Abstract
This article aims to present the case of a man affected by SARS CoV-2 and to discuss the association between this manifestation, viral infection and Open Abodmen. A 52 years old Caucasian man, affected by SARS CoV-2 infection, was admitted to the Emergency department of Arcispedale Sant’Anna of Ferrara for epigastralgia followed by syncopal episode, vomiting and diarrhea with bloody stools. The next day the patient underwent colonoscopy, which detected an ulceration proximally to the ileocecal valve without active bleeding. Subsequently an initial non-operative management and two pharyngeal swabs negative, for another rectorrhagia and hypotensive episode, underwent emerging surgery and an Open Abdomen was performed. The patient was discharged in 12th post-surgery day without complications. The IHC analysis with anti-SARS-CoV-2 nucleocapsid-protein revealed the presence of viral protein expression in epithelial cell of ulcerated intestinal mucosa.
In this case report, we showed the presence of viral inclusion in small intestinal wall after two negative pharyngeal swabs for SARS-CoV-2 RNA. We can also say that the largest amount of viral inclusions was in the tissue of ulceration of the last ileal loop. This case report showed that SARS-CoV-2 can be unseen also after clinical healing. It's probably can be expelled with stools and rectal swabs search for SARS-Cov-2 RNA after pharyngeal swabs could be mandatory for declare heled a patient. Moreover, damage control surgery and Open Abdomen as a surgical technique can be a valid alternative in case of uncertainty of the bleeding source and when a second surgical look is necessary.
Keywords: COVID-19, Rectal swabs, Gastrointestinal bleeding, Damage control surgery, Case report
Highlights
-
•
The novel SARS-CoV-2 may result in gastrointestinal (GI) symptoms in up to one-third of patients.
-
•
SARS-CoV-2 is excreted in the faeces, thus raising the possibility of faecal-oral transmission.
-
•
Two pharyngeal swabs for SARS-CoV-2 RNA were performed and were both negative.
-
•
Viral protein expression in epithelial cell of ulcerated intestinal mucosa.
-
•
Open Abodmen Technique is a valid alternative in surgical emergencies.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2, formerly known as 2019-nCoV) belongs to the family of Coronaviridae, a group of enveloped, nonsegmented, positive-sense RNA viruses which appeared in December 2019 In the province of Hubei, China. At a genetic level, 2019-nCoV is closely related to the SARS-CoV and MERS-CoV [1,2]. It is well established that most patients suffering from COVID-19, the disease correlated by SARS-CoV-2, present fever associated with respiratory signs and symptoms, such as dry cough and dyspnea [[3], [4], [5]]; In some series, traditional HCoV account for up to 30% of influenza negative upper respiratory tract infections and up to 8.1% of enteritis [6,7]. Interestingly, the novel SARS-CoV-2 may result in gastrointestinal (GI) symptoms in up to one-third of patients [8,9]. Furthermore, SARS-CoV-2 may present primarily with GI complaints such as diarrhea, abdominal pain, hematochezia and liver injury that may precede typical respiratory presentation [4,10]. Also, Zhang et al. showed that SARS-CoV-2 is excreted in the faeces, thus raising the possibility of faecal-oral transmission [11].Whereas more research is underway to elucidate the transmission mechanism of SARS-CoV-2 by the interaction of SARS-CoV-2 with the small bowel, specifically with the angiotensin-converting enzyme 2 (ACE2) receptors located on the brush border of enterocytes [12]: SARS-CoV-2 S protein binds ACE2 with 10-fold higher affinity than the previous SARS-CoV [13]. Interestingly in SARS-CoV-2, the S-spike that is formed by the S1 and S2 segments and is more disordered than in SARS-CoV, having a solvent-exposed loop (i. e. “crack”) held by polybasic RRAR bonds in between S1 and S2. This “crack” facilitates the influx of human proteases, such as the serine proteases (transmembrane protease serine 2) TMPRSS2 and furin, which cleave this polybasic RRAR site at the junction of S1 and S2 14. This cleavage results in a separation of both arms (i.e. “pinchers”) of the S-spike. S1 contains the receptor-binding domain, which directly binds to the peptidase domain of ACE2. Whereas S2 is responsible for cell membrane fusion [[13], [14], [15]]. As with TMPRSS, the enzyme furin may enable the S-spike to separate into two pinching structures.
The open abdomen non-trauma patients has been proposed to be effective in preventing or treating deranged physiology in patients with severe injuries or critical illness when no other perceived options exist. Its use, however, remains controversial as it is resource consuming and represents a non-anatomic situation with the potential for severe adverse effects [19].
This case report [20] aims to present the case of a man affected by SARS CoV-2 infection and gastrointestinal bleeding, and to discuss the association between this manifestation and viral infection. Also we discuss the importance of Open Abdomen technique.
1.1. Presentation of case
A 52 years old Caucasian man, affected by SARS CoV-2 infection in-home therapy with hydroxychloroquine and azithromycin, was admitted to the Emergency department of Arcispedale Sant’Anna of Ferrara, complaining epigastralgia followed by syncopal episode, vomiting and diarrhea with bloody stools.
1.2. The patient's medical history was silent
Rectal exploration showed blood traces on the explorer finger. Laboratory exams showed Haemoglobin level 8.7 g/dl, neutrophilic leukocytosis, no CRP elevation.
The patient underwent blood transfusion in the emergency room and was submitted to urgent oesophagus-gastroscopy, not detecting active bleeding or any abnormalities in the upper digestive tract. Thorax and abdomen contrast-enhanced TC was performed, without showing any abdominal bleeding source, but detecting worsening bilateral interstitial pneumonia. The patient was admitted to COVID-19 Medical ward.
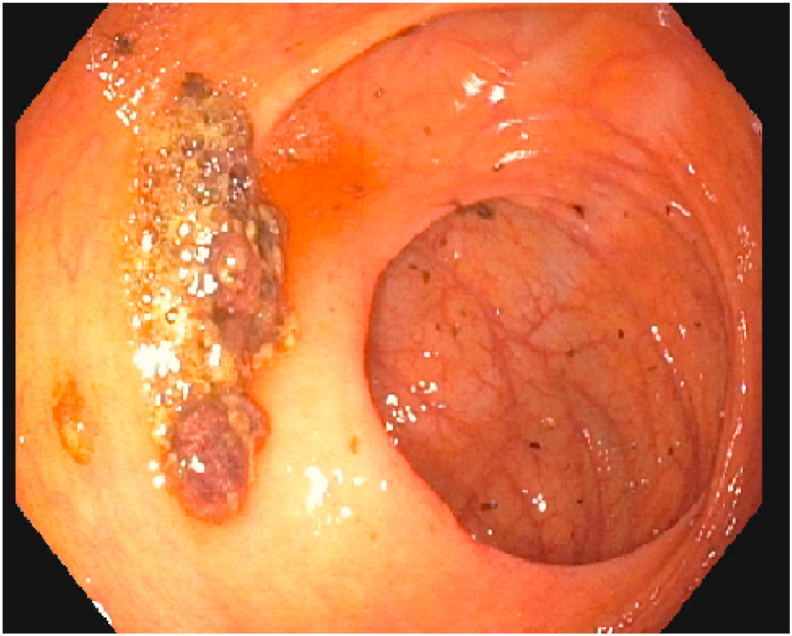
The next day the patient underwent colonoscopy, which detected an ulceration 5 cm proximally to the ileocecal valve (Fig. 1); endoscopy did not find any active bleeding and ileocolic biopsies were made. Were set therapy with ceftriaxone 2 g/die, tranexamic acid, methylprednisolone 40 mg twice a day and therapy with Mesalazine, associated with proton pump inhibitors. The patient was kept in fasting and parenteral nutrition was set. During hospitalization the patient presented several episodes of rectal bleeding, some of them were followed by symptomatic hypotension, requiring intensive rianimatory evaluation, with the indication of fluid filling and blood transfusion. A non-operative management was initially given. Further colonoscopy and another abdomen contrast-enhanced TC were performed without detecting bleeding source; even arteriography of intestinal vessels was performed and was negative for arterial active bleeding.
Fig. 1.
First colonscopy: ulceration 5 cm proximally to the ileo-cecal valve.
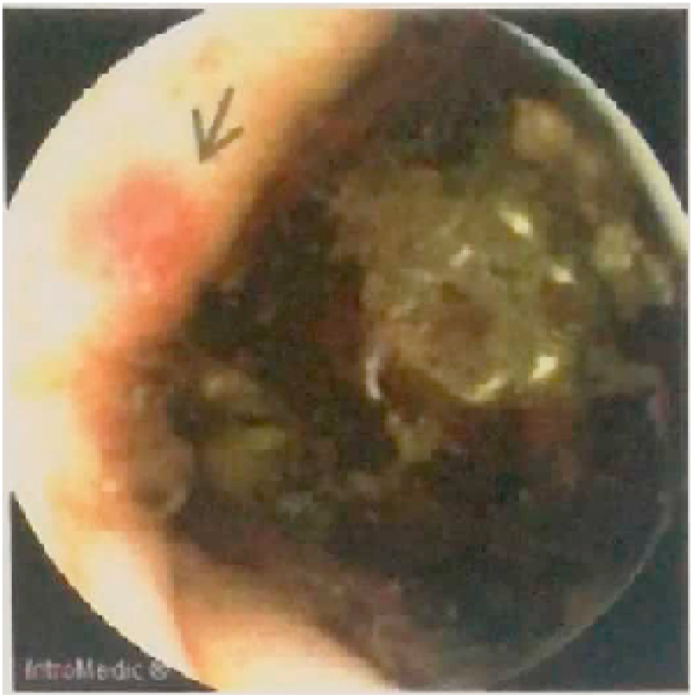
Four days later video-capsule endoscopy to study small bowel was performed, showing hyperemia of the duodenal bulb, small duodenal ulcer, ulcer covered by fibrin in the last ileal loop and small jejunal and right colon angiodysplasia (Fig. 2). The whole exam was made difficult by the presence of melaena in the intestinal tract.
Fig. 2.
Colon angiodysplasia.
Blood tumor markers were evaluated (negative). In the suspicion of inflammatory bowel disease, fecal calprotectin was tested, detecting moderately increased levels (maximum 128 mg/kg with a cut-off of 50 mg/kg in our laboratory).
Two pharyngeal swabs were performed and both of them were negative for SARS CoV-2. No fecal research of SARS CoV-2 RNA was performed and the patient was transferred to Gastroenterology Ward first and then, the day after, to our Emergency Surgery Ward for the persistence of rectorrhagia. At the day of transfer, the patient in our Unit presented further hypotensive episode with hematic stools in the evening. Hemoglobin was 8.5 g/dL (10.5 g/dL in the morning) on the hematic exam. The patient was supported with colloid infusion while waiting for blood transfusion and was taken to the surgery room for emergent surgery. The surgical finding was a small bowel and colon full of blood, Meckel diverticulum, several jejunal diverticula; no blood or effusion in the peritoneal cavity was found. Last ileal loop and cecum with Merkel diverticulum resection with ileal manual anastomosis was performed; laparostomy was made and the patient was transferred to the Intensive Care Unit. Two days later second-look surgery was performed: bowel was vital and well perfused and. Because of the presence of several jejunal diverticula, jejunal resection was decided and manual anastomosis was performed. Laparostomy was closed and the patient returned to Intensive Care Ward.
During recovery in Intensive Care Unit, Pseudomonas Aeruginosa was isolated from bronchoaspiration and broncho-alveolar lavage, so therapy with Piperacillin-Tazobactam was set. Postoperative electrocardiogram was done, detecting negative T-waves in precordial leads (V1–V3), associated with hypokalemia (3,3 mEq/l) and hypocalcemia (7,5 mg/dl); Troponin I HS was tested, finding out not significant elevation (25 ng/l). Electrolyte correction was performed; echocardiogram showed no myocardial ischemic abnormalities, mild left ventricular hypertrophy with Ejection Fraction 58%, mild mitral and tricuspidal insufficiency.
The patient was transferred to Emergency Surgery Ward two days after abdominal closure. Next days of recovery was regular without any complication, with normal blood pressure and stable hemoglobin values.
The patient was discharged in 12th post-surgery day without complications. Follow up was regular, without complications. The histological examination of small bowel showed intestinal diverticulosis with diverticulitis, chronic inflammation of the lamina propria is associated with focal superficial erosions, vascular ectasias and recent blood extravasations coexist also involving perivisceral adipose tissue and fibrinoleukocytic peritonitis. Abnormally enlarged and tortuous thick-walled veins were seen in the submucosa. IHC analysis with anti-SARS-CoV-2 nucleocapsid-protein revealed the presence of viral protein expression in epithelial cell of ulcerated intestinal mucosa (cytoplasmic staining) and a minority of lymphocytes [18].
2. Discussion
In this case report, we showed the presence of viral inclusion in small intestinal wall after two pharyngeal swabs for SARS-CoV-2 RNA were performed and were both of them negative.
We can also say that the largest amount of viral inclusions was in the tissue of ulceration of the last ileal loop.
It's only a case report. In the past months, most publications on COVID-19 have been observational studies in clinical cohorts, epidemiological investigations and forecasts, and in-silico genomic and structural analyses [16], without showing viral inclusion in vivo.
Many basic virological questions of SARS-CoV-2 remain unanswered. The SARS-CoV-2 use of human ACE2 as an entry receptor, and replication in Calu3 (pulmonary) cells. It also replicated to comparable levels in both Calu3 and Caco2 (intestinal) cells. Among other non-pulmonary cell lines, SARS-CoV-2 showed significant replication in Huh7 (hepatic) and 293T (renal) cells [16].
Clinical presentation of COVID-19 is often correlated to nausea, vomiting and abdominal pain or, sometimes, with hematochezia. In this case report, we have described important gastrointestinal bleeding. Also presented by Chen et al., the duration of viral shedding from the faces after negative conversion in pharyngeal swabs was 7 (6–10) days, regardless of COVID‐19 severity and SARS‐CoV‐2 transmission via the fecal-oral route should be seriously considered [17].
The open abdomen technique is a valid alternative in case of deranged physiology and bowel damage and necessity to perform a second look or delayed anastomosis [19].
3. Conclusion
This case report [20] showed that SARS-CoV-2 can be unseen also after clinical healing. It's probably can be expelled with stools and rectal swabs search for SARS-Cov-2 RNA after pharyngeal swabs could be mandatory for declare heled a patient. Furthermore, we reiterate how the open abdomen technique is a valid alternative in complex clinical cases.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102405.
Contributor Information
D. Lacavalla, Email: d.lacavalla@ospfe.it.
G. Santandrea, Email: sgiorsg@gmail.com.
D. Andreotti, Email: ndrdra@unife.it.
R. Stano, Email: r.stano@ospfe.it.
S. Occhionorelli, Email: savino.occhionorelli@unife.it.
Consent
The patient gives the informed consent for research on anatomical specimens and the publication of this case report.
Ethical approval
Case reports do not need to be approved by the ethics committee
Funding for research
The authors received no funding sources for this article.
Author contribution
D. Lacavalla, G. Santandrea, D. Andreotti, R. Stano, S. Occhionorelli contributed to the writing of the article.
Guarantor
D. Lacavalla accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zang R., Castro M.F.G., McCune B.T. TMPRSS2 and TMPRSS4 mediate SARS-CoV-2 infection of human small intestinal enterocytes. bioRxiv. 2020 doi: 10.1101/2020.04.21.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020 doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 3.Legido-Quigley H., Asgari N., Teo Y.Y. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020 doi: 10.1016/S0140-6736(20)30551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Totura A.L., Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expet Opin. Drug Discov. 2019 doi: 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jevšnik M., Steyer A., Pokorn M. The role of human coronaviruses in children hospitalized for acute bronchiolitis, acute gastroenteritis, and febrile seizures: a 2-year prospective study. PloS One. 2016 doi: 10.1371/journal.pone.0155555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu S.S., Hung Chan K., Wing Chu K. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 2005 doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Lou Yang X., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y., Liu P., Shi X.L. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microb. Infect. 2020 doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mönkemüller K., Fry L., Rickes S. Covid-19, Coronavirus, SARS-CoV-2 and the small bowel. Rev. Esp. Enferm. Dig. 2020 doi: 10.17235/reed.2020.7137/2020. [DOI] [PubMed] [Google Scholar]
- 13.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;80 doi: 10.1126/science.aax0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;80 doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu H., Chan J.F.-W., Yuen T.T.-T. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. The Lancet Microbe. 2020 doi: 10.1016/s2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Chen L., Deng Q. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo R., Neri L.M., Simioni C. SARS-CoV-2 nucleocapsid-protein and ultrastructural modifications in small bowel of a four week negative COVID-19 patient. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coccolini F., Roberts D., Ansaloni L. The open abdomen in trauma and non-trauma patients: WSES guidelines. World J. Emerg. Surg. 2018 doi: 10.1186/s13017-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agha R.A., Franchi T., Sohrabi C. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.