Abstract
Objectives:
To develop nomograms predicting overall survival (OS), freedom from locoregional recurrence (FFLR), and freedom from distant metastasis (FFDM) for patients receiving chemoradiation for laryngeal squamous cell carcinoma (LSCC).
Material and Methods:
Clinical and treatment data for patients with LSCC enrolled on NRG Oncology/RTOG 0129 and 0522 were extracted from the RTOG database. The dataset was partitioned into 70% training and 30% independent validation datasets. Significant predictors of OS, FFLR, and FFDM were obtained using univariate analysis on the training dataset. Nomograms were built using multivariate analysis with four a priori variables (age, gender, T-stage, and N-stage) and significant predictors from the univariate analyses. These nomograms were internally and externally validated using c-statistics (c) on the training and validation datasets, respectively.
Results:
The OS nomogram included age, gender, T stage, N stage, and number of cisplatin cycles. The FFLR nomogram included age, gender, T-stage, N-stage, and time-equivalent biologically effective dose. The FFDM nomogram included age, gender, N-stage, and number of cisplatin cycles. Internal validation of the OS nomogram, FFLR nomogram, and FFDM nomogram yielded c=0.66, c=0.66 and c=0.73, respectively. External validation of these nomograms yielded c=0.59, c=0.70, and c=0.73, respectively. Using nomogram score cutoffs, three risk groups were separated for each outcome.
Conclusions:
We have developed and validated easy-to-use nomograms for LSCC outcomes using prospective cooperative group trial data.
Keywords: Larynx Cancer, Nomograms, Chemoradiation, Prognostic Factors
Introduction
Since the VA Larynx landmark trial in 1991[1], organ preservation in selected patients with locally advanced laryngeal cancer using primary chemoradiation therapy has become a worldwide standard of care. The initial publication of the Radiation Therapy Oncology Group (RTOG) RTOG 9111 trial clarified that concomitant chemoradiation was superior to induction chemotherapy followed by radiation or radiation alone for laryngeal preservation[2]. However, long-term outcomes of RTOG 9111 showed a trend (p = 0.08) toward poorer overall survival with concomitant chemoradiation relative to induction chemotherapy despite a significantly higher laryngeal preservation rate and locoregional control with concomitant chemoradiation[3]. This unexpected finding may be related to late toxicity associated with concomitant chemoradiation, though the study was not powered to detect this.
Nomograms are useful tools for predicting time-dependent outcomes for patients. A recently published nomogram examining a Dutch cohort demonstrated that age, pre-treatment hemoglobin, T-stage, N-stage, sex, tumor location, and equivalent radiation dose in 2 Gray fractions could be used to predict long-term local control and overall survival in a population of laryngeal cancer patients treated with radiotherapy without chemotherapy[4]. As such, this nomogram does not apply to patients receiving chemoradiation.
In order to develop nomograms to predict survival and control outcomes for patients receiving chemoradiation, we analyzed laryngeal cancer patients from two recently published NRG Oncology/RTOG trials: NRG Oncology/RTOG 0129[5] and NRG Oncology/RTOG 0522[6]. RTOG 0129 randomized patients with locally advanced head and neck cancers to standard fractionation radiation (70 Gy in 35 fractions over 7 weeks) with concurrent cisplatin versus accelerated fractionation with concomitant boost with concurrent cisplatin (72 Gy in 42 fractions over 6 weeks), showing no difference in long-term overall survival, locoregional control, progression-free survival, or distant metastasis[5]. RTOG 0522 randomized a similar group of patients to accelerated fractionation with concomitant boost with concurrent cisplatin with or without cetuximab, showing similar outcomes in both groups in overall survival, locoregional failure, and distant metastasis[6]. 392 eligible patients with laryngeal cancer enrolled on these two trials, providing a large cohort of patients with laryngeal cancer receiving organ preservation with chemoradiation therapy and long-term follow-up. Utilizing these data, we developed and validated nomograms for laryngeal cancer predicting overall survival (OS), freedom from locoregional recurrence (FFLR), and freedom from distant metastasis (FFDM) after completing chemoradiation. Further, we developed a risk group stratification based on our nomograms to classify laryngeal cancer patients into low-, intermediate-, and high-risk groups for these outcomes.
Materials and Methods
Patient Population
Patients eligible for this study had squamous cell carcinoma of the larynx and were enrolled on RTOG 0129 or RTOG 0522. Detailed eligibility and study details for these trials are available in the original publications. [5, 6]
Briefly, for RTOG 0129, patients were eligible if they were 18 years or older and had newly diagnosed Stage III-IV carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx (excluding T1N+ or T2N1 disease) with adequate performance status, bone, marrow, hepatic, and renal function. Patients were randomized to one of two chemoradiation regimens. In the control arm, patients received 70 Gy in 35 fractions over 7 weeks with concurrent cisplatin given at 100 mg/m2 every 3 weeks for 3 cycles. In the experimental arm, patients received 72 Gy in 42 fractions over 6 weeks (BID radiation for the last 12 days) with concurrent cisplatin given at 100 mg/m2 every 3 weeks for 2 cycles.
For RTOG 0522, patients were eligible if they were 18 years or older and had newly diagnosed Stage III-IV (T2N2-N3 M0 or T3–4Nany M0) squamous cell carcinoma of the oropharynx, hypopharynx, or larynx with adequate performance status, bone, marrow, hepatic, and renal function. Patients all received accelerated radiotherapy (either 72 Gy in 42 fractions over 6 weeks with BID radiation for the last 12 days for patients receiving 3D-conformal radiotherapy (3D-CRT) or 70 Gy in 35 fractions over 6 weeks with BID radiation once a week for patients receiving intensity modulated radiotherapy (IMRT)) with concurrent cisplatin 100 mg/m2 on days 1 and 22 of radiotherapy. Patients were randomized to receive this regimen with (experimental arm) or without (control arm) cetuximab.
De-identified clinical data were extracted from the RTOG database for all laryngeal cancer patients enrolled on these two trials and were updated as of the most recent publications of these trials (1/3/2013 for RTOG 0129 and 6/28/2012 for RTOG 0522). All time data were converted to time after randomization. Extracted clinical data include treatment arm, age, gender, Zubrod performance status, baseline hemoglobin level, smoking history, laryngeal subsite, clinical T stage, clinical N stage, treatment institution RTOG accrual volume dichotomized as high or low in the 5 years prior to RTOG 0129, treatment duration, delivered RT dose, delivered RT fractions, delivered cisplatin cycles, survival status, time to last follow-up, time to primary recurrence, time to nodal recurrence, and time to distant metastasis.
To consolidate the effect of different radiation regimens, time-equivalent biologically effective dose (TE-BED) was calculated as:
Where α/β=10, Tk=21 days (kickoff time), T2=2 days (doubling time), and α=0.35 per the methodology modeled for RTOG 90–03[7, 8] by Fowler et al. [9, 10]
Patients receiving < 60 Gy or who had an unknown smoking history were excluded from this analysis.
Statistical Analysis, Nomogram Development and Validation
All statistical analyses and nomogram development were performed using R version 3.2.3 (R-Project, Vienna, Austria), using the following packages: Survival, RMS, pROC, survivalROC, and boot.
Patient data were partitioned randomly into a 70% subset of the total population for nomogram development (henceforth referred to as the training dataset) and a 30% subset for nomogram validation (henceforth referred to as the independent validation dataset). Univariate analysis for clinical predictors of locoregional recurrence, distant metastasis, and overall survival was performed on the training dataset without considering death as a competing risk. A log-rank test p-value of 0.1 or less was considered statistically significant for variable selection.
Nomograms for OS, FFLR, and FFDM were built using Cox proportional hazards models with four a priori variables (age, gender, T-stage, and N-stage) and additional significant predictors from univariate analysis. These nomograms were internally validated on the training dataset using 1000 bootstrapped samples to develop both observed and optimism-corrected calibration plots at a time point of 5 years. C-statistics (c) of the nomograms were calculated for both the training dataset and the independent validation dataset at a time point of 5 years. To calculate bias-corrected 95% confidence interval (CI) for each c-statistic, c-statistics were bootstrapped using 1000 resamples.
Results
Patient Characteristics
392 eligible patients with laryngeal cancer were enrolled on RTOG 0129 and RTOG 0522. Of these, 43 patients received < 60 Gy or had an unknown smoking history and were excluded from this analysis. Thus, this analysis included 349 patients. 70% of these patients were randomly selected as a training dataset (n = 244) and the remaining 30% of these patients were selected as an independent validation dataset (n = 105).
Characteristics of the entire analyzed cohort, the training dataset and the independent validation dataset are summarized in Table 1. The mean age of all analyzed patients was 56 years (range: 26 – 82 years). Patients included were equally split between RTOG 0129 (45%) and RTOG 0522 (55%). 72% of patients had supraglottic cancers, and 28% had glottic or subglottic cancers. 15% of patients had T2, 64% had T3, and 21% had T4 disease. 51% of patients had N0-N1 and 49% of patients had N2-N3 nodes. Ninety-five percent of patients received at least 2 cisplatin cycles and 79% of patients received accelerated fractionation.
Table 1.
Patient Characteristics.
| All Patients | Training Dataset | Independent Validation Dataset | |
|---|---|---|---|
| Number of Patients | 349 | 244 | 105 |
| Enrolled Study | |||
| 0129 | 157 (45%) | 112 (46%) | 45 (43%) |
| 0522 | 192 (55%) | 132 (54%) | 60 (57%) |
| Age (years) | |||
| Mean (Range) | 56.3 (26 – 82) | 56.2 (26 – 79) | 56.5 (30–82) |
| Gender | |||
| Male | 275 (79%) | 191 (78%) | 84 (80%) |
| Female | 74 (21%) | 53 (22%) | 21 (20%) |
| Zubrod Performance Status | |||
| 0 | 198 (57%) | 139 (57%) | 59 (56%) |
| 1 | 151 (43%) | 105 (43%) | 46 (44%) |
| Smoking History | |||
| Never Smoker | 22 (6%) | 18 (7%) | 4 (4%) |
| Former Smoker | 222 (64%) | 155 (64%) | 67 (64%) |
| Current Smoker | 105 (30%) | 71 (29%) | 34 (33%) |
| Primary Site | |||
| Glottis/Subglottis | 99 (28%) | 73 (30%) | 26 (25%) |
| Supraglottis | 250 (72%) | 171 (70%) | 79 (75%) |
| T Stage | |||
| T2 | 51 (15%) | 33 (14%) | 18 (17%) |
| T3 | 223 (64%) | 164 (67%) | 59 (56%) |
| T4 | 75 (21%) | 47 (19%) | 28 (27%) |
| N Stage | |||
| N0-N1 | 179 (51%) | 131 (54%) | 48 (46%) |
| N2-N3 | 170 (49%) | 113 (46%) | 57 (54%) |
| Institutional Head and Neck Accrual Volume | |||
| Low (≤ 41 patients) | 281 (81%) | 196 (80%) | 85 (81%) |
| High (> 41 patients) | 68 (19%) | 48 (20%) | 20 (19%) |
| Cisplatin Cycles | |||
| 0–1 | 19 (5%) | 15 (6%) | 4 (4%) |
| 2–3 | 330 (95%) | 229 (94%) | 101 (96%) |
| Fractionation | |||
| Standard | 73 (21%) | 48 (20%) | 25 (24%) |
| Accelerated | 276 (79%) | 196 (80%) | 80 (76%) |
| Treatment Time (days) | |||
| Mean (Range) | 47.1 (37 – 81) | 47.3 (37 – 79) | 46.6 (38–81) |
| RT Dose (Gy) | |||
| Mean (Range) | 70.8 (63.9–76.0) | 70.8 (64.5–76.0) | 70.6 (63.9–74.3) |
| Time-Equivalent Biologically Effective Dose | |||
| Mean (Range) | 77.5 (68.4–82.9) | 77.6 (68.4–82.9) | 3 (69.2–82.5) |
Survival and Recurrence Outcomes
For all 349 patients, median follow-up was 3.5 years (range: 0.1 – 10.1 years). Among 191 patients alive at last follow-up, median follow-up was 4.1 years (range: 0.1 – 10.1 years).
82 of 349 patients had locoregional recurrence at the time of failure. 65 (79%) of these patients had a failure at the primary site with or without nodal recurrence, leaving only 17 (20%) of these patients with an isolated nodal recurrence. Thus, in the overall population the rate of isolated regional recurrence was only 4.9%.
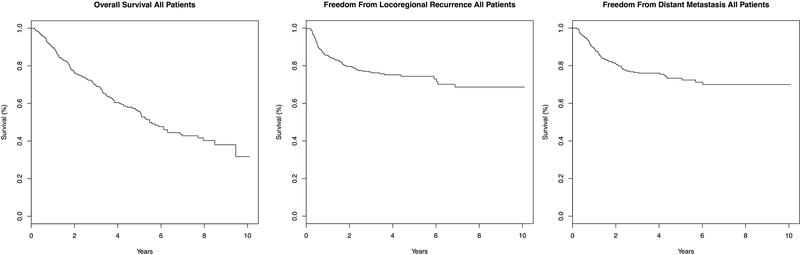
The overall survival (OS), freedom from locoregional recurrence (FFLR), and freedom from distant metastasis (FFDM), of all patients were plotted in Figure 1. Median survival of all 349 patients was 5.47 years with an estimated 3-year and 5-year overall survival of 69.6% and 55.6%, respectively. Median FFLR was not reached with an estimated 3-year and 5-year FFLR of 76.6% and 74.4%, respectively. Median FFDM was also not reached with an estimated 3-year and 5-year FFDM of 76.4% and 73.4%, respectively.
Figure 1. Kaplan-Meier Curves for all Patients.
Kaplan-Meier curves for overall survival (A), freedom from locoregional recurrence (B), and freedom from distant metastasis (C) are plotted for all patients included in this analysis.
Univariate Predictors of Survival and Recurrence Outcomes in the Training Dataset
Univariate analysis was performed on the training dataset to determine any significant predictors of overall survival, locoregional recurrence, and distant metastasis. Results of this analysis are summarized in Table 2. Older age (p < 0.01), male gender (p = 0.02), T-stage (p = 0.02), N2–3 nodes (p = 0.05), and receiving less than 2 cisplatin cycles (p < 0.01) were predictors of poor overall survival. Older age (p = 0.05), N2-N3 nodes (p < 0.01), and lower TE-BED (p < 0.02) were predictors of increased locoregional recurrence. Both male gender and T-Stage showed a trend towards increased locoregional recurrence but did not reach significance (p = 0.1). Finally, male gender and N2-N3 nodes were significant predictors of distant metastasis (p < 0.01 for both), with older age (p = 0.06) and receiving less than 2 cisplatin cycles (p = 0.10) being moderately significant predictors of distant metastasis.
Table 2.
Univariate and Multivariate P-Values for Clinical Variables for Training Dataset.
| Univariate Analysis (Log-rank testing) | |||
| OS | FFLR | FFDM | |
| Age (Continuous) | < 0.01 | 0.05 | 0.06 |
| Gender | 0.02 | 0.10 | < 0.01 |
| Zubrod (0 v. 1) | 0.41 | 0.33 | 0.77 |
| T-Stage (T2 v. T3 v. T4) | 0.02 | 0.10 | 0.29 |
| N-Stage (N0–1 v. N2–3) | 0.05 | < 0.01 | < 0.01 |
| Baseline Hemoglobin (Continuous) | 0.22 | 0.99 | 0.39 |
| Primary Site (Supraglottis v. Glottis/Subglottis) | 0.74 | 0.82 | 0.68 |
| Smoking History (Current v. Former v. Never) | 0.99 | 0.79 | 0.23 |
| Time-Equivalent Biologically Effective Dose (Continuous) | 0.21 | 0.02 | 0.50 |
| Center Head and Neck Accrual Volume (High vs. Low) | 0.91 | 0.63 | 0.59 |
| Cisplatin Cycles (0–1 v. 2+) | < 0.01 | 0.73 | 0.10 |
| Multivariate Analysis (Cox Proportional Hazards Regression) | |||
| OS | FFLR | FFDM | |
| Age (per year) | < 0.01 (HR: 1.03) | 0.07 (HR: 1.03) | 0.10 (HR: 1.03) |
| Male Gender | 0.03 (HR: 1.78) | 0.24 | < 0.01 |
| T-Stage (relative to T2) | |||
| T3 | 0.21 (HR: 1.50) | 0.87 (HR: 0.94) | 0.36 (HR: 1.41) |
| T4 | < 0.01 (HR: 2.72) | 0.35 (HR: 1.48) | 0.14 (HR: 1.89) |
| N-Stage (N2–3 v. N0-N1) | < 0.01 (HR: 1.84) | 0.01 (HR: 2.09) | < 0.01 (HR: 3.74) |
| TE-BED (per Gy) | ∼ | 0.11 (HR: 0.89) | ∼ |
| Cisplatin Cycles (0–1 v 2+) | < 0.01 (HR: 2.76) | ∼ | 0.02 (HR: 2.85) |
Nomogram Development and Validation
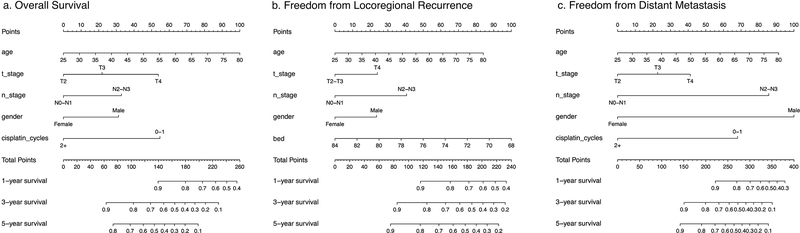
Nomograms for OS (Figure 2a), FFLR (Figure 2b), and FFDM (Figure 2c) were developed on the training dataset utilizing a multivariate Cox proportional hazards model including the aforementioned four a priori predictors (age, gender, T-stage, and N-stage) and additional predictors determined by the univariate analyses. Results of the multivariate analyses are summarized in Table 2. Internal validation of the OS nomogram, FFLR nomogram, and FFDM nomogram was performed using bootstrapped calibration plots. Each nomogram showed excellent correlation both with and without optimism correction. (Supplemental Figure 1) C-statistics obtained for the training dataset yielded c=0.66 (95% CI: 0.60–0.71), c=0.66 (95% CI: 0.59–0.72), and c=0.73 (95% CI: 0.65–0.78), for the OS nomogram, FFLR nomogram and FFDM nomogram, respectively. External validation of each nomogram was performed on the independent validation dataset using c-statistics yielding c=0.59 (95% CI: 0.48–0.68), c=0.70 (95% CI: 0.56–0.80), and c=0.73 (95% CI: 0.61–0.82), respectively.
Figure 2. Nomograms for Overall Survival (OS), Freedom from Locoregional Recurrence (FFLR) and Freedom from Distant Metastasis (FFDM).
The nomogram for OS (a) includes age, T-stage, N-stage, gender, and number of cisplatin cycles received. The nomogram for FFLR (b) includes age, T-stage, N-stage, gender, and time-equivalent BED. The nomogram for FFDM (c) includes age, T-stage, N-stage, gender, and number of cisplatin cycles received.
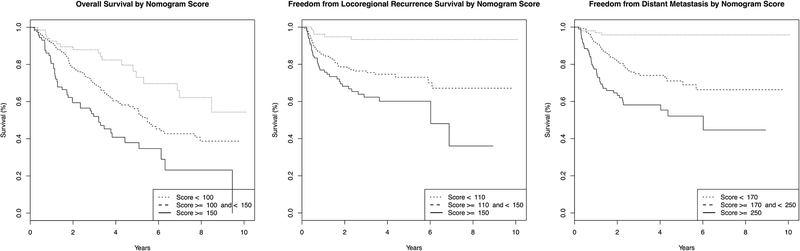
Risk Groups Stratified by Nomogram Scores
To develop practical nomogram score cutoffs, nomogram scores for OS, FFLR, and FFDM were calculated for all 349 eligible patients. Kaplan-Meier curves were plotted (not shown) for each outcome and stratified into three groups by quartile score (Table 3). Using the log-rank test, nomogram score stratification by quartile was shown to be a significant predictor for OS, FFLR, and FFDM (p < 0.001 for each outcome Bonferroni corrected for multiple comparisons). Quartile cutoffs were rounded to the nearest ten to develop Low-, Intermediate-, and High-Risk groups for each outcome (Table 3). Repeat Kaplan-Meier curves were developed utilizing this stratification scheme and plotted in Figure 3. This new risk group stratification by nomogram score remained statistically significant for OS, FFLR, and FFDM (Bonferonni-corrected p < 0.001 for each outcome).
Table 3.
Nomogram Score Cutoffs.
| OS | FFLR | FFDM | |
|---|---|---|---|
| First Quartile | < 106 | < 111 | < 166 |
| Second and Third Quartiles | ≥ 106 and < 146 | ≥ 112 and < 154 | ≥ 166 and < 253 |
| Fourth Quartile | ≥ 146 | ≥ 154 | ≥ 253 |
| Low-Risk Group | < 100 | < 110 | < 170 |
| Intermediate-Risk Group | ≥ 100 and < 150 | ≥ 110 and < 150 | ≥ 170 and < 250 |
| High-Risk Group | ≥ 150 | ≥ 150 | ≥ 250 |
Figure 3. Nomogram-Scores Stratify Patients into Low, Intermediate and High-Risk Groups for OS, FFLR and FFDM.
Utilizing the cutoffs from Supplementary Table 1, Kaplan-Meier curves stratified by risk group for OS, FFLR, and FFDM were plotted for all LSCC patients included in this analysis. The nomogram risk group stratifications for OS, FFLR, and FFDM were significant predictors of each respective outcome. (p < 0.001)
Discussion
Despite improving survival in all malignancies between the mid-1980s and 1990s, laryngeal cancer demonstrated a slight decline in overall survival across the same time period, most notably between 1990 and 1994[11]. This coincided with the 1991 publication of the VA Larynx Cancer Trial. Thus, some authors have attributed the decline in survival to a shift away from total laryngectomy to the increased use of chemoradiation therapy for laryngeal preservation[12], although the VA trial demonstrated equivalent survival with an upfront laryngeal preservation approach when compared to an upfront laryngectomy approach for locally advanced larynx cancers[1]. Others have cited inappropriate patient selection for chemoradiation therapy in patients who would not have been eligible for either the VA Larynx Trial or RTOG 9111[13], including patients with T4 disease with full thickness thyroid or cricoid cartilage, features portending poorer outcomes for laryngeal preservation.[14, 15]
Despite a large number of publications on this topic, patient selection for non-surgical laryngeal preservation remains controversial. Thus, we have developed and validated easy-to-use nomograms for predicting treatment outcomes for these patients utilizing data from two national clinical trials of chemoradiation for head and neck cancer. These nomograms provide useful tools for monitoring patients for early salvage after laryngeal preservation.
Utilizing our nomograms, we were able to divide patients into low-risk, intermediate-risk, and high-risk groups for overall survival, locoregional recurrence, and distant metastasis using individual nomogram scores. For example, a patient in our cohort with an LR nomogram score of less than 110 has less than a 10% chance of locoregional recurrence. Thus, this patient may be an ideal candidate for laryngeal preservation as the majority of locoregional recurrences have a component of failure at the primary site (79% in our population). Conversely, a patient in our cohort with an LR nomogram score of greater than 150 has a 5-year risk of locoregional recurrence of about 40%, suggesting they should be monitored closely for salvage laryngectomy. Additionally, our nomograms can be used to make individualized decisions by integrating the risk of distant metastasis and overall survival. If a patient has a high rate of distant metastasis or poor overall survival as predicted by the nomogram scores, omitting surgical salvage may be preferred to spare the morbidity of laryngectomy.
A strength of our nomogram is that it is developed from national clinical trial data with a large patient sample and rigorous follow-up. Thus, the data reflects a large variation in practice patterns. In our cohort, 21% of patients had T4 disease, patients who may be less likely to receive chemoradiation if treated at academic centers. Thus, our data reflects the actual practice patterns for the management of laryngeal cancer in the United States.[16] Conversely, patients included on cooperative group trials are generally healthier than the general population treated in the community and our outcomes likely reflect the best-case scenario for similarly healthy patients treated in the community.
A weakness of our nomogram is the lack of data relevant to functional larynx outcomes. Most notably, we were unable to predict laryngectomy-free survival from our available data given the lack of data on surgery at the primary tumor site. This weakness is reflected in the fact that a patient may have a very low risk of locoregional recurrence after chemoradiation but may ultimately require laryngectomy due to a dysfunctional larynx and/or late aspiration. Clinical factors predicting poor functional larynx outcomes have been detailed in other series.[14, 15, 17] Thus, in addition to the use of our nomogram, we recommend that clinicians assess laryngeal function and airway protection with videostroboscopy and MBS prior to making the decision to perform laryngeal preservation on an individualized patient basis.
Our nomograms demonstrate the importance of both optimal chemotherapy and radiation therapy in laryngeal cancer patients. Patients receiving less than 2 cycles of tri-weekly 100 mg/m2 cisplatin had poorer overall survival (p < 0.01) and increased rates of distant metastasis (p = 0.10) when compared to those who received 2–3 cycles of the same regimen. This supports the fact that a cumulative cisplatin dose of 200 mg/m2 is necessary in laryngeal cancer patients treated with chemoradiation, as shown in other head and neck cancers treated with concurrent chemoradiation[18]. This finding should be interpreted with caution, as only 5% of patients in our cohort received < 2 cycles of cisplatin suggesting that receiving less chemotherapy may serve as a surrogate for poor prognosis patient and/or tumor factors. This concern is mitigated by the fact that delivered cisplatin cycles was an independent predictor of both OS and DM outcomes on multivariate analysis which included many of these factors.
Further, our data point strongly to the importance of optimal radiation delivery in laryngeal preservation as TE-BED was a predictor of FFLR. Though accelerated fractionation schemes are not commonly used with chemotherapy after the negative results of RTOG 0129, this data suggests that adequate radiation dose without treatment delays is still necessary for the best outcomes with concurrent chemoradiation therapy.
Our nomogram is unique as it is exclusive to patients receiving concurrent chemoradiation therapy for laryngeal cancer. Egelmeer et al.[4] have previously developed nomograms for overall survival and local control for patients from the Netherlands receiving radiotherapy alone for laryngeal cancer. Their overall survival nomogram contained similar factors to our nomogram for overall survival including age, T-stage, N-stage, and gender, however unlike our nomogram, their nomogram also included time-adjusted EQD2, hemoglobin, and tumor location. The inclusion of time-adjusted EQD2 in their nomogram may reflect the fact that patients included in their nomogram did not receive chemotherapy. The lack of significance of EQD2 in our overall survival nomogram adds credence to the widespread belief that the importance of treatment delays is ameliorated by the use of concurrent chemotherapy. However, this belief has come under more recent scrutiny[19, 20], and it may be that there were insufficient data in our patient population to clarify the significance of EQD2 with regards to overall survival. Regarding local control, their nomogram was very similar to ours and included age, hemoglobin, T-stage, N-stage, gender, and time-adjusted EQD2. With the exception of their inclusion of hemoglobin, our locoregional recurrence nomograms are similar, providing further credence to the validity of both nomograms.
Finally, a recently published overall survival nomogram [21] examined patients receiving a variety of treatments for laryngeal cancer. This nomogram primarily examined survival at 1 and 3 years, and significant factors only included age, N stage, performance status, and type of treatment. This publication looked at patients receiving a variety of treatments for larynx cancer including postoperative radiation, thus providing little information regarding outcomes after laryngeal preservation with chemoradiation.
In conclusion, we have developed nomograms to be used by clinicians to provide prognostic insight to patients with locally advanced laryngeal cancer treated with an organ preservation approach. These nomograms can be further validated on independent datasets to confirm the important findings.
Supplementary Material
Supplemental Figure 1. Internal Validation of the Nomogram Using Boostrapped Calibration Plots For 5-Year Outcomes. Calibration plots for each nomogram using 1000 bootstrapped samples at a time point of 5 years for each nomogram show excellent correlation of model predictions to actual outcomes both without (black) and with (blue) optimism correction.
Research Highlights.
Patient selection for laryngeal preservation remains a significant clinical problem
We developed nomograms for larynx cancer outcomes using cooperative group trial data
These nomogram scores can stratify patients by both mortality and risk of recurrence
Funding:
This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (IROC) from the National Cancer Institute (NCI) and Eli Lilly.
Footnotes
Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.
References
- [1].Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–90. [DOI] [PubMed] [Google Scholar]
- [2].Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. [DOI] [PubMed] [Google Scholar]
- [3].Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Egelmeer AG, Velazquez ER, de Jong JM, Oberije C, Geussens Y, Nuyts S, et al. Development and validation of a nomogram for prediction of survival and local control in laryngeal carcinoma patients treated with radiotherapy alone: a cohort study based on 994 patients. Radiother Oncol. 2011;100:108–15. [DOI] [PubMed] [Google Scholar]
- [5].Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. [DOI] [PubMed] [Google Scholar]
- [8].Beitler JJ, Zhang Q, Fu KK, Trotti A, Spencer SA, Jones CU, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. [DOI] [PubMed] [Google Scholar]
- [10].Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. [DOI] [PubMed] [Google Scholar]
- [12].Chen AY, Fedewa S, Zhu J. Temporal trends in the treatment of early- and advanced-stage laryngeal cancer in the United States, 1985–2007. Arch Otolaryngol Head Neck Surg. 2011;137:1017–24. [DOI] [PubMed] [Google Scholar]
- [13].Forastiere AA, Weber RS, Trotti A. Organ Preservation for Advanced Larynx Cancer: Issues and Outcomes. J Clin Oncol. 2015;33:3262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wagner MM, Curé JK, Caudell JJ, Spencer SA, Nabell LM, Carroll WR, et al. Prognostic significance of thyroid or cricoid cartilage invasion in laryngeal or hypopharyngeal cancer treated with organ preserving strategies. Radiat Oncol. 2012;7:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang WY, Jen YM, Chen CM, Su YF, Lin CS, Lin YS, et al. Intensity modulated radiotherapy with concurrent chemotherapy for larynx preservation of advanced resectable hypopharyngeal cancer. Radiat Oncol. 2010;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grover S, Swisher-McClure S, Mitra N, Li J, Cohen RB, Ahn PH, et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int J Radiat Oncol Biol Phys. 2015;92:594–601. [DOI] [PubMed] [Google Scholar]
- [17].Hutcheson KA, Lewin JS. Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep. 2012;14:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ang KK. Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: are we addressing burning subjects? J Clin Oncol. 2004;22:4657–9. [DOI] [PubMed] [Google Scholar]
- [19].Cannon DM, Geye HM, Hartig GK, Traynor AM, Hoang T, McCulloch TM, et al. Increased local failure risk with prolonged radiation treatment time in head and neck cancer treated with concurrent chemotherapy. Head Neck. 2014;36:1120–5. [DOI] [PubMed] [Google Scholar]
- [20].Shaikh T, Handorf EA, Murphy CT, Mehra R, Ridge JA, Galloway TJ. The Impact of Radiation Treatment Time on Survival in Patients With Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2016;96:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Group MLW. Conditional Survival Analysis of Patients With Locally Advanced Laryngeal Cancer: Construction of a Dynamic Risk Model and Clinical Nomogram. Sci Rep. 2017;7:43928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Internal Validation of the Nomogram Using Boostrapped Calibration Plots For 5-Year Outcomes. Calibration plots for each nomogram using 1000 bootstrapped samples at a time point of 5 years for each nomogram show excellent correlation of model predictions to actual outcomes both without (black) and with (blue) optimism correction.