Abstract
Oral cancer (OC) is the sixth most commonly reported malignant disease globally, with high rates of disease-related morbidity and mortality due to advanced loco-regional stage at diagnosis. Early detection and prompt treatment offer the best outcomes to patients, yet the majority of OC lesions are detected at late stages with 45% survival rate for 2 years. The primary cause of poor OC outcomes is unavailable or ineffective screening and surveillance at the local point-of-care level, leading to delays in specialist referral and subsequent treatment. Lack of adequate awareness of OC among the public and professionals, and barriers to accessing health care services in a timely manner also contribute to delayed diagnosis. As image analysis and diagnostic technologies are evolving, various artificial intelligence (AI) approaches, specific algorithms and predictive models are beginning to have a considerable impact in improving diagnostic accuracy for OC. AI based technologies combined with intraoral photographic images or optical imaging methods are under investigation for automated detection and classification of OC. These new methods and technologies have great potential to improve outcomes, especially in low-resource settings. Such approaches can be used to predict oral cancer risk as an adjunct to population screening by providing real-time risk assessment. The objective of this study is to (1) provide an overview of components of delayed OC diagnosis and (2) evaluate novel AI based approaches with respect to their utility and implications for improving oral cancer detection.
Keywords: artificial intelligence, oral cancer, early detection, oral cancer diagnosis, diagnostic delay
Introduction
Oral cancer (OC) development is primarily considered as a continuum of epithelial alterations in the oral mucosa [1]. The term “oral cancer” includes malignant lesions of the lips, buccal mucosa, hard palate, the floor of the mouth, upper and lower gingiva, and anterior two-thirds of the tongue, whereas “oropharyngeal cancer (OPC)” describes malignancies at the posterior third of the tongue, tonsils, and soft palate. Ninety-five percent of all oral and oropharyngeal cancers are squamous cell carcinomas (SCC) [1]. During malignant transformation, aberrant lesions on the oral mucosa, termed oral potentially malignant disorders (OPMD), can emerge [2]. These mucosal disorders are defined as morphologically altered tissue in which cancer is more likely to occur than its normal counterpart [3]. Only some of these OPMDs transform into oral malignancies, thus, commonly used other terms such as “oral premalignant lesions”, “oral premalignant disease” or “oral premalignant conditions” that imply a more preconceived path of progression are no longer advocated [4]. Clinically, these lesions may present as white plaques that cannot be scraped off, velvety/fiery red patches, red and white mixed areas, chronic ulcers with induration and raised margins, and exophytic or verrucous growths [2–5] (Figure 1 a, b, c). Health care providers, mostly dental or medical practitioners and/or dental hygienists, are expected to assess potential and contributing risk factors of oral malignancy development including tobacco and alcohol use, genetic susceptibility, immunosuppression, and infections prior to clinical examination [6]. After a thorough intraoral and extraoral visual and tactile examination under incandescent light, alterations on the oral mucosa, head and neck area, and cervical lymph nodes must be recorded [5,7]. Numerous adjunctive methods and devices with ambiguous degrees of success may be used to assist the clinicians to reveal lesion characteristics [8–11]. Among these, the diagnostic value of toluidine blue staining has been reported to have variable sensitivity and specificity, mostly due to the method of staining, quality of the solution used, and investigator experience [12,13]. Autofluorescence and chemiluminescence imaging methods also present low specificity, and concerns regarding failure to help clinicians in selecting the biopsy site and relatively high cost was reported for chemiluminescence based methods [13]. Narrow band imaging was shown as a non-invasive and sensitive tool for optical biopsies of OC and oral lichen planus, with significantly reduced false positives and false negatives rates compared to conventional oral examination and white light endoscopy [14]. However, early OC lesions can be asymptomatic and may appear innocuous, which challenges their clinical differentiation from common non-neoplastic conditions. Some of these lesions may fail to cause clinical complaints until reaching an advanced stage and may go unnoticed by the patients [15,16], further delaying the diagnostic process.
Figures 1 a, b, c:
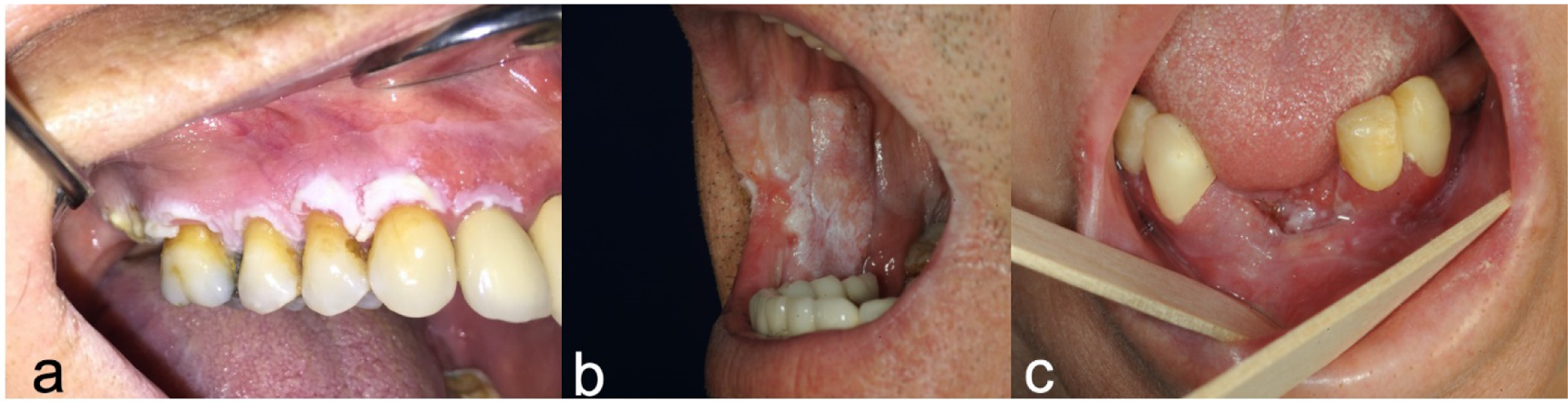
Clinical presentations of OPMD and OC a) white plaque which cannot be scraped off (bx: proliferative verrucous leukoplakia); b) mostly white colored verrucous growth (bx: verrucous carcinoma) c) red and white mixed exophytic area (bx: SCC).
Even though OC is a relatively rare malignancy [16,17], it is the sixth most commonly reported malignant disease globally [18], with uniquely high rates of disease-related morbidity and mortality due to advanced loco-regional stage at diagnosis [16–19], especially in low and middle-income countries (LMICs) such as Southern Asia, Pacific Islands, India, Sri Lanka, Eastern Europe, and Southern Africa [18,20]. Worldwide, there are 354,864 new cases and 177,384 deaths from cancer of the lip and oral cavity annually [20]. The five-year survival rate for US patients with localized disease at diagnosis is 75–83%, however, for those whose cancer has metastasized, the rate decreases to 16–32% [6,21]. Early cancers (stage I and stage II) are highly curable (nearly 90% of people survive two years) using single modality therapy, causing considerably less morbidity and mortality than advanced cancers (stage III and stage IV) which have approximately 45% survival rate over two years when treated with a combination of surgery and chemoradiotherapy [22].
The primary cause of poor OC outcomes is unavailable or ineffective screening and surveillance at the local point-of-care level - which is usually the dentist or hygienist - leading to delays in (or absence of) specialist referral and treatment. Oral cancer patients with a shorter interval from initial detection of a suspicious lesion to histological diagnosis have a higher chance of better prognosis than those with a long delay [16,19,22]. A substantial amount of time lapses between patient admission to a health care provider and initiation of appropriate treatment after final diagnosis. However, it is a major concern that this long process allows disease progression, decreases tumor control, requires more extensive and costly treatment, and reduces survival rates [18,23]. Delayed OC diagnosis is also related to poor OC awareness among the public and professionals, and to barriers to health care access [5,19]. The path between the onset of symptoms and treatment combines several complex components: a) onset of clinical symptoms when the patient first notices alterations, b) first visit to a health care professional, c) first referral to a specialist for consultation, d) first specialist examination, e) final diagnosis, f) treatment initiation [5,19,24]. This highly variable “gap” to diagnosis typical has a duration of five to six months [5,19]. Diagnostic delay can be classified as “patient related”, “professional related” and “system related” [5], which together comprise the “total delay” [5,19]. The Aarhus checklist with 4 segments (appraisal, help-seeking, diagnosis, pre-treatment) was introduced as a guide for a standard and precise methodology in early cancer-diagnosis research [25], however, widespread utilization of this guide is yet to be established.
Diagnostic Delay and Unequal Access to Health Care
Patient delay
Patient delay constitutes approximately 1.6–5.4 months [5] and is less in individuals who attend dental check-ups regularly [26]. After self-discovery of signs and symptoms of OSCC, 30% of patients wait more than 3 months to seek a professional opinion, presuming the symptoms to be related to other benign dental or mucosal conditions [27]. Individuals residing in rural areas with limited access to medical centers additionally report inability to access healthcare services in a timely manner [28]. This also applies to LMICs and underserved populations with no health insurance, including immigrants, homeless, and/or nursing home residents [29]. Thus, the individual causes of patient delay can be complex and may also include cultural and religious factors [30,31], which can hinder access to healthcare, leading to under-reporting as well as poor outcomes [32–34]. Gender preference for providers, family involvement in care, maintaining religious practices during illness, and preference for traditional remedies [35,36] are reported as additional barriers to healthcare.
Professional delay
Due to their specialty, mostly dental practitioners provide the gateway to OC care through visual and tactile examination of the head and neck, oral soft and hard tissues [5,37,38]. Even though advanced OC lesions can be rapidly recognized, general practitioners are still unable to effectively recognize early-stage OC or OPMDs [18,19]. The screening and diagnostic performance of dental practitioners are poor, with 57.8% sensitivity [39] and 31–53% specificity [37,39] for discriminating OC from OPMDs and benign oral confounders. The agreement between referring dentists and specialists averages only 40%, and dental practitioners tend to refer patients to a specialist only after 2 or 3 recall visits [38]. For general medical practitioners who do not provide routine oral mucosal examinations due to lack of training, knowledge, equipment, time, and the notion that dentists are primarily responsible for oral cancer detection, awareness of OC and screening implementation and effectiveness may be even less [19], further impairing early detection and timely treatment [27,40]. Delayed diagnosis is directly implicated in poorer treatment outcomes, higher cost of care, and increased morbidity and mortality [22,27,40].
Health care system delay
Patients and health care professionals are not the only variables that contribute to delayed OC diagnosis [23,26]. Other factors such as the time required for scheduling, workflow, treatment planning [27], as well as access to, availability, and affordability of appropriate care are also relevant [5,19,27]. The duration of health care system delay and the variables involved differ among national health care programs. In Finland and Denmark, the public health care system requires patients to contact a primary care practitioner before referral to a specialist, and patients who need immediate medical care can be referred in due time before being lost among the considerable number of patients in the hospitals [41–43]. With the implementation of a program that includes multidisciplinary team boards and joint clinics to provide rapid counseling and treatment planning, the median waiting time for treatment of OC patients has decreased significantly in Denmark [43]. Similarly, a maximum duration of 2-weeks before being examined by a specialist and a maximum of 31 days from decision to- date of definitive treatment has been targeted for all patients referred with suspected cancer symptoms in the UK [44]. The presence of a multidisciplinary team is a vital component of any cancer care which positively impacts both the diagnosis and the treatment outcomes, and the perceived quality of life of the patients [23,43] by reducing the times between consultation, patient evaluation, diagnostic biopsy, and initiation of definitive treatment [23]. Unfortunately, economic limitations and geographic differences can serve as major barriers to the implementation of the multidisciplinary teams that ensure optimal outcomes for individuals with OC risk
Delays may be common in developed and underdeveloped countries and regardless of cause, the consequences of diagnostic delays for OC are always devastating. Public awareness should be raised and continuing education on OC must be implemented in health care policies to reduce patient and professionally-related delays. Focused national programs for high risk groups, immigrants, and underserved populations may also improve access to health care and lead to a reduction in health care system delays. Additionally, integration of remote healthcare solutions and technologies such as telemedicine and teledentistry could be beneficial for bridging the gap between healthcare systems and underserved populations which are more prone to risk factors for OPMLDs and OC. Within this context, the development and incorporation of artificial intelligence (AI) to health care services deserves a special place in attempts to reduce the total delay of OC diagnosis.
Artificial Intelligence
There was a growing interest in AI techniques such as fuzzy expert systems, Bayesian networks (BNs), artificial neural networks (ANNs), and hybrid intelligent systems throughout the 80’s and 90’s. AI techniques still arouse interest as a means of improved image-based diagnosis [45], with healthcare applications constituting the largest investment share in 2016 compared to other sectors [46]. The exponential growth in science and technology has introduced different applications of AI to our daily lives including virtual/digital assistants such as Apple’s Siri and Amazon’s Alexa, self-driving cars and cognitive computing services to predict business outcomes such as IBM Watson. Today these “skills” offered by AI technologies are helping millions of people to complete their daily life tasks in a more efficient and timely way. AI is also used to address criminal justice needs, such as identifying individuals in photographs or videos related to criminal activity. The role of AI in shaping the future of modern society remains under debate for now, however it is almost certain that these technologies will dramatically improve efficiency and performance in our professional workplaces.
AI refers to the simulation of human intelligence and behavior in machines and comprises several emerging technologies that have become a part of everyday life in recent decades. Machine Learning (ML), an important aspect of AI, has enabled computers to develop problem-solving skills and to learn without being explicitly programmed. ML uses different types of classifiers such as support vector machines (SVM), ANNs, and decision trees, that require accurately categorized input data and structured hierarchical learning networks to assist computer systems in building a mathematical model to make decisions [47,48]. The improvements in computer hardware led to the computation of bigger data and models via more efficient algorithms and resulted in the breakthrough of deep learning (DL). DL uses layers of neural-network algorithms and is modeled on behavioral patterns in the layers of neurons in the neocortex of the human brain. The more layers that exist, the more the depth and performance of the model increase. DL enabled computers are able to process numerous algorithms very efficiently and are able to improve image interpretation not only by reducing the time, effort, and necessary expertise required for analysis, but also by extracting/correcting the essential features utilized for automated medical diagnosis [45,46,49]. Based on the functionality of the system, AI can be categorized into 4 main categories: reactive machines, limited memory machines, theory of mind, and self-aware AI applications. The two latter currently exist in theory and the reactive machines type of AI has been well past. Machine learning models that derive knowledge from previously learned information or stored data (limited memory type) have been studied most in the field of health care [50]. There are 3 main steps to applying AI to medical imaging: preprocessing, image segmentation, and postprocessing. Preprocessing refers to the removal of unwanted image information to overcome the noise in raw images, while the segmentation process recognizes and delineates the region of interest (e.g. distinction of pathological areas of the lesion from healthy sites) [47]. The primary function of postprocessing is to target and extract information on features of interest (e.g. borders, islands) [51,52] and can be performed with several techniques including convolutional neural networks (CNNs), recurrent neural networks (RNNs), multiscale convolutional neural networks (M-CNN), and multi-instance learning convolutional neural networks (MIL-CNN). Neural networks can perform complex computational tasks because of the nonlinear processing capabilities of their neurons (Figure 2) [45]. AI based systems can detect minor variations that cannot be observed by the human eye, develop diagnostic services by gathering data from diverse origins (genomics, radiomics, histology, patient demographics), and guide clinical decision processes. In particular, development and refinement of CNNs dramatically improved the ability for automated cancer detection [53], management decisions, and monitoring treatment responses [54–56]. AI applications have been particularly successful in screening and risk assessment for breast [57], and lung cancers [58], even outperforming human observers for the diagnosis of melanoma [59] and interpretation of screening mammograms [60]. Using digitized cervical images taken with a fixed-focus camera, AI-assisted diagnostic technologists also performed better than human experts during routine cervical cancer screening [61].
Figure 2:
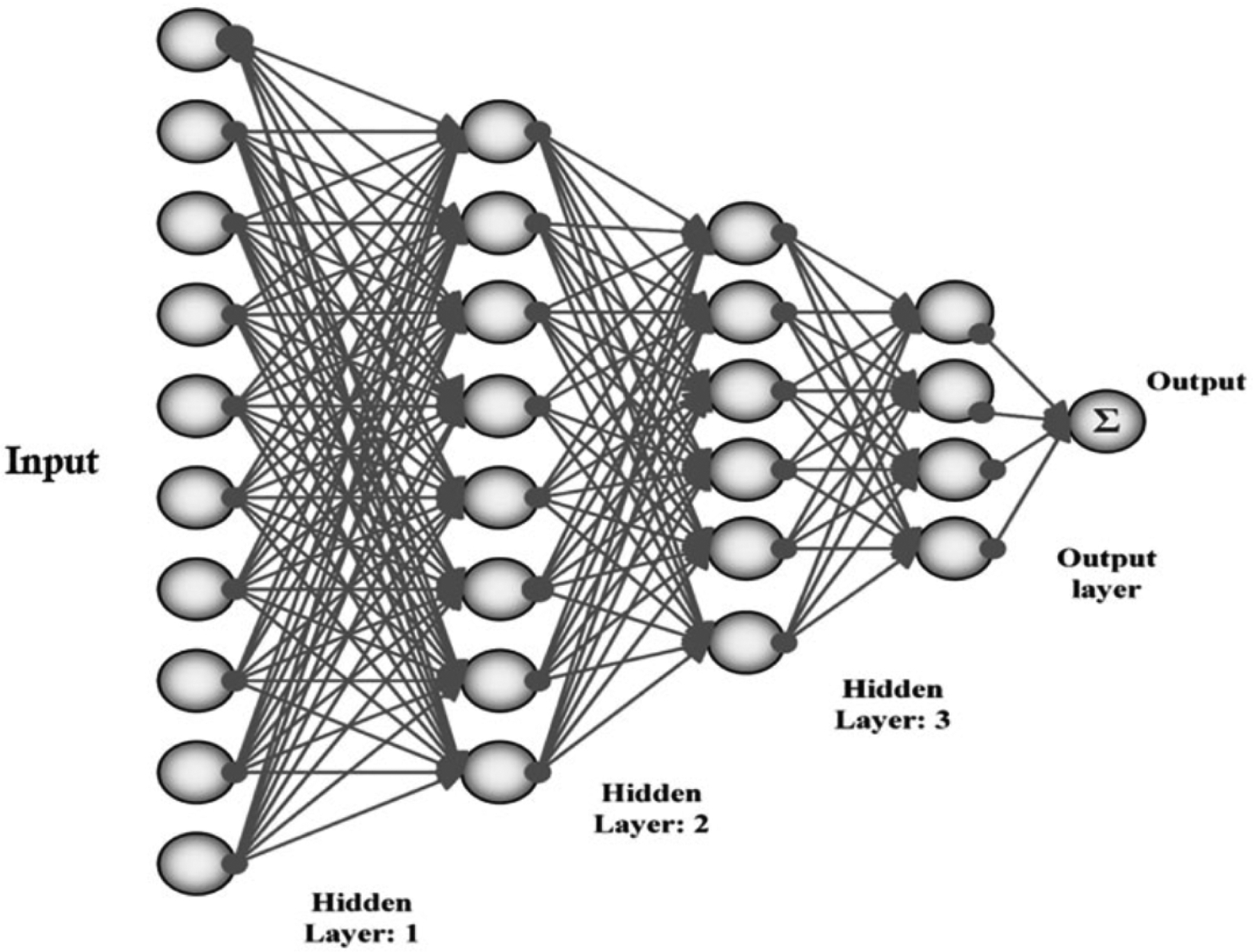
Simplified schematic of artificial neural networks (ANNs). Source: Munir K, Elahi H, Ayub A, Frezza F, Rizzi A. Cancer diagnosis using deep learning: a bibliographic review. Cancers (Basel). 2019;11(9):E1235.
Artificial Intelligence for Oral Cancer Diagnosis
AI for early diagnosis of OC is gaining attention as a tool for establishing more accurate and effective diagnostic tools and superior patient care. AI based systems/applications with clinical decision support systems for differential diagnosis of oral mucosal lesions can serve as useful modalities in screening [62], classifying suspicious mucosal changes [63], tissue diagnostics [64], prediction of lymph node involvement [65], gene expression analysis [66], and microbiome profiling [67].
AI for Oral Cancer Screening, Identification and Classification
Especially in LMICs, the lack of special centers for head and neck cancer and the scarcity of clinicians familiar with OSCC pose major logistical problems. Effective screening by community health workers using only visual examination combined with risk factors can almost halve OC and OPC-related mortality in high-risk groups [68,69]. In some studies, this type of screening was reported to be cost-effective in individuals with high OC risk [68,69]. However, other major OC screening studies demonstrated no impact on outcomes such as mortality, morbidity, and cost [70]. Doubtless, traditional oral screening should theoretically be effective in LMICs, yet limited access of large populations sectors with high OC risk to any form of health care within these countries necessitates other approaches tailored to each area’s individual constraints and parameters [50]. Several AI based algorithms and techniques have been developed in the last decade to which can increase the accuracy of oral cancer screening. They have the potential to achieve a similar or better level of screening efficacy and accuracy as conventional approaches, while overcoming the need for skilled, trained, and constantly retrained screeners.
AI was used to identify individuals with OC risk as early as 1995. The sensitivity and specificity of a trained ANN to detect oral lesions was reported as 0.80 and 0.77 respectively [62]. This approach was subsequently shown to identify high-risk individuals and recognize 80% of all lesions by screening only 25% of the population using targeted simulation modeling techniques [71,72]. An unmatched case-control study in 2010 [73] compared the ability of fuzzy regression and fuzzy neural network prediction models with that of specialist clinicians to predict oral cancer likelihood in individuals. Using sociodemographic findings, risk habits, and genomic data (GSTM1 and GSTT1) from 84 OC patients and 87 healthy subjects as input variables, the authors suggested that both AI models provide improved oral cancer susceptibility prediction compared with human experts [73]. Similarly, using data from 266 patients with oral suspicious lesions, a personalized model for the prediction of cancer risk in OPMDs was developed through a ML algorithm (random forest) [74]. The authors reported that the proposed novel ML model, which can be freely accessed from web.opmd-risk.com, could not only distinguish between high-risk and low-risk lesions with high sensitivity and specificity, but it could also predict the risk of future OC.
AI enables remote health care interactions, which may improve expedited screening reach, especially in LMICs where screening impact is greatest. In recent years interest in AI based telehealth applications has exploded, and the value of AI as a tool for remote oral screening has been emphasized. In a multistage, multicenter study, a very low-cost DL–supported smartphone-based oral cancer probe was developed for high-risk populations in remote regions with limited infrastructure [75–77] (Figure 3). The probe’s autofluorescence and polarization images were combined with OSCC risk factors for analysis by a proprietary DL-based algorithm to generate a screening output that provides triage guidance for the screener. In the first clinical study, agreement between the screening algorithm and standard-of-care diagnosis measured 80.6% [76]. After additional training, the algorithm classified intraoral lesions with sensitivities, specificities, positive predictive values, and negative predictive values ranging from 81% to 95%. In another study, automated screening accuracy approximated 85%, which is considerably higher than conventional screening accuracy by community health workers [76–78]. These results are particularly encouraging in light of the large number of global smartphone users, which is projected to total 3.5 billion by 20201. These studies demonstrate the feasibility and potential effectiveness of screening by non-specialist health care providers such as nurse/general practitioner/dentist/hygienist./community worker using AI supported applications incorporated within mobile phones, particularly in underserved and rural areas.[79]. Agreement between intraoral images of mucosal lesions recorded by mobile phones and clinical examination is reported as moderate to strong, although the degree of agreement decreases for low-resolution images [80]. Nevertheless, low-cost AI supported and smartphone-based technologies for initial screening of oral lesions can serve as an effective and inexpensive technique for reducing professional and health care system delay and allowing patients to be triaged to receive appropriate and timely treatment.
Figure 3:
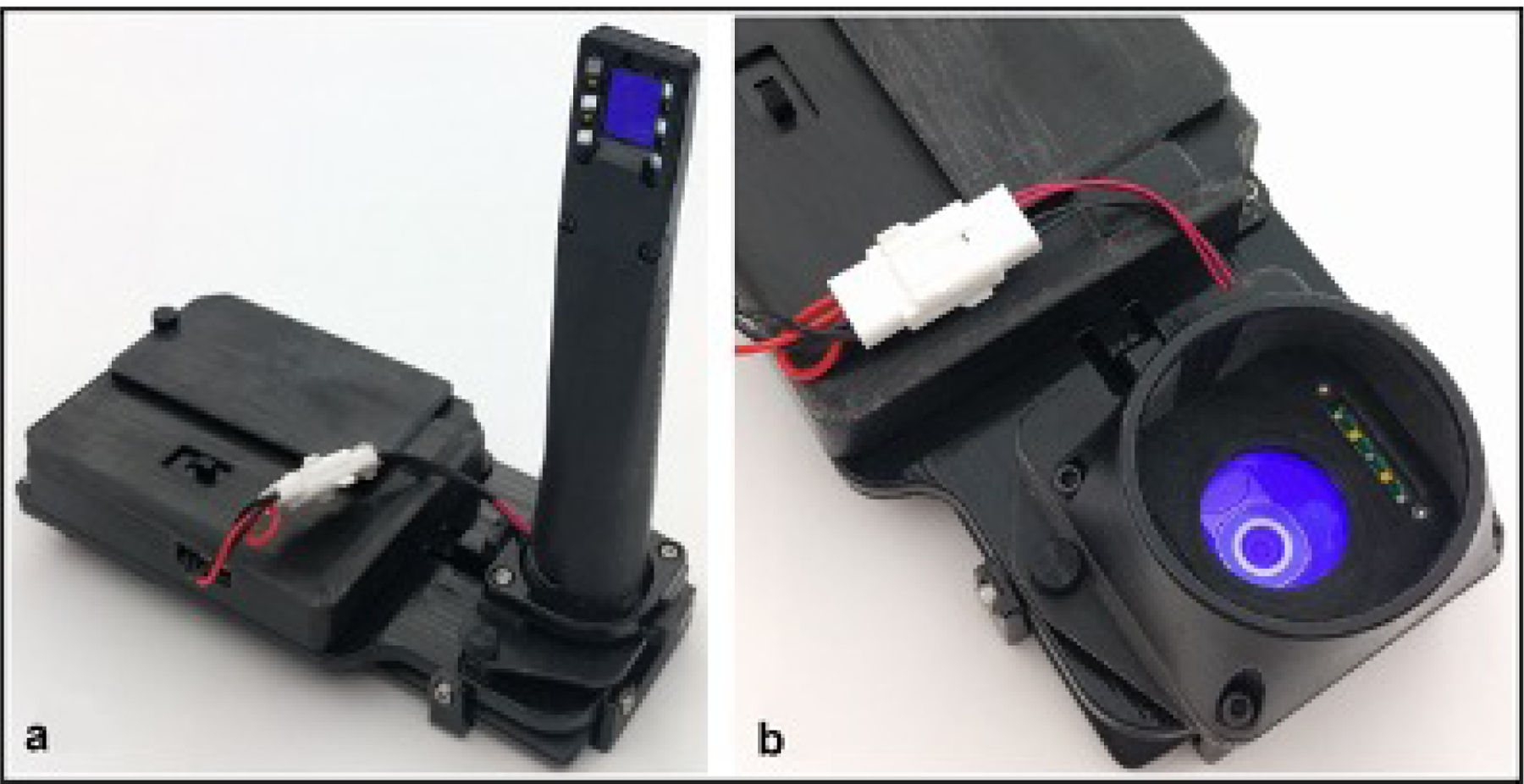
Smartphone-based oral cancer screening device using both autofluorescence imaging and white light imaging which has interchangeable modules installed on a common platform that allows for both (a) intraoral imaging and (b) whole cavity imaging. Source: Uthoff RD, Song B, Sunny S, Patrick S, Suresh A, Kolur T, Keerthi G et al. 2018. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS One. 13(12):e0207493
Using intraoral photographic images of OSCC, leukoplakia, and lichen planus lesions, a discrimination method based on fuzzy inference was able to identify OSCC and lichen planus lesions with an accuracy of 87%, while accuracy was 70% for leukoplakia [81]. Similarly, using deep CNN (DCNN) models on a limited number of photographic images of tongue lesions for training, a similar performance to humans for detecting early signs of OC was achieved [82]. In accordance with these findings, an automated DL algorithm that was recently developed using a total of 44.409 photographic images of biopsy-proven OSCC lesions and healthy mucosa achieved an AUC of 0.983 (95% CI 0.973–0.991), sensitivity of 94.9%, and specificity of 88.7% on the internal validation dataset [83].
To date, there are a number of publications on the application of AI technologies to discriminate between oral lesions based on autofluorescence spectroscopy data of the oral cavity . In an early work by van Staveren et al. [63], the performance of an ANN based classification technique for evaluating autofluorescence spectra of 22 oral leukoplakia lesions and 6 healthy mucosal sites was evaluated. The sensitivity and specificity of ANN for discrimination between abnormal and normal tissues using spectral images were reported as 86% and 100% respectively [63]. Wang et al. [84] utilized a partial least squares and artificial neural network (PLS-ANN) classification algorithm to discriminate the autofluorescence spectra of premalignant (epithelial dysplasia) and malignant (SCC) lesions from benign tissues with a sensitivity of 81%, specificity of 96%, and a positive predictive value of 88%. Higher sensitivity (96.5%) and specificity (100%) values were reported by others with the use of an ANN classifier in a similar study design [85]. Measuring autofluorescence spectra for lesion classification with ANN, de Veld et al. [86] reported that distinction of healthy mucosa from cancer was excellent whereas discrimination of benign tissue from pre-malignant lesions was poor.
To differentiate between oral leukoplakia and OSCC based on spectral-biomarker selection, a SVM based method was developed using fourier-transform infrared (FTIR) spectroscopy applied to paraffin-embedded tissue sections from 8 healthy, 16 leukoplakia, and 23 OSCC samples [87]. The authors reported that they were able to identify discriminatory spectral markers with important bio-molecular changes at qualitative and quantitative levels, which were found to be effective for classifying disease with high sensitivity and specificity [87]. Additionally, a ML based diagnostic algorithm for head and neck SCC using mass spectra correctly defined the borders of the cancerous regions in positive- and negative-ion modes with accuracies of 90.48% and 95.35%, respectively [88].
In a recent study, the performance of a DCCN based algorithm for detecting oral cancer from hyperspectral images of patients with oral cancer was evaluated [89]. The investigators reported a classification accuracy of 94.5% for differentiating between images of malignant and healthy oral tissues. Similar results were described in a recent animal study [90] and in another project that imaged human tissue specimens [91]. DL techniques were also applied to confocal laser endomicroscopy to analyze cell structure as a means of detecting OSCC and showed a mean diagnostic accuracy of 88.3% (sensitivity 86.6%, specificity 90%) [92]. A prototype low-cost optical coherence tomography (OCT) system was also used in combination with an automated diagnostic algorithm linked to an image-processing app and user interface [93]. The automated cancer screening platform differentiated between healthy versus dysplastic versus malignant tissues with a sensitivity of 87% and a specificity of 83% versus the histopathological gold standard [93].
AI for Oral Tissue Diagnostics
AI technologies can contribute to tissue diagnostics by removing subjectivity, as well as applying automation and quantification to guide diagnosis. In an early study, image analysis was applied to measure mean nuclear and mean cytoplasmic areas on oral mucosal cytological smears, and the developed algorithm was able to diagnose normal/non-dysplastic mucosa and dysplastic/malignant mucosa with a sensitivity of 0.76 and specificity of 0.82 [94]. In order to facilitate histological examination of collected cell samples, researchers recently developed a tablet-based mobile microscope that combines an iPad Mini with collection optics, LED illumination, and Bluetooth-controlled motors to scan a slide specimen and capture high-resolution images of stained brush biopsy samples [95]. According to their results, the agreement between gold standard histology, conventional cytology, and remote pathologist evaluation of images indicated that the proposed device may improve screening and referral effectiveness especially in rural areas and health care facilities with no specialists.
AI for Omics in Oral Cancer
The introduction of various new omics technologies (e.g., genomics, proteomics) made possible the collection of large amounts of cancer data. In OC and OPC omics research, AI applications were used in a number of studies with the aim of developing prognostic prediction models [66,96], determining nodal involvement [97], detecting human papillomavirus (HPV) associated biomarkers [98], as well as transcriptomic [99] and metabolite [100] signatures.
To improve the accuracy of prognostic prediction, Chang et al. [66] in a cohort study combined clinicopathologic and genomic data (p53 and p63) from 31 oral cancer patients using 4 types of classifiers (ANN, SVM, logistic regression, and adaptive neuro-fuzzy inference system [ANFIS]). The authors reported that ANFIS was the best tool for predicting oral cancer prognosis, and the 3-input features achieved the best accuracy (accuracy = 93.81%; AUC = 0.90) were alcohol habit, depth of invasion, and p63. The prediction of prognosis improved using clinicopathologic and genomic data compared to clinicopathological data alone. Similarly, in 2020, comprehensive clinicopathologic and genetic data from 334 advanced OC patients were used to validate a ML based algorithm for survival risk stratification in a 15-year cohort study [96]. The algorithm was trained using extensive data including patient characteristics, personal habits, primary cancer site, pathological T and N stages of the tumor, histopathological findings, surgical findings, and ultra-deep sequencing of 44 cancer related gene variant profiles of tumor tissue samples. The predictive model using the aforementioned clinicopathologic and genetic data outperformed those using clinicopathologic data alone. Exarchos et al. [101] used a multitude of heterogeneous data based on clinical examination, imaging, and gene expression data to identify the factors that anticipate OC progression and predict potential relapses of the disease. A separate classifier was trained from each data source, as well as an overall one that combines the individual classifiers. Additionally, employing a Dynamic Bayesian Network (DBN), genomic data was used to develop a Disease Evolution Monitoring system. The authors reported an accuracy of 86% by feeding the DBN data from the baseline visit which enabled them to provide a more personalized treatment by identifying patients with high/low risk of reoccurrence.
Others used fuzzy neural networks (FNN), a subtype of ML, to investigate the association between human papillomavirus (HPV) infection and the presence of apoptotic and proliferative markers in patients with oral leukoplakia [98]. Data from the clinical and immunohistochemical examination, personal characteristics, and habits from 21 patients with oral leukoplakia was used as ‘input’ variables, while presence/absence of HPV was used as ‘output’ variable by the FNN system. The developed method revealed that HPV infection was associated with survival while proliferating cell nuclear antigen was related to HPV positivity with smoking habit in oral leukoplakia patients. In a case control study, an SVM classifier was employed to investigate the regulatory network of long non-coding RNAs, microRNAs, and messenger RNAs in OSCC, which was able to identify transcriptomic biomarkers through bioinformatics analysis and revealed potential therapeutic targets for OSCC [99].
Recently, conductive polymer spray ionization mass spectrometry (CPSI-MS) was employed on saliva samples from 124 healthy subjects, 124 patients with premalignant, and 125 patients with OC lesions to discover and validate dysregulated metabolites and determine altered metabolic pathways [100]. According to the study results, the combination of Lasso approach and CPSI-MS enabled a molecular diagnosis of 86.7% accuracy, which indicates that adaptation of ML to CPSI-MS of saliva samples can provide a simple, fast, affordable noninvasive tool for OC diagnosis. AI was also used to determine salivary microbiome changes during oral carcinogenesis. Chen et al. [67] compared salivary microbiome from patients with that in patients with oral submucous fibrosis (OSF) and OSF+OSCC using high-throughput sequencing of the bacterial 16S rRNA gene. By combining features of the bacterial species with the host’s clinical findings and lifestyle, ML analysis efficiently discriminated the OSF and OSF+OSCC groups with 85.1% mean 5-fold cross-validation accuracy and an AUC of 0.88.
AI applications in omics target tasks that are impractical to perform using human intelligence and error prone when addressed with standard statistical approaches. Advanced and combined interpretation of omics data with clinicopathologic and imaging features through AI and ML approaches could improve clinical practice and also provide a deeper understanding of OC.
Conclusion
The rapid evolution of AI based applications promises new opportunities for screening and diagnostic approaches to OPMLs and OC. These technologies are still in their infancy and need to be developed better before they can find widespread use as adjuncts for predicting cancer risk and population screening. Nevertheless, considering that detection of OPMDs and early stage OC is challenging for general practitioners, AI algorithms incorporated with telemedicine technologies and AI supported and smartphone-based technologies for the initial screening of oral lesions can serve as effective and valuable methods that may decrease both professional and health care system delay and allow patients to be triaged accordingly to receive appropriate and timely treatment in future. This would be most beneficial to underserved populations and high-risk groups in rural/remote areas with limited access to health-care services.
Highlights.
Early diagnosis is the most important determinant of oral cancer (OC) outcomes.
The primary cause of poor outcomes is unavailable/ineffective screening.
AI technologies promise new opportunities for diagnostic processes of OC.
These technologies can serve as effective and valuable methods and decrease diagnostic delay
The incorporation of AI to screening of OC would be most beneficial to underserved populations
Funding:
This research was supported in part by funding from LAMMP NIH/NIBIB P41EB05890; NIH/NIBIB UH3EB022623 ; NIH/NCI P30CA062203; NIH R03 EB014852; T31IR1825; the Arnold and Mabel Beckman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/, accessed on Nov 2020
References
- [1].Speight PM, Farthing PM. The pathology of oral cancer. Br Dent J 2018;225:841–47. [DOI] [PubMed] [Google Scholar]
- [2].Carreras-Torras C, Gay-Escoda C. Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med Oral Patol Oral Cir Bucal 2015;20:e305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575–80. [DOI] [PubMed] [Google Scholar]
- [4].Goyal D, Goyal P, Singh HP, Verma C. An Update on precancerous lesions of oral cavity. Int J Med and Dent Sci 2013;2:70–5. [Google Scholar]
- [5].Güneri P, Epstein JB. Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol 2014;50:1131–6. [DOI] [PubMed] [Google Scholar]
- [6].Messadi DV, Wilder-Smith P, Wolinsky L. Improving oral cancer survival: the role of dental providers. J Calif Dent Assoc 2009;37:789–98. [PMC free article] [PubMed] [Google Scholar]
- [7].MacCarthy D, Flint SR, Healy C, Stassen LF. Oral and neck examination for early detection of oral cancer--a practical guide. J Ir Dent Assoc 2011;57:195–9. [PubMed] [Google Scholar]
- [8].Wilder-Smith P, Holtzman J, Epstein J, Le A. Optical diagnostics in the oral cavity: an overview. Oral Dis 2010;16:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Çankaya H, Güneri P, Epstein JB. Adjunctive methods and devices for clinical detection of oral squamous cell carcinoma. Oral Health Prev Dent 2015;13:29–39. [DOI] [PubMed] [Google Scholar]
- [10].Güneri P, Epstein JB, Kaya A, Veral A, Kazandı A, Boyacioglu H. The utility of toluidine blue staining and brush cytology as adjuncts in clinical examination of suspicious oral mucosal lesions. Int J Oral Maxillofac Surg 2011;40:155–61. [DOI] [PubMed] [Google Scholar]
- [11].Messadi DV. Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci 2013;5:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Epstein JB, Feldman R, Dolor RJ, Porter SR. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck 2003; 25:911–21. [DOI] [PubMed] [Google Scholar]
- [13].Piazza C, Bon FD, Peretti G, Nicolai P. ‘Biologic endoscopy’: optimization of upper aerodigestive tract cancer evaluation. Curr Opin Otolaryngol Head Neck Surg 2011;19:67–6. [DOI] [PubMed] [Google Scholar]
- [14].Piazza C, Bon FD, Paderno A, Grazioli P, Perotti P, Barbieri D. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur Arch Otorhinolaryngol 2016;273:3347–53. [DOI] [PubMed] [Google Scholar]
- [15].Feller L, Lemmer J. Cell transformation and the evolution of a field of precancerization as it relates to oral leukoplakia. Int J Dent 2011;2011:321750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Güneri P, Epstein JB. Why are we still unable to accurately determine the malignant potential or the behavior of oral mucosal lesions? Oral Oncol 2017;71:177–79. [DOI] [PubMed] [Google Scholar]
- [17].Dhanuthai K, Rojanawatsirivej S, Thosaporn W, Kintarak S, Subarnbhesaj A, Darling M et al. Oral cancer: A multicenter study. Med Oral Patol Oral Cir Bucal 2018;23:e23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Varela-Centelles P, Lopez-Cedrun JL, Fernandez-Sanroman J, Seoane-Romero JM, Santos de Melo N, Alvarez-Novoa P et al. Key points and time intervals for early diagnosis in symptomatic oral cancer: a systematic review. Int J Oral Maxillofac Surg 2017;46:1–10. [DOI] [PubMed] [Google Scholar]
- [19].van der Waal I Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med Oral Patol Oral Cir Bucal 2013;18:e33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [21].Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people--a comprehensive literature review. Oral Oncol 2001; 37:401–18. [DOI] [PubMed] [Google Scholar]
- [22].Scully C, Kirby J. Statement on mouth cancer diagnosis and prevention. Br Dent J 2014;216:37–8. [DOI] [PubMed] [Google Scholar]
- [23].Coca-Pelaz A, Takes RP, Hutcheson K, Saba NF, Haigentz M Jr, Bradford CR et al. Head and Neck Cancer: A Review of the Impact of Treatment Delay on Outcome. Adv Ther 2018;35:153–60. [DOI] [PubMed] [Google Scholar]
- [24].Stefanuto P, Doucet JC, Robertson C. Delays in treatment of oral cancer: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:424–9. [DOI] [PubMed] [Google Scholar]
- [25].Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu T, Wood RE, Tenenbaum HC. Delays in diagnosis of head and neck cancers. J Can Dent Assoc 2008;74:61. [PubMed] [Google Scholar]
- [27].van der Waal I, de Bree R, Brakenhoff R, Coebergh JW. Early diagnosis in primary oral cancer: is it possible?. Med Oral Patol Oral Cir Bucal 2011;16:e300–5. [DOI] [PubMed] [Google Scholar]
- [28].Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, et al. Cancer Survival in Africa, Asia, and Central America: A Population-Based Study. Lancet Oncol 2010;11:165–173. [DOI] [PubMed] [Google Scholar]
- [29].Gómez I, Warnakulasuriya S, Varela-Centelles PI, López-Jornet P, Suárez-Cunqueiro M, Diz-Dios P et al. Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay? Oral Dis 2010;16:333–42. [DOI] [PubMed] [Google Scholar]
- [30].Costa MC, Silva EB, Soares JSF, Borth LC, Honnef F. Rural women and violence situation: access and accessibility limits to the healthcare network. Rev Gaúcha Enferm 2017;38:e59553. [DOI] [PubMed] [Google Scholar]
- [31].Rizvi Narjis, Khan Kausar S and Shaikh Babar T. Gender: shaping personality, lives and health of women in Pakistan. BMC Women’s Health 2014;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crimmins EM, Kim JK, Sole-Auro A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health 2010;21:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duetz MS, Abel T, Niemann S. Health. Health measures differentiating associations with gender and socio-economic status. Eur J Public Health 2003;13:313–9. [DOI] [PubMed] [Google Scholar]
- [34].Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD).Lancet Oncol 2008;9:730–56. [DOI] [PubMed] [Google Scholar]
- [35].Tackett S, Young HJ, Putman S, Wiener C, Deruggiero K, Bayram JD. Barriers to healthcare among Muslim women: A narrative review of the literature. Women’s Studies International Forum 2018;69:190–194. [Google Scholar]
- [36].Hammoud MM, White CB, Fetters MD. Opening cultural doors: Providing culturally sensitive healthcare to Arab American and American Muslim patients. Am J Obstet and Gynecol 2005;193:1307–11. [DOI] [PubMed] [Google Scholar]
- [37].Epstein JB, Güneri P, Boyacioglu H, Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc 2012; 143:1332–42. [DOI] [PubMed] [Google Scholar]
- [38].Grafton-Clarke C, Chen KW, Wilcock J. Diagnosis and referral delays in primary care for oral squamous cell cancer: a systematic review. Br J Gen Pract 2019;69:e112–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Seoane J, Warnakulasuriya S, Varela-Centelles P, Esparza G, Dios P. Oral cancer: experiences and diagnostic abilities elicited by dentists in North-western Spain. Oral Dis 2006;12:487–92. [DOI] [PubMed] [Google Scholar]
- [40].Epstein JD, Knight TK, Epstein JB, Bride MA, Nichol MB. Cost of care for early and late-stage oral and pharyngeal cancer in the California Medicaid population. Head Neck 2008;30:178–86. [DOI] [PubMed] [Google Scholar]
- [41].Teppo H, Alho OP. Relative importance of diagnostic delays in different head and neck cancers. Clin Otolaryngol 2008;33:325–30. [DOI] [PubMed] [Google Scholar]
- [42].Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer 2009;101 Suppl 2(Suppl 2):S5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lyhne NM, Christensen A, Alanin MC, Bruun MT, Jung TH, Bruhn MA, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer 2013;49:1627–33. [DOI] [PubMed] [Google Scholar]
- [44].Gigliotti J, Madathil S, Makhoul N. Delays in oral cavity cancer. Int J Oral Maxillofac Surg 2019;48:1131–37. [DOI] [PubMed] [Google Scholar]
- [45].Munir K, Elahi H, Ayub A, Frezza F, Rizzi A. Cancer Diagnosis Using Deep Learning: A Bibliographic Review. Cancers (Basel) 2019;11:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Amisha, Malik P, Pathania M, Rathaur VK. Overview of artificial intelligence in medicine. J Family Med Prim Care 2019;8: 2328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ilhan B, Lin K, Guneri P, Wilder-Smith P. Improving Oral Cancer Outcomes with Imaging and Artificial Intelligence. J Dent Res 2020;99:241–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schwendicke F, Samek W, Krois J. (2020). Artificial Intelligence in Dentistry: Chances and Challenges J Dent Res, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436–44. [DOI] [PubMed] [Google Scholar]
- [50].Kar A, Wreesmann VB, Shwetha V, Thakur S, Rao VUS, Arakeri G et al. Improvement of oral cancer screening quality and reach: The promise of artificial intelligence. J Oral Pathol Med 2020;49:727–30. [DOI] [PubMed] [Google Scholar]
- [51].Sikorski J 2004. Identification of malignant melanoma by wavelet analysis in Student/Faculty Research Day. New York: CSIS, Pace University. [Google Scholar]
- [52].Zhou H, Chen M, Rehg JM. 2009. Dermoscopic interest point detector and descriptor. Paper presented at: Proceedings of the 6th IEEE International Symposium on Biomedical Imaging: From Nano to Macro (ISBIâTM09); 2009 Oct 11; Boston, MA. http://www.howardzzh.com/research/publication/2009.ISBI/2009.ISBI.Zhou.DIP.pdf [Google Scholar]
- [53].Xu B, Wang N, Chen T, Li M. Empirical evaluation of rectified activations in convolutional network. arXiv 2015; https://arxiv.org/abs/1505.00853 [Google Scholar]
- [54].Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J 2014;13:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khalvati F, Wong A, Haider MA. Automated prostate cancer detection via comprehensive multi-parametric magnetic resonance imaging texture feature models. BMC Med Imaging 2015;15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol 2017;30:1655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Abbasi J Artificial Intelligence improves breast cancer screening in study. JAMA 2020;323:499. [DOI] [PubMed] [Google Scholar]
- [58].Ardila D, Kiraly AP, Bharadwaj S, Choi B, Reicher JJ, Peng L et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nature Med 2019;25:954–61. [DOI] [PubMed] [Google Scholar]
- [59].Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM et al. Dermatologist-level classification of skin cancer with deep neural networks Nature 2017;542:115–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H et al. International evaluation of an AI system for breast cancer screening. Nature 2020;577:89–94. [DOI] [PubMed] [Google Scholar]
- [61].Hu L, Bell D, Antani S, Xue Z, Yu K, Horning MP et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. J Natl Cancer Inst 2019;111:923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Speight PM, Elliott A, Jullien JA, Downer MC, Zakzrewska JM. The use of artificial intelligence to identify people at risk of oral cancer and precancer. Br Dent J 1995;179: 382–87. [DOI] [PubMed] [Google Scholar]
- [63].van Staveren HJ, van Veen RL, Speelman OC, Witjes MJ, Star WM, Roodenburg JL. Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: a pilot study. Oral Oncol 2000;36:286–93. [DOI] [PubMed] [Google Scholar]
- [64].Paul RR, Mukherjee A, Dutta PK, Banerjee S, Pal M, Chatterjee J et al. A novel wavelet neural network based pathological stage detection technique for an oral precancerous condition. J Clin Pathol 2005;58:932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kann BH, Aneja S, Loganadane GV, Kelly JR, Smith SM, Decker RH et al. Pretreatment identification of head and neck cancer nodal metastasis and extranodal extension using deep learning neural networks. Sci Rep. 2018;8:14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chang SW, Abdul-Kareem S, Merican A, Zain R. Oral cancer prognosis based on clinicopathologic and genomic markers using a hybrid of feature selection and machine learning methods. BMC Bioinformatics 2013;14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen MY, Chen JW, Wu LW, Huang KC, Chen JY, Wu WS et al. (2020). Carcinogenesis of Male Oral Submucous Fibrosis Alters Salivary Microbiomes. J Dent Res, [DOI] [PubMed] [Google Scholar]
- [68].Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B et al. Trivandrum Oral Cancer Screening Study Group. 2005. Effect of screening on oral cancer mortality Kerala, India: a cluster-randomised controlled trial. Lancet 365:1927–33. [DOI] [PubMed] [Google Scholar]
- [69].Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G, Mathew B et al. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bull World Health Organ 2009;87:200–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kuriakose MA. Strategies to improve oral cancer outcome in high-preva- lent, low-resource setting. J Head Neck Physicians Surg 2018;6:63–8. [Google Scholar]
- [71].Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: 2. Developing a model of population screening. Community Dental Health 1997;14:227–32. [PubMed] [Google Scholar]
- [72].Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: 3. Preselecting high risk individuals. Community Dental Health 1998;15:72–6. [PubMed] [Google Scholar]
- [73].Rosma MD, Sameemii AK, Basir A, Mazlipahiv IS, Norzaidi MD. The use of artificial intelligence to identify people at risk of oral can- cer: empirical evidence in Malaysian University. Int J Sci Res Edu 2010;3:10–20. [Google Scholar]
- [74].Wang X, Yang J, Wei C, Zhou G, Wu L, Gao Q et al. A personalized computational model predicts cancer risk level of oral potentially malignant disorders and its web application for promotion of non-invasive screening. J Oral Pathol Med 2020;49:417–26. [DOI] [PubMed] [Google Scholar]
- [75].Song S, Sunny S, Uthoff RD, Patrick S, Suresh A, Kolur T et al. Automatic classification of dual-modality, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed Opt Express 2018;9:5318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Uthoff RD, Song B, Birur P, Kuriakose MA, Sunny S, Suresh A et al. 2018. Development of a dual-modality, dual-view smartphone-based imaging system for oral cancer detection. Proceedings of SPIE 10486, Design and Quality for Biomedical Technologies XI. 10486. doi: 10.1117/12.2296435. [DOI] [Google Scholar]
- [77].Uthoff RD, Song B, Sunny S, Patrick S, Suresh A, Kolur T et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS One 2018;13:e0207493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cleveland JL, Robison VA. 2013. Clinical oral examinations may not be predictive of dysplasia or oral squamous cell carcinoma. J Evid Based Dent Pract 2013;13:151–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].de Almeida Geraldino R, Rezende LVML, da-Silva CQ, Almeida JCF. Remote diagnosis of traumatic dental injuries using digital photographs captured via a mobile phone. Dent Traumatol 2017;33:350–57. [DOI] [PubMed] [Google Scholar]
- [80].Haron N, Zain RB, Nabillah WM, Saleh A, Kallarakkal TG, Ramanathan A et al. Mobile phone imaging in low resource settings for early detection of oral cancer and concordance with clinical oral examination. Telemed J E Health 2017;23:192–99. [DOI] [PubMed] [Google Scholar]
- [81].Nishi Y, Horio K, Saitrapo K, Habu M, Tominaga K. Discrimination of oral mucosal disease inspired by diagnostic process of specialist. JOMB 2013;2:57–61. [Google Scholar]
- [82].Shamim MZM, Syed S, Shiblee M, Usman M, Ali S. Automated detection of oral precancerous tongue lesions using deep learning for early diagnosis of oral cavity cancer. arXiv 2019; arXiv:1909.08987. [Google Scholar]
- [83].Fu Q, Chen Y, Li Z, Jing Q, Hu C, Liu H et al. (2020). A deep learning algorithm for detection of oral cavity squamous cell carcinoma from photographic images: A retrospective study. Clin Med, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang CY, Tsai T, Chen HM, Chen CT, Chiang CP. PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg Med 2003;32:318–26. [DOI] [PubMed] [Google Scholar]
- [85].Nayak GS, Kamath S, Pai KM, Sarkar A, Ray S, Kurien J et al. Principal component analysis and artificial neural network analysis of oral tissue fluorescence spectra: classification of normal premalignant and malignant pathological conditions. Biopolymers 2006;82:152–66. [DOI] [PubMed] [Google Scholar]
- [86].de Veld DC, Skurichina M, Witjes MJ, Duin RP, Sterenborg HJ, Roodenburg JL. Clinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopy. J Biomed Opt 2004;9:940–50. [DOI] [PubMed] [Google Scholar]
- [87].Banerjee S, Pal M, Chakrabarty J, Petibois C, Paul RR, Giri A et al. Fourier-transform-infrared-spectroscopy based spectral-biomarker selection towards optimum diagnostic differentiation of oral leukoplakia and cancer. Anal Bioanal Chem 2015;407:7935–43. [DOI] [PubMed] [Google Scholar]
- [88].Ashizawa K, Yoshimura K, Johno H, Inoue T, Katoh R, Funayama S et al. Construction of mass spectra database and diagnosis algorithm for head and neck squamous cell carcinoma. Oral Oncol 2017;75:111–19. [DOI] [PubMed] [Google Scholar]
- [89].Jeyaraj PR, Samuel Nadar ER. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J Cancer Res Clin Oncol 2019; 145:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lu G, Wang D, Qin X, Muller S, Wang X, Chen AY et al. Detection and delineation of squamous neoplasia with hyperspectral imaging in a mouse model of tongue carcinogenesis. J Biophotonics 2018;11:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fei B, Lu G, Wang X, Zhang H, Little JV, Patel MR et al. Label-free reflectance hyperspectral imaging for tumor margin assessment: a pilot study on surgical specimens of cancer patients. J Biomed Opt 2017;22:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Aubreville M, Stoeve M, Oetter N, Goncalves M, Knipfer C, Neumann H et al. Deep learning-based detection of motion artifacts in probe-based confocal laser endomicroscopy images. Int J Comput Assist Radiol Surg 2019;14:31–42. [DOI] [PubMed] [Google Scholar]
- [93].Heidari E, Sunny S, James BL, Lam TM, Tran AV, Yu J et al. Optical coherence tomography as an oral cancer screening adjunct in a low resource settings. IEEE J Sel Top Quant 2019;25:1–8. [Google Scholar]
- [94].Brickley MR, Cowpe JG, Shepherd JP. Performance of a computer simulated neural network trained to categorise normal, premalignant and malignant oral smears. J Oral Pathol Med 1996;25:424–28. [DOI] [PubMed] [Google Scholar]
- [95].Skandarajah A, Sunny SP, Gurpur P, Reber CD, D’Ambrosio MV, Raghavan N et al. Mobile microscopy as a screening tool for oral cancer in India: A pilot study. PLoS One 2017;12:e0188440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tseng YJ, Wang HY, Lin TW, Lu JJ, Hsieh CH, Liao CT. (2020). Development of a machine learning model for survival risk stratification of patients with advanced oral cancer. JAMA Netw Open, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Stepp WH, Farquhar D, Sheth S, Mazul A, Mamdani M, Hackman TG et al. RNA oncoimmune phenotyping of HPV-positive p16-positive oropharyngeal squamous cell carcinomas by nodal status. JAMA Otolaryngol Head Neck Surg 2018;144:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Campisi G, Di Fede O, Giovannelli L, Capra G, Greco I, Calvino F et al. Use of fuzzy neural networks in modeling relationships of HPV infection with apoptotic and proliferation markers in potentially malignant oral lesions. Oral Oncol 2005;41:994–1004. [DOI] [PubMed] [Google Scholar]
- [99].Li S, Chen X, Liu X, Yu Y, Pan H, Haak R et al. Complex integrated analysis of lncRNAs-miRNAs-mRNAs in oral squamous cell carcinoma. Oral Oncol 2017;73:1–9. [DOI] [PubMed] [Google Scholar]
- [100].Song X, Yang X, Narayanan R, Shankar V, Ethiraj S, Wang X et al. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc Natl Acad Sci USA 2020;117:16167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Exarchos K, Goletsis Y, Fotiadis D. A multiscale and multiparametric approach for modeling the progression of oral cancer. BMC Med Inform Decis Mak 2012;12:136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


