Abstract
Asthma is a heterogeneous disease, characterised by different phenotypes and endotypes. Precision medicine in asthma refers to the implementation of a targeted therapy for each individual child, based on the identification of treatable traits, including environmental, immunological and genetic factors. Severe asthma in children is associated with increased hospitalisation rates, a lower quality of life, increased healthcare costs and an increased mortality. In the era of new molecular biologics treatments, it is essential to improve deep phenotyping of children with severe asthma in order to deliver the most effective treatment to each individual child. In this review, we discuss the personalised approach to the assessment and management of severe asthma. We explore the indications and use of the currently licensed biologics, as well as the potential of other emerging treatments.
Keywords: child, omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab
Introduction
Childhood asthma is a global problem with substantial variation in asthma prevalence worldwide.1 In the USA, it is estimated that 6.2 million children <18 years have asthma,2 the equivalent of 8.4% or 1 in 12 children. The prevalence of childhood asthma in the European Union is very similar with an estimated 9.4% of children diagnosed and treated for the condition.3
Childhood asthma frequently starts in the early years as preschool wheeze. Several epidemiological phenotypes have been described in North America4 and Europe.5–7 Three epidemiological phenotypes are recognised in both continents:8 (a) transient early wheeze which starts before the age of 3 years and resolves by age 6, (b) late-onset wheeze which starts 3 years of age and persists in childhood, and (c) persistent wheeze which starts before 3 years of age and persists into school age. The clinical phenotypes are described as episodic viral wheeze and multiple trigger wheeze,9,10 but there is considerable overlap and movement between the clinical phenotypes over time.11 Children with preschool episodic viral wheeze often become asymptomatic when reaching school age.5 However, these children still show lung function deficits as young adults and low level eosinophilic airway inflammation.12 Preschool disease markers predicting the development of severe asthma in older children are lacking; however, a recent study suggests that early sensitisation to thermotolerant fungi is associated with worse lung function in children with asthma.13
Asthma severity is a spectrum but most children with asthma are controlled with regular use of low-to-medium doses of inhaled corticosteroids (ICS) with or without additional controller drugs. Severe asthma constitutes the extreme end of the asthma spectrum. The European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force defines severe asthma in people ≥6 years in the following way; asthma that requires treatment with high dose ICS and long-acting beta-2 agonist (LABA) or leukotriene receptor antagonists (LTRA) or theophylline at GINA steps 4–514 for the previous year or the need for systemic corticosteroids (CS) for ≥50% of the previous year to prevent the asthma from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy.15 Uncontrolled asthma is defined by the presence of one of: a) poor symptom control score, b) ≥3 bursts of systemic corticosteroids, c) one or more hospitalisations in the previous 12 months, or d) airflow limitation (forced expiratory volume in 1st second (FEV1) <80% predicted and the Tiffeneau-Pinelli index (FEV1/forced vital capacity (FVC)) < lower limit of normal).
These diagnostic criteria for severe asthma are fairly rigid and do not take into account that only a small proportion of children with asthma is followed in specialist care settings. Asthma severity in non-specialist settings is probably underestimated in a significant number of children.16 Estimating the number of children with severe asthma therefore is difficult and it is not surprising that the data varies from country to country and in different healthcare settings.1 Recent reports from Europe17 and North America18 suggest that the prevalence of severe asthma is approximately 1% to 5% of the child asthma population.1
Children with severe asthma have a disproportionately high healthcare burden due to the frequency of asthma attacks experienced by this group of children in combination with the cost of high-dose ICS and other preventer and rescue medication.19 High-cost therapies such as the newly available monoclonal antibodies are playing their part in the escalating cost of severe childhood asthma.
Classification of Severe Childhood Asthma
Problematic Severe Asthma
The term problematic severe asthma has been used to describe children with a poor clinical response despite prescription of high dose preventer medication.20,21 After careful clinical evaluation, which includes confirmation that asthma is the correct diagnosis, these children fall broadly into two categories although there can be considerable overlap; children with difficult-to-treat asthma with or without comorbidities and children with severe therapy resistant asthma.
Difficult-to-Treat Asthma
This group includes children where modifiable factors including comorbidities contribute to asthma severity. These children often become controlled when these factors are addressed. Modifiable factors include poor medication adherence,22 exposure to environmental factors such as pet allergens23 and other indoor allergens such as fungi,13 exposure to tobacco smoke,24 air pollution,25 and psychosocial factors.26
Comorbidities
Important comorbidities include dysfunctional breathing,27 allergic rhinitis,28 sinusitis,29 anxiety and depression,30 and obesity.31 The evidence however suggests that treatment of allergic rhinitis does not improve asthma symptoms in either children or adults where both conditions co-exist.32
Home visits are important because they allow the asthma team to get an impression of the home environment. The visit can identify ongoing allergen exposure to dust, moulds, pets, exposure to second hand tobacco smoke or vaping and other pollutants.33 The Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED) is a European project involving 16 centres in 11 countries in Europe34 conducted over five years between 2010 and 201435 found that 23% of children with severe asthma had evidence of exposure to environmental tobacco smoke.19 Identification of this trigger is important and a change in parent or young person behavior should be encouraged.36 Recent data from Scotland highlight the benefits of a smoke-free home intervention to decreases asthma attacks in children.37
Severe Therapy-Resistant Asthma (STRA)
This term describes children who remain uncontrolled on high dose preventer medication after modifiable factors including comorbidities have been addressed.38 Importantly, good adherence to preventer medication using technology is required to confirm the presence of STRA.39 These children may be considered for biologics as an add-on therapy.
Adherence
Poor adherence to asthma preventer medication is common and contributes substantially to the number of children labelled as severe asthma that have in fact mild-moderate asthma. A recent study of 93 children39 attending a specialist severe asthma clinic found that 58% of children showed poor adherence (<80%) despite the fact that the children and families knew that they were monitored, which by itself may have boosted adherence. In addition, 14% dropped out from the original cohort of 108 children.
There is poor correlation between reported and actual adherence and therefore some form of electronic monitoring is essential in the assessment of children with problematic severe asthma. The World Health Organisation40 defines poor adherence as erratic nonadherence, unwitting nonadherence and intelligent nonadherence. Interventions to improve adherence need to be tailored to the pattern of non-adherence41 and to the needs of families and children in order to be effective.
Inhaler Technique
Children and young people should be prescribed pressurized metered dose inhalers (pMDI) with spacers or dry powder inhalers. Inhaler technique must be demonstrated and practiced with the child each time a new device is prescribed39 and yearly thereafter.42 Poor inhaler technique is common in patients with asthma including children43,44 and can be the reason for poor control despite high doses of prescribed ICS.45 A recent study found inhaler technique found mistakes in the majority of children attending a severe asthma clinic and it took on average three weeks of daily observedMobile Remote Video Directly Observed Therapy (MDOT) with coaching before good inhaler technique was established.46 The asthma symptoms improved in all children but the effect of adherence monitoring and improved inhaler technique is difficult to disentangle. In children, directly observed administration of morning dose ICS in school settings is also linked with better asthma control.47
Phenotyping of Severe Pediatric Asthma Patients to Identify Treatable Traits
Precision Medicine
This refers to a personalised approach to patient care by choosing a treatment for the individual patient based on his/her specific characteristics or treatable traits, rather than adopting a “one size fits all” attitude. It requires the identification of treatable traits using deep phenotyping, including genetic, immunological, environmental, and lifestyle factors to identify relevant targeted treatments.48,49
Asthma Phenotypes
Five biologic therapies are now available to treat adults with asthma50 and two for children. This number is likely to increase and accurate phenotyping to identify responder profiles to these biologics is essential for precision medicine. Type 2 (T2) inflammation characterised by the mediators Interleukin (IL)-4, 5 and 13 is present in the vast majority of children with asthma and most adult asthmatics. Biomarkers used in the clinic to diagnose T2 asthma are blood eosinophils51 and fraction of exhaled nitric oxide (FeNO). Sputum eosinophils are useful T2 markers, but due to the semi-invasive nature of obtaining sputum eosinophils, this test is rarely available outside specialist settings, particularly in children.
T2-low asthma is probably rare in children. It is characterized by the absence of T2 mediators and there are no established biomarkers for T2-low asthma.52
The use of blood eosinophil counts as a surrogate marker of airway T2 inflammation has allowed widespread identification of T2 driven asthma and the application of biologics targeting T2 pathways. Newer biologics targeting upstream inflammatory mediators, such as thymic stromal lymphopoietin (TSLP) show clinical benefit and may provide additional options for patients who are not responding to current T2 biologics.53
Fraction of Exhaled Nitric Oxide
FeNO is a marker of T2 asthma and steroid responsiveness.54 It can be easily measured in the clinic from a breath sample.55 Levels less than 20 parts per billion (ppb) are deemed to be normal, levels 20–35 ppb are intermediate and greater than 35 ppb are considered high in children.55 Elevated FeNO levels in breath are present in patients with asthma and allergic rhinitis independent of asthma.55 FeNO is often regarded as an indirect marker of eosinophilic airway inflammation,55 however there is a poor relationship between the degree of sputum eosinophilia and FeNO56 and this may explain why FeNO directed asthma management has not resulted in significantly improved clinical outcomes.54 A raised FeNO is however associated with a greater risk of subsequent asthma attacks57 and it is a marker of non-adherence to ICS.58
Eosinophilic Asthma
Blood and sputum eosinophils above threshold levels are the clinical hallmark of T2 driven asthma, present in the majority of children and adults with asthma. Allergic asthma in adults and older children is characterised by increased numbers of circulating blood eosinophils.59 The recognition in the 1950s that the presence of sputum eosinophils identifies patients who are responsive to corticosteroids marked the beginning of a growing interest in precision medicine in asthma. The initial non-selective study of systemic corticosteroids for asthma concluded that corticosteroids had no benefit for chronic disease management.60 Subsequently however, Harry Morrow Brown,61 an asthma and allergy physician at Derby hospital (UK), showed that corticosteroids improved asthma control in patients with demonstrable sputum eosinophilia and he later showed similar benefits of ICS treatment in children and adults with allergic asthma.62,63
Sputum eosinophils promote airway remodelling, subepithelial membrane thickening, goblet cell metaplasia, altered mucin composition in sputum64 and perpetuate chronic airway inflammation65 (see Figure 1). IL-5 is the key mediator necessary for the development, differentiation, recruitment, activation, and survival of circulating eosinophils.66 Blood and sputum IL-5, eosinophil numbers and their secreted products correlate with the severity and frequency of asthma exacerbations.57,67–69
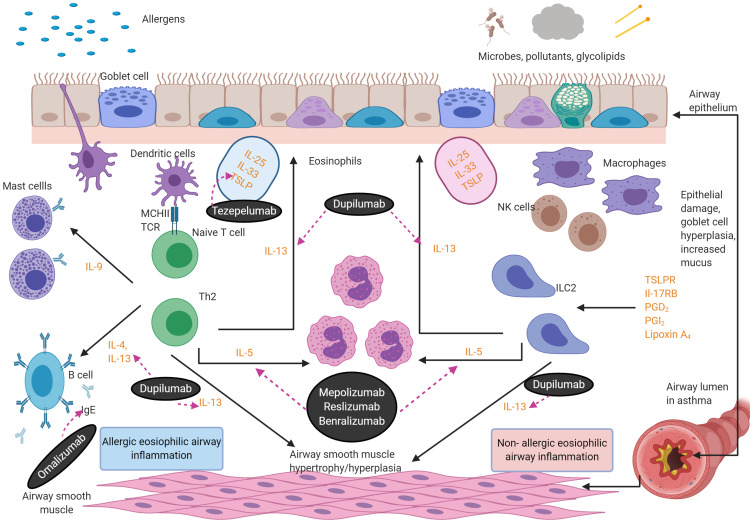
Figure 1.
Eosinophilic asthma inflammatory pathways. There are two aetiologies for eosinophilic inflammation in asthma: an allergic pathway triggered by allergens and a non-allergic mechanism triggered by microbes, pollutants and glycolipids. The key mediators in the pathways are depicted below. Eosinophils release cationic proteins,57 which lead to bronchial epithelial tissue damage, thus causing airways hyper-responsiveness. Eosinophils also lead to airway smooth muscle cell proliferation through increased eosinophils adhesion caused by the release of cationic proteins and the eosinophilic effect on transforming growth factor-β1 and gene coding of wingless/integrase-1 signaling. IL-13 triggers mucus hyper-secretion. Figure created with BioRender.com.
Abbreviations: IgE, immunoglobulins E; IL, interleukins; ILC2, type 2 innate lymphoid cells; TCR, T-cell receptors; NK, natural killer T cell; TSLPR, thymic stromal lymphopoietin receptor; PG, prostaglandin; ECP, eosinophil cationic protein; EPX, eosinophil protein X; EPO, eosinophil peroxidase; MBP, major basic protein.
Blood Eosinophils
Peripheral blood eosinophil numbers correlate with symptom severity,70 degree of airflow limitation71 and airways responsiveness to direct72 and indirect bronchial challenge73 testing.
Sputum Eosinophils
The presence of airway eosinophils predicts a response to corticosteroid therapy in adult patients with asthma.74 Elevated sputum eosinophils are also an important feature of uncontrolled asthma75,76 and correspond to an increased risk of asthma attacks.77 Treatment strategies in adults with asthma, based on regular monitoring and titrating of corticosteroid medication based on sputum eosinophils reduce exacerbations and lower sputum eosinophils.75,76
The effectiveness of a management strategy based on sputum eosinophils in children has not been confirmed. One small study in older children with severe asthma found little benefit in titrating corticosteroids based on sputum eosinophil counts.78 A number of limitations including lack of run-in period limit conclusions that can be drawn from this study.
Current Therapies for T2-High Asthma
Life-Markers
There is a need for shared decision-making between healthcare professionals, and children and their parents. Before even considering a biological therapy, healthcare professionals must assess whether the children and their carers are able to cope with injections and the burden of this treatment.79
IgE Blockers
Immunoglobulin E (IgE) is a class of antibody discovered in 1966, which is increased in patients with atopic illnesses such as asthma.80 IgE production is mediated by T-helper 2 cells through the release of IL-4 and IL-13 cytokines.81 In asthma, IgE exerts its immunological effects by binding to the high-affinity receptor for IgE (FcεRI) expressed on mast cells, basophils, dendritic cells, airway smooth muscle cells, and eosinophils.82 This leads to the release of pro-inflammatory mediators including histamine, tryptase, heparin, and chymase, which in turn trigger the early and late phase asthma reactions.83
Omalizumab
Omalizumab is a humanized monoclonal antibody, which binds to free systemic IgE. It was approved in 2003 by the US Food and Drug Administration as the first biologic for the treatment of asthma. The resulting Omalizumab–IgE complex does not bind to FcεRI,84 thus reducing the downstream inflammatory cascade seen in allergic asthma.
Numerous RCTs have demonstrated the beneficial effects of omalizumab as an adjunct to asthma management, summarised in a 2014 Cochrane review.85 The authors calculated that in eligible patients receiving omalizumab, asthma attacks are reduced (OR 0.55 CI 0.42–0.60), hospital admissions decreased (OR 0.16 CI 0.06–0.42), and SABA usage is reduced (mean difference −0.39 puffs per day, CI 0.55–0.2).85 Only 3 of the 25 trials included in this review were paediatric studies.86–88
A recent meta-analysis of 86 observational studies confirms real-world efficacy of omalizumab at reducing asthma attacks, and improving patient-reported outcomes and lung function in patients with severe allergic asthma.89 Discontinuation of omalizumab is associated with a decline in asthma control.90
Omalizumab reduced the dose of ICS needed to control symptoms and the frequency of asthma attacks during the steroid-reduction phase (18.2 vs 38.5% for placebo) in a double-blind, placebo-controlled RCT in children aged 6–12 years with moderate to severe allergic asthma. Children also reported better quality of life.87 Another RCT in children aged 6 to 11 years with uncontrolled moderate-to-severe persistent allergic asthma similarly showed a reduction in asthma attacks by 43%.86 The substantial reduction in asthma attacks in children on omalizumab has also been confirmed in other real-world settings.91
The multicentre ICATA study88 demonstrated the seasonal beneficial effects of omalizumab in children and young people aged six to 20 years with persistent allergic asthma. Children were randomised to subcutaneous placebo or omalizumab and the study showed a doubling of asthma attacks in the placebo group in the autumn and spring which was not seen in the omalizumab arm.88 Adherence issues in the placebo group may have influenced the findings from this trial.
Omalizumab is generally well tolerated. Upper respiratory tract infection, headache and urticaria are the most frequently reported side effects.92
Anti-IL-5 Therapy
Mepolizumab
Mepolizumab was the first licensed monoclonal antibody developed targeting the IL-5 signalling pathway. It is a humanised monoclonal antibody, belonging to the IgG subclass, which blocks the binding of IL-5 to its receptor on eosinophils.
The initial findings from two early trials of mepolizumab were disappointing. Patients with mild asthma were randomised to intravenous mepolizumab or placebo. Although both blood and sputum eosinophil counts fell significantly in the treatment groups, no significant improvement in FEV1, or airway hyper-responsiveness were observed between the groups.93 A second RCT in adults with moderate-to-severe asthma on daily ICS similarly showed a fall in blood and sputum eosinophils in the treatment groups, but no significant changes in lung function or symptoms.94
However, both these early trials included an unselected cohort of asthma patients, without stratifying for phenotype. The growing interest in precision medicine prompted further investigations into the potential use of anti-IL5 therapy in patients with a known treatable trait, ie, evidence of eosinophilic asthma.95 Two subsequent small studies, which included only patients with a history of recurrent asthma attacks and raised sputum eosinophils, found a substantial reduction by 43% in asthma attacks over 12 months in the mepolizumab arm96 and a reduction in daily OCS.97
These results have since been replicated in several large Phase 3 trials.98–101 In adults with severe eosinophilic asthma, there was a reduction in asthma attacks,98,102 an improvement in quality of life,101 and a reduction in the need for OCS.100 The efficacy of mepolizumab is sustained over the whole 4-week dosing period95 and is proportional to the baseline blood eosinophil count.98 Patient reported quality of life indices were no different between the mepolizumab and placebo groups and there was a limited impact on lung function.98
In children, although patients ≥12 years were eligible to participate in the phase 3 mepolizumab trials, fewer than 40 adolescents were recruited across all studies.103 In the 6-to-11 year age group, there is only one small trial including 30 children, which report mainly on safety and pharmacokinetic data.104 The limited efficacy data reported in this small study demonstrated similar findings to those from the adult studies in terms of reduction in frequency of asthma attacks.
The license for mepolizumab was initially for adults and then extended to children ≥6 years in 2018 by the EMA, and 2019 by the FDA (US Food and Drug Administration).
GINA recommends the consideration of anti-IL5 therapy for adults and children with above threshold blood eosinophils, severe asthma and frequent asthma attacks.14
Reslizumab
Reslizumab is an IgG4/k monoclonal antibody that binds to IL-5 with high specificity. It is used for the treatment of severe uncontrolled eosinophilic asthma, improves asthma control, quality of life and FEV1.105 It is licensed in patients >18 years with baseline blood eosinophils ≥400 cells/μL.106 Studies of reslizumab in children have ended early because no benefits were found in the 12 to 17 year age group.107
There are still concerns about the route of administration and the use of this drug in the wider context.34
Benralizumab
Benralizumab is an anti-eosinophil monoclonal antibody which is an IgG1/k antagonist of the α-chain of the human IL-5 receptor. Benralizumab is the only drug that induces eosinophil apoptosis through cellular toxicity mechanisms (antibody-dependent cell-mediated cytotoxicity) thereby reducing eosinophils in tissues.108 Its clinical effect is independent of circulating levels of IL-5, raised during an asthma attack.109 Benralizumab reduces asthma attacks and leads to improvement in FEV1.110 Benralizumab is an effective steroid-sparing agent in adults.111 Benralizumab is licensed for use in children aged 12 years and older in the USA, but only for adults in Europe, pending clinical trials in children.112
Anti-IL-4/IL-13 Therapy
Dupilumab
IL-4 and IL-13 are two closely related drivers of T2 inflammation, activating the same alpha subunit of the IL-4 receptor (IL-4Rα).113 Both cytokines are secreted by TH2 and type-2 innate lymphoid cells (ILC-2). IL-4, and to a lesser extent IL-13, upregulate IgE synthesis by inducing B-cell class switching; whilst IL-13 plays a key role in airway hyper-responsiveness, increasing mucus production, and airway remodelling.
Dupilumab is a fully humanized monoclonal antibody, which selectively binds to IL-4Rα, thus acting as a dual antagonist for both IL-4 and IL-13.114
In an early phase IIa trial, 104 adults (≥18 years) with moderate-severe asthma and elevated eosinophils in blood or sputum were randomised to receive dupilumab or placebo for up to 12 weeks. Fewer asthma attacks occurred in the treatment arm (odds ratio 0.08) together with lung function improvements and better asthma control.113 These results were replicated in three subsequent larger RCTs; a phase IIb trial which included 776 adults, and two phase 3 trials which also included adolescents (≥12 years of age).115,116 Fewer asthma attacks (~50%),115 and reduced oral corticosteroid dependence were observed in patients receiving dupilumab when compared with placebo.117
Dupilumab appears to be effective in patients with or without evidence of allergic asthma (raised IgE or specific IgE to aeroallergens),118 and regardless of baseline ICS dose or lung function. However, greater benefit is observed in patients with higher baseline eosinophil and FeNO levels.115,118
Of 1902 patients enrolled into the phase 3 dupilumab trial,115 107 were adolescents aged 12–17 years. In a post hoc analysis of this adolescent sub-group, significant improvements in lung function and frequency of attacks, at a similar rate seen in adults, were observed using the 200 mg dosing regimen.119 Dupilumab is licensed for children with asthma aged ≥12 years by the FDA.
In the 6–11 year age group, a randomised controlled trial of dupilumab versus placebo in children with uncontrolled persistent asthma has recently been completed (NCT02948959).120 Although no published data is currently available, an early company press release suggests that the efficacy of dupilumab in children aged 6–11 with asthma is favourable.
For dosing and licensing of the five licensed biologics, please refer to Table 1. See Figure 2 for the mechanism of action of the biologics.
Table 1.
Licensed Biologics Used for Severe Asthma
| Drug | Licensing age Groups | Year of First Approval by US Food and Drug Adminstraion (FDA) | Mechanism of Action | Eligibility Criteria | Published Efficacy Results | Predictors of Response | Off-License Use | Side-Effects | Route and Frequency of Administration | Cost to the NHS |
|---|---|---|---|---|---|---|---|---|---|---|
| Omalizumab | Age 6 and above | 2003 | IgE Blocker | IgE serum level between 30UI/mL to 1500 UI/mL | Better symptom control Fewer attacks |
High FeNO (≥20 ppb) High blood eosinophils (≥2%) Body mass index (≥25 kg/m2) |
IgE levels higher than 1500 UI/mL Different IgE/bodyweight combinations than on the product monograph |
Headache, skin reactions, risk of anaphylaxis- 0.3% | Subcutaneous injection once every 2–4 weeks | £256.15 for a 150-mg vial |
| Mepolizumab | Age 6 and above | 2016 | Anti-IL-5 | Blood eosinophils of ≥300 cells/µL (within 12 months of dosing) + ≥ 4 attacks needing OCS in last 12 months or daily OCS (≥5mg/day) for last 6 months | Better symptom control Fewer attacks |
Pyrexia, headaches, upper abdominal pain, hypersensitivity | Subcutaneous injection once every 4 weeks | £840 per dose | ||
| Reslizumab | Age 18 and above | 2016 | Anti-IL-5 | Blood eosinophilia ≥400 cells/μL | Better symptom control Improvement in FEV1 |
Risk of anaphylaxis, myalgia | Intravenous injection once every 4 weeks | £499.99 per 100-mg vial | ||
| Benralizumab | Age 12 and above | 2017 | Anti-IL-5 | Eosinophilic phenotype | Fewer attacks Improvement in FEV1 |
Pyrexia, headaches and hypersensitivity | Subcutaneous injection once every 4–8 weeks | £1955 per 30-mg pre-filled syringe | ||
| Dupilumab | Age 12 and above | 2018 | Anti-IL-4, Anti- IL-13 | FeNO of more than 25 ppb and a concentration of eosinophils of more than 300/mm3 | Better symptom control Fewer attacks |
Eye inflammation, eye pruritus, headache, hypersensitivity | Subcutaneous injection once every 2 weeks | £1264.89 per pack of 2×2mL syringes of 150 mg/1 mL solution |
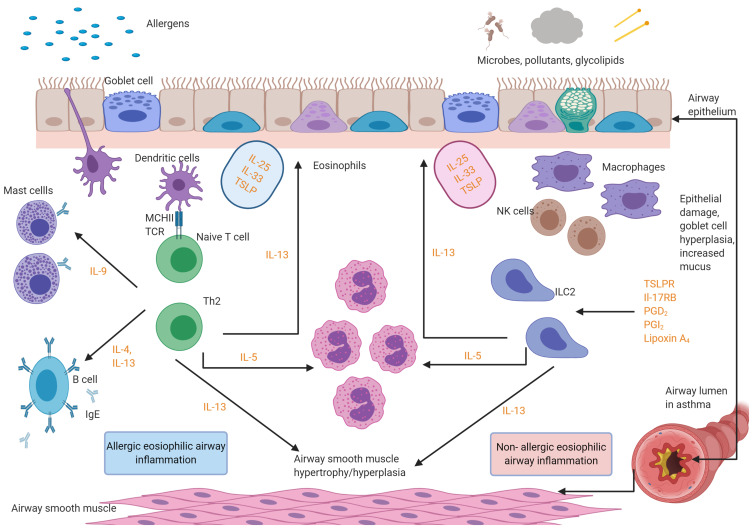
Figure 2.
Licensed mediator cascade of biologics used in eosinophilic asthma. The figure below shows the targets for the licensed biologics. Omalizumab is an IgE-blocker. Mepolizumab, reslizumab and benralizumab are anti IL-5 agents. Dupilumab is an Anti-IL-4/anti-IL-13 agent. Figure created with BioRender.com.
Abbreviations: IgE, Immunoglobulins E; IL, interleukins; ILC2, type 2 innate lymphoid cells; TCR, T-cell receptors; NK, natural killer T cell; TSLPR, thymic stromal lymphopoietin receptor; PG, prostaglandin.
Targeting Upstream Mediators
Currently licenced biologic treatments for severe asthma target downstream mediators. Monoclonal antibodies against upstream mediators that may have an effect in T2-low asthma are in development. Of particular interest are biologics against the alarmins TSLP, IL-25 and IL-33. Alarmins are mediators originating from airway epithelial cells in response to triggers such as allergens, pathogens including viruses and environmental triggers. There are no clinical trials targeting IL-25 or 33, but the monoclonal antibody tezepelumab that blocks TSLP has shown promise and is currently evaluated in phase 3 trials that have been completed.
Thymic Stromal Lymphopoietin (TSLP)
Tezepelumab is a human monoclonal antibody that binds to TSLP, blocking it from interacting with its high-affinity receptor complex composed of TSLP-receptor and IL-7Rα.121,132,133 TSLP is an airway epithelial cytokine released in response to environmental triggers such as viruses and aeroallergens.122,123 Several cells involved in asthma pathophysiology express the TSLP receptor including eosinophils, mast cells, innate type-2 lymphoid cells, lymphocytes, dendritic cells and airway smooth muscle cells.124 In children, TSLP has been implicated in the pathobiology of allergic rhinitis.125
TSLP strongly stimulates a T2 cytokine response but due to its upstream position evidence emerges that it may be useful in T2-low asthma. Interestingly, the results were similar among T2 high and T2-low asthmatics in a Phase 2 study in adults with severe asthma.126 Based on the positive results of the phase 2 study, tezepelumab is now the subject of two phase 3 studies. The NAVIGATOR study is assessing the effect of tezepelumab on the annual rate of asthma exacerbation in patients aged 12–80 years53 while the SOURCE study is an OCS dose reduction study in patients aged 18–80 years. The data is not published but initial press releases suggest that tezepelumab reduces the annual rate of asthma exacerbations in adults with severe asthma,127 but is ineffective at reducing OCS requirement without loss of asthma control.128 No data relating to adolescents is currently available.
Treatment for T2-Low Severe Asthma
There are currently no effective targeted treatments for patients with severe, T2-low asthma. Targeting upstream mediators such as TSLP offers some hope for this group.53
Macrolide Antibiotics
The macrolide antibiotics azithromycin and clarithromycin have been used to treat neutrophilic asthma.34 A trial of azithromycin in pauci-inflammatory asthma in adults also showed a significant reduction in number of attacks.129 Oral azithromycin add-on therapy reduced asthma attacks in adults with persistent uncontrolled eosinophilic and non-eosinophilic asthma in the AMAZES trial130 and is recommended as a treatment by the ERS/ATS guidelines on severe asthma in adults.15 The guidelines advise against the use of chronic macrolides in children with severe asthma.131
Other Precision Treatments for Severe Asthma
Fevipiprant
This prostaglandin D2 receptor 2 antagonist showed promise in phase 2 clinical trials,132 but the recent phase 3 trials LUSTER-1 and LUSTER-2 showed no statistically significant reduction in asthma exacerbations in adults.133
Bronchial Thermoplasty
This treatment delivers targeted heat to the airway wall to reduce airway smooth muscle mass. It also modulates mucosal inflammatory responses and collagen deposition.134 There are several trials of BT in asthma including Asthma Intervention Research (AIR)-1135 and AIR-2136 showing that BT can lead to a reduction in asthma attacks, an improvement in lung function and an improvement in the quality of life. The benefits appear to be sustained over 10 years following the procedure.137 This treatment is not approved for children.
Future Directions
Notwithstanding some geographical variation, approximately one child in every 100 to 150 children has severe asthma that is uncontrolled on high dose ICS or regular OCS. These children require careful assessment and phenotyping and many would benefit from treatment with biologics. A few monoclonal antibodies are already currently licensed for the treatment of severe asthma in children and this number is likely to increase. Biologics are frequently only evaluated in small numbers of children as part of adult focussed asthma studies, a situation that is inadequate. Future development of targeted biologic treatments should include separate high-quality studies in children to test efficacy and safety.
Conclusion
Severe asthma in children places a substantial burden on children, their carers and healthcare services. It is a heterogeneous condition and as our knowledge of phenotypes, endotypes and the genetics of asthma improves, we will increasingly be able to deliver personalised precision care to children, rather than using a “one size fits all” approach. Precision medicine in asthma allows the clinical teams to tailor treatment plans and use the most appropriate biological treatment where standard care is not adequately controlling the asthma. The increasing availability of biologics for children with severe asthma presents an opportunity to improve the lives of children with severe asthma. Ongoing challenges include the need for better phenotyping of severe childhood asthma with ideally minimally invasive or non-invasive tests and the recruitment of more children to phase 3 studies of novel targeted biological treatments.
Disclosure
Dr Erol A Gaillard reports consultancy work for Boehringer Ingelheim with money paid to the institution (University of Leicester), investigator led research grants from Chiesi, Gilead,and Circassia, and non-financial support from Medimmune, outside the submitted work. The authors reported no other potential conflicts of interest for this work.
References
- 1.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahran H, Bailey C, Damon S, Garbe P, Breysse P Vital signs: asthma in children — United States, 2001–2016; 2018. [DOI] [PMC free article] [PubMed]
- 3.Gibson GJ, Loddenkemper R, Lundbäck B, Sibille Y. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J. 2013;42(3):559. doi: 10.1183/09031936.00105513 [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301 [DOI] [PubMed] [Google Scholar]
- 5.Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J. 2008;31(5):974. doi: 10.1183/09031936.00153507 [DOI] [PubMed] [Google Scholar]
- 6.Kuehni CE, Brooke AM, Strippoli M-PF, Wels BD, Davis A, Silverman M. Cohort profile: the Leicester Respiratory Cohorts. Int J Epidemiol. 2007;36(5):977–985. doi: 10.1093/ije/dym090 [DOI] [PubMed] [Google Scholar]
- 7.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974–980. doi: 10.1136/thx.2007.093187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fainardi V, Santoro A, Caffarelli C. Preschool wheezing: trajectories and long-term treatment. Front Pediatr. 2020;8:240. doi: 10.3389/fped.2020.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand PLP, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32(4):1096. doi: 10.1183/09031936.00002108 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Marcos L, Martinez FD. Multitrigger versus episodic wheeze in toddlers: new phenotypes or severity markers? J Allergy Clin Immunol. 2010;126(3):489–490. doi: 10.1016/j.jaci.2010.06.037 [DOI] [PubMed] [Google Scholar]
- 11.Spycher BD, Cochrane C, Granell R, et al. Temporal stability of multitrigger and episodic viral wheeze in early childhood. Eur Respir J. 2017;50(5):1700014. doi: 10.1183/13993003.00014-2017 [DOI] [PubMed] [Google Scholar]
- 12.Marshall L, Beardsmore CS, Pescatore AM, Kuehni CE, Gaillard EA. Airway eosinophils in older teenagers with outgrown preschool wheeze: a pilot study. Eur Respir J. 2015;46(5):1486. doi: 10.1183/13993003.00174-2015 [DOI] [PubMed] [Google Scholar]
- 13.Welsh KG, Holden KA, Wardlaw AJ, et al. Fungal sensitization and positive fungal culture from sputum in children with asthma are associated with reduced lung function and acute asthma attacks respectively. Clin Exp Allergy. 2020. doi: 10.1111/cea.13799 [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma (GINA); 2019. Difficult-to-treat and severe asthma guide. Available from: https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed May 7, 2021
- 15.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 16.Lo DK, Beardsmore CS, Roland D, et al. Lung function and asthma control in school-age children managed in UK primary care: a cohort study. Thorax. 2020;75(2):101. doi: 10.1136/thoraxjnl-2019-213068 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed H, Turner S. Severe asthma in children—a review of definitions, epidemiology, and treatment options in 2019. Pediatr Pulmonol. 2019;54(6):778–787. doi: 10.1002/ppul.24317 [DOI] [PubMed] [Google Scholar]
- 18.Guilbert TW, Bacharier LB, Fitzpatrick AM. Severe asthma in children. J Allergy Clin Immunol Pract. 2014;2(5):489–500. doi: 10.1016/j.jaip.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015;46(5):1322. doi: 10.1183/13993003.00780-2015 [DOI] [PubMed] [Google Scholar]
- 20.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376(9743):814–825. doi: 10.1016/S0140-6736(10)61054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush A, Hedlin G, Carlsen K-H, de Benedictis F, Lodrup-Carlsen K, Wilson N. Severe childhood asthma: a common international approach? Lancet. 2008;372(9643):1019–1021. doi: 10.1016/S0140-6736(08)61422-1 [DOI] [PubMed] [Google Scholar]
- 22.McDonald VM, Yorke J. Adherence in severe asthma: time to get it right. Eur Respir J. 2017;50(6):1702191. doi: 10.1183/13993003.02191-2017 [DOI] [PubMed] [Google Scholar]
- 23.Mendy A, Wilkerson J, Salo PM, Zeldin DC, Thorne PS. Endotoxin clustering with allergens in house dust and asthma outcomes in a U.S. national study. Environl Health. 2020;19(1):35. doi: 10.1186/s12940-020-00585-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2017;195(7):854–859. doi: 10.1164/rccm.201606-1213PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffer PE, Mudway IS, Grigg J. Air pollution and asthma: mechanisms of harm and considerations for clinical interventions. Chest. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Osman LM. Psychological factors in asthma control and attack risk. Thorax. 2002;57(3):190. doi: 10.1136/thorax.57.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker N, Thevasagayam R, Ugonna K, Kirkby J. Pediatric dysfunctional breathing: proposed components, mechanisms, diagnosis, and management. Front Pediatr. 2020;8:379. doi: 10.3389/fped.2020.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Jong HJI, Voorham J, Scadding GK, et al. Evaluating the real-life effect of MP-AzeFlu on asthma outcomes in patients with allergic rhinitis and asthma in UK primary care. World Allergy Organ J. 2020;13(12):100490. doi: 10.1016/j.waojou.2020.100490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon AE. Rhinosinusitis and asthma: the missing link. Curr Opin Pulm Med. 2009;15(1):19–24. doi: 10.1097/MCP.0b013e32831da87e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saragondlu Lakshminarasappa D, Chandrasekaran V, Kandasamy P. Co-morbid anxiety and depression in childhood asthma and its effect on symptom control: a cross sectional study. Pediatr Pulmonol. 2021;56(2):378–383. doi: 10.1002/ppul.25180 [DOI] [PubMed] [Google Scholar]
- 31.Bantulà M, Roca-Ferrer J, Arismendi E, Picado C. Asthma and obesity: two diseases on the rise and bridged by inflammation. J Clin Med. 2021;10(2):169. doi: 10.3390/jcm10020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith H, Horney D, Jones C, Goubet S, Mukhopadhyay S, Frew A. Pragmatic randomized controlled trial of an allergy intervention for children aged 6–16 with asthma and rhinitis in general practice. Clin Exp Allergy. 2016;46(9):1227–1235. doi: 10.1111/cea.12781 [DOI] [PubMed] [Google Scholar]
- 33.Gold DR, Adamkiewicz G, Arshad SH, et al. NIAID, NIEHS, NHLBI, and MCAN Workshop Report: the indoor environment and childhood asthma—implications for home environmental intervention in asthma prevention and management. J Allergy Clin Immunol. 2017;140(4):933–949. doi: 10.1016/j.jaci.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galeone C, Scelfo C, Bertolini F, et al. Precision medicine in targeted therapies for severe asthma: is there any place for “Omics” technology? Biomed Res Int. 2018;2018:1–15. doi: 10.1155/2018/4617565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Lung Foundation; 2021. Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED). Available from: https://europeanlung.org/en/projects-and-campaigns/past-projects/u-biopred. Accessed May 7, 2021
- 36.Bush A, Bhatt J, Grigg J. E cigarettes: tar wars: the (Tobacco) empire strikes back. Arch Dis Child. 2019;104(11):1027–1039. doi: 10.1136/archdischild-2018-315820 [DOI] [PubMed] [Google Scholar]
- 37.Turner S, Mackay D, Dick S, Semple S, Pell JP. Associations between a smoke-free homes intervention and childhood admissions to hospital in Scotland: an interrupted time-series analysis of whole-population data. Lancet Public Health. 2020;5(9):e493–e500. doi: 10.1016/S2468-2667(20)30178-X [DOI] [PubMed] [Google Scholar]
- 38.Fainardi V, Saglani S. An approach to the management of children with problematic severe asthma. Acta Biomed. 2020;91(3):e2020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jochmann A, Artusio L, Jamalzadeh A, et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J. 2017;50(6):1700910. doi: 10.1183/13993003.00910-2017 [DOI] [PubMed] [Google Scholar]
- 40.WHO; 2003. Adherence to long-term therapies. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Accessed May7, 2021.
- 41.van Boven JFM, Trappenburg JCA, van der Molen T, Chavannes NH. Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim Care Respir Med. 2015;25:15046. doi: 10.1038/npjpcrm.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sleath B, Ayala GX, Gillette C, et al. Provider demonstration and assessment of child device technique during pediatric asthma visits. Pediatrics. 2011;127(4):642–648. doi: 10.1542/peds.2010-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchis J, Gich I, Pedersen S. Systematic review of errors in inhaler use: has patient technique improved over time? CHEST. 2016;150(2):394–406. doi: 10.1016/j.chest.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 44.Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. 2016;16(7):605–615. doi: 10.1016/j.acap.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 45.Kocks JWH, Chrystyn H, van der Palen J, et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim Care Respir Med. 2018;28(1):43. doi: 10.1038/s41533-018-0110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shields MD, ALQahtani F, Rivey MP, McElnay JC. Mobile direct observation of therapy (MDOT) - A rapid systematic review and pilot study in children with asthma. PLoS One. 2018;13(2):e0190031. doi: 10.1371/journal.pone.0190031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington CB, Langhans E, Shelef DQ, Savitz M, Whitmore C, Teach SJ. A pilot randomized trial of school-based administration of inhaled corticosteroids for at-risk children with asthma. J Asthma. 2018;55(2):145–151. [DOI] [PubMed] [Google Scholar]
- 48.Papaioannou AI, Diamant Z, Bakakos P, Loukides S. Towards precision medicine in severe asthma: treatment algorithms based on treatable traits. Respir Med. 2018;142:15–22. doi: 10.1016/j.rmed.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 49.Vijverberg SJH, Brinkman P, Rutjes NWP, Maitland-van der Zee AH. Precision medicine in severe pediatric asthma: opportunities and challenges. Curr Opin Pulm Med. 2020;26(1):77–83. doi: 10.1097/MCP.0000000000000633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzel SE. Severe adult asthmas: integrating clinical features, biology and therapeutics to improve outcomes. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson RK, Bush A, Stokes J, Nair P, Akuthota P. Eosinophilic Asthma. J Allergy Clin Immunol Pract. 2020;8(2):465–473. doi: 10.1016/j.jaip.2019.11.024 [DOI] [PubMed] [Google Scholar]
- 52.Fitzpatrick AM, Chipps BE, Holguin F, Woodruff PG. T2-“Low” asthma: overview and management strategies. J Allergy Clin Immunol Pract. 2020;8(2):452–463. doi: 10.1016/j.jaip.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 53.Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):266. doi: 10.1186/s12931-020-01526-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018;73(12):1110. doi: 10.1136/thoraxjnl-2018-211540 [DOI] [PubMed] [Google Scholar]
- 55.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 2013;188(3):400–402. doi: 10.1164/rccm.201212-2156LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol. 2013;132(4):821–827.e5. doi: 10.1016/j.jaci.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 58.Heaney LG, Busby J, Bradding P, et al. Remotely monitored therapy and nitric oxide suppression identifies nonadherence in severe asthma. Am J Respir Crit Care Med. 2018;199(4):454–464. doi: 10.1164/rccm.201806-1182OC [DOI] [PubMed] [Google Scholar]
- 59.Grol MH, Postma DS, Vonk JM, et al. Risk factors from childhood to adulthood for bronchial responsiveness at age 32–42 yr. Am J Respir Crit Care Med. 1999;160(1):150–156. doi: 10.1164/ajrccm.160.1.9707103 [DOI] [PubMed] [Google Scholar]
- 60.CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the Medical Research Council by the subcommittee on clinical trials in asthma. Lancet. 1956;271:803–806.doi: 10.1016/S0140-6736(56)92241-3 [DOI] [PubMed] [Google Scholar]
- 61.Morrow Brown H. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958;272(7059):1245–1247. doi: 10.1016/S0140-6736(58)91385-0 [DOI] [PubMed] [Google Scholar]
- 62.Brown HM, Storey G, George WH. Beclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. 1972;1(5800):585–590. doi: 10.1136/bmj.1.5800.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown HM, Storey G, Jackson FA. Beclomethasone dipropionate aerosol in long-term treatment of perennial and seasonal asthma in children and adults: a report of five-and-half years’ experience in 600 asthmatic patients. Br J Clin Pharmacol. 1977;4 Suppl 3(Suppl3):259S–267S. doi: 10.1111/j.1365-2125.1977.tb04517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welsh KG, Rousseau K, Fisher G, et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 2017;152(4):771–779. doi: 10.1016/j.chest.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saglani S, Lloyd CM. Eosinophils in the pathogenesis of paediatric severe asthma. Curr Opin Allergy Clin Immunol. 2014;14(2). doi: 10.1097/ACI.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 66.O’Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J Allergy Clin Immunol. 2001;108(4):503–508. doi: 10.1067/mai.2001.119149 [DOI] [PubMed] [Google Scholar]
- 67.Sur S, Gleich GJ, Swansonb MC, Bartemesb KR, Broide DH. Eosinophilic inflammation is associated with elevation of interleukin-5 in the airways of patients with spontaneous symptomatic asthma.J. Allergy Clin Immunol. 1995;96(5):661–668. doi: 10.1016/S0091-6749(95)70265-2 [DOI] [PubMed] [Google Scholar]
- 68.Gaillard EA, McNamara PS, Murray CS, Pavord ID, Shields MD. Blood eosinophils as a marker of likely corticosteroid response in children with preschool wheeze: time for an eosinophil guided clinical trial? Clin Exp Allergy. 2015;45(9):1384–1395. doi: 10.1111/cea.12535 [DOI] [PubMed] [Google Scholar]
- 69.Holden KA, Roland D, Welsh KG, Gaillard EA. Comparison of blood eosinophil numbers between acute asthma and stable disease in children with preschool wheeze. Pediatr Allergy Immunol Pulmonol. 2017;30(4):210–217. doi: 10.1089/ped.2017.0802 [DOI] [PubMed] [Google Scholar]
- 70.Nadif R, Siroux V, Oryszczyn M-P, et al. Heterogeneity of asthma according to blood inflammatory patterns. Thorax. 2009;64(5):374. doi: 10.1136/thx.2008.103069 [DOI] [PubMed] [Google Scholar]
- 71.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4):1702536. doi: 10.1183/13993003.02536-2017 [DOI] [PubMed] [Google Scholar]
- 72.Taylor KJ, Luksza AR. Peripheral blood eosinophil counts and bronchial responsiveness. Thorax. 1987;42(6):452–456. doi: 10.1136/thx.42.6.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh Y, Choi S. Blood eosinophil counts for the prediction of the severity of exercise-induced bronchospasm in asthma. Respir Med. 2002;96(2):120–125. doi: 10.1053/rmed.2001.1238 [DOI] [PubMed] [Google Scholar]
- 74.Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000;55(3):232–234. doi: 10.1136/thorax.55.3.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34(2):129–139. doi: 10.1177/147323000603400202 [DOI] [PubMed] [Google Scholar]
- 76.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27(3):483. doi: 10.1183/09031936.06.00137704 [DOI] [PubMed] [Google Scholar]
- 77.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161(3):769–774. doi: 10.1164/ajrccm.161.3.9809071 [DOI] [PubMed] [Google Scholar]
- 78.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67(8):675. doi: 10.1136/thoraxjnl-2011-201064 [DOI] [PubMed] [Google Scholar]
- 79.Tan L, Chupp G, Castro M, Kraft M. Going beyond “Bio-markers,” Think “Life-markers”. Chest. 2020;157(3):503–505. doi: 10.1016/j.chest.2019.08.2210 [DOI] [PubMed] [Google Scholar]
- 80.Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19(1):113. doi: 10.1186/s12931-018-0813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Punnonen J, Yssel H, de Vries JE. The relative contribution of IL-4 and IL-13 to human IgE synthesis induced by activated CD4+ or CD8+ T cells. J Allergy Clin Immunol. 1997;100(6 Pt 1):792–801. doi: 10.1016/S0091-6749(97)70276-8 [DOI] [PubMed] [Google Scholar]
- 82.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072 [DOI] [PubMed] [Google Scholar]
- 83.Pelaia C, Calabrese C, Terracciano R, de Blasio F, Vatrella A, Pelaia G. Omalizumab, the first available antibody for biological treatment of severe asthma: more than a decade of real-life effectiveness. Ther Adv Respir Dis. 2018;12:175346661881019. doi: 10.1177/1753466618810192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bousquet J, Wahn U, Meltzer EO, et al. Omalizumab: an anti-immunoglobulin E antibody for the treatment of allergic respiratory diseases. Eur Respir Rev. 2008;17(107):1. doi: 10.1183/09059180.00010701 [DOI] [Google Scholar]
- 85.Normansell R, Walker S, Milan S, Walters E, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma.J. Allergy Clin Immunol. 2009;124(6):1210–1216. doi: 10.1016/j.jaci.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 87.Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (Omalizumab). Pediatrics. 2001;108(2):e36. doi: 10.1542/peds.108.2.e36 [DOI] [PubMed] [Google Scholar]
- 88.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bousquet J, Humbert M, Gibson PG, et al. Real-world effectiveness of omalizumab in severe allergic asthma: a meta-analysis of observational studies. The Journal of Allergy and Clinical Immunology: In Practice. 2021. doi: 10.1016/j.jaip.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 90.Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–169.e2. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 91.Deschildre A, Marguet C, Salleron J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42(5):1224. doi: 10.1183/09031936.00149812 [DOI] [PubMed] [Google Scholar]
- 92.Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003;91(2):182–188. doi: 10.1016/S1081-1206(10)62175-8 [DOI] [PubMed] [Google Scholar]
- 93.Leckie MJ, Brinke AT, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi: 10.1016/S0140-6736(00)03496-6 [DOI] [PubMed] [Google Scholar]
- 94.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–1071. doi: 10.1164/rccm.200701-085OC [DOI] [PubMed] [Google Scholar]
- 95.Pavord ID, Menzies-Gow A, Buhl R, et al. Clinical development of mepolizumab for the treatment of severe eosinophilic asthma: on the path to personalized medicine. J Allergy Clin Immunol;2020. S2213219820308710. doi: 10.1016/j.jaci.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 96.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nair P, Kjarsgaard M, Efthimiadis A, Hargreave FE. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009. doi: 10.1056/NEJMoa0805435 [DOI] [PubMed] [Google Scholar]
- 98.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 99.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 100.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 101.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 102.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.EMA; 2018. Assessment report Nucala. Available from: https://www.ema.europa.eu/en/documents/variation-report/nucala-h-c-3860-ii-0013-g-epar-assessment-report-variation_en.pdf. Accessed May7, 2021.
- 104.Gupta A, Ikeda M, Geng B, et al. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J Allergy Clin Immunol. 2019;144(5):1336–1342.e7. doi: 10.1016/j.jaci.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 105.Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a Randomized Phase 3 Study. CHEST. 2016;150(4):789–798. doi: 10.1016/j.chest.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 106.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 107.Pijnenburg MW, Fleming L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir Med. 2020;8(10):S2213260020303994. doi: 10.1016/S2213-2600(20)30399-4 [DOI] [PubMed] [Google Scholar]
- 108.Menzella F, Lusuardi M, Galeone C, Facciolongo N, Zucchi L. The clinical profile of benralizumab in the management of severe eosinophilic asthma. Ther Adv Respir Dis. 2016;10(6):534–548. doi: 10.1177/1753465816667659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nowak RM, Parker JM, Silverman RA, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015;33(1):14–20. doi: 10.1016/j.ajem.2014.09.036 [DOI] [PubMed] [Google Scholar]
- 110.Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2(11):879–890. doi: 10.1016/S2213-2600(14)70201-2 [DOI] [PubMed] [Google Scholar]
- 111.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 112.ClinicalTrials.gov. PK/PD and long term safety study of benralizumab in children with severe eosinophilic asthma (TATE). Available from: https://clinicaltrials.gov/ct2/show/NCT04305405. Accessed May7, 2021.
- 113.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048 [DOI] [PubMed] [Google Scholar]
- 114.Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi: 10.3389/fimmu.2020.603312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 116.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 117.Agache I, Song Y, Rocha C, et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines—Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1058–1068. doi: 10.1111/all.14268 [DOI] [PubMed] [Google Scholar]
- 118.Castro M, Rabe KF, Corren J, et al. Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma. ERJ Open Res. 2020;6(1):00204–02019. doi: 10.1183/23120541.00204-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35:737–748. doi: 10.1007/s12325-018-0702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.ClinicalTrials.gov. VOYAGE. Available from: https://clinicaltrials.gov/ct2/show/NCT02948959. Accessed May7, 2021.
- 121.Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019;28(11):931–940. [DOI] [PubMed] [Google Scholar]
- 122.Mitchell PD, O’Byrne PM. Epithelial-derived cytokines in asthma. Chest. 2017;151(6):1338–1344. doi: 10.1016/j.chest.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 123.Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat Immunol. 2019;20(12):1603–1609. doi: 10.1038/s41590-019-0524-9 [DOI] [PubMed] [Google Scholar]
- 124.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bunyavanich S, Melen E, Wilk JB, et al. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin Mol Allergy. 2011;9:1. doi: 10.1186/1476-7961-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 127.AstraZeneca; 2020. Tezepelumab NAVIGATOR Phase III trial met primary endpoint of a statistically significant and clinically meaningful reduction in exacerbations in a broad population of patients with severe asthma . Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/tezepelumab-navigator-phase-iii-trial-met-primary-endpoint.html. Accessed May7, 2021.
- 128.AstraZeneca; 2020. Update on SOURCE Phase III trial for tezepelumab in patients with severe, oral corticosteroid-dependent asthma.Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-source-phase-iii-trial-for-tezepelumab-in-patients-with-severe-oral-corticosteroid-dependent-asthma.html. Accessed May7, 2021.
- 129.Bush A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? PRR. 2020;34:67–74. [DOI] [PubMed] [Google Scholar]
- 130.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3 [DOI] [PubMed] [Google Scholar]
- 131.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 132.Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699–707. doi: 10.1016/S2213-2600(16)30179-5 [DOI] [PubMed] [Google Scholar]
- 133.Brightling CE, Gaga M, Inoue H, et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir Med. 2021;9(1):43–56. doi: 10.1016/S2213-2600(20)30412-4 [DOI] [PubMed] [Google Scholar]
- 134.Christensen MA, Ott M. Innovative Therapies for Severe Asthma. Fed Pract. 2017;34(12):25–31. [PMC free article] [PubMed] [Google Scholar]
- 135.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707 [DOI] [PubMed] [Google Scholar]
- 136.Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–124. doi: 10.1164/rccm.200903-0354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaudhuri R, Rubin A, Sumino K, et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomised controlled trials. Lancet Respir Med. 2021;9(5):457–466. doi: 10.1016/S2213-2600(20)30408-2 [DOI] [PubMed] [Google Scholar]