Abstract
Introduction
With the increase of chronic diseases as a consequence of the population's eating habits, there is also a growing interest in foods rich in bioactive compounds capable of mitigating these diseases. Thus, this study aimed to evaluate the effects of supplementation with kombucha and green banana flour (GBF) on Wistar rats fed with cafeteria diet (CAF).
Methods
The animals were randomized into five groups of seven animals each, which were fed with the following diets: Treatment 1 (T1): Control treatment/commercial feed; Treatment 2 (T2): cafeteria diet (CAF); Treatment 3 (T3): CAF + kombucha; Treatment 4 (T4): CAF + green banana flour (GBF); Treatment 5 (T5): CAF + GBF + kombucha. Daily weight gain, daily food consumption, feed conversion, blood glucose, total cholesterol and fractions, triglycerides, liver enzymes, antioxidant activity, and body composition were evaluated.
Results
T5 presented lower feed intake and less weight gain. Liver histology revealed vacuolization in all treatments except T1, which was confirmed by the results of liver enzymes. There was no increase in blood glucose, and changes were observed in the lipid profile of the animals. T1 had the lowest body fat and the highest protein levels. Differences were observed for the antioxidant capacity in the liver of animals among treatments.
Conclusion
The intake of cafeteria diet altered the lipid and liver profile of the animals and the consumption of kombucha and GBF did not prevent these changes. The high polyphenols level of kombucha did not exert a hepatoprotective effect as an antioxidant. However, this supplementation generated greater satiety in the animals, leading to less weight gain until the end of the experiment.
Keywords: Functional foods, Cafeteria diet, Hepatic steatosis, Antioxidant activity, Hypercaloric diet
Functional foods; Cafeteria diet; Hepatic steatosis; Antioxidant activity; Hypercaloric diet
1. Introduction
Noncommunicable diseases (NCDs) are the leading cause of death in the world, triggered by several factors, including lifestyle, economic transition, urbanization of the population, and inadequate food intake (Balakumar et al., 2016).
However, scientific literature demonstrates the relationship between chronic non-communicable diseases and an imbalance in the body composition of an individual that can lead to death (Censin et al., 2019).
Diabetes mellitus (DM) is one of the most frequent NCDs in Brazil and worldwide and refers to a metabolic disorder that generates recurrent hyperglycemia resulting from defects in insulin secretion, insulin action, or both (Mahan and Raymond, 2018; Ross et al., 2016). An increase in the production of free radicals and the impairment of the physiological structure of the body antioxidant defense is also observed in this situation (Vona et al., 2019).
NCDs are also associated with liver diseases such as hepatic steatosis (Cruz et al., 2016), where there is an imbalance between the formation of triglycerides in the liver and the mobilization of very-low-density lipoprotein (VLDL) to tissues of the body. Excess triglycerides are due to the excess of macromolecules from the diet, mainly simple carbohydrates that are converted into fatty acids (Ferrier, 2018).
Due to the increasing prevalence of NCDs and the limitations of modern medicine, there is a growing interest in alternative or complementary tools for the treatment of these diseases. Foods with physiological and functional properties stand out as a nutritional strategy (Diederich, 2020), especially those containing antioxidant compounds (Abenavoli et al., 2020), probiotics, and prebiotics (Mahboobi et al., 2018).
According to scientific literature, kombucha is a probiotic drink that results from fermentation of a tea sweetened with sugar by a symbiotic community of bacteria, fungi and yeasts, which is characterized by having bioactive compounds such as glucuronic acid, cellulose, acetic acid and water-soluble vitamins, that demonstrate in in-vitro and biological studies, to have an immunomodulatory effect that would exert a prophylactic action for the health and treatment of NCDs. However, there is no evidence of these effects in humans yet (Villareal-Soto et al., 2018; Kapp and Sumner, 2018).
Green banana flour (FGB) is described in the literature as a prebiotic ingredient because it has a high content of resistant starch, which is not absorbed in the intestine but metabolized by intestinal microbiota bacteria, which, in turn, produce short-chain fatty acids (SCFA) with consequent beneficial (Farzaneh and Carvalho, 2015) decrease in colon pH, leading to systemic effects on glucose and lipid metabolism (Falcomer et al., 2019).
In recent times, the use of natural products as therapy for chronic non-communicable diseases is being used more and more. However, studies need to be carried out to determine the effectiveness of the products in addition to establishing how safe quantities to consume (Farzaneh and Carvalho, 2015).
It is known that the CAF high in saturated fats and simple sugars is similar to the diet responsible for dyslipidemia, obesity, and other NCDs in humans (Costa et al., 2014). Considering the growing increase in non-communicable chronic diseases in the world, such as obesity, diabetes, fatty liver due to the population's dietary pattern.
Thus, this study aimed to induce metabolic disorders in Wistar rats using an experimental diet and to evaluate the effects of dietary supplementation with kombucha and GBF alone or in combination.
2. Materials and methods
2.1. Kombucha and green banana flour (GBF)
To define the kombucha formulation for the biological assay, preliminary tests of total phenols and antioxidant activity were carried out on kombucha produced from dried green tea (Camellia sinensis) and mate tea (Ilex paraguariensis) leaves, which were acquired in a storehouse in the city of Cascavel - PR. Kombucha beverages were characterized for total phenols (Folin Ciocalteu assay) as described by Swain and Hillis (1959); Ferric Reducing Antioxidant Power (FRAP) as described by Benzie and Strain (1996), and Oxygen Radical Absorbance Capacity (ORAC) as described by Ou et al. (2013).
The kombucha beverages were prepared as proposed by Fu et al. (2014), with modifications. To prepare the kombucha culture, a symbiotic colony of bacteria and yeasts (homemade scoby), acquired by donation, was soaked in a sucrose-tea solution. For the preparation of green tea, 15 g of leaves were immersed in 1 L of boiled water for 20 min. Then, the tea was filtered and 50 g of sucrose was added. The tea was stored in a sterilized glass bottle containing 100 mL of the liquid phase of the kombucha culture. The beverage was kept under aerobic fermentation for 15 d at room temperature and in the absence of light, followed by anaerobic fermentation for 2 d at room temperature.
The tea for the production of the kombucha beverages was selected according to the results of total phenols, FRAP, and ORAC. Then, the beverage was prepared for use in the biological assay. The production process was repeated every 2 d, to standardize the fermentation characteristics of kombucha administered to the animals, using the scoby from the mother culture previously produced.
The GBF was produced by the Company of Agricultural Research and Rural Extension (EPAGRI), Itajai - SC, Brazil. For this purpose, green bananas of maturity 1 of the cultivar BRS SCS Belluna were used, which were washed in running water and sanitized in chlorinated solution, peeled, cut into slices (5 mm thick) and kept for 10–15 min in 10 L of a water solution containing 1 g of citric acid and 10 g of ascorbic acid to prevent browning. Then, the banana slices were removed from the solution and dried in a dehydrator at 50 °C and an airspeed of 1.5 m/s for 19 h. The dried samples were ground (30 mesh) in a knife mill, packed in plastic bags, and stored under refrigeration (5 °C). Each 100 g of flour contained 70 g carbohydrates (14 g dietary fiber, and 56 g resistant starch), 4.3 g protein, and 0.8 g fat (Reis et al. 2017).
The green tea kombucha and GBF for use in the biological assay were subjected to analysis of antioxidant activity by the 2,2′-azino-bis (3-ethylbenzothiazolin) 6-sulfonic acid radical cation (ABTS) assay, as described by Boroski et al. (2015), using different concentrations of Trolox as a standard. For that, 3.0 mL of ABTS solution was mixed with 30 μL of Trolox solutions (100, 500, 1000, 1500, and 2000 μmol/L), and the absorbance readings were performed at 734 nm after 6 min protected from light. A blank was made by replacing the Trolox solution by ethanol (analytical grade). For the evaluation of the samples, GBF and kombucha ethanolic extracts were prepared in the concentrations of 2.5 mg/mL and 100 mg/mL, respectively, and the antioxidant activity was determined as previously described, using 3.0 mL of ABTS solution and 30 μL of the extracts, with absorbance readings at 734 nm after 6 min of reaction. The entire procedure was carried out in triplicate.
2.2. Biological assay
The experiment was carried out in accordance with the ARRIVAL guidelines and in accordance with the National Institutes of Health guide for the care and use of laboratory animals – NIH Publications No. 8023, revised in 1978) and in accordance the Brazilian Federal Law on Animal Experimentation (Law No. 11,794, of October 8, 2008), and after approval by the Ethics Committee on the Use of Animals of the Centro Universitario Fundação Assis Gurgacz (CEUA -FAG), under protocol # 058/2018.
For the experiment, 35 Wistar rats supplied by the Bioterio of the Centro Universitario Fundação Assis Gurgacz (Cascavel-PR, Brazil) were used. The animals were kept in the same place during the experimental period, in individual cages under controlled temperature (22 ± 2 °C), with a 12 h light/dark cycle, randomly distributed in 5 groups. The biological assay lasted 55 d, with ad libitum access to water and feed. The first 10 d corresponded to the adaptation period to the CAF, in which 4 of the 5 experimental groups received CAF without supplementation, while the control group received commercial feed (Biotec Ratos e Camundongos, Biobase, Águas Frias - SC, Brazil). The remaining 45 d corresponded to the experimental period, in which each group received a different treatment, as follows: Treatment 1 (T1) Control treatment/commercial feed (Biotec Ratos e Camundongos, Biobase, Águas Frias-SC, Brazil); Treatment 2 (T2) CAF; Treatment 3 (T3) CAF + kombucha; Treatment 4 (T4) CAF + 20% GBF; Treatment 5 (T5) CAF + 20% GBF + kombucha.
The CAF was prepared according to the methodology described by Costa et al. (2014), with modifications. The diet consisted of condensed milk, potato chips, bacon, stuffed cookie, milk chocolate and commercial feed (Biotec Rats and mice, Manufacturer Biobase, Águas Frias - SC, Brazil) and in the case of the FBV CAF, were used the same ingredients, but there was an increase of 20% FBV. The diets were prepared in the Nutrition Laboratory of the Centro Universitário Fundação Assis Gurgacz (Cascavel-PR, Brazil) and the ingredients were purchased in the local market, with the exception of GBF, which was purchased from the Company of Agricultural Research and Rural Extension (EPAGRI) in Santa Catarina, Brazil.
The ingredients were weighed, milled, and mixed by hand, with the addition of distilled water until reaching adequate consistency for the pellet format. GBF was added directly to the formulation at a concentration of 20% for the treatments T4 and T5. After molding, the feed was subjected to a drying process in an air circulation oven (Ethik, series 420-TD, Vargem Grande Paulista-SP, Brazil) at 60–65 °C for 96 h, and stored under refrigeration (5 °C).
The CAF had 11.2% protein, 27.4% lipids and 58% carbohydrates in its nutritional composition, 4.1% of which correspond to fiber. The CAF with FBV added 10.2% of proteins and 22.6% of lipids and 64.2% of carbohydrates, of which 16% were fibers.
Kombucha was administered by gavage using an orogastric tube for the treatments T3 and T5, and a saline solution was provided to the treatments T1, T2, and T4 so that the experimental rats were exposed to the same stress. The amounts of kombucha and saline were determined weekly, according to Bellassoued et al. (2015), considering 5 mL/kg of body weight.
For the analysis of food consumption and weight gain, the animals were weighed at the beginning of the induction period, weekly throughout the experiment, and 1 d before euthanasia. To determine feed consumption, leftovers were weighed every 2 d, along with feed consumed. The weighing data provided the daily weight gain (DWG) of the animals, the daily feed intake (DFI) and feed conversion (FC), which was calculated using the formula FC = DFI/DWG.
After 55 d of the experiment, the animals were euthanized under anesthesia (isoflurane). Then, the nasoanal length was measured to calculate the Lee Index (cube root of body weight (g)/nasoanal length (mm) x 1000) (Bernardis, 1970) and euthanasia was conducted by decapitation using the guillotine, with subsequent collection of biological samples.
Blood samples were placed in polyethylene tubes without anticoagulant and centrifuged at 3000 rpm for 20 min to separate serum, which was divided into two fractions. The first fraction was stored in liquid nitrogen until the analysis of antioxidant activity by the ABTS assay (Boroski et al., 2015). The second fraction was stored under refrigeration for further biochemical analysis using commercial kits (Gold Analisa Diagnósticos Ltda, Belo Horizonte-MG, Brazil) and an SX-140 automatic analyzer for biochemical testing (Sinnowa Brasil, Ribeirão Preto - SP, Brazil). The following diagnostic kits were used: liver-specific enzymes Aspartate aminotransferase-AST (commercial kit MS 80022230086; UV-kinetic assay), Alanine Aminotransferase-ALT (commercial kit MS 80022230083, UV-kinetic assay), total cholesterol quantification-TC (Commercial kit MS 80022230064, enzymatic-colorimetric assay), triglycerides - TG (Commercial kit MS 80022230062, enzymatic-colorimetric assay - Trinder) and LDL-cholesterol (Commercial kit MS 80022230072, direct enzymatic-colorimetric assay). The HDL-cholesterol levels were determined using Friedewald's formula: LDL = (TC) - (HDL) - (TG/5) described by Friedewald et al. (1972).
Another portion of blood samples was placed in tubes with anticoagulant (potassium fluoride + EDTA) for biochemical analysis, using commercial kits (Gold Analisa Diagnósticos Ltda, Belo Horizonte-MG, Brazil) and an SX-140 automatic analyzer for biochemical testing (Sinnowa Brasil, Ribeirão Preto - SP, Brazil). The following parameters were determined: glycemia (commercial kit MS 80022230067, enzymatic-colorimetric method) and pancreatic amylase (commercial kit MS 80022230145, kinetic-colorimetric-Caraway assay).
After euthanasia, the animals were placed in the dorsal decubitus position for the pectoral incision procedure, to remove the organs from the carcass, and the liver was weighed to obtain the organ weight/body weight ratio of the animals, using the formula (weight organ x 100)/body weight.
Liver tissue samples were stored in liquid nitrogen to determine the antioxidant activity by the ABTS assay (Boroski et al., 2015). For that, a homogenate was prepared, using 2.5 mg of liver tissue and 1 mL of ethanol. Concomitantly, liver segments were kept in vials containing 30 mL of paraformaldehyde and stored at 4 °C for 24 h. Then, the fixative solution was replaced by 70% alcohol for 12 h, which was discarded and replaced to remove the paraformaldehyde solution (Beçak and Paullete, 1976). The liver segments were subjected to histological study using increasing series of ethanol (70%, 80%, 90%, and 95%; I, II, III), alcohol-xylene, xylene I, xylene II, and xylene III for 5 min for dehydration and later the inclusion in paraffin wax (Junqueira and Junqueira, 1983). The material was sectioned in an Olympus CUT4055 microtome, with a thickness of 5 μm. The cuts were fixed to the slides, which were submitted to xylene I, II, and III, alcohol-xylol, absolute alcohol I, II, and III; 95%, 90%, 80%, and 70%. The slides were stained using the Hematoxylin and Eosin (HE) technique (Junqueira and Junqueira, 1983) for analysis of liver morphology. All slides were visualized using an Olympus CBA light microscope and photographed on equipment in the clinical analysis laboratory at Centro Universitário FAG, Cascavel - PR, Brazil.
The eviscerated carcass of the animals was characterized for proximate composition, determining the protein, moisture, and ash levels according to the methodologies of the Association of Official Analytical Chemists (AOAC, 1995). The lipids content was determined according to the method described by Bligh and Dyer (1959).
2.3. Statistical analysis
The statistical analysis was performed using RStudio statistical software. Data were subjected to descriptive analysis, Pearson's correlation test, and analysis of variance (ANOVA). The normality of the residues was assessed by the histogram of residuals, normal QQ plot, Shapiro-Wilk test and Bartlett test to determine the homogeneity of variance. The independence of residuals was measured through the plot of residuals and the predicted residual values. The presence of outliers was verified using a box plot. When assumptions were violated, Box-cox transformation was applied to the response variable. F test was used to determine the significant differences between treatments, and Tukey's test was used for multiple comparisons between means. The non-parametric Kruskal-Wallis test was used when assumptions in ANOVA seemed impossible even after Box-Cox transformation.
3. Results and discussion
3.1. Selection of kombucha and antioxidant activity of kombucha and green banana flour
Table 1 shows the results of the determination of total phenols and antioxidant activity of green tea and mate tea kombucha before the selection of kombucha for the biological assay, and the antioxidant activity of green tea kombucha and GBF used for animal supplementation.
Table 1.
Total phenols and antioxidant properties of green tea kombucha, and mate tea kombucha before animal supplementation, and antioxidant activity of green tea kombucha and green banana flour used for the supplementation.
| Total phenols and antioxidant activity of kombucha | |||
|---|---|---|---|
| Green tea kombucha | Mate tea kombucha | p-value | |
| Phenols (μg GAE/L) | 1519.2 ± 16.96 | 779.9 ± 1.85 | 0.01214 |
| FRAP∗ (μMol TE/L) | 22399.5 ± 138.12 | 9281.5 ± 138.12 | <0.001 |
| ORAC∗ (μMol TE/L) |
23784.0 ± 684.78 |
18159.3 ± 1.514.11 |
0.01214 |
| Antioxidant activity of kombucha and green banana flour used in the biological assay | |||
| Green tea kombucha |
Green banana flour |
||
| ABTS∗ (μMol TE/L) | 50111.75 ± 1052.02 | 0 ± 2.04 | |
Results are expressed as means and standard deviations (mean ± standard deviations).
FRAP: Ferric Reducing Antioxidant Power; ORAC: Oxygen Radical Absorbance Capacity; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid.
The results prior to the biological assay (Table 1) showed that both kombucha beverages presented high phenols levels and antioxidant properties, with better results for the green tea kombucha when compared to mate tea kombucha. According to Jayabalan et al. (2007), the enzymes released by the microorganisms present in kombucha lead to the biotransformation of epicatechins from green tea, favoring the release of catechins, thus improving the antioxidant activity of the final beverage.
Amarasinghe et al. (2018) evaluated a chemical composition and antioxidant activity in kombucha fermented from different varieties of black tea in periods of aerobic fermentation that varied from 1 to 224 d, thus, they proved that, both the antioxidant activity and the concentration of graduated acids, dissipation an increase directly proportional to the amount of black tea used. However, after 15 d of fermentation, the antioxidant activity of the drinks began to decline, regardless of the amount of tea used.
Therefore, green tea was selected for kombucha fermentation to be used in the animal diet for the biological test.
According to the results of the antioxidant activity by the ABTS assay of the green tea kombucha and GBF (Table 1) used for supplementation in the biological assay, the green tea kombucha exhibited a potent antioxidant activity, as expected, corroborating the previous discussion. On the other hand, the GBF had no antioxidant effect, which is consistent with the results found by Barros et al. (2018), who reported that although the GBF has a significant content of phenolic compounds, it has a reduced antioxidant activity (4.75 μMol TE/L) when evaluated by the ABTS assay, that is, a low capacity to capture the ABTS radical.
3.2. Biological assay
3.2.1. Food consumption and weight gain of animals
Table 2 presents the results of the daily feed consumption and daily weight gain of the animals, as well as the feed conversion during the adaptation and experimental periods.
Table 2.
Daily feed consumption, daily weight gain, and feed conversion of the animals during the adaptation period and experimental period.
| T1∗ | T2∗ | T3∗ | T4∗ | T5∗ | p-value | |
|---|---|---|---|---|---|---|
| DFC a∗ (g) | 13.12 ± 4.23 | 9.15 ± 2.65 | 9.03 ± 2.84 | 9.72 ± 2.53 | 9.12 ± 2.84 | 0.08576 |
| DFC e∗ (g) | 14.52a ± 2.86 | 7.64a ± 2.57 | 7.46a ± 1.9 | 7.05a ± 1.87 | 6.37b ± 1.55 | <0.0001 |
| DWG a∗ (g) | 3.53a ± 1.12 | 1.87b ± 0.82 | 1.92b ± 0.36 | 1.68b ± 0.78 | 1.64b ± 0.51 | 0.000248 |
| DWG e∗ (g) | 2.76a ± 0.73 | 1.38ab ± 0.59 | 1.30b ± 0.40 | 0.92b ± 0.33 | 0.68c ± 0.19 | <0.0001 |
| FC a∗ | 3.75 ± 0.63 | 5.66 ± 3.58 | 4.69 ± 1.05 | 6.74 ± 3.54 | 5.73 ± 1.63 | 0.08943 |
| FC e∗ | 5.46a ± 1.24 | 5.72a ± 0.60 | 5.94ab ± 0.93 | 7.99bc ± 1.47 | 9.80c ± 2.92 | <0.0001 |
Results are expressed as means and standard deviations (mean ± standard deviations).
T1: Control treatment/commercial feed; T2: cafeteria diet (CAF); T3: CAF + kombucha; T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF. Daily feed consumption in the adaptation period (DFC a) and in the experimental period (DFC e), Daily weight gain of the animals in the adaptation period (DWGa) and in the experimental period (DWG e), Feed conversion in the adaptation period (FCa) and in the experimental period (FCe). Values in the column followed by different letters differ significantly by the Tukey test (p < 0.05).
During the adaptation period (Table 2), the experimental diets did not affect the daily feed consumption for all treatments; however, the CAF presented a lower daily weight gain when compared to the group subjected to the treatment with a commercial diet. However, the results of feed conversion showed that the difference in daily weight gain was not enough to impact feed conversion during the adaptation period.
On the other hand, in the experimental period (Table 2), the intake of CAF + kombucha + GBF generated the lowest feed intake and weight gain of the animals. According to the results of feed conversion, CAF associated with kombucha and GBF proved to be the least effective in promoting weight gain in rats.
In a study Kim et al. (2020), proved that rodents fed a high-fat diet underwent changes in the profile of the microbiota causing vagal damage and decreased satiety, which triggered a greater weight gain in relation to rodents fed a low-fat diet.
As reported by Roberfroid et al. (2010), one of the factors that determine satiety is an adequate production of short-chain fatty acids (SCFA) in the intestine, as they have the ability to bind to receptors that trigger the production of YY peptide, a gut hormone that inhibits the action of orexigenic neurons in the central nervous system. In addition, the production of SCFA can be favored by the intake of prebiotics and probiotics.
3.2.2. Biochemical parameters
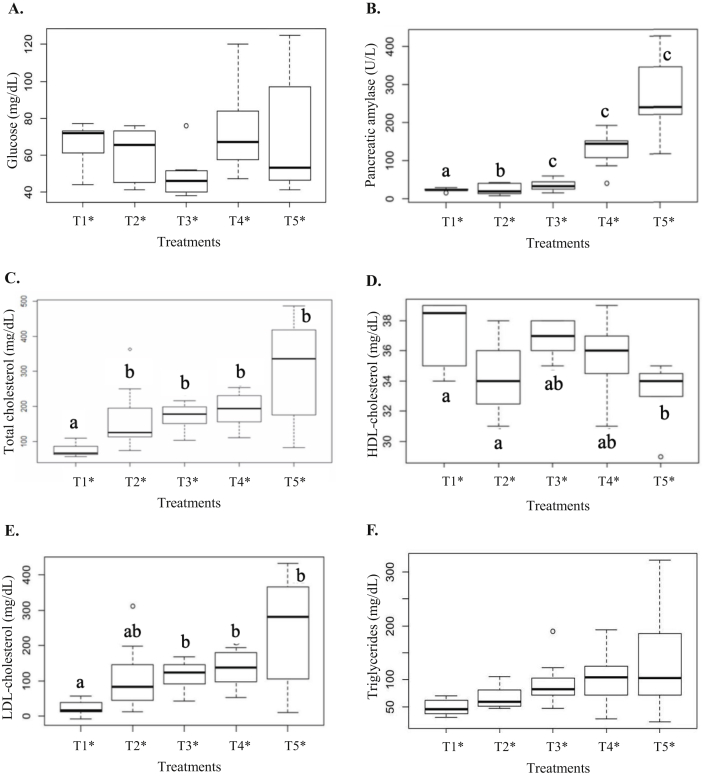
Figure 1 shows the results of the statistical analysis of the biochemical parameters evaluated in animal blood.
Figure 1.
Results of blood glucose (A), pancreatic amylase (B), total cholesterol (C), HDL-cholesterol (D), LDL-cholesterol (E) and blood triglycerides (F) in the animals. ∗T1: Control treatment/commercial feed; T2: cafeteria diet (CAF); T3: CAF + kombucha; T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF. Values followed by different letters differ significantly by the Tukey test (p < 0.05).
As shown in Figure 1A, there was no significant difference in fast blood glucose among treatments (p = 0.1362). In contrast, Bhattacharya et al. (2013) studied rats induced by alloxan diabetes and supplemented for 14 d with 150 mg/kg of body weight of lyophilized extract of black tea kombucha, and observed a 56% decrease in glycemia when compared to the group of diabetic animals without supplementation.
The consumption of kombucha containing GBF or the combination of both led to an increase in the concentration of plasma pancreatic amylase (Figure 1B) when compared to T1 and T2 (p < 0.001). However, Pearson's correlation data showed a moderate correlation between the concentration of this enzyme and total cholesterol (p = 0.6), LDL-cholesterol (p = 0.6), and liver enzyme AST (p = 0.4) levels.
Concerning the total cholesterol (Figure 1C) and LDL-cholesterol (Figure 1D) levels in the animal serum, the consumption of kombucha and GBF were not effective (p = 0.1644) in reversing the damage caused by CAF, which contained a high content of saturated fat in its composition. According to Pearson's correlation coefficient, there was a strong relationship (p = 1.00) between total cholesterol and LDL-cholesterol fractions.
The CAF and the consumption of kombucha and GBF alone were able to reduce the HDL-cholesterol concentrations (Figure 1C), with a greater decrease for the supplementation with kombucha + GBF (p = 0.01065).
The treatments had no significant effect on blood triglycerides levels (Figure 1E) of the animals (p = 0.1644).
High plasma levels of pancreatic enzymes such as amylase are associated with acute pancreatitis, due to the leakage of this enzyme into the bloodstream. When this evidence is associated with changes in the concentrations of liver enzymes such as AST, the biliary etiology can be considered (Santos et al., 2003). Pozo et al. (2017) evaluated the effects of a standard commercial diet administered to Wistar rats, compared to a diet rich in saturated fat for 60 d, and reported that excess saturated fat intake caused an increase in total cholesterol levels and LDL-cholesterol fraction, increased bile cholesterol concentration, and abnormal formation of vesicular transporters, which are triggers for cholelithiasis. A significant increase in HDL-cholesterol was also observed, with no effect on triglyceride levels when compared to the group fed with a balanced diet.
Therefore, Aloulou et al. (2012), proved that certain metabolites of kombucha, have an inhibitory effect of the enzymes α-amylase and pancreatic lipase, a fact that would hinder a rapid absorption of glucose and fats from food intake.
3.2.3. Liver enzymes and histology in animal liver sections
Table 3 shows the concentration of the serum liver enzymes Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) and the ratio of liver weight and body weight of animals.
Table 3.
Concentration of serum liver enzymes Alanine Aminotransferase and Aspartate Aminotransferase and ratio of liver weight and body weight of animals.
| T1∗ | T2∗ | T3∗ | T4∗ | T5∗ | p-value | |
|---|---|---|---|---|---|---|
| ALT∗(U/L) | 41.57 ± 7.5 | 47.57 ± 25.51 | 45.57 ± 6.19 | 45.29 ± 9.3 | 50.57 ± 20.59 | 0.496 |
| AST∗(U/L) | 227.14a±14.51 | 181.57ab±73.49 | 235.86ab±24.79 | 248.71ab±90.17 | 332.57b ± 94.34 | 0.042 |
| % Liver Weight | 2.42 ± 0.34 | 2.49 ± 0.34 | 2.54 ± 0.34 | 2.52 ± 0.34 | 2.69 ± 0.34 | 0.63 |
Results are expressed as means and standard deviations (mean ± standard deviations).
ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ∗T1: Control treatment/commercial feed; T2: cafeteria diet (CAF); T3: CAF + kombucha; T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF. Values in the column followed by different letters differ significantly by the Tukey test (p < 0.05).
The consumption of kombucha and GBF did not affect the activity of the liver enzyme ALT. On the other hand, the AST results demonstrate that the diets caused different effects between treatments since the supplementation of kombucha + GBF + CAF increased the ALT activity, while the separated consumption caused similar effects to those observed after the consumption of CAF alone (Table 3).
It is known that plasma activity of liver enzymes increases with the consumption of a diet with a high lipid profile (Lasker et al., 2019). Although tea fermentation with kombucha can provide protective health effects, the consumption of inadequate concentrations can cause hepatotoxicity due to the action of the beverage or its interaction with other substances consumed, and this hepatic alteration can be detected by variations in the biochemical parameters, including the liver enzymes (Kovacevic et al., 2014).
Table 3 shows that the ratio of liver weight and body weight of animals was not affected by the consumption of experimental diets. According to Reis Júnior (2016), there is a direct relationship between liver weight and the degree of liver steatosis since the more advanced this pathology, the greater the degree of organ compensation.
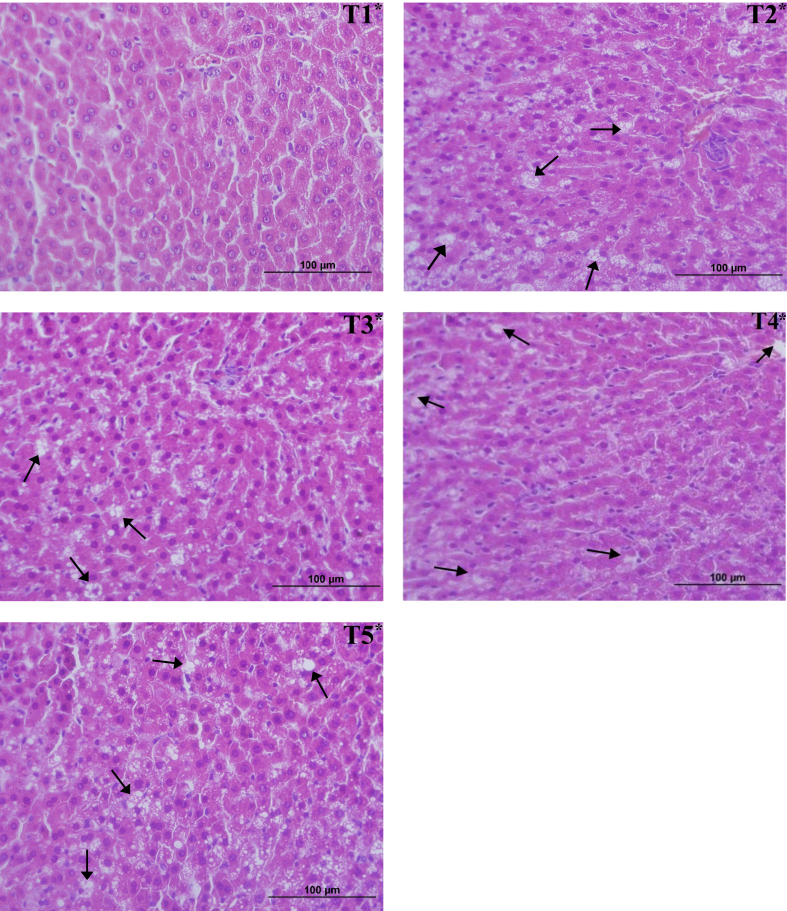
Figure 2 shows the photomicrograph of the histological sections of the liver of all treatments, which show vacuolization of hepatocytes in CAF rats (T2, T3, T4, and T5). As reported by Reis Júnior (2016), this phenomenon is characteristic of hepatic steatosis and occurs due to an abnormal accumulation of triglycerides in liver cells.
Figure 2.
Photomicrograph of histological sections of the liver of experimental animals. ∗T1: Control treatment/commercial feed, ∗T2: cafeteria diet (CAF), ∗T3: CAF + kombucha T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF.
In a biological study with mice Lee et al. (2019) induced liver steatosis in animals using a diet low in methionine and applied the supplementation of kombucha fermented from black tea, which was able to prevent vacuolization when compared to the group that did not receive this supplementation.
3.2.4. Body composition of animals
Table 4 shows the results of the Lee index and the body composition of the animals for fat, protein, ash, and moisture levels of the eviscerated carcasses.
Table 4.
Lee index and body composition of the animals.
| T1∗ | T2∗ | T3∗ | T4∗ | T5∗ | p-value | |
|---|---|---|---|---|---|---|
| Lee index | 379.29a ± 54.14 | 263.44a ± 69.36 | 259.95a ± 59.49 | 240.89a ± 57.93 | 222.4b ± 50.9 | <0.001 |
| Moisture (%) | 64.01a ± 1.78 | 61.67ab ± 3.82 | 59.01b ± 1.45 | 61.00ab± 1.37 | 60.31b ± 1.92 | <0.001 |
| Protein (%) | 21.25a ± 1.21 | 18.35ab ± 3.10 | 19.74ab ± 3.19 | 17.69b ± 1.99 | 16.01b ± 2.02 | 0.002 |
| Lipids (%) | 11.75c ± 2.74 | 18.80ab ± 4.63 | 21.12a ± 3.01 | 16.10bc ± 1.96 | 16.04ab ± 4.66 | <0.001 |
| Ash (%) | 3.35 ± 0.73 | 3.60 ± 0.60 | 3.49 ± 0.39 | 4.03 ± 1.47 | 3.68 ± 0.43 | 0.07955 |
Results are expressed as means and standard deviations (mean ± standard deviations).
T1: Control treatment/commercial feed; T2: cafeteria diet (CAF); T3: CAF + kombucha; T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF. Values in the column followed by different letters differ significantly by the Tukey test (p < 0.05).
The intake of kombucha and GBF led to the lowest Lee index (Table 4) among all treatments. According to the Pearson's correlation, the Lee index showed a very strong correlation (p = 1.00) with the daily feed intake of the animals in the experimental period (Table 2), with the lowest average for T5.
On the other hand, Pearson's correlation identified a moderate correlation (p = 0.4) between the Lee index and the protein contents of the carcasses (Table 4), indicating that the higher the Lee index, the higher the protein content. It is noteworthy that the GBF supplementation (T4 and T5) led to the lowest protein contents in the carcasses.
As expected, higher fat contents (Table 4) were observed for the consumption of CAF between treatments, which was not observed with the supplementation of GBF alone, which presented a fat content similar to T1. The Pearson's correlation indicated that the fat content correlated strongly and negatively (p = -0.7) with the moisture content of the carcasses (Table 4), that is, the higher the moisture, the lower the fat content.
Regarding the ash levels, as shown in Table 4, the CAF did not affect the mineral content of the animal body when compared to those that received the nutritionally balanced diet (T1).
According to Malafaia et al. (2013), although the Lee index represents a tool for the rapid diagnosis of obesity in rats, it should be associated with the results of the body composition of animals for a better diagnosis.
In the present study, the lower feed consumption led to a lower weight gain, thus a lower Lee index. However, the analysis of the body composition of the rats revealed that the treatments with rats with lower weight presented higher fat and lower protein contents.
3.2.5. Serum and liver antioxidant activity of animals
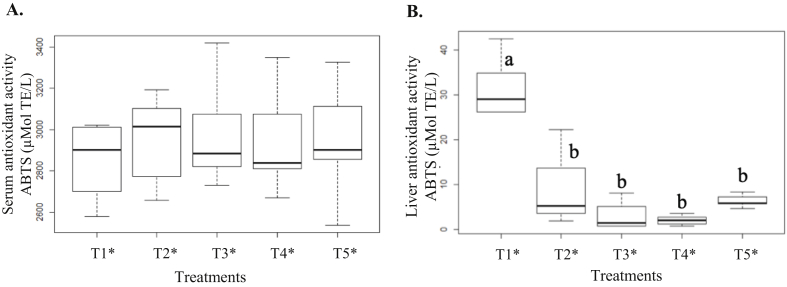
Figure 3 shows the results of serum and liver antioxidant activity in the animals.
Figure 3.
Serum (A) and liver (B) antioxidant activity (ABTS) in the experimental animals. ∗T1: Control treatment/commercial feed; T2: cafeteria diet (CAF); T3: CAF + kombucha; T4: CAF + green banana flour (GBF); T5: CAF + kombucha + GBF. Values followed by different letters differ significantly by the Tukey test (p < 0.05).
As can be seen in Figure 3A, no changes in the serum antioxidant activity of animals were observed for all diets (p = 0.8615). However, the intake of CAF led to a significant reduction (p < 0.001) of the liver antioxidant activity (Figure 3B) while the consumption of kombucha and GBF did not prevent this alteration.
The consumption of hypercaloric diets due to excess saturated fats generates oxidative stress, which compromises the functioning of the endogenous antioxidant system. In addition, dyslipidemia is also triggered by excessive fat intake, which when in synergy with oxidative stress, stimulates irregular liver fat accumulation (Lasker et al., 2019).
In this study, kombucha and GBF were not able to mitigate the deleterious effects of oxidative stress generated by the CAF, which, is related to changes in total cholesterol and the LDL and HDL fractions (Figure 1D, E) that together originated the accumulation of fat in hepatocytes for the CAF rats, as shown in the photomicrograph of the animal liver (Figure 2).
4. Conclusions
Although a positive effect of the supplementation of kombucha in combination with GBF was observed both in food consumption and weight gain of the animals, this supplementation generated hepatotoxicity.
The functional foods investigated in the present study were not able to prevent the health consequences of a diet high in fat and sugar, characteristic of the western population.
Declarations
Author contribution statement
Marianela Andrea Díaz Urrutia, Amanda Gemelli Ramos, Rafaela Beatriz Menegusso, Rafael Dewes Lenz, Sóstenez Alexandre Vessaro da Silva, Daniela Miotto Bernardi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mateus Gemelli Ramos: Analyzed and interpreted the data.
Adriana Gadioli Tarone: Performed the experiments.
Cinthia Baú Betim Cazarin, Solange Maria Cottica: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Research and Extension Coordination (COOPEX) of the Centro Universitário Fundação Assis Gurgacz; Veterinary Hospital of the Centro Universitário Fundação Assis Gurgacz; Company of Agricultural Research and Rural Extension (EPAGRI); University of Campinas (UNICAMP); Federal Technological University of Paraná (UTFPR). Adriana G. Tarone was supported by CNPQ (140942/2016-5).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Cinthia Baú Betim Cazarin; [is an Associate Editor for Heliyon Food Science and Nutrition].
Additional information
No additional information is available for this paper.
References
- Abenavoli L., Boccuto L., Federico A., Dallio M., Loguercio C., Renzo L.D., Lorenzo A.D. Diet and non-alcoholic fatty liver disease: the mediterranean way. Int. J. Environ. Res. Public Health. 2020;16(17):3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloulou A., Hamden K., Elloumi D., Ali B.A., Hargafi K., Jaouadi B., Ayadi F., Elfeki A., Ammar E. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Compl. Alternative Med. 2012;12(1):12–63. doi: 10.1186/1472-6882-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe H., Weerakkody N.S., Waisundara V.Y. Evaluation of physicochemical properties and antioxidant activities of kombucha “Tea Fungus” during extended periods of fermentation. Food Sci. Nutr. 2018;6(3):659–665. doi: 10.1002/fsn3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists – AOAC . sixteenth ed. Association of Official Analytical Chemists; Washington: 1995. Official Methods of Analysis of the Association of Official Analytical Chemists International. [Google Scholar]
- Balakumar P., Maung U.K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016;113(PtA):600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- Barros H.E.A., Santos M.M.O., Natarelli C.V.L., Carvalho E.E.N., Vilas Boas E.V.B., Franco M. Anais Digitais, 4a SEALIM/I SIMPECAL – UESB, Itapetinga, BA. 2018, November. Avaliação de Compostos Fenólicos Totais e Atividade Antioxidante de Farinha de Banana Verde. [Google Scholar]
- Bhattacharya S., Gachhui R., Sil P.C. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013;60:328–340. doi: 10.1016/j.fct.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Beçak W., Paulete J. Vol. I. Livros Técnicos e Científicos; Rio de Janeiro: 1976. Técnicas de citologia e histologia. [Google Scholar]
- Bellassoued K., Ghrab F., Makni-Avadi F., Pelt J.V., Elfeki A., Emna A. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharmaceut. Biol. 2015;53(11):1699–1709. doi: 10.3109/13880209.2014.1001408. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bernardis L.L. Prediction of carcass fat, water and lean body mass from Lee’s “nutritive ratio” in rats with hypothalamic obesity. Experientia. 1970;26(7):789–790. doi: 10.1007/BF02232553. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boroski M., Visentainer J.V., Cottica S.M., Morais D.R. 1th ed. Appris; Curitiba: 2015. Antioxidantes: Princípios e métodos analíticos. [Google Scholar]
- Censin J.C., Peters S.A.E., Bovijn J., Ferreira T., Pulit S.L., Mägi R., Mahajan A., Holmes M.V., Lindgren M. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019;15(10) doi: 10.1371/journal.pgen.1008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa N.M.B., Peluzio M.C.G., Martino H.S.D., Henriques G.S. Rubio; Rio de Janeiro: 2014. Nutrição Experimental: teoria e prática. (Chapter: 3) [Google Scholar]
- Cruz J.F., Rezende K.F., da Silva P.M.C., Cruz M.A.F., de Santana D.S., Oliveira C.C.C., Lima S.O. Relação entre a esteatose hepática não alcoólica e as alterações dos componentes da síndrome metabólica e resistência à insulina. Revista Sociedade Brasileira Clínica Médica. 2016;14(2):79–83. [Google Scholar]
- Diederich M. Natural products target the hallmarks of chronic diseases. Biochem. Pharmacol. 2020;173:113828. doi: 10.1016/j.bcp.2020.113828. [DOI] [PubMed] [Google Scholar]
- Falcomer A.L., Resende R.R.F., Lima B.R., Ginani V.C., Zandonadi R.P. Health benefits of green banana consumption: a systematic review. Nutrients. 2019;11(6):1222. doi: 10.3390/nu11061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh Vahid, Carvalho Isabel S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crop. Prod. 2015;65:247–258. doi: 10.1016/j.indcrop.2014.10.057. [DOI] [Google Scholar]
- Ferrier Denise R. Bioquímica ilustrada. 7th. Artmed; 2018. [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Fu C., Yan F., Cao Z., Xie F., Lin L. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014;34(1):123–126. [Google Scholar]
- Jayabalan R., Marimuthu S., Swaminathan K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007;102(1):392–398. [Google Scholar]
- Junqueira L.C., Junqueira L.M.M.S. Santos; São Paulo: 1983. Técnicas básicas de citologia e histologia. [Google Scholar]
- Kapp J.M., Sumner W. Kombucha: a systematic review of the empirical evidence of human health benefit. Annals of epidemiology. Ann. Epidemiol. 2019;30:66–70. doi: 10.1016/j.annepidem.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Kim J., Kirkland R., Lee S., Cawthon C., Rzepka K., Minaya D.…de La Serre C.B. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol. Behav. 2020:113082. doi: 10.1016/j.physbeh.2020.113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic Z., Davidovic J., Vuckovic-Filipovic J., Janicijevic-Petrovic M.A., Janicijevic K., Popovic A. A toxic hepatitis caused the kombucha tea – case report. Macedonian J. Med. Sci. 2014;2(1):128–131. [Google Scholar]
- Lasker S., Rahman M.M., Parvez1 F., Zamila M., Miah P., Nahar K., Kabir F., Sharmin S.B., Subhan N., Ahsan G.U., Alam M.D.A. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019;9(1):20026. doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Kim J., Wang S., Sung S., Kim N., Lee H., Seo Y., Jung Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019;20(9):2369. doi: 10.3390/ijms20092369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan L.K., Raymond J.L. fourteenth ed. Elsevier; São Paulo: 2018. Krause Alimentos, Nutrição e Dietoterapia. (Chapter 30) [Google Scholar]
- Mahboobi S., Rahimi F., Jafarnejad S. Effects of prebiotic and synbiotic supplementation on glycaemia and lipid profile in Type 2 diabetes: a meta-analysis of randomized controlled trials. Adv. Pharmaceut. Bull. 2018;8(4):565–574. doi: 10.15171/apb.2018.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafaia A.B., Nassif P.A.N., Ribas C.A.P., Ariede B.L., Sue K.N., Cruz M.A. Obesity induction with high fat sucrose in rats. Arquivos Brasileiros de Cirurgia Digestiva. 2013;26(1):17–21. doi: 10.1590/s0102-67202013000600005. [DOI] [PubMed] [Google Scholar]
- Ou B., Chang T., Huang D., Prior R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: first action 2012.23. J. AOAC Int. 2013;96(6):1372–1376. doi: 10.5740/jaoacint.13-175. [DOI] [PubMed] [Google Scholar]
- Pozo R., Mardones L., Villagrán M., Muñoz K.C., Roa S., Rozas F., Ormazábal V., Muñoz M. Efecto de una dieta alta en grasas en el proceso de formación de cálculos biliares de cholesterol. Rev. Med. Chile. 2017;145:1099–1105. doi: 10.4067/s0034-98872017000901099. [DOI] [PubMed] [Google Scholar]
- Reis-Júnior P.M. Catálogo USP; São Paulo: 2016. Frequência de esteatose e esteato-hepatite em necropsias por morte violenta em população adulta. [Google Scholar]
- Reis R.C., Viana E.S., Amorim E.P., Maro L.A.C. Embrapa; Cruz das Almas: 2017. Farinha de Banana Verde: alimento nutritivo e rico em amido resistente. [Google Scholar]
- Roberfroid M., Gibson G.R., Hoyles L., Maccartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., Guarner F., Respondek F., Whelan K., Coxam V., Davicco M.J., Léotoing L., Wittrant Y., Delzenne N.M., Cani P.D., Neyrinck A.M., Meheust A. Prebiotic concept and health. Br. J. Nutr. 2010;104(2):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Ross A.C., Caballero B., Cousins R.J., Tucker K.L., Ziegler T.R. eleventh ed. Manole; Barueri: 2016. Nutrição Moderna de Shils na Saúde e na Doença. (Chapter 61) [Google Scholar]
- Santos J.S., Júnior J.E., Scarpelini S., Sankarankutty A.K. Pancreatite aguda: atualização de conceitos e condutas. Medicina. 2003;36:266–282. [Google Scholar]
- Swain T., Hillis W.E. The phenolic constituents of Prunus domestica. I.-The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10(1):63–68. [Google Scholar]
- Villarreal-Soto S.A., Beaufort S., Bouajila J., Souchard J., Taillandier P. Understanding kombucha tea fermentation: a review. J. Food Sci. 2018;83(3):580–588. doi: 10.1111/1750-3841.14068. [DOI] [PubMed] [Google Scholar]
- Vona R., Gambardella L., Cittadini C., Straface E., Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell Longev. 2019:8267234. doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.