Abstract
Moyamoya disease is a chronic, progressive intracranial arteriopathy. It is characterised by progressive stenosis/occlusion of distal intracranial carotid and cerebral arteries. It is associated with a high risk of ischaemic and haemorrhagic stroke. Hereditary, infectious and inflammatory factors have been found to be associated with this condition; however, its aetiology is still unclear. The estimation of disease prevalence is approximately 1.6 to 16.1 in 100 000 live births. This article presents the case of a 5-year-old girl child diagnosed with moyamoya disease, placing emphasis on the clinical and radiographic orofacial characteristics of the case and its dental management.
Keywords: paediatrics (drugs and medicines), healthcare improvement and patient safety, moyamoya, stroke, oral and maxillofacial surgery
Background
Moyamoya disease (MMD) is a chronic, progressive arteriopathy of intracranial vessels. It carries a high risk of ischaemic and haemorrhagic stroke. The aetiology of this condition is still unclear; however, hereditary, infectious and inflammatory factors have been found to be associated with this.1 The ring finger protein (RNF) 213 gene (RNF213) which is located at 17q25.3 has been implicated in this disease, in a study conducted in an East Asian population.2 Similarly, the results from patients of Japanese origin highlighted a single-nucleotide polymorphism in the RNF213 gene among 95% of familial and 79% of sporadic cases of MMD.3 It may manifest as a unilateral or bilateral disease, characterised by progressive stenosis and/or occlusion of distal intracranial internal carotid arteries (ICAs) along with the proximal branches of the anterior and middle cerebral arteries. Cerebral angiography is considered the gold standard for diagnosing MMD and assessing its progression. In most cases, there is a dilation of basal collateral vessels, which arise from the intracranial ICAs and supply the ischaemic area of the brain distal to the occlusion. This results in the characteristic ‘puff of smoke’ appearance in an angiograph.4
MMD was first described by Takeuchi and Shimizu. It was a case of underdeveloped bilateral ICAs.5 Suzuki and Takaku coined the term moyamoya, which stands for something which appears hazy, resembling the puff of cigarette smoke.6 The incidence and prevalence rate of MMD in Japan was reported to be 0.35 and 3.16 per 100 000 individuals, respectively, in 1995, which increased to 0.54 and 6.03 in 2003.7 In South Korea, the prevalence was reported to have increased from 6.3 per 100 000 in 2004 to 9.1 per 100 000 in 2008, while the annual incidence rate had increased from 1.7 to 2.3 per 100 000 individuals.8 9 Similarly, the prevalence in China was reported as 3.92 per 100 000 individuals,10 and the incidence in the USA was reported as 0.086 per 100 000 individuals with a bimodal age of distribution and the peaks in the first and fourth decades of life. A female predisposition with a female-to-male ratio of 2.2 was also reported.
With the advent of non-invasive diagnostic tools such as MR angiography or CT angiography, there has been an increase in the number of newly diagnosed patients with MMD.11 In addition, an increase in the number of survivors with improvements in their management could also have led to an increase in the reported prevalence of MMD worldwide.
The literature regarding the dental management of MMD is still scarce. The previously published reports have not reported coherent clinical signs and symptoms leading to a lack of characteristic manifestations of disease in the oral region. To the best of our knowledge, none of the papers were found to have attempted any analysis about the orofacial features of the disease. This article reports the case of a 5-year-old girl child with MMD with an
objective to elucidate the orofacial characteristics and dental management under local anaesthesia.
Case presentation
A 5-year-old girl child was referred from the Department of Paediatrics for her oral examination due to irregularly placed teeth. Intraoral examination revealed mixed dentition with multiple carious teeth involving maxillary and mandibular primary molars. The child has retained maxillary primary incisors (Federation Dentaire Internationale (FDI) notation-51,52,61,62) with palatally erupting maxillary permanent central incisors (FDI notation-11,21). There has been an increased overjet with class II molar relationship. Facial examination reveals a convex facial profile with prominent maxilla and retruded mandible (figure 1). The family history was unremarkable with non-consanguineous marriage. The medical history was significant with right-side hemiparesis at the age of 2 years. Angiography and MRI were done that revealed left central atrophy with middle cerebral artery territory involvement and multiple tiny collaterals around the circle of Willis (figure 2). The patient is taking aspirin 75 mg daily since then. The child had excessive weight gain with increased appetite and had pain in the abdomen. Contrast-enhanced CT of the abdomen was advised by concerned neurologist, which revealed a hypodense lesion indicative of a simple cyst. Intraoral examination revealed the light pink colour of gingiva with melanin pigmentation and slightly pale hard palate with prominent rugae in the anterior region of palate. Dental hard tissue examination revealed involvement of teeth (FDI notation-51,52,61,62,54,64,65,84,85,75) with dental caries.
Figure 1.
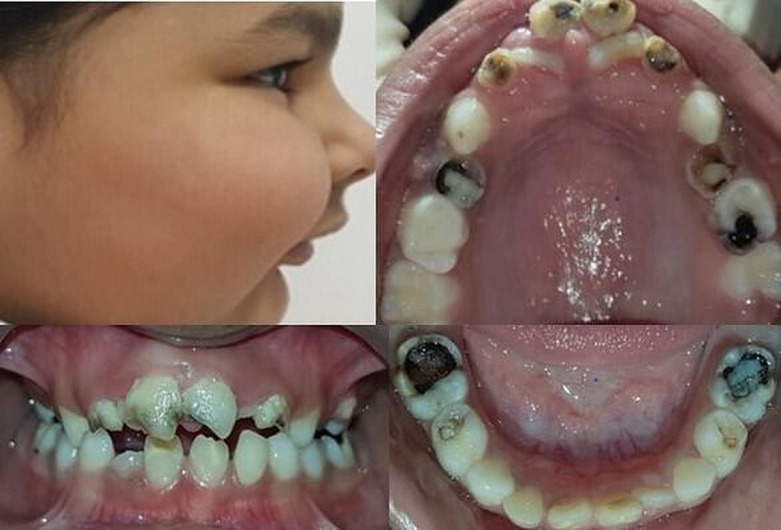
Preoperative clinical photographs.
Figure 2.
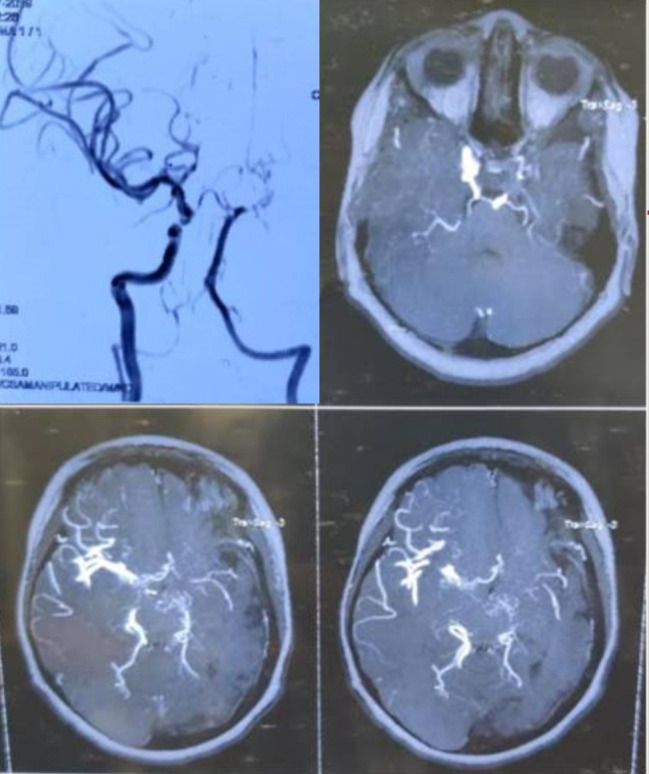
Diagnostic angiogram and MRI.
Investigations
Radiographic examination comprised an orthopantomogram (OPG) and lateral cephalogram. OPG reveals normal tooth development and the presence of developing permanent teeth expected of the dental age (figure 3A). Cephalometric examination reveals a prominent maxilla and retrognathic mandible with increasing angle of convexity (figure 3B). The cephalometric findings of the case are given in table 1. An assessment of Cervical Vertebrae Maturity Indicator (CVMI) staging reveals cervical vertebrae maturation stage 2 which is suggestive of peak interval of mandibular growth that would begin approximately within a year after this stage (figure 3B).
Figure 3.
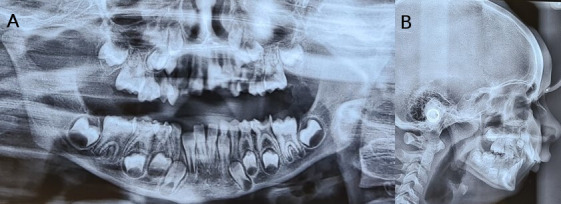
(A) Preoperative orthopantomogram. (B) Preoperative lateral cephalogram.
Table 1.
Dental and skeletal parameters on lateral cephalogram
| Parameter | Normal value | Patient value |
| Tweed analysis | ||
| FMA | 25 | 21 |
| FMIA | 65 | 67 |
| IMPA | 90 | 92 |
| Steiner’s analysis (skeletal parameters) | ||
| SNA | 82 | 89 |
| SNB | 80 | 75 |
| ANB | 2 | 14 |
| Occlusal plane angle | 14 | 15 |
| Mandibular plane angle | 32 | 24 |
| Steiner’s analysis (dental parameters) | ||
| U-NA angle | 22 | 17 |
| U-NA linear | 4 MM | −2 MM |
| L-NB angle | 25 | 17 |
| L-NB linear | 4 MM | −3 MM |
| Interincisal angle | 132 | 133 |
| Down’s analysis (skeletal parameter) | ||
| Facial angle | 82 to 95 | 84 |
| Angle of convexity | −8.5 to 10 | 28 |
| Mandibular plane angle | 17 to 28 | 17 |
| Y-axis | 53 to 66 | 56 |
| A–B plane angle | −9 to 0 | −22 |
| Down’s analysis (dental parameters) | ||
| Cant of occlusal plane | 1.5 to 14 | 7 |
| Interincisal angle | 130 to 150.5 | 133 |
| Incisor occlusal plane angle | 3.5 to 20 | 17 |
| Incisor mandibular plane angle | −8.2 to 7 | 5 |
| Upper incisor to A-Pog line | 1 to 5 | 3 |
ANB, A point to B Point Angle; FMA, Frankfort Mandibular Plane Angle; FMIA, Frankfort Mandibular Incisor Angle; IMPA, Incisor Mandibular Plane Angle; L-NB angle, Angle between lower incisor to NB line; L-NB linear, Distance from lower incisor to NB line; SNA, Sella-Nasion to A Point Angle; SNB, Sella-Nasion to B Point Angle; U-NA angle, Angle between upper incisor to NA line; U-NA linear, Distance from upper incisor to NA line.
Differential diagnosis
The various differential diagnoses based on clinical presentation, haematological findings and familial transmission/genetics are explained in table 2.
Table 2.
Differential diagnosis of moyamoya disease
| Conditions | Clinical presentation | Haematological findings | Family transmission/genetic alteration |
| Homocystinuria |
|
Thromboembolismor cardiovascular disease | Cystathionine beta-synthase gene mutation |
| Homocystienemia | Vascular thrombotic events (with or without the traditional risk factors for a stroke) | Increased carotid plaque thickness or carotid stenosis | Alteration of methylene tetrahydrofolate reductase |
| Hyperglycaemia |
|
Low Hb concentrations or accelerated ageing of RBCs | |
| Hypoglycaemia |
|
Reduced level of blood glucose |
Hb, haemoglobin; RBC, red blood cell.
Treatment
The concerned paediatrician was consulted before the commencement of the treatment. The child was taking aspirin 75 mg daily as prophylaxis to prevent cerebrovascular ischaemic incidents. It was stopped 5 days prior to the procedure (extraction of teeth) as suggested by the paediatrician. The avoidance of stress is crucial to avoid any cerebrovascular accidents during the treatment procedure. The routine behaviour management measures comprising systematic desensitisation, tell–show–do technique and live modelling for management of the present case were used. The efforts were made to keep the length of duration of the procedure as short as possible. Extraction of retained primary teeth (FDI notation-51,61,52,62) and root stump (64) was carried out under local anaesthesia (lignocaine 2% with epinephrine 1:100 000 dilution), and haemostasis was achieved. Carious lesions with tooth 75 and 85 were managed with indirect pulp capping, whereas the other carious teeth were managed with restorations (figure 4).
Figure 4.
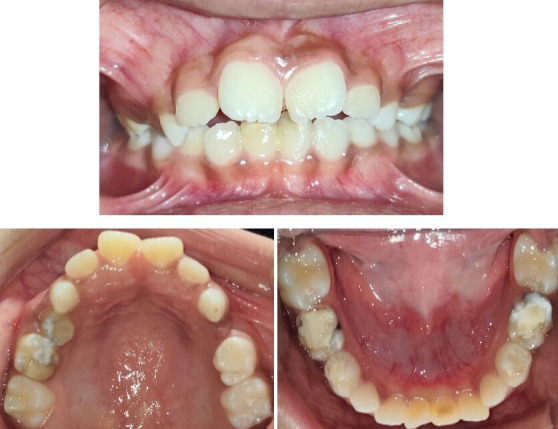
Postoperative clinical photographs.
Follow-up
The patient is under periodic follow-up every 3 months and advised to maintain oral hygiene. There was no further worsening of systemic or oral signs and symptoms.
Discussion
MMD is a rare progressive disease of the proximal cerebral blood vessels with unknown cause, which is strongly associated with genetic factors. MMD tends to affect children between the ages of 5 and 10 years, and adults in the third to fourth decades of life. It is characterised by narrowing of the ICA, middle and anterior cerebral arteries, leading to irreversible blockage of the main blood vessels to the brain. The disease signs include paralysis of part of the body, disability or loss of consciousness. MMD is an arteriopathy with no previously described characteristic oral manifestations. The haemodynamic control during procedure/sedation/anaesthesia is very crucial because hypertension can lead to haemorrhage from friable vessels. Similarly, hypotension resulting in hypoperfusion may further increase the chances of brain ischaemia.12 The dentist should consider the management plan with emphasis on reducing the risk of recurrent stroke. Therefore, management of anxiety and stress is of utmost importance when planning for dental treatment in these cases.
Oral management of cases with MMD should be carried out carefully as the dental procedures can lead to stress, which might be a precipitating factor for ischaemic attacks.13 Table 3 summarises the oral findings and dental management of cases with MMD reported in the literature.
Table 3.
Clinical characteristics and management of cases reported in the literature
| Author/year/ country |
Number of cases | Oral findings | Anaesthesia used (local/sedation/general anaesthesia) |
Treatment rendered |
| Van Camp et al/2019/Belgium14 | 1 | Agenesis of an incisor and multiple impacted third molars | Conscious sedation (midazolam and fentanyl) |
Extraction of multiple teeth including impacted molars |
| Alkeshan et al/2019/South Korea15 | 1 | Multiple carious teeth | General anaesthesia | Oral rehabilitation with full coverage crowns for posterior teeth and composite restorations for anterior teeth |
| Ko and Unkel/2018/USA16 | 1 | Multiple carious primary first molars | General anaesthesia | Formocresol pulpotomy, stainless steel crown and resin-based composite on carious primary molars |
| Bo et al /2017/USA17 | 1 | Poor oral hygiene. Pain with draining sinus with carious teeth. Class I molar relation with an anterior crossbite and negative overjet. Midline diastema and mandibular shift on left side. Tooth size arch length discrepancy in maxillary and mandibular arches | Multiple procedures under local and general anaesthesia | Oral prophylaxis, pit and fissure sealant application, composite resin restorations, extractions of multiple teeth, stainless steel crowns and space maintainers |
| Seto et al/2013/Japan18 | 1 | Multiple impacted third molars | Intravenous sedation | Extraction of multiple impacted third molars |
| Ishikawa et al/2004/Japan19 | 1 | Tactile sensation throughout the jaw and marked collapse of the crown reaching the pulp cavity with multiple teeth | General anaesthesia | Extraction, pulp therapy using formocresol and crown restoration with photopolymerised resin |
It has been found that almost all the cases documented in the literature regarding the dental management of patients with MMD have used pharmacological means in the form of sedation and general anaesthesia.14–19 However, in the present case, the child was potentially cooperative and was managed using non-pharmacological behaviour management techniques. Treatment was initiated with procedures comprising topical fluoride application and application of pit and fissure sealant with shorter duration to improve the child’s behaviour and acceptance of the future dental treatment. This was followed by extraction of primary maxillary central incisors under local anaesthesia as atraumatically as possible and restoration of multiple carious teeth, avoiding the need for general anaesthesia.
Learning points.
There are no characteristic oral manifestations as such in patients with moyamoya disease.
Care should be taken to avoid the stress during dental procedures and to carry them out as atraumatically as possible to avoid any stress-induced ischaemic episodes.
Pharmacological means such as general anaesthesia and sedation shall be available as a treatment option when treating these cases, especially those which cannot be managed by non-pharmacological means or undergoing dental procedures which might be stressful.
Footnotes
Contributors: MR was involved in concept, design, review of literature and preparation. FS was involved in patient care, preparation of the manuscript and revision of the manuscript. NT and VM are involved in review of literature, preparation of the manuscript and critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226–37. 10.1056/NEJMra0804622 [DOI] [PubMed] [Google Scholar]
- 2.Kamada F, Aoki Y, Narisawa A, et al. A genome-wide association study identifies RNF213 as the first moyamoya disease gene. J Hum Genet 2011;56:34–40. 10.1038/jhg.2010.132 [DOI] [PubMed] [Google Scholar]
- 3.Kim E-H, Yum M-S, Ra Y-S, et al. Importance of Rnf213 polymorphism on clinical features and long-term outcome in moyamoya disease. J Neurosurg 2016;124:1221–7. 10.3171/2015.4.JNS142900 [DOI] [PubMed] [Google Scholar]
- 4.Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004;100:142–9. 10.3171/ped.2004.100.2.0142 [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve 1957;9:37–43. [Google Scholar]
- 6.Suzuki J, Takaku A. Cerebrovascular 'moyamoya' disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99. 10.1001/archneur.1969.00480090076012 [DOI] [PubMed] [Google Scholar]
- 7.Wakai K, Tamakoshi A, Ikezaki K, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg 1997;99:S1–5. 10.1016/S0303-8467(97)00031-0 [DOI] [PubMed] [Google Scholar]
- 8.Im SH, Yim SH, Cho CB, SH I, Joo WI, et al. Prevalence and epidemiological features of moyamoya disease in Korea. J Cerebrovasc Endovasc Neurosurg 2012;14:75–8. 10.7461/jcen.2012.14.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn IM, Park D-H, Hann HJ, et al. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke 2014;45:1090–5. 10.1161/STROKEAHA.113.004273 [DOI] [PubMed] [Google Scholar]
- 10.Miao W, Zhao P-L, Zhang Y-S, et al. Epidemiological and clinical features of moyamoya disease in Nanjing, China. Clin Neurol Neurosurg 2010;112:199–203. 10.1016/j.clineuro.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases . Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir 2012;52:245–66. 10.2176/nmc.52.245 [DOI] [PubMed] [Google Scholar]
- 12.Parray T, Martin TW, Siddiqui S. Moyamoya disease: a review of the disease and anesthetic management. J Neurosurg Anesthesiol 2011;23:100–9. 10.1097/ANA.0b013e3181f84fac [DOI] [PubMed] [Google Scholar]
- 13.Balkaya M, Prinz V, Custodis F, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke 2011;42:3258–64. 10.1161/STROKEAHA.110.607705 [DOI] [PubMed] [Google Scholar]
- 14.Van Camp P, Vercruysse M, Gemels B, et al. Prophylactic measures during oral surgery in patients with moyamoya. Oral Maxillofac Surg Cases 2019;5:100101. 10.1016/j.omsc.2019.100101 [DOI] [Google Scholar]
- 15.Alkeshan MM, Song JS, Shin TJ. Dental treatment in a child with moyamoya disease. Acta Scientific Dental Sci 2019;11:57. [Google Scholar]
- 16.Ko BL, Unkel JH. Dental management of a pediatric patient with moyamoya syndrome: a rare clinical entity. Pediatr Dent 2018;40:56–8. [PubMed] [Google Scholar]
- 17.Bo H, Avenetti D, Kratunova E. Dental management considerations in a pediatric patient with moyamoya disease. J Dent Child 2017;84:100–5. [PubMed] [Google Scholar]
- 18.Seto M, Aoyagi N, Koga S, et al. Wisdom teeth extraction in a patient with moyamoya disease. J Korean Assoc Oral Maxillofac Surg 2013;39:289–91. 10.5125/jkaoms.2013.39.6.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa N, Nakamura H, Sugano A. For children with Moya-Moya disease with widespread severe caries. A case of intensive dental treatment under general anaesthesia. In: Small child dentistry miscellaneous magazine. 42, 2004: 689–93. [Google Scholar]


