diversity and Abundance in Makame Wildlife Management Area (WMA) by Camera Trap Survey.https://www.honeyguide.org/wp-content/uploads/2020/04/Makame-camera-trap-report-2018.pdf [Google Scholar]
- 27.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing.http://www.R-project.org [Google Scholar]
- 28.Our Stories - Honeyguide, Honeyguide. 2020. https://www.honeyguide.org/stories/ [Google Scholar]
- 29.McCabe J.T., Leslie P.W., DeLuca L. Adopting cultivation to remain pastoralists: the diversification of Maasai livelihoods in northern Tanzania. Hum. Ecol. 2010;38:321–334. doi: 10.1007/s10745-010-9312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe J.T., Smith N.M., Leslie P.W., Telligman A.L. Livelihood diversification through migration among a pastoral people: contrasting case studies of Maasai in northern Tanzania. Hum. Organ. 2014;73:389–400. doi: 10.17730/humo.73.4.vkr10nhr65g18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craft M.E. Infectious disease transmission and contact networks in wildlife and livestock. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140107. doi: 10.1098/rstb.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWaal K.L., Atwill E.R., Isbell L.A., McCowan B. Quantifying microbe transmission networks for wild and domestic ungulates in Kenya. Biol. Conserv. 2014;169:136–146. [Google Scholar]
- 33.VanderWaal K., Enns E.A., Picasso C., Packer C., Craft M.E. Evaluating empirical contact networks as potential transmission pathways for infectious diseases. J. R. Soc. Interface. 2016;13:20160166. doi: 10.1098/rsif.2016.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moritz M., Ewing D., Garabed R. On not knowing zoonotic diseases: pastoralists’ ethnoveterinary knowledge in the Far North Region of Cameroon. Hum. Organ. 2013 doi: 10.17730/humo.72.1.72672642576gw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasanjani Roushan M.R., Ebrahimpour S. Human brucellosis: an overview, Casp. J. Intern. Med. 2015;6:46–47. https://pubmed.ncbi.nlm.nih.gov/26221498 [PMC free article] [PubMed] [Google Scholar]
- 36.Abdi I.H., Affognon H.D., Wanjoya A.K., Onyango-Ouma W., Sang R. Knowledge, attitudes and practices (KAP) on rift valley fever among pastoralist communities of ijara district, north eastern Kenya. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hundal J.S., Sodhi S.S., Gupta A., Singh J., Chahal U.S. Awareness, knowledge, and risks of zoonotic diseases among livestock farmers in Punjab. Vet. World. 2016;9:186–191. doi: 10.14202/vetworld.2015.186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail A., Josephat P. Knowledge and perception on tuberculosis transmission in Tanzania: multinomial logistic regression analysis of secondary data. Tanzan. J. Health Res. 2014;16 doi: 10.4314/thrb.v16i1.5. [DOI] [PubMed] [Google Scholar]
- 39.Shabani S.S., Ezekiel M.J., Mohamed M., Moshiro C.S. Knowledge, attitudes and practices on Rift Valley fever among agro pastoral communities in Kongwa and Kilombero districts, Tanzania. BMC Infect. Dis. 2015;15 doi: 10.1186/s12879-015-1099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archambault C.S. ‘The pen is the spear of today’’: (re)producing gender in the Maasai schooling setting. Gend. Educ. 2017;29:731–747. [Google Scholar]
- 41.Caudell M.A., Quinlan M.B., Quinlan R.J., Call D.R. Medical pluralism and livestock health: ethnomedical and biomedical veterinary knowledge among East African agropastoralists. J. Ethnobiol. Ethnomed. 2017;13 doi: 10.1186/s13002-017-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Right to Education Country Factsheet: United Republic of Tanzania, Right to Educ. Proj. 2015. https://www.right-to-education.org/sites/right-to-education.org/files/resource-attachments/RTE_Country_Facsheet_Tanzania_January_2015.pdf [Google Scholar]
- 43.Katale B.Z., Mbugi E.V., Kendal S., Fyumagwa R.D., Kibiki G.S., Godfrey-Faussett P., Keyyu J.D., van Helden P., Matee M.I. Bovine tuberculosis at the human-livestock-wildlife interface: is it a public health problem in Tanzania?: a review. Onderstepoort J. Vet. Res. 2012;79:e1–e8. doi: 10.4102/ojvr.v79i2.463. [DOI] [PubMed] [Google Scholar]
- 44.Århem K. Maasai food symbolism: the cultural connotations of milk, meat, and blood in the pastoral Maasai diet. Anthropos. 1989;84:1–23. http://www.jstor.org/stable/40461671 [Google Scholar]
- 45.Kioko J., Baker J., Shannon A., Kiffner C. Ethnoecological knowledge of ticks and treatment of tick-borne diseases among Maasai people in Northern Tanzania. Vet. World. 2015;8:755–762. doi: 10.14202/vetworld.2015.755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kioko J., Smith D., Kiffner C. Uses of birds for ethno medicine among the Maasai people in Monduli district, northern Tanzania. Int. J. Ethnobiol. Ethnomedicine. 2015;1:1–13. www.advancejournals.org [Google Scholar]
- 47.Jacob M.O., Farah K.O., Ekaya W.N. Indigenous knowledge: the basis of the Maasai ethnoveterinary diagnostic skills. J. Hum. Ecol. 2004;16:43–48. [Google Scholar]
- 48.Sachedina H. Univ. Oxford St. Antony’s Coll.; 2006. Conservation, Land Rights and Livelihoods in the Tarangire Ecosystem of Tanzania: Increasing Incentives for Non-conservation Compatible Land Use Change through Conservation Policy.https://cgspace.cgiar.org/bitstream/handle/10568/79447/Sachedina_2006.pdf?sequence=1&isAllowed=y [Google Scholar]
- 49.Jahnke H.E. Kieler Wissenschaftsverlag Vauk, Kiel. 1982. Livestock production systems and livestock development in tropical Africa. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.