Abstract
Background:
Obesity has multifactorial effects on lung function and exercise capacity. The contributions of obesity-related inflammatory pathways to alterations in lung function remain unclear.
Research Question:
To examine the association of obesity-related inflammatory pathways with pulmonary function, exercise capacity, and pulmonary-specific contributors to exercise intolerance.
Method:
We examined 695 patients who underwent cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring at Massachusetts General Hospital between December 2006–June 2017. We investigated the association of adiponectin, leptin, resistin, IL-6, CRP, and insulin resistance (HOMA-IR) with pulmonary function and exercise parameters using multivariable linear regression.
Results:
Obesity-related inflammatory pathways were associated with worse lung function. Specifically, higher CRP, IL-6, and HOMA-IR were associated with lower percent predicted FEV1 and FVC with a preserved FEV1/FVC ratio suggesting a restrictive physiology pattern (P≤0.001 for all). For example, a 1-SD higher natural-logged CRP level was associated with a nearly 5% lower percent predicted FEV1 and FVC (beta −4.8, s.e. 0.9 for FEV1; beta −4.9, s.e. 0.8 for FVC; P<0.0001 for both). Obesity-related inflammatory pathways were associated with worse pulmonary vascular distensibility (adiponectin, IL-6, and CRP, P<0.05 for all), as well as lower pulmonary artery compliance (IL-6 and CRP, P≤0.01 for both).
Interpretation:
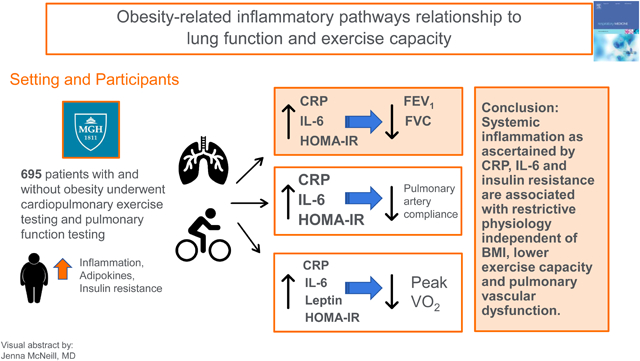
Our findings highlight the importance of obesity-related inflammatory pathways including inflammation and insulin resistance on pulmonary spirometry and pulmonary vascular function. Specifically, systemic inflammation as ascertained by CRP, IL-6 and insulin resistance are associated with restrictive pulmonary physiology independent of BMI. In addition, inflammatory markers were associated with lower exercise capacity and pulmonary vascular dysfunction.
Graphical Abstract
Introduction:
The prevalence of obesity continues to increase on a global scale and is viewed as a risk factor for the development of many pulmonary disease processes such as asthma, obstructive sleep apnea and pulmonary hypertension.1 Obesity can adversely affect respiratory mechanics via direct effects on the diaphragm and chest wall, which can lead to decreased compliance, altered ventilation to perfusion ratio, and hypoxemia. Further, increased demand for ventilation may uncover respiratory muscle inefficiency and contribute to reduced exercise capacity.1 Beyond mechanical effects on lung function, however, other obesity-related pathways including inflammation may underlie alterations in lung function and exercise capacity.2–4
Obesity is associated with multiple systemic derangements that lead to widespread organ dysfunction, including systemic inflammation, insulin resistance, and elaboration of adipose-derived hormones that have multiple metabolic effects.5 However, previous studies examining the effect of obesity-related adipokines on lung function have shown conflicting results.2 In this context, we hypothesized that obesity-associated inflammatory pathways would be associated with lung dysfunction. Specifically, we sought to examine the association of obesity-related pathways including systemic inflammation as ascertained by interleukin-6 (IL-6) and C-reactive protein (CRP), insulin resistance as measured by homeostatic model assessment of insulin resistance (HOMA-IR), and circulating adipokines including leptin, adiponectin and resistin with pulmonary function. Given that lung disease is a significant contributor to exercise intolerance, we additionally sought to examine the association of obesity-related markers of inflammation, insulin resistance, and adipokines with pulmonary vascular function during cardiopulmonary exercise testing.
Methods:
Study Sample
We included consecutive patients with chronic dyspnea on exertion who underwent clinically indicated pulmonary function testing and cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring at Massachusetts General Hospital between December 2006 and June 2017. Of the 830 patients, we excluded participants with a clinical history of heart failure with reduced ejection fraction (<50%, n=74), a history of heart and/or lung transplant (n=17), complex adult congenital heart disease (n=17), mitochondrial disorder (n=14), or undergoing lung transplant evaluation (n=13), leaving 695 patients for analysis. The participants in the study provided informed consent and the study was approved by the institutional review board.
Clinical Variables and Biomarkers
Patients underwent comprehensive medical history and assessment of blood pressure and body mass index (BMI) at the time of the CPET. Pulmonary function tests were performed on the day of the CPET or within a 3-month time interval of the CPET and were performed by certified technicians at Massachusetts General Hospital. Pulmonary function tests were reviewed to confirm they were in agreement with the American Thoracic Society (ATS) definition for acceptability, usability and repeatability.6, 7 The medical history was collected from review of the medical chart. Patients were additionally identified as having COPD based upon Global Obstructive Lung Disease (GOLD) criteria or COPD within medical chart review.8 Restrictive lung disease was defined as FEV1/FVC>70 along percent predicted FVC<80% and percent predicted TLC<80% or evidence of interstitial lung disease on CT imaging or medical chart review.
Fasting blood samples were obtained and immediately processed and stored at −80C. A simple plex assay was utilized for the following biomarkers: adiponectin (ProteinSimple; intra-assay coefficient of variation (CV), 6.5%–8.9%), IL-6 (ProteinSimple, intra-assay CV 2.4%–3.9%), leptin (ProteinSimple; intra-assay CV, 3.3%–9.3%), and resistin (ProteinSimple; intra-assay CV, 6.4%–9.9%). For CRP (hsCRP; intra-assay CV, 0.4%–8.4%) an immunoturbidimetric (Roche) assay was utilized. Insulin resistance was measured using HOMA-IR (calculated by: [insulin (uIU/mL) × glucose (mg/dL)/405]).
Cardiopulmonary Exercise Testing
Patients underwent cardiopulmonary exercise testing with the assistance of trained exercise laboratory staff. Patients underwent right heart catheterization with access through the right internal jugular vein and performed upright cycle ergometry via a ramp protocol (3 minutes of unloaded exercise followed by a 5–20 watt/min continuous ramp).9 Hemodynamic variables were measured at rest followed by minute-by-minute of exercise. Breath-to-breath respiratory gas exchange was measured throughout exercise using a metabolic cart as previously described.10 Pulmonary artery compliance (PAC) was measured at rest and was defined as stroke volume (SV) divided by pulmonary pulse pressure at end expiration (PAC=SV/Pulmonary pulse pressure). Pulmonary vascular distensibility was calculated using previous described equation (Linehan et al.) with mPAP, PAWP and pulmonary blood flow(Q; L/Min) at a minimum of four time points to calculate the pulmonary vascular pressure-flow relationship.9, 11, 12
Statistical Analysis
Baseline clinical characteristics were summarized as percentages for dichotomous variables and mean (SD) or medians (IQR) for continuous variables. Biomarkers were natural log-transformed due to right-skewed distributions. We examined the association of each biomarker with measures of pulmonary function, including percent predicted FEV1, FVC, FEV1/FVC, TLC and DLCO. Models were adjusted for age, sex, BMI, diabetes mellitus, prior myocardial infarction, prior heart failure (as defined by medical chart review), and smoking status (current or former). Models were standardized to express the change in response variable (lung function) per 1-standard deviation change in biomarker.. In secondary analyses we examined the association of obesity-related inflammatory pathways with pulmonary functions among subgroups of individuals based on the presence or absence of lung disease. Analyses using HOMA-IR excluded patients with underlying diabetes (n=109). In addition, we performed quartile analysis examining the association of biomarkers with lung function in a multivariable adjusted model.
We next examined the association of each biomarker with exercise parameters and pulmonary vascular function using multivariable linear regression. Measures of pulmonary distensibility and compliance were natural log-transformed and standardized. In exploratory analyses, we examined sex-stratified models and tested for multiplicative interaction terms (sex*biomarker) given known sex differences in inflammation and metabolic disease.13, 14 Primary analyses were considered significant at a Bonferroni-corrected P-value threshold of 0.05/(6 biomarkers * 3 spirometry measures)=0.003, and suggestive at a P<0.05. Analyses were performed using Stata version 15.1 (StataCorp).
Results
We studied a total of 695 patients with mean age 57±16 years and comprising 60% women. Clinical characteristics are shown in Table 1. In brief, the average BMI was 29±7 with 40% classified as having obesity (BMI>30),16% had diabetes mellitus and 21% had obstructive sleep apnea. A total of 3% were current smokers and 38% were past smokers. Most individuals had mild abnormalities in spirometry, with mean FEV1 87±20 % predicted, FVC 91±19%, FEV1/FVC 95±11%, TLC 95±17%, and DLCO 73±21%. In addition, 25% met clinical criteria or carried a diagnosis of at least moderate COPD (4% patients had FEV1/FVC<70 and FEV1<50%), and 14% carried a diagnosis of restrictive lung disease.
Table 1:
Baseline Clinical Characteristics
| Characteristic | Total N=695 |
|---|---|
| Age, mean years | 57 (16) |
| Female sex, n (%) | 414 (60) |
| Past smoking, n (%) | 262 (38) |
| Current Smoking, n (%) | 22 (3) |
| Total cholesterol, mg/dl | 177 (45) |
| Prevalent MI, n (%) | 28 (4) |
| Hypertension, n (%) | 364 (52) |
| BMI, mean (SD) | 29 (7) |
| Diabetes Mellitus, n (%) | 109 (16) |
| Atrial Fibrillation, n (%) | 101 (15) |
| Obstructive Sleep Apnea, n (%) | 146 (21) |
| Restrictive lung disease, n (%) | 98 (14) |
| COPD, n (%) | 174 (25) |
| Connective tissue disease, n (%) | 60 (9) |
| Obese, n (%) | 278 (40) |
| Obesity related inflammatory Biomarkers | |
| Adiponectin, ng/mL | 6556 (4232–9485) |
| Leptin, pg/mL | 17128 (7291–32249) |
| Resistin, pg/mL | 11575 (9075–15294) |
| IL-6, pg/mL | 4.5 (2.9–7.7) |
| CRP, mg/L | 1.9 (0.8–4.6) |
| HOMA-IR, mg·IU/dL·mL | 2.1 (1.1–3.7) |
| Pulmonary Parameters | |
| FEV1, % predicted | 87 (20) |
| FVC, % predicted | 91 (19) |
| FEV1/FVC, % predicted | 95 (11) |
| TLC, % predicted | 95 (17) |
| DLCO, % predicted | 73 (21) |
| Gas Exchange Parameters | |
| Peak VO2, ml/kg/min | 16 (13–20) |
| % predicted peak VO2, wasserman | 73 (62–86) |
| Work, watts | 98 (73–127) |
| Peak RER | 1.17 (1.09–1.24) |
| PETCO2 at AT, mmHg | 35 (31–38) |
| Breathing reserve, % | 37 (22–48) |
| PV Parameters | |
| PAP/CO slope, mm Hg/L/min | 2.60 (1.8–4.0) |
| PAC, mL/mmHg | 0.004 (0.003–0.006) |
| Pulm distensibility, % per mmHg | 0.01 (0.008–.002) |
| RVEF(peak exercise), % | 53 (47–59) |
Values are means (standard deviations) or medians (inter-quartile ranges) unless otherwise noted. Abbreviations: MI, myocardial infarction; BMI, body mass index; CRP, C-reactive protein; IL-6, interleukin-6; HOMA-IR, homeostatic model assessment of insulin resistance; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity of lungs for carbon monoxide; VO2, maximum oxygen consumption; VE/VCO2 slope, minute ventilation/carbon dioxide production; RER, respiratory exchange ratio; PETCO2 at AT, Postapneic End-Tidal Carbon Dioxide Pressure at Anaerobic Threshold; PAP/CO; pulmonary artery pressure/cardiac output; PAC, pulmonary artery compliance; RVEF, Right ventricular ejection fraction
BMI, obesity and lung function
We observed higher BMI was associated with lower lung volumes including FVC and TLC (p≤0.007 for both) with but not FEV1/FVC ratio, with little effect on DLCO (beta 0.02, s.e 0.15, p=0.19). In sensitivity analyses after exclusion of participants with restrictive lung disease and COPD, we found that higher BMI remained associated with lower FEV1, FVC, FEV1/FVC and TLC (p≤0.03 for all). Similar findings were seen in the whole sample after adjusting for lung disease in the multivariable model. We further examined the association with obesity with lung function and found similar results with lower FVC and TLC (p≤0.02 for both). Specifically, obese individuals had 4% lower FVC (beta −3.55, s.e. 1.46, p=0.02) and TLC (beta −4.11, s.e.1.61, p=0.01).
Inflammatory markers and lung function
Obesity-related inflammatory pathways including circulating levels of adipokines, inflammatory markers, and insulin resistance were associated with worse lung function (Table 2). Specifically, CRP, IL-6, and HOMA-IR were associated with lower percent predicted FEV1 and FVC with a preserved FEV1/FVC ratio (P≤0.001 for all) consistent with restrictive physiology. For example, a 1-SD higher natural logged CRP level was associated with a nearly 5% lower percent predicted FEV1 and FVC (beta −4.8, s.e. 0.9 for FEV1; beta −4.9, s.e. 0.8 for FVC; P<0.0001 for both). In addition, higher CRP and HOMA-IR were significantly associated with worse TLC and DLCO, supporting spirometry findings of a restrictive pattern (P≤0.008 for all), with similar suggestive associations for IL-6 and worse TLC and DLCO.
Table 2:
Association of obesity-related inflammatory pathways with lung function
| Adiponectin | Leptin | Resistin | IL-6 | CRP | HOMA-IR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | |
| FEV1 | −1.84 | 0.89 | 0.04 | −2.98 | 1.30 | 0.02 | −0.82 | 0.82 | 0.32 | −3.75 | 0.82 | <0.0001 | −4.80 | 0.86 | <0.0001 | −3.96 | 1.21 | 0.001 |
| FVC | −1.09 | 0.82 | 0.19 | −2.72 | 1.20 | 0.02 | −0.84 | 0.76 | 0.27 | −3.02 | 0.76 | <0.0001 | −4.85 | 0.80 | <0.0001 | −3.92 | 1.13 | 0.001 |
| FEV1/FVC | −1.04 | 0.51 | 0.04 | −0.38 | 0.75 | 0.61 | 0.16 | 0.47 | 0.73 | −1.29 | 0.48 | 0.007 | −0.12 | 0.51 | 0.82 | −0.13 | 0.71 | 0.86 |
| TLC | −0.13 | 0.90 | 0.88 | −4.48 | 1.25 | <0.0001 | 0.11 | 0.84 | 0.89 | −1.45 | 0.82 | 0.08 | −3.94 | 0.86 | <0.0001 | −3.74 | 1.24 | 0.003 |
| DLCO | −0.62 | 1.02 | 0.55 | −3.55 | 1.44 | 0.01 | −2.24 | 0.96 | 0.02 | −2.23 | 0.94 | 0.02 | −4.47 | 1.01 | <0.0001 | −3.79 | 1.42 | 0.008 |
Multivariable model adjusted for age, sex, smoking status, previous MI, heart failure, and BMI. Units indicate difference in % predicted PFT per 1-SD change in log-transformed biomarker.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity of lungs for carbon monoxide; IL-6, interleukin-6; CRP, C-Reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance
Of the adipokines, leptin showed a worse FEV1 and FVC (P=0.02 for both), worse TLC (P<0.0001) and DLCO (P=0.01), with no association with FEV1/FVC ratio. By contrast, adiponectin had worse FEV1 and FEV1/FVC ratio (P=0.04 for both).
In quartile analyses, we found that participants in the upper quartile of CRP had a 14% lower percent predicted FEV1 and FVC compared with individuals in the lowest quartile (beta −14.1, s.e. 2.4 for FEV1, and beta −13.8, s.e. 2.2, P<0.0001 for both, Figure 1). In addition, TLC and DLCO declined across CRP quartiles while FEV1/FVC did not. Similarly, individuals in the upper quartile of IL-6 had a 9% lower percent predicted FEV1 and 6% lower FVC compared with individuals in the lowest quartile (beta −8.53, s.e. 2.28, p<0.0001 for FEV1, and beta −5.96, s.e. 2.12, p=0.0005 for FVC).
Figure 1:
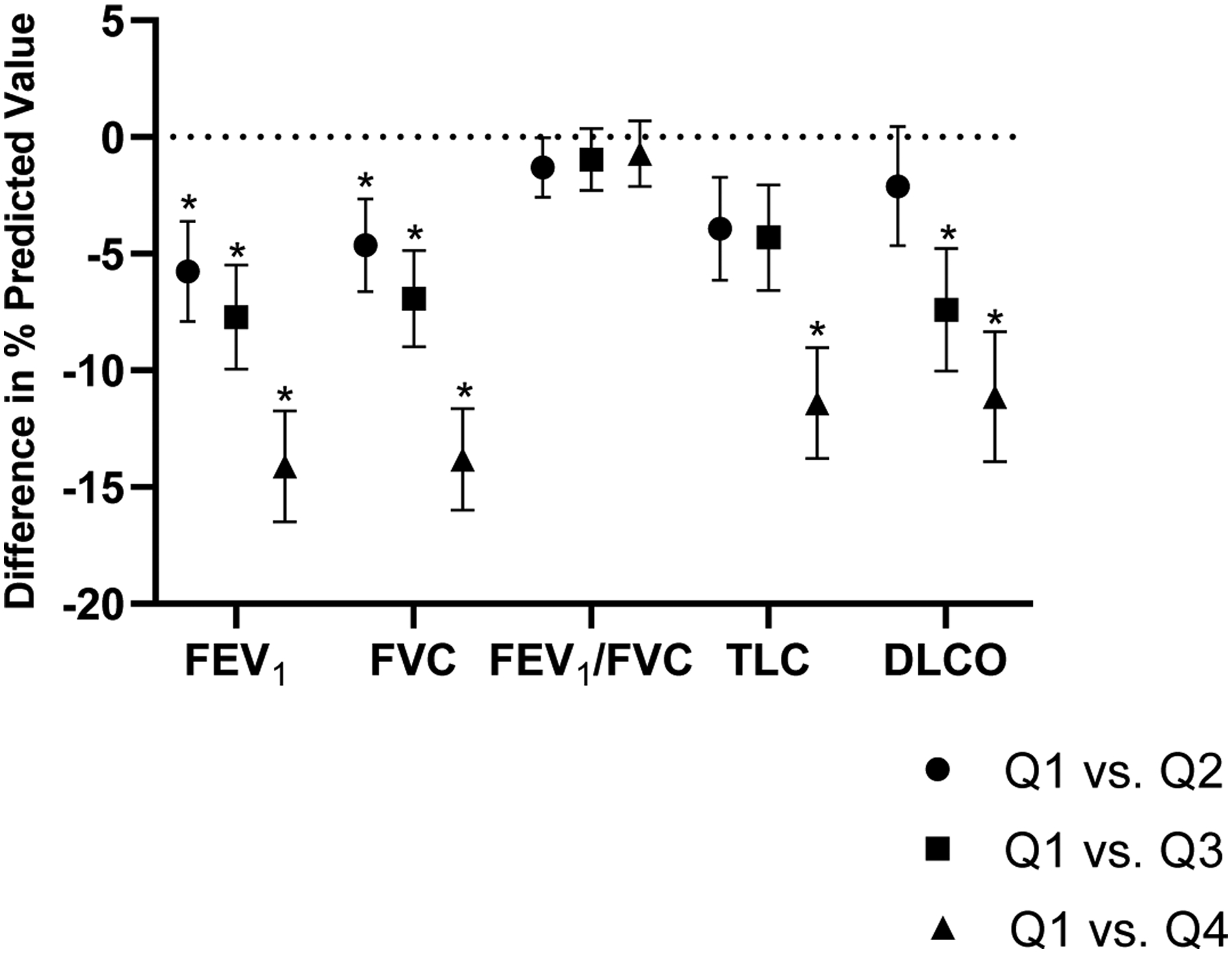
Lung function by CRP quartiles. Patients in the highest CRP quartile had lower FEV1, FVC, TLC and DLCO in comparison to the lowest quartile. * denotes p<0.05
When restrictive lung disease was used as a dichotomous trait, patients with higher levels of CRP had a 66% higher likelihood for restrictive lung disease (OR 1.66, 95% CI 1.29–2.25, p<0.001). When COPD was used as a dichotomous trait, patients with higher levels of IL-6 had a 28% higher likelihood for COPD (OR 1.28, 95% CI 1.05–2.56, p=0.01). In secondary analyses we evaluated the association of obesity-related inflammatory pathways with lung function among individuals with lung disease (Table 3). In individuals without lung disease, findings were similar to primary results with attenuated effect estimates. Among n=174 patients with COPD, we found that IL-6 and CRP remain associated with FEV1 and FVC whereas previous association of HOMA-IR and lung function were attenuated. Among n=98 with restrictive lung disease, we find that the association of obesity-related biomarkers with lung function was attenuated.
Table 3:
Subgroup analyses of association of obesity-related inflammatory pathways with lung function
| Adiponectin | Leptin | Resistin | IL-6 | CRP | HOMA-IR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | |
| Without lung disease N=421 | ||||||||||||||||||
| FEV1 | 0.05 | 0.77 | 0.95 | −4.04 | 1.11 | <0.0001 | −0.30 | 0.81 | 0.71 | −1.46 | 0.76 | 0.06 | −1.76 | 0.81 | 0.03 | −2.07 | 1.02 | 0.04 |
| FVC | 0.39 | 0.76 | 0.61 | −3.50 | 1.11 | 0.002 | −0.99 | 0.80 | 0.21 | −1.36 | 0.75 | 0.07 | −2.22 | 0.80 | 0.006 | −2.10 | 1.03 | 0.04 |
| FEV1/FVC | −0.26 | 0.35 | 0.46 | −0.46 | 0.52 | 0.38 | 0.72 | 0.37 | 0.05 | −4E-4 | 0.35 | 0.99 | 0.68 | 0.37 | 0.07 | −0.01 | 0.48 | 0.98 |
| TLC | 0.44 | 0.76 | 0.56 | −5.16 | 1.02 | <0.0001 | 0.31 | 0.81 | 0.70 | −0.95 | 0.76 | 0.21 | −1.29 | 0.81 | 0.11 | −3.01 | 1.05 | 0.005 |
| DLCO | 0.11 | 1.10 | 0.92 | −5.40 | 1.51 | <0.0001 | −1.76 | 1.18 | 0.14 | −1.95 | 1.10 | 0.08 | −2.53 | 1.18 | 0.03 | −2.34 | 1.53 | 0.13 |
| Restrictive lung disease N=98 | ||||||||||||||||||
| FEV1 | −3.12 | 2.04 | 0.13 | −1.79 | 3.16 | 0.57 | −0.13 | 1.59 | 0.93 | −1.98 | 1.84 | 0.29 | −3.76 | 1.99 | 0.62 | −6.84 | 3.22 | 0.04 |
| FVC | −4.10 | 1.98 | 0.04 | −0.77 | 3.1 | 0.80 | 0.13 | 1.56 | 0.93 | −2.8 | 1.79 | 0.12 | −3.04 | 1.96 | 0.13 | −5.31 | 3.15 | 0.10 |
| FEV1/FVC | 1.47 | 1.10 | 0.19 | −1.85 | 1.69 | 0.28 | 0.13 | 0.86 | 0.87 | 1.04 | 0.99 | 0.30 | −0.77 | 1.09 | 0.48 | −1.80 | 1.82 | 0.33 |
| TLC | −1.71 | 1.96 | 0.39 | −5.09 | 3.00 | 0.10 | 0.87 | 1.54 | 0.57 | −1.19 | 1.62 | 0.46 | −1.72 | 1.79 | 0.34 | −5.00 | 3.21 | 0.13 |
| DLCO | −0.76 | 1.94 | 0.69 | −1.65 | 3.06 | 0.59 | −1.94 | 1.56 | 0.22 | −0.05 | 1.65 | 0.98 | 1.04 | 1.89 | 0.60 | −3.03 | 3.28 | 0.36 |
| COPD N=174 | ||||||||||||||||||
| FEV1 | 0.37 | 1.95 | 0.85 | −2.87 | 2.58 | 0.27 | 0.58 | 1.44 | 0.69 | −5.51 | 1.54 | <0.001 | −3.98 | 1.67 | 0.02 | −4.73 | 2.57 | 0.07 |
| FVC | 0.80 | 1.86 | 0.67 | 2.48 | 2.46 | 0.32 | 0.44 | 1.37 | 0.75 | −5.01 | 1.47 | 0.001 | −5.05 | 1.57 | 0.001 | −4.75 | 2.46 | 0.06 |
| FEV1/FVC | −1.26 | 1.18 | 0.29 | −1.10 | 1.57 | 0.48 | 0.61 | 0.87 | 0.49 | −2.24 | 0.96 | 0.02 | 0.42 | 1.03 | 0.69 | −0.63 | 1.55 | 0.69 |
| TLC | 1.20 | 2.27 | 0.60 | −1.90 | 2.96 | 0.52 | 0.25 | 1.66 | 0.88 | −1.71 | 1.76 | 0.33 | −4.54 | 1.82 | 0.01 | −3.81 | 2.79 | 0.18 |
| DLCO | −0.35 | 2.38 | 0.88 | −0.96 | 3.11 | 0.76 | −0.26 | 1.74 | 0.88 | −1.16 | 1.87 | 0.54 | −4.28 | 2.00 | 0.03 | −2.73 | 2.87 | 0.34 |
Multivariable model adjusted for age, sex, smoking status, previous MI, heart failure, and BMI. Units indicate difference in % predicted PFT per 1-SD change in log-transformed biomarker.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity of lungs for carbon monoxide; IL-6, interleukin-6; CRP, C-Reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance
Inflammatory markers and pulmonary vascular responses to exercise
When examining the pulmonary vascular response to exercise, pulmonary distensibility was lower in patients with higher adiponectin, IL-6 and CRP levels (Table 4). In addition, patients with higher IL-6 and CRP had lower PAC.
Table 4:
Association of obesity-related inflammatory pathways with cardiopulmonary exercise test (CPET) and pulmonary vascular (PV) parameters
| Adiponectin | Leptin | Resistin | IL-6 | CRP | HOMA-IR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPET parameters | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value |
| Peak V02, ml/kg/min | 0.08 | 0.18 | 0.67 | −1.88 | 0.25 | <0.0001 | −0.53 | 0.16 | 0.001 | −0.57 | 0.16 | <0.0001 | −0.87 | 0.17 | <0.0001 | −1.02 | 0.24 | <0.0001 |
| % pred VO2 | 0.20 | 0.74 | 0.79 | −6.11 | 1.05 | <0.0001 | −2.36 | 0.67 | <0.0001 | −2.58 | 0.67 | <0.0001 | −3.54 | 0.72 | <0.0001 | −3.67 | 1.00 | <0.0001 |
| Work, watts | −2.31 | 1.55 | 0.14 | −7.68 | 2.24 | 0.001 | −4.16 | 1.42 | 0.003 | −4.01 | 1.42 | 0.005 | −5.90 | 1.52 | <0.0001 | −3.87 | 2.12 | 0.07 |
| Peak RER | −0.005 | 0.005 | 0.33 | 0.02 | 0.008 | 0.009 | 0.003 | 0.005 | 0.52 | −0.01 | 0.005 | 0.003 | 0.001 | 0.005 | 0.86 | 0.001 | 0.007 | 0.92 |
| PETCO2 at AT | 0.45 | 0.35 | 0.19 | −0.43 | 0.54 | 0.42 | −0.42 | 0.34 | 0.22 | 0.42 | 0.31 | 0.18 | 0.35 | 0.36 | 0.33 | 0.28 | 0.43 | 0.52 |
| Breathing reserve | −1.34 | 0.88 | 0.13 | 0.23 | 1.29 | 0.86 | 1.44 | 0.81 | 0.08 | 0.31 | 0.83 | 0.71 | −0.46 | 0.88 | 0.60 | −0.77 | 1.22 | 0.53 |
| Oxygen pulse | −0.28 | 0.11 | 0.01 | −0.61 | 0.16 | <0.0001 | −0.12 | 0.10 | 0.24 | −0.19 | 0.10 | 0.07 | −0.40 | 0.11 | <0.0001 | −0.35 | 0.15 | 0.02 |
| Adiponectin | Leptin | Resistin | IL-6 | CRP | HOMA-IR | |||||||||||||
| PV Parameters | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value | beta | s.e. | p-value |
| PAP/CO slope | 0.31 | 0.14 | 0.03 | −0.25 | 0.21 | 0.23 | 0.06 | 0.13 | 0.66 | 0.24 | 0.13 | 0.07 | 0.44 | 0.14 | 0.002 | 0.05 | 0.16 | 0.78 |
| PCWP/CO slope | 0.28 | 0.10 | 0.007 | −0.19 | 0.15 | 0.21 | 0.03 | 0.10 | 0.73 | 0.12 | 0.09 | 0.20 | 0.30 | 0.10 | 0.003 | −0.07 | 0.12 | 0.55 |
| TPG/CO slope | 0.03 | 0.09 | 0.71 | −0.06 | 0.14 | 0.64 | 0.03 | 0.09 | 0.71 | 0.12 | 0.09 | 0.17 | 0.15 | 0.09 | 0.11 | 0.12 | 0.09 | 0.20 |
| Pulm disten (alpha) | −0.09 | 0.04 | 0.05 | 0.03 | 0.06 | 0.62 | −0.03 | 0.04 | 0.42 | −0.12 | 0.04 | 0.003 | −0.09 | 0.04 | 0.04 | −0.08 | 0.06 | 0.21 |
| PAC | −0.06 | 0.04 | 0.12 | −0.11 | 0.06 | 0.05 | −0.04 | 0.04 | 0.24 | −0.12 | 0.04 | 0.001 | −0.11 | 0.04 | 0.006 | −0.13 | 0.05 | 0.02 |
| RVEF (peak) | −1.70 | 0.40 | <0.0001 | 1.68 | 0.58 | 0.004 | −0.24 | 0.38 | 0.53 | −0.76 | 0.36 | 0.04 | −0.51 | 0.40 | 0.20 | 0.41 | 0.55 | 0.46 |
Multivariable model adjusted for age, sex, smoking status, previous MI, heart failure, and BMI
Abbreviations: VO2, peak oxygen consumption; VE/VCO2 slope, minute ventilation/carbon dioxide production; RER, respiratory exchange ratio; PETCO2 at AT, Postapneic End-Tidal Carbon Dioxide Pressure at Anaerobic Threshold; PAP/CO; pulmonary artery pressure/cardiac output; PCWP/CO; pulmonary capillary wedge pressure/cardiac output; TGP/CO, transpulmonary gradient pressure/cardiac output; PAC, pulmonary artery compliance; RVEF, Right ventricular ejection fraction; IL-6, interleukin-6; CRP, C-Reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance
Higher levels of leptin, resistin, IL-6, CRP and HOMA-IR were associated with lower peak VO2 and percent predicted VO2 (Table 4). In addition, patients with higher levels of leptin, resistin, IL-6 and CRP achieved lower total work during exercise (p<0.005 for all).
Sex differences and lung function
We examined the association of biomarkers with lung function and exercise parameters stratified by sex and adjusted for BMI in secondary analyses. When testing multiplicative interaction terms, we did not find that sex was a significant effect modifier of biomarker effects on lung function (P>0.05 for all interaction terms, Supplemental Table 2a). While we may have been underpowered to detect a statistical interaction, it is notable that effect sizes were higher among women. For example, higher CRP was associated with a 6.5% lower percent predicted FEV1 among women, and 3.2% lower FEV1 among men (FEV1: women beta −6.47, s.e. 1.16, p<0.0001 vs. men beta −3.18, s.e. 1.29 p=0.01) with similar findings for FVC and DLCO in stratified analyses (Figure 2, Supplemental Table 2a). These observations extended to IL6 as well. Lastly, women with higher levels of CRP and IL-6 had worse pulmonary vascular responses to exercise as demonstrated by lower pulmonary artery distensibility and compliance (Supplemental Table 3a).
Figure 2:
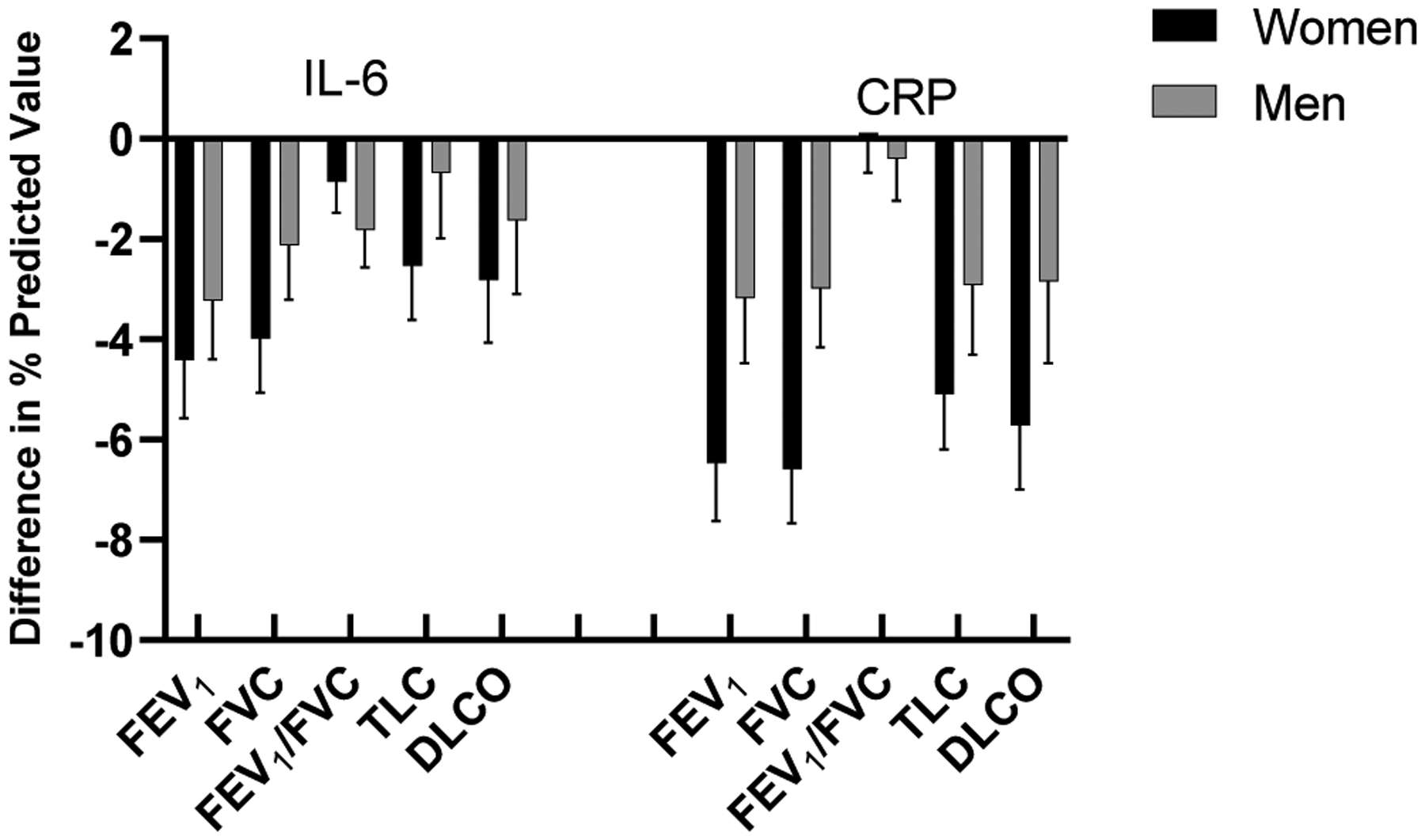
Sex-specific effects of IL-6 and CRP on lung function. Women with higher levels of CRP and IL-6 had greater reduction in FEV1, FVC, TLC and DLCO in comparison to men.
Discussion
While obesity may have considerable effects on lung and chest wall mechanics, other biological pathways dysregulated in obesity may also have detrimental effects on lung function, including systemic inflammation, adipokines, and metabolic dysfunction. We leveraged a rigorously-phenotyped sample with comprehensive cardiopulmonary assessment, in order to investigate the association ofmarkers of inflammation, insulin resistance, and adiposity with pulmonary spirometry and pulmonary vascular function. Our principal findings are as follows: First, systemic inflammation as ascertained by circulating CRP and IL-6 levels, and insulin resistance as measured by HOMA-IR, were associated with worse lung function primarily marked by restrictive physiology, including lower FEV1, FVC and DLCO, with preserved FEV1/FVC ratio. By contrast, adipokines were less clearly related to lung function, with suggestive associations of leptin demonstrating a with restrictive physiology (lower FEV1, FVC, TLC and DLCO), and adiponectin (lower FEV1 and FEV1/FVC) with obstructive physiology. Second, we found obesity-related inflammatory pathways were broadly associated with worse pulmonary vascular function. When examining cardiopulmonary contributions to exercise physiology, we found that both CRP and IL-6 were associated with worse pulmonary artery compliance and distensibility. Lastly, in sex-stratified analyses, we found that inflammatory markers appeared to have more pronounced effects on lung function among women although no significant effect modification by sex was detected upon formal testing.
The role of inflammation in various lung diseases is well known, and further evidenced by the use of anti-inflammatory medications to treat a spectrum of pulmonary diseases and prevent further decline in lung function. In addition, obesity and specifically visceral adiposity are thought to incite systemic inflammation, and have been previously associated with a decline in lung function.15 However, prior studies examining CRP and IL-6 as markers of inflammation have yielded conflicting results.16 For example, Fogarty et al. demonstrated no association between longitudinal measurements of FEV1 and FVC and CRP in individuals aged 17–80 years old while in contrast Ahmadi-Abhari et al. demonstrated higher CRP levels were associated with a baseline lower FEV1 and FVC as well as a decrease in FEV1 and FVC at 13 year follow-up in a multivariable analysis that included BMI.16, 17 In an adult asthma cohort, higher IL-6 was associated with higher needs for inhaled corticosteroid (ICS) use and reduced lung function, while the same was not found with CRP.18 In our study, we found that both IL-6 and CRP were associated with a restrictive physiology pattern. These findings are in agreement with a prior study demonstrating associations of higher IL-6 and CRP with lower FEV1 and FVC in a healthy cohort.19 We now extend these findings by demonstrating congruous effects of IL-6 and CRP on TLC and DLCO to substantiate a restrictive pattern. Further, we show that these findings are observed across a broad spectrum of cardiopulmonary disease.
Obesity and associated chronic low grade inflammation is thought to lead to insulin resistance with prior evidence of higher CRP and IL-6 as risk factors for insulin resistance.20 Furthermore, diabetes has previously been associated with worse lung function as demonstrated by lower FEV1 and FVC as well as a higher likelihood for a restrictive ventilatory defect.21, 22 In addition, pulmonary function is known to decrease prior to the diagnosis of diabetes, suggesting that early detection of insulin resistance may predict future pulmonary decline.23 Our findings substantiate the association of insulin resistance as measured by HOMA-IR with worse FEV1, FVC, TLC and DLCO among non-diabetic individuals. These results are in agreement with a prior study examining HOMA-IR in a young Korean cohort, where individuals in the highest quartile of HOMA-IR had a greater reduction in FEV1 and FVC (p<0.0001) with little effect on FEV1/FVC (p=0.71) as well as an obese female cohort in which individuals with high HOMA-IR> 3.8 had lower FEV1 and FVC in comparison to controls.24, 25 Whether targeting insulin resistance may have salutary benefits on lung function remains to be studied.26
In addition, adipose tissue is known to have widespread metabolic effects by secreting adipokines. These adipokines have been studied in asthma and COPD cohorts with mixed results.2 In our study we found that leptin was associated with a lower FEV1, FVC, TLC, and DLCO, suggesting a restrictive physiology while in contrast adiponectin was associated with a lower FEV1 and FEV1/FVC, suggesting obstructive physiology. These results are in agreement with prior data demonstrating patients with higher leptin levels had a reduced FEV1 independent of weight, and adiponectin levels were higher in COPD patients in comparison to controls.2 Prior evidence has suggested leptin could play a role in pulmonary fibrosis through increasing the activity of transforming growth factor (TGF) beta-1 and decreasing the activity of anti-fibrotic activity of PPAR-gamma.27 It is notable that we find greater associations of inflammatory markers and insulin resistance with restrictive physiology, suggesting that adipokines may not be the primary drivers of lung dysfunction associated with obesity among a diverse sample with a spectrum of cardiopulmonary disease.
Pulmonary function decline has been associated with a reduction in cardiorespiratory fitness.28, 29 We thus interrogated obesity-associated pathways in relation to functional capacity as well as pulmonary-specific contributors to exercise intolerance. We found that IL-6 and CRP were associated both with worse peak VO2 and pulmonary vascular dysfunction as measured by worse PAC and pulmonary distensibility. PAC and pulmonary vascular distensibility have emerged as early diagnostic clues to the development of reduced exercise capacity as well as pulmonary hypertension, as PAC and pulmonary distensibility decrease prior to increases in PVR.30, 31 Both lower PAC and worse pulmonary distensibility have also been associated with worse clinical outcomes including cardiovascular mortality among patients with pulmonary hypertension and heart failure.32–34 While prior studies show that IL-6 and CRP are markers of reduced cardiorespiratory fitness independent of BMI, and predict disease severity, outcomes and response to therapy in pulmonary arterial hypertension (PAH) patients, we now extend these findings to show that IL-6 and CRP are also associated with early indicators of pulmonary vascular function among a broad spectrum of individuals.3, 4, 35, 36
Lastly, given prior studies demonstrating important sex differences in obesity-related inflammatory pathways and their relationship to lung function as well as pulmonary vascular disease, we examined sex-stratified analyses.37 We found that the association of inflammatory markers and insulin resistance with restrictive lung physiology, lower functional capacity, and pulmonary vascular dysfunction was more pronounced among women compared with men, though formal interaction tests were not significant. These findings are in agreement with a prior study demonstrating women with higher CRP regardless of body fat composition demonstrated a greater degree of restrictive lung disease in comparison to men, and are also in keeping with female-predominant prevalence of PAH.38, 39 Women may also be more susceptible to exercise induced arterial hypertension in comparison to men placing a larger strain on the pulmonary vasculature.40
Our study has several limitations. The study sample included patients who were referred to a tertiary center for underlying dyspnea who met clinical criteria to undergo a CPET and may be subject to referral bias. Although these patients may have been symptomatic, they overall had a low clinical burden of comorbidities and had baseline preserved pulmonary function. We acknowledge further that inflammatory biomarkers studied are not specific to obesity and other underlying factors may have contributed to our findings. While sex-stratified analyses indicate differences in effect estimates, multiplicative sex interaction terms were not significant. The study was likely underpowered to identify a true sex related cause; however, the trend was suggestive of sex related differences. Additional information on the effect of gender related hormones, for example estrogen and progesterone, on pulmonary function and vasculature should be explored. Finally, we did not have information on specific adipose depots; therefore, we are not able to comment on the degree of visceral vs. subcutaneous adipose tissue. Future studies examining adipose tissue composition and the effect on the relationship of adipokines and inflammatory biomarkers to lung function and pulmonary vascular response would be interesting, as visceral adipose tissue is known specifically to be associated with elevations in IL-6 and CRP.38
Conclusion
Our findings highlight the importance of obesity-related pathways including inflammation and insulin resistance on lung function, cardiorespiratory fitness and the pulmonary vascular system. Specifically, systemic inflammation as ascertained by CRP, IL-6 and insulin resistance as measured by HOMA-IR are associated with restrictive pulmonary physiology independent of BMI. In addition, inflammatory markers were associated with lower exercise capacity and pulmonary vascular dysfunction. Whether targeting specific obesity-related inflammatory pathways may improve lung function will need further study.
Supplementary Material
Highlights.
Obesity has multifactorial effects on lung function and exercise capacity
Higher CRP, IL-6, and insulin resistance were associated with worse lung function
Higher CRP and IL-6 were associated with lower pulmonary artery compliance
Higher levels of CRP, IL-6, leptin, HOMA-IR were associated with lower peak VO2
Funding:
NIH grants: 5T32HL094301-07 (E.S.L), K23 HL138260 (M.N.); R01 HL142809 (R.M.); R01 HL134893 (J.E.H.); R01 HL140224 (J.E.H.); K24 HL153669 (J.E.H.)
Declarations of Interest:
Dr. Ho has received research support from Gilead Sciences and Bayer AG, and research supplies from EcoNugenics.
Abbreviations:
- IL-6
interleukin-6
- CRP
C- reactive protein
- HOMA-IR
homeostatic model assessment of insulin resistance
- CPET
cardiopulmonary exercise test
- FEV1
Forced Expiratory volume in one second
- FVC
Forced Vital Capacity
- TLC
Total Lung Capacity
- DLCO
Diffusion capacity of the lungs for carbon monoxide
- PAC
pulmonary artery compliance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Dixon AE and Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018;12:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol (1985). 2010;108:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams MJ, Milne BJ, Hancox RJ and Poulton R. C-reactive protein and cardiorespiratory fitness in young adults. Eur J Cardiovasc Prev Rehabil. 2005;12:216–20. [DOI] [PubMed] [Google Scholar]
- 4.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL and Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–76. [DOI] [PubMed] [Google Scholar]
- 5.Mafort TT, Rufino R, Costa CH and Lopes AJ. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med. 2016;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE, Rosenfeld M, Stanojevic S, Swanney MP and Thompson BR. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, Hallstrand TS, Hankinson JL, Kaminsky DA, MacIntyre NR, McCormack MC, Rosenfeld M, Stanojevic S, Weiner DJ and Laboratories ATSCoPSfPF. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med. 2017;196:1463–1472. [DOI] [PubMed] [Google Scholar]
- 8.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Lopez Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA and Agusti A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra R, Bakken K, D’Elia E and Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016;4:607–16. [DOI] [PubMed] [Google Scholar]
- 10.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD and Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. [DOI] [PubMed] [Google Scholar]
- 11.Linehan JH, Haworth ST, Nelin LD, Krenz GS and Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985). 1992;73:987–94. [DOI] [PubMed] [Google Scholar]
- 12.Reeves JT, Linehan JH and Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–25. [DOI] [PubMed] [Google Scholar]
- 13.Chella Krishnan K, Mehrabian M and Lusis AJ. Sex differences in metabolism and cardiometabolic disorders. Curr Opin Lipidol. 2018;29:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdts E and Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 15.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L and Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–16. [DOI] [PubMed] [Google Scholar]
- 16.Fogarty AW, Jones S, Britton JR, Lewis SA and McKeever TM. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007;62:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadi-Abhari S, Kaptoge S, Luben RN, Wareham NJ and Khaw KT. Longitudinal association of C-reactive protein and lung function over 13 years: The EPIC-Norfolk study. Am J Epidemiol. 2014;179:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilmarinen P, Tuomisto LE, Niemela O, Danielsson J, Haanpaa J, Kankaanranta T and Kankaanranta H. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J. 2016;48:1052–1062. [DOI] [PubMed] [Google Scholar]
- 19.Gimeno D, Delclos GL, Ferrie JE, De Vogli R, Elovainio M, Marmot MG and Kivimaki M. Association of CRP and IL-6 with lung function in a middle-aged population initially free from self-reported respiratory problems: the Whitehall II study. Eur J Epidemiol. 2011;26:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yudkin JS, Stehouwer CD, Emeis JJ and Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. [DOI] [PubMed] [Google Scholar]
- 21.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, Selvin E and Brancati FL. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2008;31:741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawlor DA, Ebrahim S and Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia. 2004;47:195–203. [DOI] [PubMed] [Google Scholar]
- 23.Davis WA, Knuiman M, Kendall P, Grange V, Davis TM and Fremantle Diabetes S. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27:752–7. [DOI] [PubMed] [Google Scholar]
- 24.Lee YB, Kim YS, Lee DH, Kim HY, Lee JI, Ahn HS, Sohn TS, Lee TK, Song JY, Yeo CD, Hong M, Han K, Jeong SC and Chae HS. Association between HOMA-IR and Lung Function in Korean Young Adults based on the Korea National Health and Nutrition Examination Survey. Sci Rep. 2017;7:11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecube A, Sampol G, Munoz X, Lloberes P, Hernandez C and Simo R. Insulin resistance is related to impaired lung function in morbidly obese women: a case-control study. Diabetes Metab Res Rev. 2010;26:639–45. [DOI] [PubMed] [Google Scholar]
- 26.Tunkamnerdthai O, Auvichayapat P, Donsom M and Leelayuwat N. Improvement of pulmonary function with arm swing exercise in patients with type 2 diabetes. J Phys Ther Sci. 2015;27:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain M, Budinger GR, Lo A, Urich D, Rivera SE, Ghosh AK, Gonzalez A, Chiarella SE, Marks K, Donnelly HK, Soberanes S, Varga J, Radigan KA, Chandel NS and Mutlu GM. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-gamma. Am J Respir Crit Care Med. 2011;183:1490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto M, Shipley MJ, Wilkinson IB, McEniery CM, Valencia-Hernandez CA, Singh-Manoux A, Kivimaki M and Brunner EJ. Does Poorer Pulmonary Function Accelerate Arterial Stiffening?: A Cohort Study With Repeated Measurements of Carotid-Femoral Pulse Wave Velocity. Hypertension. 2019;74:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullo IJ, Khaleghi M and Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol (1985). 2007;102:1374–9. [DOI] [PubMed] [Google Scholar]
- 30.Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K and Weir EK. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh I, Oliveira RKF, Naeije R, Rahaghi FN, Oldham WM, Systrom DM and Waxman AB. Pulmonary Vascular Distensibility and Early Pulmonary Vascular Remodeling in Pulmonary Hypertension. Chest. 2019;156:724–732. [DOI] [PubMed] [Google Scholar]
- 32.Mahapatra S, Nishimura RA, Sorajja P, Cha S and McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R and Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. [DOI] [PubMed] [Google Scholar]
- 34.Al-Naamani N, Preston IR, Paulus JK, Hill NS and Roberts KE. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart Fail. 2015;3:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quarck R, Nawrot T, Meyns B and Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53:1211–8. [DOI] [PubMed] [Google Scholar]
- 36.Prins KW, Archer SL, Pritzker M, Rose L, Weir EK, Sharma A and Thenappan T. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson L, Vonk JM, Lofdahl CG, Pride NB, Pauwels RA, Laitinen LA, Schouten JP, Postma DS and European Respiratory Society Study on Chronic Obstructive Pulmonary D. Predictors of lung function and its decline in mild to moderate COPD in association with gender: results from the Euroscop study. Respir Med. 2006;100:746–53. [DOI] [PubMed] [Google Scholar]
- 38.Tsao YC, Lee YY, Chen JY, Yeh WC, Chuang CH, Yu W and Li WC. Gender- and Age-Specific Associations Between Body Fat Composition and C-Reactive Protein with Lung Function: A Cross-Sectional Study. Sci Rep. 2019;9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mair KM, Johansen AK, Wright AF, Wallace E and MacLean MR. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol. 2014;171:567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol. 2006;151:124–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


