Abstract
Capecitabine is a well-established agent for adjuvant chemotherapy in breast and colorectal cancers. However, one of the limiting adverse events of this therapy is severe diarrhea, which is reported with increasing frequency as of late. Capecitabine-induced ileitis should be suspected in cases with severe, treatment-refractory diarrhea. We present a case of capecitabine-induced terminal ileitis in a patient who received the medication as adjuvant therapy for previously resected colon adenocarcinoma. Capecitabine-induced diarrhea secondary to ileitis is a severe adverse drug event, which occurs during adjuvant chemotherapy and does not respond to conservative treatment with antidiarrheals, often necessitating permanent drug withdrawal. A high index of suspicion is critical as the complications, such as dehydration and the associated electrolyte derangements, may be life-threatening if diagnosis and cause-specific treatment are delayed.
Keywords: capecitabine, ileitis, diarrhea, gastrointestinal injury
Introduction
Fluoropyrimidines (e.g., capecitabine, 5-fluorouracil (5-FU), trifluridine-tipiracil) are chemotherapy agents, which are cytotoxic and/or cytostatic antimetabolites - they antagonize pyrimidine analog production to halt deoxyribonucleic acid synthesis and induce cell death. The oral agent capecitabine emerged as an attractive alternative to the parenteral 5-FU due to ease of administration, a more favorable side effect profile, and matched efficacy [1]. Capecitabine is frequently used as an adjunctive or palliative agent for locally advanced or metastatic colon and breast cancer. The most commonly reported side effects are myelosuppression, palmar-plantar erythrodysesthesia, and diarrhea.
Chemotherapy-related diarrhea (CRD) is a serious dose-limiting adverse drug reaction, which can be life-threatening and require hospitalization for supportive care. This is particularly relevant for capecitabine, as randomized trial data reported the frequency of severe diarrhea (grade 3 or 4) to be as high as 47% in certain regimens [2]. In rare cases, fluoropyrimidines have been associated with various gastrointestinal tract injuries, such as mucositis, enterocolitis, and, rarely, ileitis [2]. We report a rare case of capecitabine-induced terminal ileitis. We further conduct a literature review to identify patient characteristics and treatment considerations, as the management approach for severe diarrhea secondary to terminal ileitis poses a significant clinical challenge.
Case presentation
A 68-year-old male presented to the emergency room (ER) of “Elias” Emergency University Hospital in Bucharest with severe watery diarrhea (grade 4), associated with nausea, vomiting, and malaise. Ten days prior, treatment with adjuvant capecitabine was initiated for colon adenocarcinoma. His medical history was significant for diabetes mellitus type 2 treated with long-acting insulin and oral antidiabetic agents and arterial hypertension treated with calcium channel blockers and beta-blockers. The patient denied smoking or illicit drug use. He suffered from a bilateral inguinal hernia, and he was previously diagnosed with moderately differentiated ascending colon adenocarcinoma, which was treated with a right hemicolectomy and ileal-transverse anastomosis.
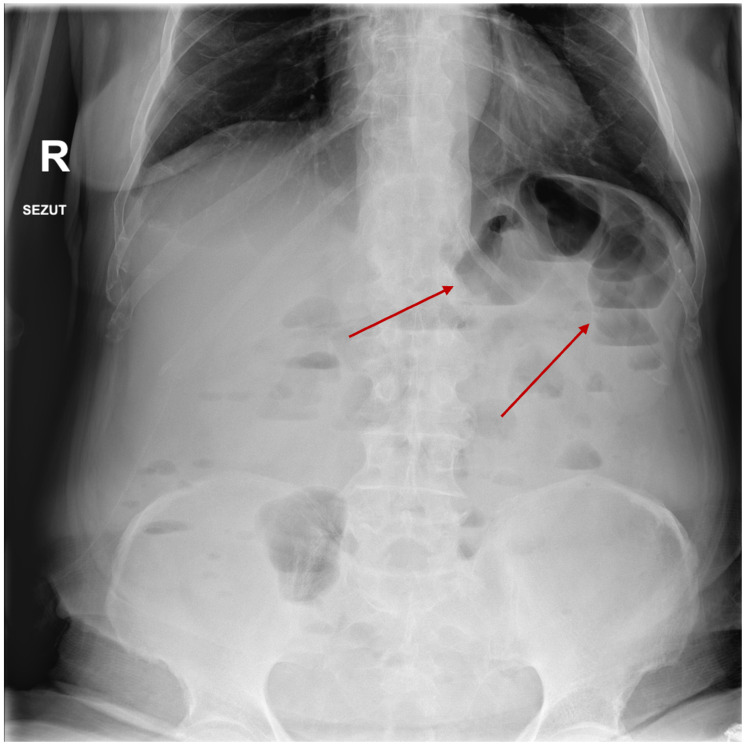
His clinical examination was significant for mild but diffuse abdominal pain exacerbated by palpation, without peritoneal signs. Laboratory examinations were significant for mild hypernatremia (146 mmol/L, reference 135-145 mmol/L), hypokalemia (2.5 mmol/L, reference 3.5-5 mmol/L), serum osmolarity of 299 mOsm/kg, hypoalbuminemia (2.8 mg/dL, reference 3.3-5 mg/dL), slightly elevated blood urea nitrogen, mild anemia (10.5 g/dL, reference 12-15 g/dL) on complete blood count, elevated inflammatory markers (C reactive protein of 46 mg/dL, normal value <5 mg/dL), normal procalcitonin, and normal liver function tests. Exhaustive stool workup did not identify any pathogens, with significantly elevated fecal calprotectin (1053 mcg/g, normal value <50 mcg/g). The abdominal ultrasound at the ER was inconclusive, while abdominal plain radiographs showed several non-specific, small bowel air-fluid levels (Figure 1).
Figure 1. Abdominal radiography conducted at admission showing air-fluid levels (red arrows).
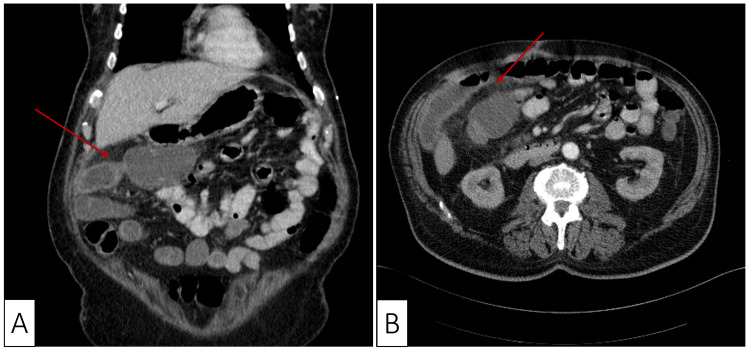
The patient was transferred to the oncology department where a computed tomography (CT) scan of the abdomen was performed to further evaluate the cause of the air-fluid levels. The result showed ileal wall thickening with contrast enhancement of the adjacent fat and intraluminal vessel enhancement suggestive of a local inflammatory process (Figure 2).
Figure 2. Coronal (A) and axial (B) computed tomography scan demonstrating thickening of the terminal ileum and mural thickening (red arrows).
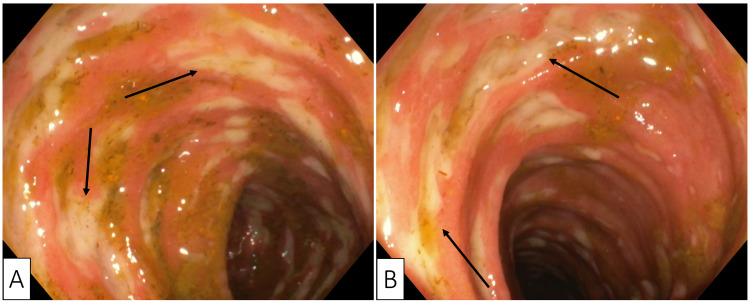
Colonoscopy revealed a normal ileocolonic anastomosis; however, multiple large ulcers and diffuse erythematous ileitis were discovered, extending approximately 10 cm proximal to the anastomosis (Figure 3). Antidiarrheal drugs were prescribed, consisting initially with loperamide and later with octreotide. However, after three days of treatment there was no significant reduction in stool frequency or improvement of stool consistency. Therefore, capecitabine was withdrawn while continuing loperamide. The patient began to improve clinically. Three days later, gastrointestinal transit was normalized, and the patient was discharged with recommendations to taper down the dose of loperamide.
Figure 3. Colonoscopy showing the ileum at the ileo-colonic anastomosis (A) and the terminal ileum (B) with diffusely erythematous mucosa covered with multiple large ulcerations (black arrows).
The patient was tested in the outpatient care clinic for dihydropyrimidine dehydrogenase (DPD) deficiency being heterozygous for the splice-site mutation affecting dihydropyrimidine dehydrogenase gene (DPYD)*2A. Due to the associated costs, the patient declined preemptive DPD screening. Given the relapse risk associated with retreatment in the setting of DPD deficiency, and the adjuvant role of chemotherapy in this case, we decided to discontinue capecitabine permanently and closely monitor the patient further.
Discussion
Diarrhea is a well-established side effect of capecitabine treatment, usually starting after two to three weeks from the first drug administration, is mostly mild in severity, and rarely necessitates antimotility agents, such as loperamide, for symptomatic control. However, grade 3-4 diarrhea can occur in 11.4% of patients and can be a life-threatening complication in the setting of DPD deficiency [3,4]. Capecitabine causes ileitis presumably through inducing loss of surface epithelium secondary to acute mucosal injury through a mechanism that involves interference with crypt cell mitoses. This leads to a high-volume fluid loss in the small bowel, which exceeds the colonic capacity for reabsorption [2,5].
In order to further identify patient characteristics, management considerations, and outcome trajectories, we conduct a search of the PubMed and Scopus databases for cases describing terminal ileitis induced by capecitabine. A set of keywords was identified either within the title/abstract or as Medical Subject Heading terms. In addition to the eight published articles that were reviewed, four cases of capecitabine-induced terminal ileitis were reported as posters in congresses. Although they were excluded from our review due to being unpublished, they suggest that the prevalence of capecitabine-induced ileitis may be underreported. Colonoscopy and histopathology findings were included in six and four studies, respectively. A total of eight studies was included in our review, totaling 13 patients - our results are presented in Table 1.
Table 1. Published literature describing capecitabine-induced ileitis.
CT: computed tomography; IVF: intravenous fluids; MRE: magnetic resonance enterography; CAPOX: capecitabine with oxaliplatin; CAPIRI: capecitabine with irinotecan; TPN: total parenteral nutrition
| Author and year | Patient gender and age (years) | Chemotherapy regimen prior to diagnosis | Symptoms | Diagnostic findings | Treatment and outcome |
| Our patient | Male, 68 | Adjuvant capecitabine | Severe watery diarrhea (grade 4) accompanied by nausea, vomiting, and malaise | CT - ileal wall thickening with adjacent fat stranding; endoscopy - multiple large ulcers of the ileum with and diffusely erythematous mucosa | IVF, antidiarrheal drugs; diarrhea did not respond to antidiarrheal therapy, but improved within three days of capecitabine discontinuation |
| Dao et al., 2019 [6] | Female, 72 | Neoadjuvant capecitabine | Severe watery diarrhea | CT - mildly dilated fluid-filled bowel loops with mesenteric edema; endoscopy - ulceration of ileum/ileocecal valve and granular erythematous mucosa of ascending colon | IVF, broad-spectrum antibiotics, loperamide, diphenoxylate-atropine, octreotide, cholestyramine; diarrhea resolved after four weeks |
| Female, 42 | Neoadjuvant capecitabine | Severe voluminous bloody diarrhea (grade 3) | CT - marked wall thickening distal small bowel loops with luminal fluid; endoscopy - severe ileal ulceration with necrosis, bleeding, and pseudomembrane formation | IVF, broad-spectrum antibiotics, loperamide, diphenoxylate/atropine, octreotide, cholestyramine, dietary changes; diarrhea resolved after four weeks | |
| Van Hellemond et al., 2018 [7] | Female, 69 | CAPOX | Severe watery diarrhea (grade 4) | MRE - 5 mm thickening of the distal 15 cm of the ileum; endoscopy - superficial, yet extensive ulceration of the terminal ileum; histopathology - extensive inflammation consistent with ileitis | IVF, budesonide; diarrhea resolved |
| Nicosia et al., 2018 [8] | Female, 71 | Combined radiotherapy and capecitabine | Severe watery diarrhea (grade 3) | CT - diffuse edema of the distal ileum with luminal narrowing | IVF, broad-spectrum antibiotics; diarrhea resolved after 15 days |
| Female, 54 | Combined radiotherapy and capecitabine | Severe watery diarrhea (grade 2) | CT - ileal distension with edematous thickening and perivisceral effusion | IVF, broad-spectrum antibiotics; diarrhea resolved after seven days | |
| Lee et al., 2015 [9] | Female, 61 | CAPIRI and bevacizumab | Watery diarrhea, right lower quadrant abdominal pain, vomiting, fever | CT - extensive submucosal ileal edema with adjacent fat stranding | IVF, dietary modifications; diarrhea resolved after 12 days |
| Female, 59 | Adjuvant capecitabine | Severe watery diarrhea (grade 4), palmar-plantar erythrodysesthesia, stomatitis, and severe abdominal pain | CT - diffuse submucosal ileal edema with adjacent fat stranding and pneumatosis intestinalis | IVF, TPN, broad-spectrum antibiotics; diarrhea resolved after 29 days | |
| Mokrim et al., 2014 [10] | Female, 66 | Adjuvant capecitabine | Severe watery diarrhea (grade 3), fatigue, emesis, altered mental status | CT - submucosal ileal edema with mural thickening; endoscopy - inflammatory changes in the ileal mucosa; histopathology - inflammatory changes without intraepithelial lymphocytic infiltrates | IVF, broad-spectrum antibiotics; diarrhea resolved after several days |
| Female, 67 | Adjuvant capecitabine | Severe watery diarrhea (grade 3), fever, fatigue, reduced appetite | CT - parietal thickening of the distal ileal loops | IVF, broad-spectrum antibiotics; diarrhea resolved after several days | |
| Al-Gahmi et al., 2012 [11] | Male, 65 | Adjuvant capecitabine with radiotherapy | Watery diarrhea, fever, abdominal pain, vomiting | Endoscopy - isolated ulceration of the terminal ileum; histopathology - inflammatory changes with eosinophilic infiltrate | IVF, conservative treatment; diarrhea resolved after some time |
| Radwan et al., 2012 [12] | Male, 67 | Adjuvant capecitabine | Watery diarrhea (grade 3), reduced appetite, lower abdominal discomfort, and vertigo precipitating falls | CT - distended loops of small bowel, wall thickening, and inflammatory changes | IVF, broad-spectrum antibiotics, TPN, and loperamide; diarrhea resolved after some time |
| Bouma and Imholz, 2011 [13] | Male, 73 | Capecitabine with oxaliplatin and bevacizumab | Watery diarrhea, nausea, vomiting, abdominal pain | CT - ileal mural thickening | IVF, clear liquid diet; diarrhea resolved after several days |
The average age of the patients was 64 (range 42-73), and of the 13 patients, nine were female, and four were male. In most cases, capecitabine was administered for either metastatic breast or colon cancer, while in a minority of cases, the indication was primary but locally advanced rectal or colon adenocarcinoma. The clinical picture was dominated by gradually increasing frequency of bowel movements with loosening of stool consistency accompanied by nausea, vomiting, and abdominal pain. In five cases and three cases, the diarrhea was grade 3 and grade 4, respectively. Bloody diarrhea was noted in one patient. In two patients, the degree of dehydration was severe enough to precipitate altered mental status and vertigo with falls. In two patients, concomitant occurrence of terminal ileitis and palmar-plantar erythrodysesthesia was noted, suggesting a potential hypersensitivity reaction. Typical workup began with excluding infectious causes of diarrhea, and in eight patients, prophylaxis with broad-spectrum antibiotics was initiated.
Endoscopic studies may be helpful in further supporting the diagnosis, especially in evaluating potential anastomotic complications in patients who underwent colorectal surgery. Typical findings included ileal ulceration with associated inflammatory changes - in one patient, ulceration was severe enough to precipitate focal hemorrhages with pseudomembrane formation. A cause of refractory CRD is neutropenic enterocolitis, which precludes endoscopic investigations - although evident, potential contraindications must be respected before proceeding with invasive studies in patients taking capecitabine. Reported histopathologic findings include inflammatory changes, eosinophilic infiltrate, mucosal erosion with necrotic debris, without the presence of intraepithelial lymphocytic infiltrates, granulomas, or viral inclusions.
Approach to management presented as a clinical challenge, and in our review, the treatment strategy differed across studies. Three patients were managed with intravenous fluids (IVFs) and bowel rest - dietary modifications (clear liquid diet or low-lactose, low-fat, high-protein diet) appeared to contribute to recovery. Four patients were managed with IVFs and broad-spectrum antibiotics without antidiarrheal therapy. The rest of the patients were managed with IVFs, usually broad-spectrum antibiotics, and some form of step-up antidiarrheal therapy depending on response and other clinical symptoms - loperamide, diphenoxylate-atropine, octreotide, and budesonide. In one patient who previously underwent a right hemicolectomy, cholestyramine was also trialed to address a possible biliary etiology of diarrhea - however, the patient did not improve. Empiric antibiotics were typically discontinued after infectious etiology of the diarrhea was ruled out and instead replaced with antidiarrheal therapy, which few patients responded to adequately. Two patients required total parental nutrition to correct a protein deficiency.
Resolution time of gastrointestinal upset varied from several days up to four weeks, and it is unclear if the severity and the protracted recovery courses are related to the cumulative dose of capecitabine received. Regardless, the backbone of managing these patients included the withdrawal of capecitabine. The safety of resuming capecitabine after recovery is unclear - five patients went on to restart capecitabine (two patients at a reduced dosage), with acceptable tolerability of the regimen. For the rest of the cases, the managing physicians were wary of provoking repeat complications and chose to switch to alternative regimens.
Nicosia et al. described a patient who developed capecitabine-induced terminal ileitis during his 16th fraction of a pelvic irradiation protocol [8]. Within the combinatorial radiochemotherapy treatment regimen, the administered dose of capecitabine was lower than in other cases, leading the authors to speculate that pelvic irradiation may increase the risk of developing ileitis in patients receiving neoadjuvant capecitabine. In a retrospective review of 188 patients who received abdominal or pelvic radiotherapy, Daly et al. found the incidence of chronic ileitis to range from 2.2% to 14.3%, possibly due to radiation enteritis [14]. In order to mitigate this rare complication, meticulous identification and constrained contouring of organs at risk is especially critical to ensure both safety and efficacy of treatment delivery. Al-Gahmi et al. also described ileitis in a patient on combinatorial therapy, but radiation-induced ileitis was ruled unlikely due to low exposure of the ileum to the radiation field [11].
The mechanism behind capecitabine-induced diarrhea, and potentially ileitis, is complex and multifaceted. As mentioned previously, early studies identified capecitabine causing mitotic arrest of crypt cells in the G2 phase, impairing their subsequent migration and differentiation into mature enterocytes [5]. The colon's diminished absorptive capacity works in tandem with goblet cell hyperplasia and subsequent excessive mucin secretion from the small bowel to cause secretory diarrhea. However, the mechanism may be considerably more complex, as fluoropyrimidines may increase gene expression of inflammatory cytokines and cause decreased expression of colonic aquaporin channels through neutrophilic inflammation [15]. Mitotic arrest in smooth muscle cells, leading to apoptosis and reduction in contractility through actin cytoskeleton reorganization, has also been documented [16]. Finally, fluoropyrimidines are directly toxic to the endothelium through increased generation of reactive oxygen species - this toxicity can lead to thrombosis or vasospasm via release of sequestered vasoactive substances [17]. It is conceivable that all of these pathologic processes contribute to the clinical phenotype.
Capecitabine is metabolized to the active metabolite 5-FU through several enzymatic reactions, which preferentially occur in solid tumors and the liver. Genetic polymorphism of drug-metabolizing enzymes through the variable expression of single nucleotide (SNPs) is well described and may explain why only select patients suffer from adverse drug reactions, such as terminal ileitis. The majority of 5-FU is catabolized by DPD, which is encoded by the highly polymorphic DPYD gene [18]. Deficiency of DPD is reported in approximately 5-9% of patients, and administering fluoropyrimidines in the context of depressed enzyme activity can be fatal [4,19].
In our review, DPD genotype testing was done in three patients, and a mutation was found in two of them. In both cases, there was only a partial reduction in DPD activity. Curiously, the patient described by Mokrim et al. only developed symptoms of ileitis after completing an entire cycle of capecitabine despite having diminished DPD activity [10]. Recommendations are in place advocating for routing screening for four most common DPD variants before initiating treatment with capecitabine, especially in regions with accentuated prevalence [19]. A recent genome-wide association study identified three novel SNPs, which can be used as germline genetic predictors of capecitabine-associated diarrhea and, potentially, ileitis [20]. The toxicity capture approach was used in patients being treated with capecitabine for breast cancer, with further research being required to identify if cancer type influences SNP effects on enzyme activity and adverse drug event incidence. The application of genetic screens in identifying patients who are likely to develop capecitabine toxicity is promising and may aid in reducing morbidity.
Conclusions
Capecitabine-induced terminal ileitis is rare but may be life-threatening - treating physicians should be aware of this potential complication and intervene early when persistent, high-grade diarrhea is reported. CT scans showing edematous mural thickening and distention of the terminal ileum should raise suspicion for ileitis. The cornerstone of conservative management includes the withdrawal of capecitabine, antidiarrheal therapy, and broad-spectrum antibiotics, and dietary modifications are acceptable treatment modalities. Pelvic irradiation may represent a risk factor, which increases the likelihood of developing terminal ileitis with capecitabine. Restarting capecitabine may be attempted if the patient harbors normal DPD function, although further research is required to validate safety outcomes.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.The role of pharmacogenetics in capecitabine efficacy and toxicity. Lam SW, Guchelaar HJ, Boven E. Cancer Treat Rev. 2016;50:9–22. doi: 10.1016/j.ctrv.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Guidance on the management of diarrhoea during cancer chemotherapy. Andreyev J, Ross P, Donnellan C, et al. Lancet Oncol. 2014;15:0–460. doi: 10.1016/S1470-2045(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 3.Incidence and relative risk of grade 3 and 4 diarrhoea in patients treated with capecitabine or 5-fluorouracil: a meta-analysis of published trials. Iacovelli R, Pietrantonio F, Palazzo A, Maggi C, Ricchini F, de Braud F, Di Bartolomeo M. Br J Clin Pharmacol. 2014;78:1228–1237. doi: 10.1111/bcp.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Is capecitabine safe in patients with gastrointestinal cancer and dihydropyrimidine dehydrogenase deficiency? Saif MW, Diasio R. Clin Colorectal Cancer. 2006;5:359–362. doi: 10.3816/CCC.2006.n.007. [DOI] [PubMed] [Google Scholar]
- 5.Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. Ikuno N, Soda H, Watanabe M, Oka M. J Natl Cancer Inst. 1995;87:1876–1883. doi: 10.1093/jnci/87.24.1876. [DOI] [PubMed] [Google Scholar]
- 6.Role of colonoscopy in diagnosis of capecitabine associated ileitis: two case reports. Dao AE, Hsu A, Nakshabandi A, et al. World J Gastrointest Endosc. 2019;11:383–388. doi: 10.4253/wjge.v11.i5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capecitabine-associated terminal ileitis. van Hellemond IEG, Thijs AM, Creemers GJ. Case Rep Oncol. 2018;11:654–659. doi: 10.1159/000492781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Two cases of capecitabine-induced ileitis in patients treated with radiochemotherapy to the pelvis and review of the literature. Nicosia L, Russo I, De Sanctis V, Minniti G, Valeriani M, Osti MF. J Gastrointest Cancer. 2018;49:538–542. doi: 10.1007/s12029-017-9955-4. [DOI] [PubMed] [Google Scholar]
- 9.Severe ileitis associated with capecitabine: two case reports and review of the literature. Lee SF, Chiang CL, Lee AS, Wong FC, Tung SY. Mol Clin Oncol. 2015;3:1398–1400. doi: 10.3892/mco.2015.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breast cancer, DPYD mutations and capecitabine-related ileitis: description of two cases and a review of the literature. Mokrim M, Aftimos PG, Errihani H, Piccart-Gebhart M. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-203647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capecitabine-induced terminal ileitis. Al-Gahmi AM, Kerr IG, Zekri JM, Zagnoon AA. Ann Saudi Med. 2012;32:661–662. doi: 10.5144/0256-4947.2012.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ileitis secondary to oral capecitabine treatment? Radwan R, Namelo WC, Robinson M, Brewster AE, Williams GL. Case Rep Med. 2012;2012:154981. doi: 10.1155/2012/154981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.[Ileitis following capecitabine use] Bouma G, Imholz AL. https://europepmc.org/article/med/21486509. Ned Tijdschr Geneeskd. 2011;155:0. [PubMed] [Google Scholar]
- 14.The incidence of severe chronic ileitis after abdominal and/or pelvic external irradiation with high energy photon beams. Daly NJ, Izar F, Bachaud JM, Delannes M. Radiother Oncol. 1989;14:287–295. doi: 10.1016/0167-8140(89)90140-0. [DOI] [PubMed] [Google Scholar]
- 15.5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. Sakai H, Sagara A, Matsumoto K, et al. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0054788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effects of 5-fluorouracil in nuclear and cellular morphology, proliferation, cell cycle, apoptosis, cytoskeletal and caveolar distribution in primary cultures of smooth muscle cells. Filgueiras Mde C, Morrot A, Soares PM, Costa ML, Mermelstein C. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0063177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. Focaccetti C, Bruno A, Magnani E, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pharmacogenomics of fluorouracil-based chemotherapy toxicity. Matsusaka S, Lenz HJ. Expert Opin Drug Metab Toxicol. 2015;11:811–821. doi: 10.1517/17425255.2015.1027684. [DOI] [PubMed] [Google Scholar]
- 19.Dihydropyrimidine dehydrogenase testing prior to treatment with 5-fluorouracil, capecitabine, and tegafur: a consensus paper. Wörmann B, Bokemeyer C, Burmeister T, et al. Oncol Res Treat. 2020;43:628–636. doi: 10.1159/000510258. [DOI] [PubMed] [Google Scholar]
- 20.Clinical evaluation of germline polymorphisms associated with capecitabine toxicity in breast cancer: TBCRC-015. O'Donnell PH, Trubetskoy V, Nurhussein-Patterson A, et al. Breast Cancer Res Treat. 2020;181:623–633. doi: 10.1007/s10549-020-05603-8. [DOI] [PubMed] [Google Scholar]