Abstract
Background
MMR proficient (pMMR) colorectal cancer (CRC) is usually unresponsive to immunotherapy. Recent data suggest that ibrutinib may enhance the anti-tumour activity of anti-PD-1 immunotherapy. In this study, we evaluated the safety and efficacy of ibrutinib plus pembrolizumab in refractory metastatic CRC.
Methods
This was a phase 1/2 study in patients with refractory metastatic pMMR CRC. The primary endpoints for phases 1 and 2 were maximum tolerated dose (MTD) and disease control rate, respectively. The secondary endpoints were safety, progression-free survival (PFS) and overall survival (OS).
Results
A total of 40 patients were enrolled. No dose-limiting toxicity was observed, and MTD was not identified. The highest tested dose of ibrutinib, 560 mg once daily, was combined with a fixed dose of pembrolizumab 200 mg every 3 weeks for the phase 2 portion. The most common grade 3/4 treatment-related adverse events were anaemia (21%), fatigue (8%) and elevated alkaline phosphatase (8%). Among 31 evaluable patients, 8 (26%) achieved stable disease, and no objective response was observed. The median PFS and OS were 1.4 and 6.6 months, respectively.
Conclusion
Ibrutinib 560 mg daily plus pembrolizumab 200 mg every 3 weeks appears to be well tolerated with limited anti-cancer activity in metastatic CRC.
ClinicalTrials.gov identifier
Subject terms: Cancer immunotherapy, Colorectal cancer
Background
Colorectal cancer (CRC) is one of the most common malignancies worldwide, with nearly 50% of patients ultimately developing metastatic disease.1 Despite the introduction of new cytotoxic and targeted drugs in recent decades, median survival remains lower than 3 years among patients with stage IV disease.2,3 Pembrolizumab, a human immunoglobulin G4 monoclonal antibody blocking programmed cell death 1 protein (PD-1), has demonstrated significant anti-cancer activity in patients with treatment-naive and refractory microsatellite instability-high (MSI-H)/mismatch repair deficiency (dMMR) metastatic CRC.4,5 However, fewer than 5% of stage IV patients have MSI-H/dMMR disease, and pembrolizumab is relatively inactive in microsatellite stable (MSS)/MMR proficient (pMMR) metastatic CRC.4 Therefore, there is an urgent need for the development of new therapeutic approaches to improve clinical outcomes in patients with pMMR CRC.
Ibrutinib, a first-in-class inhibitor of Bruton’s tyrosine kinase (BTK) inhibitor, is approved for the treatment of various B-cell malignancies and chronic graft-versus-host-disease. BTK is a cytoplasmic tyrosine kinase and has an essential role in B lymphocyte development, differentiation and signalling.6 Interestingly, BTK is expressed in up to 90% of colorectal cancers,7,8 overexpression of BTK in colorectal cancer is associated with unfavourable clinical outcome,7 and inhibition of BTK using ibrutinib induces significant cytotoxic effect in colon cancer cell lines8 suggesting that BTK may be a potential therapeutic target in CRC. In addition, preclinical studies reported that the combination of ibrutinib and PD-1 blockade immunotherapy induced synergistic anti-cancer activity in a mouse model of colorectal cancer by enhancement of anti-tumour effector and memory T cell response and inhibition of interleukin-2 inducible T cell kinase (ITK) which potentiates T helper type 1 (Th1) based cancer immune response.9,10 Based on the preclinical data supporting the rationale of combining ibrutinib with pembrolizumab, we conducted a phase 1/2 study to evaluate the safety and efficacy of ibrutinib and pembrolizumab for patients with refractory metastatic pMMR CRC.
Methods
This study was an open-label, single-arm phase 1/2 open-label trial to assess the safety, tolerability, and efficacy of pembrolizumab plus ibrutinib in patients with refractory metastatic pMMR CRC. This study was done in accordance with Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Boards. All patients provided written, informed consent.
Patients selection and study design
Pertinent eligibility criteria were as follows: histologically confirmed MSS or pMMR metastatic colorectal adenocarcinoma, refractory or intolerant to fluoropyrimidine, oxaliplatin and irinotecan, and if RAS wild type, cetuximab or panitumumab containing therapies, age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and adequate organ function.
Patients were enrolled in 3 + 3 dose-escalation design with 3–6 patients at each cohort for the phase 1 portion. All patients were administered oral ibrutinib once daily and a fixed dose of pembrolizumab 200 mg intravenously every 3 weeks until disease progression or unacceptable toxicity. The starting dose of ibrutinib was 420 mg daily (Cohort 1), and the ibrutinib dose was escalated up to 560 mg (Cohort 2) based on the safety and tolerability profile of the combination in Cohort 1. Dose-limiting toxicities (DLTs) were considered during the first 42 days on the study. DLTs included any grade ≥4 immune-related adverse events (irAEs), any grade ≥3 non-infectious pneumonitis, any grade ≥ 3 irAEs that did not downgrade to grade ≤2 within 3 days or grade ≤1 within 14 days, any grade ≥2 pneumonitis or interstitial lung disease that did not resolve to grade 1 within 3 days and any grade ≥3 non-hematologic toxicity that did not resolve within 4 days.
Per protocol, no dose escalation of ibrutinib beyond 560 mg was permitted. The recommended phase 2 dose would be 560 mg if the maximal tolerated dose (MTD) is not identified on the phase 1 portion of the trial.
Study endpoint and assessment
The primary endpoint of the phase 1 portion was the MTD of ibrutinib (established as no more than 560 mg) in combination with pembrolizumab. The primary endpoint of the phase 2 part was disease control rate (DCR) at 4 months. Secondary endpoints of phases 1 and 2 included safety profile, objective response rates, overall survival (OS) and progression-free survival (PFS). Toxicities were monitored according to common terminology criteria for adverse events (CTCAE) criteria, version 4.0. Tumour assessment was performed with computed tomography (CT) and/or magnetic resonance imaging (MRI) at baseline and every 9 weeks until disease progression or treatment discontinuation. The objective response rate (ORR) was evaluated using RECIST 1.1 criteria. Survival was monitored every 12 weeks after discontinuation of the treatment.
Biomarkers
Neutrophil to lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count obtained from complete blood count (CBC) with differential. The change of NLR between baseline and on-treatment (week 3) was obtained for the biomarker study. RAS mutation status of each patient was also collected for evaluation of the potential prognostic value.
Statistical methods
For the phase 2 portion, Simon two-stage design was used to assess disease control rate based on radiologic assessment, with null and alternative hypothesis disease control rates of 5 and 20%, with α = 0.10 and 90% power. In the first stage, 18 patients were accrued and if at least one patient had disease control, the plan was to accrue an additional 14 patients, with a target of at least 4 patients with disease control among 32 patients enrolled and treated. PFS and OS were estimated using the Kaplan–Meier method. All statistical analyses were performed using IBM SPSS Statistics 24.
Results
Baseline characteristics
A total of 40 patients were enrolled between January 2018 and July 2019 and received pembrolizumab plus ibrutinib 420 mg (N = 4) and ibrutinib 560 mg (N = 3) in phase 1 portion and pembrolizumab plus ibrutinib 560 mg (N = 31) in phase 2. Two patients did not receive any treatment due to clinical deterioration after enrolment. Baseline characteristics of 38 patients who received at least 1 dose of pembrolizumab and ibrutinib are summarised in Table 1. The median age of the patients was 59 years (range: 24–73), and 22 (58%) patients were male. The median number of prior lines of systemic therapy was 3 (range: 1–7), and 22 (58%) patients had an ECOG performance status of 1. A majority of patients (76%) had left-side colon or rectum as primary sites and liver (71%) and/or lung (68%) as metastatic sites. RAS mutations were identified in 19 (50%) patients.
Table 1.
Patients characteristics.
| Phase I | Phase II | Total (N = 38) | ||
|---|---|---|---|---|
| Ibrutinib 420 mg + Pembrolizumab (N = 4) | Ibrutinib 560 mg + Pembrolizumab (N = 3) | Ibrutinib 560 mg + Pembrolizumab (N = 31) | ||
| Median age (range) | 62 (50–67) | 49 (47–58) | 59 (24–73) | 59 (24–73) |
| Gender | ||||
| Male | 4 (100%) | 2 (66.7%) | 16 (51.6%) | 22 (57.9%) |
| Female | 0 | 1 (33.3%) | 15 (48.4%) | 16 (42.1%) |
| Race | ||||
| White | 4 (100%) | 3 (100%) | 23 (74.2%) | 30 (78.9%) |
| Hispanic | 0 | 0 | 1 (3.2%) | 1 (2.6%) |
| Blacks | 0 | 0 | 5 (16.1%) | 5 (13.2%) |
| Asian | 0 | 0 | 1 (3.2%) | 1 (2.6%) |
| Unknown | 0 | 0 | 1 (3.2%) | 1 (2.6%) |
| ECOG | ||||
| 0 | 2 (50%) | 3 (100%) | 11 (35.5%) | 16 (42.1%) |
| 1 | 2 (50%) | 0 | 20 (64.5%) | 22 (57.9%) |
| Site of primary tumour | ||||
| Right | 1 (25%) | 0 | 8 (25.8%) | 9 (23.7%) |
| Left | 3 (75%) | 3 (100%) | 23 (74.2%) | 29 (76.3%) |
| Previous chemotherapy | ||||
| 1 line | 0 | 0 | 3 (9.7%) | 3 (7.9%) |
| 2 lines | 1 (25%) | 0 | 9 (29%) | 10 (26.3%) |
| ≥3 lines | 3 (75%) | 3 (100%) | 19 (61.3%) | 25 (65.8%) |
| Site of metastasis | ||||
| Liver | 3 (75%) | 1 (33.3%) | 23 (74.2%) | 27 (71.1%) |
| Lung | 4 (100%) | 2 (66.7%) | 20 (64.5%) | 26 (68.4%) |
| Lymph node | 2 (50%) | 3 (100%) | 19 (61.3%) | 22 (57.9%) |
| Peritoneum | 2 (50%) | 1 (33.3%) | 11 (35.5%) | 14 (36.8%) |
| KRAS/NRAS | ||||
| Wild type | 1 (25%) | 2 (66.7%) | 16 (51.6%) | 19 (50%) |
| Mutant | 3 (75%) | 1 (33.3%) | 15 (48.4%) | 19 (50%) |
Treatment
A total of 38 patients received at least 1 dose of pembrolizumab and ibrutinib. In phase 1 (dose escalation) portion, 4 patients were enrolled at dose level 1 (ibrutinib 420 mg) due to consent withdrawal of 1 patient before completion of DLT evaluation period, and 3 patients received ibrutinib 560 mg (dose level 2). No DLTs were observed with 420 or 560 mg of ibrutinib in the phase 1 dose-escalation cohort. MTD of ibrutinib was not identified, and 560 mg of ibrutinib, the higher protocol-defined dose, was used for the phase 2 (dose expansion) portion. The median number of treatment cycles was 2 (range: 1–17) in all cohorts, 5 (range: 1–17) in the phase 1 portion and 2 (range: 1–9) in phase 2. Thirty-one patients discontinued treatment due to disease progression including clinical progression (N = 5). Two patients discontinued treatment due to an adverse event (AE) including treatment-related rash, treatment-unrelated acute renal insufficiency, and five discontinued treatment due to consent withdrawal. While dose interruption was required in four patients on ibrutinib 560 mg due to AEs, dose modification was not required in this study.
Safety
The most common drug-related AEs were fatigue (45%) followed by anaemia (42%), diarrhoea (32%), elevated alkaline phosphatase (29%) and rash (26%) (Table 2). Grades 3 and 4 drug-related AEs occurred in 16 patients including 2 patients at a dose of 420 mg ibrutinib. The observed grade 3 or 4 AEs were anaemia (21%), fatigue (8%), elevated alkaline phosphatase (8%), rash (5%) and elevated bilirubin (5%) (Table 2). There were no drug-related Grade 5 events. Grade 3 immune-mediated AE was observed in 4 patients including rash (N = 2), elevated amylase (N = 1) and elevated aspartate aminotransferase (AST) (N = 1). Two of them received systemic steroid for rash and elevated AST, and ibrutinib and pembrolizumab were discontinued.
Table 2.
Treatment-related adverse events.
| Phase I | Phase II | Total (N = 38) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ibrutinib 420 mg + Pembrolizumab (N = 4) | Ibrutinib 560 mg + Pembrolizumab (N = 3) | Ibrutinib 560 mg + Pembrolizumab (N = 31) | ||||||
| All grades | Grades ≥ 3 | All grades | Grades ≥ 3 | All grades | Grades ≥ 3 | All grades | Grades ≥ 3 | |
| Fatigue | 1 (25%) | 1 (25%) | 2 (66.7%) | 0 | 14 (45.2%) | 2 (6.5%) | 17 (44.7%) | 3 (7.9%) |
| Anaemia | 1 (25%) | 1 (25%) | 2 (66.7%) | 1 (33.3%) | 13 (41.9%) | 6 (19.4%) | 16 (42.1%) | 8 (21.1%) |
| Diarrhoea | 1 (25%) | 0 | 0 | 0 | 11 (35.5%) | 1 (3.2%) | 12 (31.6%) | 1 (2.6%) |
| Alkaline phosphatase increased | 0 | 0 | 1 (33.3%) | 1 (33.3%) | 10 (32.3%) | 2 (6.5%) | 11 (28.9%) | 3 (7.9%) |
| Rash | 1 (25%) | 0 | 0 | 0 | 9 (29%) | 2 (6.5%) | 10 (26.3%) | 2 (5.3%) |
| Blood bilirubin increased | 0 | 0 | 1 (33.3%) | 0 | 8 (25.8%) | 2 (6.5%) | 9 (23.7%) | 2 (5.3%) |
| Alanine aminotransferase increased | 0 | 0 | 1 (33.3%) | 0 | 7 (22.6%) | 0 | 8 (21.1%) | 0 |
| Aspartate aminotransferase increased | 0 | 0 | 0 | 0 | 8 (25.8%) | 1 (3.2%) | 8 (21.1%) | 1 (2.6%) |
| Nausea | 0 | 0 | 2 (66.7%) | 0 | 6 (19.4%) | 0 | 8 (21.1%) | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 7 (22.6%) | 0 | 7 (18.4%) | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 6 (19.4%) | 0 | 6 (15.8%) | 0 |
| Anorexia | 0 | 0 | 1 (33.3%) | 0 | 4 (12.9%) | 0 | 5 (13.2%) | 0 |
| Headache | 0 | 0 | 0 | 0 | 5 (16.1%) | 0 | 5 (13.2%) | 0 |
| Arthralgia | 0 | 0 | 0 | 0 | 4 (12.9%) | 0 | 4 (10.5%) | 0 |
| Constipation | 1 (25%) | 0 | 0 | 0 | 3 (9.7%) | 0 | 4 (10.5%) | 0 |
| Lymphocyte count decreased | 1 (25%) | 1 (25%) | 1 (33.3%) | 0 | 2 (6.5%) | 0 | 4 (10.5%) | 1 (2.6%) |
| Serum amylase increased | 0 | 0 | 0 | 0 | 4 (12.9%) | 1 (3.2%) | 4 (10.5%) | 1 (2.6%) |
| Lipase increased | 0 | 0 | 0 | 0 | 3 (9.7%) | 0 | 3 (7.9%) | 0 |
| Platelet count decreased | 0 | 0 | 0 | 0 | 3 (9.7%) | 0 | 3 (7.9%) | 0 |
| QTc prolongation | 1 (25%) | 0 | 0 | 0 | 1 (3.2%) | 0 | 2 (5.3%) | 0 |
| Hypertension | 0 | 0 | 0 | 0 | 2 (6.5%) | 0 | 2 (5.3%) | 0 |
| Pruritus | 0 | 0 | 0 | 0 | 2 (6.5%) | 0 | 2 (5.3%) | 0 |
| Sinusitis | 0 | 0 | 0 | 0 | 2 (6.5%) | 0 | 2 (5.3%) | 0 |
Efficacy
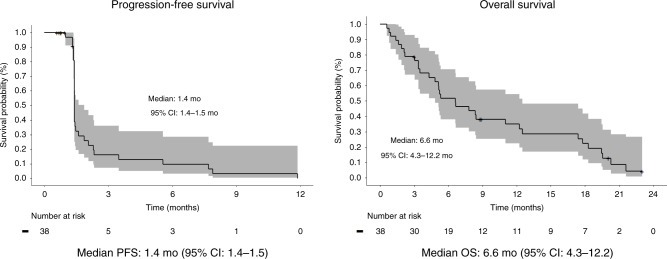
While 31 patients reached the first set of scans to evaluate disease response, 7 patients were not evaluable for tumour response due to clinical progression (N = 5) and consent withdrawal (N = 2). 8 patients (26%) achieved stable disease, and no objective response was observed (Table 3). Disease control at 4 months was achieved in 3 patients in phase 1 and 1 patient in phase 2 (Table 3). The median PFS and OS were 1.4 months (95% CI: 1.4–1.5) and 6.6 months (95% CI: 4.3–12.2) with survival rates of 52% at 6 months, 35% at 12 months and 22% at 18 months (Fig. 1).
Table 3.
Best overall response and disease control rate.
| Best overall response of evaluable patients | Phase I | Phase II | Total Evaluable (N = 31) | |
|---|---|---|---|---|
| Ibrutinib 420 mg + Pembrolizumab 200 mg (N = 3) | Ibrutinib 560 mg + Pembrolizumab 200 mg (N = 3) | Ibrutinib 560 mg + Pembrolizumab 200 mg (N = 25) | ||
| CR (%) | 0 | 0 | 0 | 0 |
| PR (%) | 0 | 0 | 0 | 0 |
| SD (%) | 2 (66.7%) | 1 (33.3%) | 5 (20%) | 8 (25.8%) |
| PD (%) | 1 (33.3%) | 2 (66.7%) | 20 (80%) | 23 (74.2%) |
| DCR (CR + PR + SD) | 2 (66.7%) | 1 (33.3%) | 5 (20%) | 8 (25.8%) |
| Primary endpoint | Ibrutinib 420 mg + Pembrolizumab 200 mg (N = 4) | Ibrutinib 560 mg + Pembrolizumab 200 mg (N = 3) | Ibrutinib 560 mg + Pembrolizumab 200 mg (N = 31) | Total treated (N = 38) |
| DCR at 4 months of treated patients | 2 (50%) | 1 (33.3%) | 1 (3.2%) | 4 (10.5%) |
CR complete response, PR partial response, PD progressive disease, SD stable disease, DCR disease control rate, evaluable patients patients’ tumour response is evaluable with scans, treated patients patients received at least 1 dose of study drugs.
Fig. 1. Kaplan–Meier survival curves.
Kaplan–Meier survival curves of progression-free survival and overall survival and Kaplan–Meier survival curves. CI confidence interval, OS overall survival, PFS progression-free survival.
Biomarkers
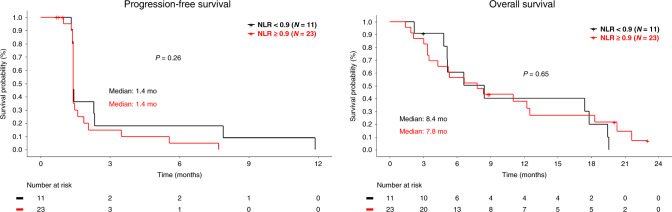
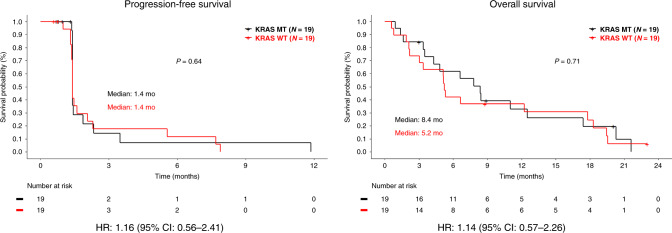
Baseline neutrophil to lymphocyte ratio (NLR), the change of NLR between baseline and on-treatment (week 3) and KRAS mutation status of each patient were obtained to identify potential prognostic values of these markers in this study. Patients were separated in two categories of NLR < 5 vs ≥5 or change of NLR between baseline and week 3 of <0.9 vs ≥0.9, as previously reported.11,12 No correlation was observed between clinical outcome and baseline NLR (Supplementary Figs. 1 and 2), change of NLR (Fig. 2) or KRAS mutations (Fig. 3).
Fig. 2. Clinical outcome by neutrophil to lymphocyte ratio change.
Kaplan–Meier survival curves of progression-free survival and overall survival by change of neutrophil to lymphocyte ratio between baseline and week 3 of <0.9 vs ≥0.9. NLR neutrophil to lymphocyte ratio.
Fig. 3. Clinical outcome by KRAS mutation status.
Kaplan–Meier survival curves of progression-free survival and overall survival by KRAS mutation status. CI confidence interval, HR hazard ratio, MT mutation, WT wild type.
Discussion
Although PD-1 blockade immunotherapy demonstrated remarkable anti-cancer activity in dMMR CRC, a majority of patients with CRC have pMMR which does not lead to a favourable response to anti-PD-1 immunotherapy. Extensive efforts have been made to enhance the anti-tumour activity of PD-1 blockade immunotherapy by conversion of immunosuppressive ‘cold tumour’ to immunogenic ‘hot tumour’ in pMMR CRC. In this study, ibrutinib was evaluated to boost anti-tumour immunity of pembrolizumab in patients with pMMR CRC based on the preclinical data demonstrating cytotoxic and immunomodulatory effects of ibrutinib in CRC. Our study did not meet the primary endpoint. The primary endpoint of phase 2 part was disease control rate at 4 months with a target of at least 4 patients with disease control among 32 patients enrolled and treated. Only one of 31 patients archived disease control over 4 months in the phase 2 portion. Despite encouraging preclinical data, the anti-cancer activity of ibrutinib combined with pembrolizumab was not clinically meaningful. The disappointing clinical outcome may be explained by the dose of ibrutinib. In this study, we evaluated 420 mg and 560 mg doses of ibrutinib based on the studies reporting that BTK occupancy ≥95% is achieved with these doses in peripheral blood mononuclear cells (PBMCs).13,14 However, a 560 mg dose of ibrutinib may not sufficiently inhibit BTK in colorectal cancer or ITK on immune cells in the tumour microenvironment. Another possible explanation is inhibition of a Tec family protein tyrosine kinase (TXK), which is expressed in T cells and acts as a Th1 cell-specific transcription factor, regulating IFN-ɣ gene expression.15 Inhibition of TXK by ibrutinib has been reported as an off-target effect,16 and ibrutinib may suppress anti-tumour immunity of pembrolizumab by inhibition of TXK and Th1 response in this study. Unfortunately, we could not confirm these possibilities with correlative studies due to insufficient tumour samples.
In our study, we did not observe any DLT at the highest tested dose of ibrutinib 560 mg daily combined with pembrolizumab 200 mg every 3 weeks. No clinically relevant safety findings were observed. Overall, 16 (42%) patients experienced a grade 3/4 AEs. There were no treatment-related deaths. The most common grade 3/4 AEs included anaemia, fatigue, increased alkaline phosphatase, rash and increased blood bilirubin. The most common adverse events of any grade included fatigue, anaemia, diarrhoea, increased alkaline phosphatase, rash and increased bilirubin. Fatigue, diarrhoea and nausea are all commonly reported with ibrutinib17 and pembrolizumab,4 so their presence with the combination therapy is not surprising. We did observe a significant number of treatment-related hepatotoxicity (21–29%) including increased aspartate transferase, alanine transferase, alkaline phosphatase and bilirubin. However, this observation is more likely due to the majority of patients in our study having liver metastases (71%) which may predispose them to develop hepatic toxic effects. Recent data demonstrated the correlation between hepatic metastasis and hepatoxicity from pembrolizumab,18 which is consistent with our finding.
Baseline neutrophil to lymphocyte ratio (NLR) and change in NLR during immunotherapy treatment have been reported as a potential predictive biomarker of immune checkpoint inhibitors in diverse cancers,11,19,20 and pre-treatment NLR and change of NLR were evaluated as potential biomarkers in this study. We also evaluated the potential prognostic value of KRAS mutations in this study since it has been reported that BTK is a potent oncoprotein acting downstream of the RAS pathway in colorectal cancer,8 and inhibition of BTK induces significant anti-cancer activity in preclinical models of KRAS mutated colorectal cancer.8 We did not observe a statistical correlation between clinical outcome and NLR or KRAS mutations in this study (Figs. 2 and 3), although this may be related to the small sample size and limited anti-cancer activity.
Interestingly, 3 patients achieved stable disease >6 months with median PFS and OS of 7.9 and 17.4 months. All 3 patients had metastatic rectal adenocarcinoma mainly involving the lung. Two of them were on regorafenib, and one received local radiation as subsequent therapy with stable disease >4 months after the trial. Unfortunately, we could not find any potential markers to explain the response in these patients and selection bias can easily explain these findings.
In conclusion, the combination of ibrutinib and pembrolizumab has an acceptable safety, which was consistent with known profiles for these agents in pMMR patients with advanced CRC. However, limited anti-cancer activity does not warrant further study of this combination.
Supplementary information
Acknowledgements
We thank all the patients who agreed to participate in this trial.
Author contributions
Study concept: R.K. Study design: D.K. and R.K. Acquisition, analysis, or interpretation of data: D.K, E.T., J.Z., M.S., M.M., J.Y, E.C. R.M., J.S., I.I. and R.K. Statistical analysis: M.S. and J.Z. Manuscript preparation: D.K. and R.K. Manuscript editing and revision: D.K, E.T., J.Z., M.S., M.M., J.Y, E.C. R.M., J.S., I.I. and R.K. Manuscript review and approval: D.K, E.T., J.Z., M.S., M.M., J.Y, E.C. R.M., J.S., I.I. and R.K.
Ethics approval and consent to participate
The protocol and informed consent forms were approved by the Institutional Review Boards at Moffitt Cancer Center (IRB# 00000790). Informed consent was obtained from all subjects prior to participating in the study. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent to participate
Not applicable.
Data availability
All authors had access to the data published in this paper. The anonymised dataset may be available from the corresponding author on reasonable request.
Competing interests
Rutika Mehta reported advisory/consulting for Taiho Oncology, Bristol Myers Squibb and Eli Lilly. Jonathan Strosberg reported consulting for Novartis. Richard Kim received an honorarium from Eli Lilly, Bristol Myers Squibb and Bayer. The remaining authors declare no competing interests.
Funding information
This work was supported by Janssen Oncology and Merck.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01368-z.
References
- 1.Van Cutsem E, Nordlinger B, Cervantes A, Group EGW. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann. Oncol. 2010;21(Suppl 5):v93–v97. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 2.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre, T., Shiu, K., Kim, T., Jensen, B. V., Jensen, L. H., Punt, C. J. A. et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J. Clin. Oncol.10.1200/JCO.2020.38.18_suppl.LBA4 (2020).
- 6.Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 7.Basile, D., Gerratana, L., Buonadonna, A., Garattini, S. K., Perin, T., Grassilli, E. et al. Role of Bruton’s tyrosine kinase in stage III colorectal cancer. Cancers11, 880 (2019). [DOI] [PMC free article] [PubMed]
- 8.Grassilli E, Pisano F, Cialdella A, Bonomo S, Missaglia C, Cerrito MG, et al. A novel oncogenic BTK isoform is overexpressed in colon cancers and required for RAS-mediated transformation. Oncogene. 2016;35:4368–4378. doi: 10.1038/onc.2015.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J. Cancer Res. Clin. Oncol. 2019;145:2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin. Lung Cancer. 2018;19:280–288.e284. doi: 10.1016/j.cllc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlett NL, Costello BA, LaPlant BR, Ansell SM, Kuruvilla JG, Reeder CB, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood. 2018;131:182–190. doi: 10.1182/blood-2017-09-804641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwakura J, Suzuki N, Nagafuchi H, Takeno M, Takeba Y, Shimoyama Y, et al. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J. Exp. Med. 1999;190:1147–1154. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglof A, Hamasy A, Meinke S, Palma M, Krstic A, Mansson R, et al. Targets for ibrutinib beyond B cell malignancies. Scand. J. Immunol. 2015;82:208–217. doi: 10.1111/sji.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah-Korang A, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment. Pharm. Ther. 2019;50:800–808. doi: 10.1111/apt.15413. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) may predict the outcomes of advanced non-small-cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs) Front. Oncol. 2020;10:654. doi: 10.3389/fonc.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All authors had access to the data published in this paper. The anonymised dataset may be available from the corresponding author on reasonable request.