Abstract
Objectives:
While preliminary evidence suggests that noninvasive vagal nerve stimulation (nVNS) may enhance cognition, to our knowledge no study has directly assessed the effects of nVNS on brain function and cognitive performance in healthy individuals. The aim of this study was therefore to assess if nVNS enhances complex visuospatial problem solving in a normative sample. Functional Magnetic Resonance Imaging (fMRI) was used to examine underlying neural substrates.
Material and Methods:
Participants received transcutaneous cervical nVNS (N=15) or sham (N=15) stimulation during a 3T fMRI scan. Stimulation lasted for 2 minutes at 24 V for nVNS and at 4.5 V for sham. Subjects completed a matrix reasoning (MR) task in the scanner and a forced-choice recognition task outside the scanner. An Analysis of Variance (ANOVA) was used to assess group differences in cognitive performance. And linear mixed effects (LME) regression analysis was used to assess main and interaction effects of experimental groups, level of MR task difficulty and recall accuracy on changes in BOLD signal.
Results:
Subjects who received nVNS showed higher accuracy for easy (p=.017) and hard (p=.013) items of the MR task, slower reaction times for hard items (p=.014) , and fewer false negative errors during the forced-choice recognition task (p=.047). MR task difficulty related to increased activation in frontoparietal regions (p < .001). No difference between nVNS and sham stimulation was found on BOLD response during performance of the MR task.
Conclusions:
We hypothesize that nVNS increased attention compared to sham, and that this effect led to enhanced executive functions, and consequently to better performance on visuospatial reasoning and recognition tasks. Results provide initial support that nVNS may be a low risk, low cost treatment for cognitive disorders.
Keywords: Cervical noninvasive vagal nerve stimulation, functional magnetic resonance imaging, cognition, attention
Introduction
Vagal nerve stimulation (VNS) is an effective treatment for several neurological and psychiatric disorders1–3. In addition to robust findings of anticonvulsant and antidepressant effects, there is evidence that VNS may also enhance cognition4–7. A promising new direction of VNS is consequently the treatment of cognitive disorders such as dementia and the rehabilitation of cognitive deficits following stroke or traumatic brain injury8–10. Traditionally, VNS is surgically implanted limiting its use and research studies to patients treated for intractable seizures or depression. The potential impact of VNS on cognition in other patient populations including healthy controls therefore remains unclear, especially in a wider clinical and mechanistic context. In recent years, however, VNS has been gaining increased popularity largely due to the development of noninvasive applications, via transcutaneous stimulation of the neck (cervical) or the ear (auricular). While it remains unclear whether improved cognitive function can also be achieved with noninvasive VNS (nVNS), whether these effects translate to healthy subjects, how long these effects last, and what the mechanism of action is, these novel devices permit low-risk study designs to address these research gaps.
Early work characterizing potential adverse effects of VNS in patients treated for depression and epilepsy focused on cognition and its potential impairment11. Interestingly, these studies reported better neuropsychological performances following the VNS implant11, which suggested cognitive enhancement was due either to VNS mediated symptom reduction or supplementary VNS pathways. While studies using sham control conditions provide support that VNS can improve cognitive functions independent of its effect on disease symptoms (see review in Vonck et al 201412), others report correlations between symptom reduction and improvements in cognition11. It is therefore crucial to reproduce and expand cognition specific findings of VNS in healthy subjects without underlying neurological or psychiatric conditions.
In recent years, there has been a growing interest in the effects of transcutaneous VNS on cognition in healthy subjects. Based on the neural pathways of nVNS action, these studies have been focusing mainly on cognitive processes that rely on prefrontal brain regions (i.e. executive function13–15) and on the hippocampus (i.e. memory function16–18) . Study findings provide initial evidence for enhanced action control15, post error slowing19, divergent thinking20, cognitive flexibility14, as well as improved memory performance10,16 in healthy adults following transcutaneous VNS. However, others did not confirm nVNS mediated improvements in cognition or reported mixed findings16,21,22.
Likewise, underlying neurobiological substrates of functional changes in cognition following nVNS remain unclear as neuroimaging studies of nVNS and cognition are lacking. A possible mechanism of action is the modulation of neurotransmitters (e.g., norepinephrine, serotonin, gamma-aminubutryic acid/GABA) and brain regions (e.g., frontal cortex and hippocampus) critical for cognitive functions23 following VNS. Foundational neuroimaging work to improve the understanding of nVNS mechanisms on healthy cognition is therefore needed and may translate into therapeutic VNS for cognitive disorders.
Matrix reasoning (MR) and forced-choice recognition are useful tasks to measure cognitive functioning that is often impaired in dementia, traumatic brain injury, stroke, as well as disorders that are treated by VNS24–28. In addition, these tests are widely used measures of complex problem solving and memory recognition, are largely dependent on executive functions, and are regulated by prefrontal and hippocampal brain regions29,30. Ameliorative effects of nVNS of these cognitive functions would have substantial clinical implications and significant potential to enhance the quality of life in these patients. In order to gain a better understanding of the impact of nVNS on cognition and associated brain activation patterns in healthy adults, the present study assessed performance on a matrix reasoning task as well as incidental learning and later recall following nVNS. In addition to assessing memory function, the forced-choice recognition task serves as a validity check to ensure subjects are engaged in the MR task during the scan. Functional Magnetic Resonance Imaging (fMRI) was used to examine potential neural substrates of nVNS and cognitive functioning. In particular, we investigated whether subjects who received transcutaneous cervical nVNS versus sham stimulation showed different brain activation patterns during performance of the MR task, and if this brain response predicted which items were later recalled.
Materials and Methods
Subjects
A total of 30 healthy, right-handed subjects (11 females and 19 males; age range 18–54 years; mean ± SD: 27 ± 8.4 years) were included in the present study (Table 1). Since this was a pilot study, no a priori power calculation was conducted. This research was a sub-study of another study31. Study visits lasted approximately 2 hours. See figure 1 for a flowchart of the study protocol and a timeline. Exclusion criteria were prior surgery or abnormal anatomy in the anterior cervical neck region, history of neurologic disease, implanted neurostimulator or cardiac pacemaker, cardiovascular disease or carotid artery disease, metallic implants that are not MRI-safe as well as Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DMS-IV) Axis I psychiatric disorders. The study was approved by the University of California San Diego, Institutional Review Board (IRB#150943), and all subjects provided written informed consent prior to participation.
Table 1.
Demographics study sample
| Sham | VNS | Total | Difference (p) | |
|---|---|---|---|---|
| Male/female (N/%) | 8 (53%)/7 (47%) | 11 (73%)/4 (27%) | 19 (63%)/11 (37%) | .225a |
| Age in yrs. mean (SD) | 30 (10.9) | 25 (3.7) | 27 (18–54) | .109c |
| Race (N/%) | .460b | |||
| Asian | 5 (33%) | 7 (47%) | 12 (40%) | |
| White | 9 (60%) | 7 (47%) | 16 (53%) | |
| Black | 1 (7%) | 0 (0%) | 1 (3%) | |
| Other | 0 (0%) | 1 (7%) | 1 (3%) | |
| BAI mean (range) | 2.1 (0–12) | 2.6 (0–13) | 2.3 (0–13) | .671c |
| BDI-2 mean (range) | 2.3 (0–14) | 3.1 (0–17) | 2.7 (0–17) | .618c |
Notes. BAI = Beck Anxiety Inventory, BDI-2 = Beck Depression Inventory. 2nd Edition,
Fishers exact test,
Pearson Chi-Square,
Two-sampled t-Test
Figure 1.
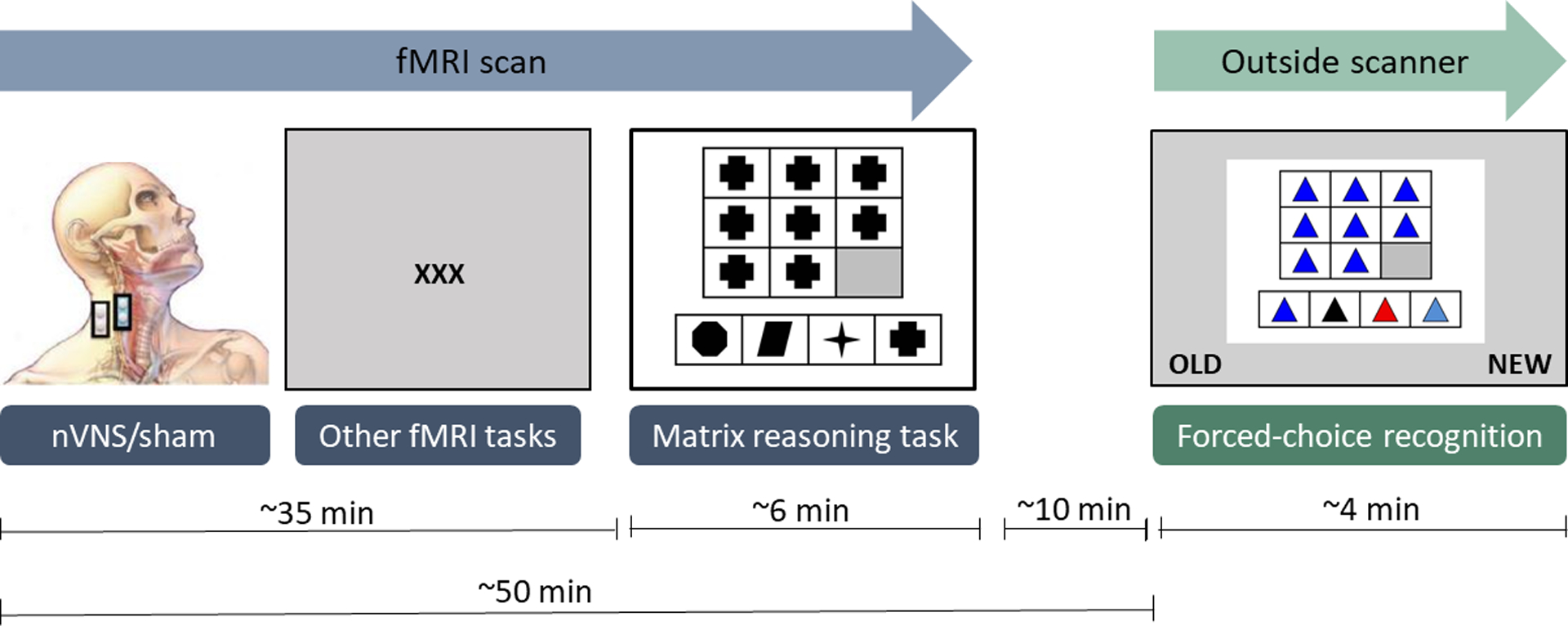
Timeline of study illustrating order and timing of stimulation and tasks. Graphic adapted with permission from Lerman et al. 2019.31
Stimulation paradigm
Subjects were randomly assigned to receive either transcutaneous cervical nVNS (n=15) or sham (n=15) stimulation during an fMRI scan. Subjects were blinded to which condition they received. Sham and nVNS devices were identical in appearance and produced similar sensations on the skin. In our previous work (pilot extension of parent study31), subjects were unable to differentiate sham from active nVNS. A pair of electrodes was placed on the neck and secured with a Velcro strap; the electrodes were placed on the right lateral cervical area for the sham condition or on the right anterior cervical area for the nVNS condition. Electrodes were 1cm in diameter and made of stainless-steel. The stimulation unit, which was connected to the electrodes via a shielded, grounded cable (6m long), was controlled by the experimenter outside the MRI magnet room.
In both conditions, stimulation lasted for 2 minutes at 5,000 Hz with a repetition rate of 25 Hz (24 V for nVNS and 4.5 V for sham). The stimulation protocol for both nVNS and sham included an initial ramp-up period of 30 seconds, followed by a peak stimulation of the remaining 90 seconds. Maximum tolerable amplitude for sham stimulation, which typically produces greater discomfort than nVNS because it stimulates the neck muscles, is typically between 2–8 V31–33. In addition, low voltage stimulation of the lateral neck area does not activate the vagus nerve31–33. Active 20 V nVNS placed over the carotid artery, on the other hand, has been shown to result in activation of the vagus nerve34. We therefore used 4.5 V applied to the lateral neck area for sham stimulation and 24 V applied to the carotid artery for nVNS stimulation. Voltage settings were the same for all subjects (depending on their condition). For more detailed information regarding the study design see Lerman et al., 201931.
Matrix reasoning and forced-choice recognition tasks
Stimulation (nVNS/sham) was carried out approximately 35 minutes prior to completing the MR task, both took place in the scanner. The MR task is comprised of 30 different matrices including geometric shapes arranged in specific patterns in a 3×3 panel (Figure 2). Fifteen shapes have one dimension of difficulty (shape, color, or orientation) and 15 shapes have two dimensions of difficulty (color and shape, orientation and color, etc.). In each matrix, the bottom right panel is left blank. Subjects were asked to complete each pattern by selecting one of four shape options and pushing the corresponding button on a handheld button box, which subsequently sent the responses to the research computer. No feedback was provided regarding response accuracy and items varied in level of difficulty (easy and hard). After a response is recorded, the task advances to the next item. If no response is detected, easy items timed out after 16.8 seconds and hard items timed out after 26 seconds. Responses were scored as error, correct, or timed-out. Subjects were not told they would be asked to recall the items later to allow assessment of group differences in incidental learning.
Figure 2.
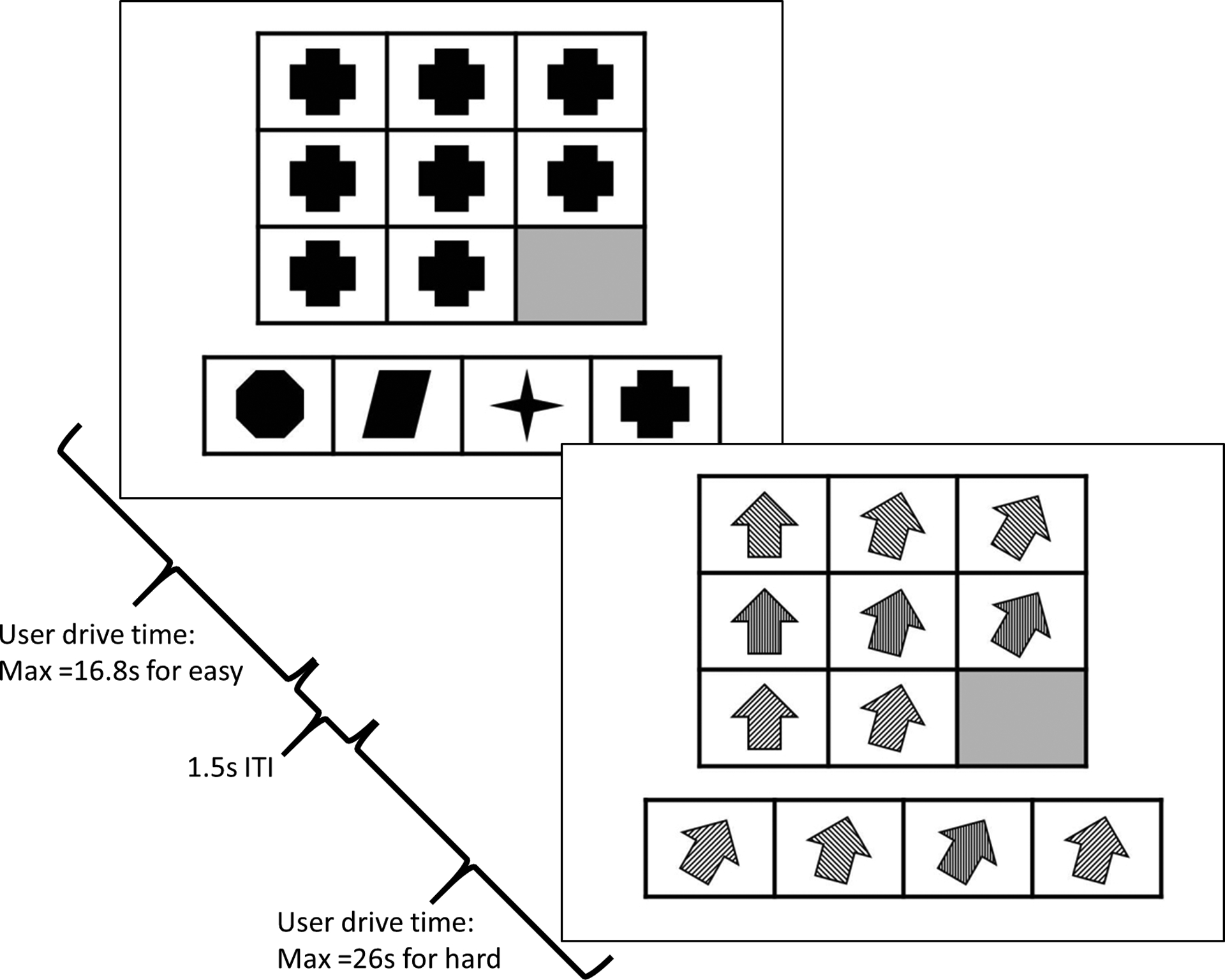
Hard and easy sample items of Matrix Reasoning task. Task would automatically advance upon selection or when time had expired (easy=16.8s, hard=26s). Task were separated by an intertrial interval (ITI) of 1.5s and fixation time was provided sporatically through the task.
Following the neuroimaging session and approximately 10 minutes after completing the MR task in the scanner, subjects participated in a forced-choice recognition task outside the scanner in a well-lit exam room. Task items were presented on a laptop and subjects were instructed to indicate which stimuli they remembered seeing during the scan by pressing one of two buttons on the laptop keyboard corresponding to “old” and “new”. Items timed out after 12 seconds. Forced-choice response items had the same appearance as MR task items and included 30 stimuli seen during the MR task (targets) and 30 new but similar stimuli (foils). The number of correctly/incorrectly recognized items as well as types of errors (false positives/ false negatives) were scored. For both tasks, subjects did not receive feedback about whether their responses were correct or incorrect.
Neuroimaging
Neuroimaging scans were acquired on a 3 Tesla General Electric Discovery MR 750 MRI scanner using an 8-channel head coil. A high resolution T1-weighted FSPGR scan sequence (172 sagittal 1mm slices, flip angle = 12°, 256 × 256 matrix, TR = 8.1 s, TE = 3.17 s, 1 × 1mm in-plane resolution) was acquired for anatomical and functional localization. T2*-weighted echo-planar images were acquired for functional analyses (flip angle = 80°, FOV = 24 cm, 64 × 64 matrix, 3.75 × 3.75 mm in plane resolution, 30 3mm (1mm gap) ascending interleaved axial slices, TR = 1.5 s, TE = 30 ms) runs averaged ~355 seconds (~237 rep; ranging from 181 to 390).
MRI preprocessing
Analyses of functional and structural MRI data was performed with Analysis of Functional NeuroImages (AFNI)35 and R statistical software. Echo planar images were corrected for slice-time and motion and aligned to anatomical scans. Using 3dToutcount, volumes with more than 20% voxels identified as outliers, were dropped from the analysis. In addition, for all groups data points 1.5% data censor were used as outliers. Using 3dDespike, outlying voxels in the time series were interpolated. Anatomical scans were aligned to Montreal Neurological Institute (MNI) atlas and functional scans were then aligned to standard space through alignment with anatomical scans (ANTsRCore::antsRegistration). Functional images were whitened to reduce effects of autocorrelation, residualized for motion regressors, and key noise components were removed (ANTsR::compcor) resampled to 4-mm isotropic voxels. Using line interpolation, hemodynamics during the MR task were modeled over time (easy and hard by correct and incorrect). Group differences in the time course blood oxygen level-dependent (BOLD) responses over the course of task completion were measured.
Statistical analyses
Differences between groups in terms of demographics and behavioral characteristics, as well as performance on the MR task independent of difficulty level were assessed with two-sample t-tests. Further analysis of MR task performance was included as reaction time and accuracy for easy items and for hard items, respectively. Reaction time was calculated as the sum of (number correct * reaction time correct) and (number errors * reaction time errors) divided by the sum of number correct and number incorrect. Accuracy was calculated as total correct divided by the sum of total correct and total errors. An Analysis of Variance (ANOVA) was used to assess differences in accuracy and reaction times for easy and hard MR items between groups. In addition, an ANOVA was used to assess group differences for false positives and false negatives as well as correctly identified and correctly rejected items on the recognition task.
A linear mixed effects (LME) regression analysis using AFNI’s 3dLME was used to assess main and interaction effects of experimental groups (nVNS/sham), level of MR task difficulty (easy and hard) and recall accuracy on changes in BOLD signal. Multi-voxel multiple comparisons were performed using Monte Carlo simulations (3dClustSim [bug fixed] with 3-parameter noise modeling) in order to reduce potential false positives. To achieve cluster-wise threshold of p<.05 a per-voxel thresholds of p < .001 were set along with a minimum of 14 voxels per cluster. Statistical analyses were performed with R version 3.4.3 and SPSS version 26.
Results
Subject characteristics
Subjects were healthy men and women with an average age of 27 years (±8.4 years). The nVNS and sham groups did not differ significantly in terms of age, race, or sex. Subjects did not report elevated levels of depression (BDI-II mean (SD): 2.7 (3.9)) or anxiety (BAI: 2.3 (3.4)) and there was no significant difference between groups. See Table 1 for demographics.
Cognitive performance
Analyses showed that during the MR task in the scanner, the nVNS group solved overall significantly more MR items correctly (26.36 +/− 1.3) compared to the sham group (23.08 +/− 3.9), t(13)=2.786, p=0.015, regardless of item difficulty. More specifically, subjects who received nVNS showed higher accuracy for both easy (F(1,24) = 6.6, p=.017) and hard (F(1,24) = 7.3, p=.013) MR items. In addition, the nVNS group showed slower reaction times for solving hard MR items (F(1,24) = 6.9, p=.014). The group difference did not reach statistical significance for easy MR items (F(1,24) = 4.2 p=.052) (Table 2).
Table 2.
Group differences during forced-choice recognition (FC) and Matrix Reasoning (MR) task
| Variable | Sham | nVNS | F-Stat | P-value |
|---|---|---|---|---|
| True negative, mean (SD) | 22.7 (2.0) | 23.9 (1.3) | 3.735 | 0.064 |
| True positive, mean (SD) | 23.5 (3.9) | 23.2 (2.7) | 0.058 | 0.812 |
| FC false negative, mean (SD) | 7.3 (2.0) | 6.0 (1.3) | 4.318 | 0.047 |
| FC false positive, mean (SD) | 6.5 (4.0) | 6.6 (2.7) | 0.006 | 0.937 |
| MR items correct, mean (SD) | 23.1 (3.9) | 26.4 (1.3) | 10.763 | 0.015 |
| MR easy accuracy, % mean (SD) | 88.3 (8.3) | 94.6 (3.7) | 6.569 | 0.017 |
| MR hard accuracy, % mean (SD) | 54.2 (25.7) | 74.3 (10.2) | 7.270 | 0.013 |
| MR easy reaction time in sec., mean (SD) | 7.1 (0.7) | 7.62 (0.7) | 4.191 | 0.052 |
| MR hard reaction time in sec., mean (SD) | 7.9 (1.9) | 9.8 (1.9) | 6.987 | 0.014 |
Notes. Recall errors during forced-choice recognition and accuracy and reaction times during MR task performance. nVNS = noninvasive vagal nerve stimulation.
During the post-scan forced-choice recognition task, the nVNS group correctly rejected more items than the sham group. However, this difference did not reach statistical significance (F(1,27) = 3.735, p=0.064). However, there was a significant group difference in error type. The nVNS group showed significantly fewer false negatives than the sham group (F(1,27)=4.3, p=.047; Table 2), i.e. incorrectly indicating they had not seen an item, which they had seen previously. There was no group difference in false positive rates (F(1,27)=0.006, p=0.937; Table 2), i.e. incorrectly indicating they had not seen an item that was previously seen.
Neuroimaging
A main effect for task activation for easy items is shown in Figure 3. Analyses showed a main effect for MR task difficulty (Table 3) and a main effect for recall accuracy (Table 4) on BOLD response. In particular, harder items were linked to increased BOLD signal in frontoparietal regions, with the largest clusters found in the left postcentral, right superior frontal, and left cingulate gyri (p < .001; Table 3). In addition, post hoc comparison of the BOLD signal during the MR task and items that were later remembered versus forgotten, revealed a main effect for recall accuracy during the post scan recognition task. Increased activation patterns in more visual processing areas, with the largest clusters in the precuneus, lingual and middle occipital gyri (p < .002), during performance of the MR task predicted recall accuracy during the forced-choice task, i.e. correctly recognized items (Table 4). There were no main or interaction effects of group on BOLD response, indicating that nVNS did not significantly affect brain activation during performance on the MR task.
Figure 3.
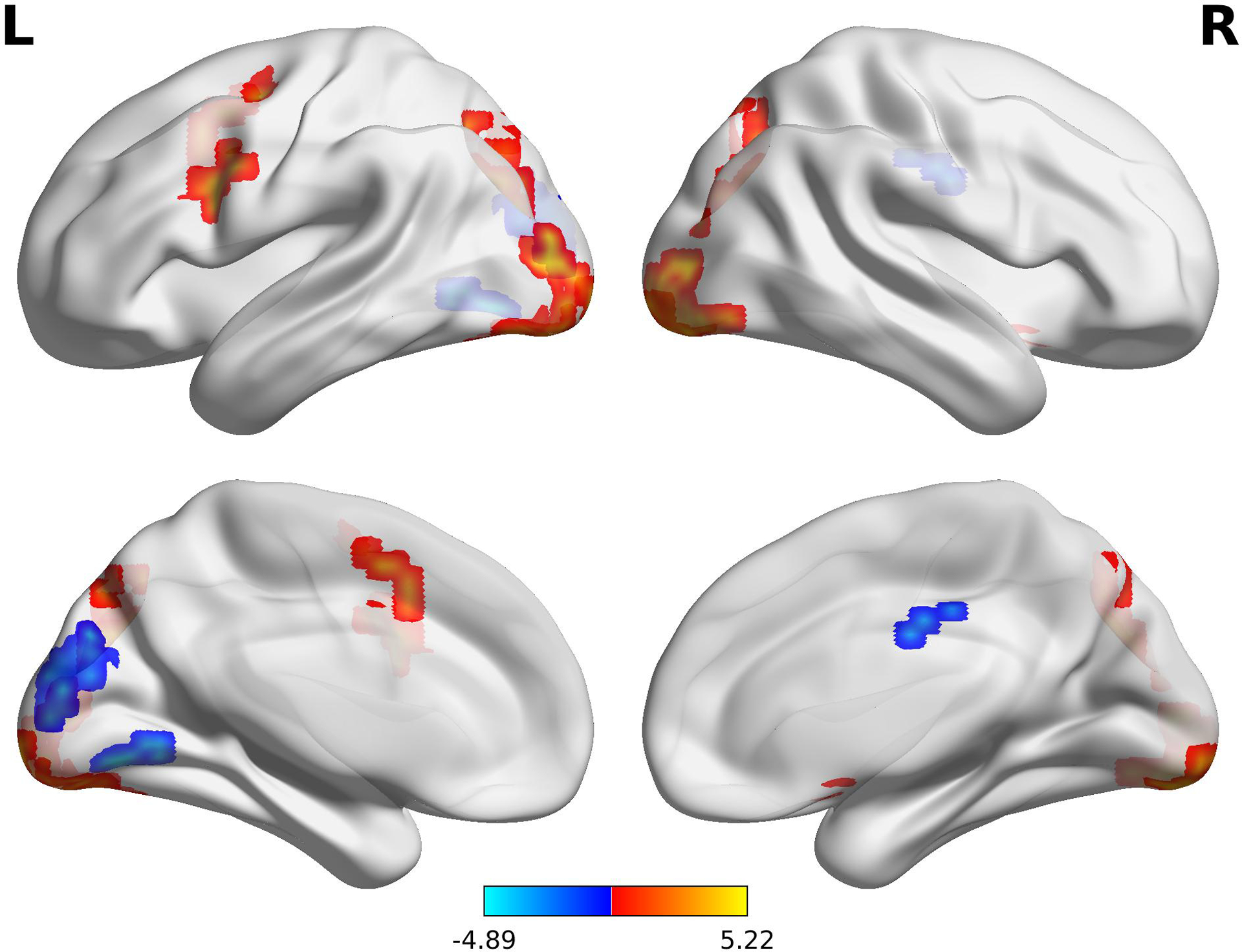
Lateral view of the brain showing main effect for task activation for easy items. L = left hemisphere, R = right hemisphere. Color bar represents t statistic.
Table 3.
Main effect of item difficulty level on BOLD response
| voxels | X | Y | Z | F-Stat | Region |
|---|---|---|---|---|---|
| 175 | −42 | −25 | 49 | 17.859 | Left Postcentral Gyrus (BA 2) |
| 60 | 13 | 49 | 27 | 15.108 | Right Superior Frontal Gyrus (BA 9) |
| 48 | −5 | −87 | −8 | 14.883 | Left Lingual Gyrus (BA 18) |
| 36 | −2 | −19 | 41 | 15.491 | Left Cingulate Gyrus (BA 24) |
| 21 | 25 | −53 | −27 | 15.516 | Right Culmen |
| 11 | 10 | −89 | 8 | 17.616 | Right Cuneus (BA 17) |
| 8 | 57 | −47 | 9 | 14.714 | Right Superior Temporal Gyrus (BA 21) |
| 7 | 5 | 27 | 56 | 14.033 | Right Superior Frontal Gyrus (BA 8) |
| 7 | −37 | 4 | 59 | 15.669 | Left Middle Frontal Gyrus (BA 6) |
Notes. ROIs with significant change in BOLD signal. BA=Brodmann’s Area
Table 4.
Main effect of recognition accuracy on BOLD response
| voxels | X | Y | Z | F-Stat | Region |
|---|---|---|---|---|---|
| 41 | −23 | −70 | 33 | 15.387 | Left Precuneus (BA 7) |
| 35 | −19 | −94 | −4 | 16.045 | Left Lingual Gyrus (BA 17) |
| 34 | 24 | −64 | 39 | 14.909 | Right Precuneus (BA 7) |
| 24 | 26 | −92 | −2 | 18.590 | Right Middle Occipital Gyrus (BA 18) |
| 14 | −3 | 7 | 47 | 14.092 | Left Cingulate Gyrus (BA 32) |
| 11 | 2 | −82 | 32 | 15.947 | Right Cuneus (BA 19) |
| 8 | −40 | −2 | 30 | 18.318 | Left Precentral Gyrus (BA 6) |
| 7 | −23 | −7 | 49 | 13.109 | Left Middle Frontal Gyrus (BA 6) |
Notes. ROIs with significant change in BOLD signal. BA=Brodmann’s Area
Discussion
The aim of this study was to assess whether nVNS improves cognition in healthy adults, which was measured with a complex visuospatial problem-solving task followed by a test of forced-choice recognition of the visual stimuli. In addition, we examined whether nVNS affects brain activation patterns during performance of the problem-solving task. The key findings of this study suggest the existence of cognitive effects following cervical nVNS compared to sham stimulation in the context of no significant difference in brain activation patterns during performance of a reasoning task between groups. Specifically, nVNS was linked to better overall performance on the MR task, including higher accuracy for both easy and hard items and slower reaction times for hard items of the MR task. The nVNS group also made fewer false negative errors during later recognition of the stimuli.
Neuroimaging findings were two-fold. Firstly, we observed that MR item difficulty was linked to incremental changes in BOLD signal in regions implicated in spatial cognition, working memory, conflict anticipation, and response inhibition36,37. Secondly, we observed that later recognition accuracy was linked to change in BOLD signal during the MR task. Items that were later correctly recognized were linked to increased activation during performance of the MR task in regions involved in visuospatial processing, visual attention, and encoding of complex visual images38,39. Together, fMRI findings suggest that solving more complex matrices required increased activation in brain regions that are involved in abstract thinking, and that those items that were visually inspected in more detail during the MR task, were subsequently better encoded, predicting recall accuracy during post-scan forced-choice recognition. Brain activation patterns were not affected by nVNS compared to sham stimulation.
Effect of nVNS on cognitive performance
The present study shows that nVNS has a beneficial effect on cognitive performance. Compared to sham stimulation, subjects who received nVNS spent more time on hard MR items, which was linked to higher accuracy. In addition, nVNS led to fewer false negative errors during later recognition of items, i.e. subjects less often failed to recognize items which they had previously seen. Based on these behavioral observations, we hypothesize that nVNS increased alertness and improved attention, which led to more engagement in the tasks and consequently enhanced higher order executive processes, such as visuospatial problem solving. It is possible that this effect lasted for at least 50 minutes following stimulation and led to more efficient recognition and less errors compared to sham. However, it is also possible that increased attention during the MR task led to better encoding of the items, and consequently to less misses during the recognition. Further research is necessary to disentangle the exact cognitive functions that may be enhanced by nVNS and the duration of these effects.
These findings are in line with previous research reporting enhanced visual attention40 response inhibition11,41, working memory40, as well as increased daytime alertness42 in patients with surgically implanted VNS. More recent studies provide support that these effects can also be achieved with noninvasive VNS, including improved attention43, response selection15, cognitive flexibility14, and enhanced response inhibition13. It is important to point out that the majority of noninvasive VNS studies use auricular VNS. To our knowledge, the present study is the first to suggest that cervical noninvasive VNS can improve performance on visuospatial problem solving and recognition tasks by enhancing alertness and attention.
Potential mechanisms of cognition enhancing effects following nVNS
Previous research has established a link between alertness and attention and improved cognition (i.e., executive functions and memory) via activation of brainstem structures (locus coeruleus) by the autonomic nervous system43,44. This response typically involves threatening or salient stimuli in the environment, that trigger the release of several neurotransmitters with noradrenaline (NE) playing a central role. NE levels can also be increased experimentally with VNS in the prefrontal cortex and hippocampus, as well as other brain regions45–47. Vagal fibers send mostly sensory information from the autonomic nervous system to the central nervous system. Its fibers project to the locus coeruleus (and other brainstem nuclei), which is the main production and release site of NE known to activate a number of cortical and subcortical brain regions critical for attention, executive function, and memory, including the prefrontal cortex, amygdala, and hippocampus10,12.
In addition to increased levels of NE in the prefrontal cortex, nVNS raises GABA levels48. Both NE and GABA are neurotransmitter that play a critical role in attention and executive functions, such as conflict monitoring, response selection, and response inhibition13,48–51. It is consequently likely that the increase in these neurotransmitters following nVNS, leads to enhanced attention and executive functions, which results in a more focused mind and better cognitive performance that is characterized by less errors, better reasoning, and higher response accuracy. However, it is important to point out that since NE and GABA levels were not measured in the present study, the mechanism proposed to underlie the observed cognitive effects cannot be confirmed based on these results.
Limitations and future directions
Several methodological limitations and future directions should be considered when drawing conclusions regarding potential effects of nVNS on cognition, especially since the number of studies in this field is still limited. For instance, it should be pointed out that the absolute difference in accuracy for MR items was relatively small albeit statistically significant. Moreover, caution is needed when interpreting slower reaction times during the MR task as a direct effect of nVNS. An alternative explanation could be that the nVNS group consisted of more “contemplating” individuals, resulting in higher accuracy. Another important consideration is that performance on the MR is associated with intelligence. However, level of education or estimate of intelligence were not obtained in this study and were therefore not controlled for.
Furthermore, due to differing stimulation parameters between studies, it is unclear which settings are ideal to accomplish optimal outcomes of cognitive improvement. Early reports showing increased memory function in patients treated with VNS for epilepsy suggest that the intensity of the stimulation plays a key factor as lower intensities improved word recognition while higher intensities were found to have a negative effect on memory5,12. In addition, stimulation parameters and intensities differ due to different stimulation sites, i.e. invasively with a cuff electrode on the vagus nerve, or noninvasively by sending electrical currents through the skin (neck/cervical or ear/auricular), limiting generalizability of findings. Other design considerations include adding a third, no stimulation control group in order to ensure sham stimulation does not have any effects on outcome measures. Systematic research will require normalization of stimulation parameters such as intensity as well as experimental setup (stimulation prior to or following learning or stimulus presentation for memory tasks, repeated stimulation, duration of stimulation) before nVNS can be established as a treatment for cognitive disorders.
Other study design considerations with regards to the recognition task include that beneficial effects of VNS may be limited to different stages of memory function, such as encoding, storage, recall, recognition, or different types of information (i.e., verbal versus non-verbal, emotional versus neutral, implicit versus explicit). In the context of the recognition task in the present study, it is therefore important to point out that only effects of nVNS on incidental learning were assessed as subjects were not instructed to learn or try to remember the stimuli presented during the scan. Optimal timing of vagus nerve stimulation during the learning task is likely critical to eventual discrimination of short and long-term learning effects. For instance, it is possible that nVNS only improves memory function if it is administered immediately prior to stimuli presentation to affect encoding. Instead, increased attention may be sustained following nVNS, leading to better focus during recognition. Noise of retrieval false negatives may consequently affect the non-focused mind more than the focused mind. An alternative explanation for the observed cognitive effects could also be that less errors during recognition and higher accuracy during the MR task was due to subjects in the nVNS group spending more time on MR items as opposed to nVNS per se.
Limitations of the present study include the moderate sample size, demographic differences between the groups, and the cross-sectional versus cross-over design. It is important to point out that, despite randomization, the female to male ratio as well as the mean age of the two groups were different. Although this difference did not reach statistical significance, it is possible that these differences were not detected due to the moderate sample size and consequently limited power. When interpreting cognitive performance and brain activation patterns following nVNS versus sham stimulation, it is therefore relevant to consider these group characteristics. For instance, males tend to show superior visuospatial functioning52,53, which may be due to sex differences in hemispheric laterality during visuospatial tasks54,55. In addition, cognitive processing slows with increasing age56,57. However, since only two subjects in the sham group were 54 years old and slower reaction times were found in the nVNS group and slower reaction time was linked to higher accuracy, it is unlikely that age played a significant role in the present findings.
Further limitations of the present study include that cognitive effects were measured only twice within approximately one hour and cannot be generalized to long-term effects. In addition, cognitive differences between groups were relatively small, however statistically significant. It is also important to stress that the present study did not use a comprehensive neuropsychological assessment battery to characterize the effect of nVNS on a broad number of cognitive functions. For instance, administration of a free recall memory task would have added valuable information regarding the effect of nVNS on memory function. Limitations of the study design include that subjects did not participate in an fMRI scan during the recognition task, which could have provided important information regarding activation patterns indicative of different recall strategies, or recruitment of different brain regions or mechanisms. Further research is needed to address these methodological considerations and to evaluate whether VNS has only short-term effects or whether long-term improvements in cognition can be achieved, and whether the effects translate to clinical populations.
Conclusion
Taken together, nVNS was linked to better performance on visuospatial reasoning and memory recognition tasks than sham stimulation, which was likely mediated by enhanced attention. However, these are proof-of-principle findings and further research is needed before nVNS can be implemented in clinical practice. This is the first study investigating the effect of cervical nVNS on cognition in young, healthy subjects and results provide initial support that nVNS may be a low risk, low cost device with the capability of improving cognitive function. It cannot be concluded whether nVNS has ameliorative effects in individuals with cognitive deficits. Further studies with larger cohorts, patient populations, and study designs considering the above discussed stimulation parameters are warranted. Although the present study included healthy adults to address the above described research gap, replicating the current design with a cohort with cognitive impairments may yield more detectable improvement in cognitive function as the present cohort showed a ceiling effect. Therapeutic use of noninvasive neuromodulation opens a novel field of research, which has the potential for important clinical implications for the treatment and management of cognitive disorders due to stroke, traumatic brain injury, or dementia.
Supplementary Material
Sources of financial support:
This work was funded by the VA San Diego Center of Excellence for Stress and Mental Health (CESAMH). Ruth Klaming is supported by a National Institute of Alcohol Abuse and Alcoholism T32 fellowship [T32 AA013525]. Alan Simmons is supported by a VA Clinical Science Research and Development Merit Award [I01 CX001542]. Andrea Spadoni is supported by a VA Clinical Science Research and Development Merit Award [I01 CX001762]. Imanuel Lerman is supported by a VA Rehabilitation Research and Development CDA Award [1IK2RX002920-01A1].
Footnotes
Conflict of interest: The authors declare no competing financial interests.
References
- 1.Ben-Menachem E Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 2002;1(8):477–482. doi: 10.1016/S1474-4422(02)00220-X [DOI] [PubMed] [Google Scholar]
- 2.Silberstein SD, Calhoun AH, Lipton RB, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 2016;87(5):529–538.dDoi: 10.1212/WNL.0000000000002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry 2005;58(5):364–373. doi: 10.1016/j.biopsych.2005.07.028 [DOI] [PubMed] [Google Scholar]
- 4.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cogn Behav Neurol 2006;19(3):119–122. doi: 10.1097/01.wnn.0000213908.34278.7d [DOI] [PubMed] [Google Scholar]
- 5.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999;2(1):94–98. [DOI] [PubMed] [Google Scholar]
- 6.Liu A-f, Zhao F-b, Wang J, et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J Transl Med 2016;14(1):101. doi: 10.1186/s12967-016-0858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broncel A, Bocian R, Kłos-Wojtczak P, Kulbat-Warycha K, Konopacki J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull 2019. doi: 10.1016/j.brainresbull.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Qiao P, Li Q, et al. Vagus nerve stimulation as a promising adjunctive treatment for ischemic stroke. Neurochem Int 2019:104539. doi: 10.1016/j.neuint.2019.104539 [DOI] [PubMed] [Google Scholar]
- 9.Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care 2016;24(2):308–319. doi: 10.1007/s12028-015-0203-0 [DOI] [PubMed] [Google Scholar]
- 10.Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging 2015;36(5):1860–1867. doi: 10.1016/j.neurobiolaging.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 11.Sackeim HA, Keilp JG, Rush AJ, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Cogn Behav Neurol 2001;14(1):53–62. [PubMed] [Google Scholar]
- 12.Vonck K, Raedt R, Naulaerts J, et al. Vagus nerve stimulation… 25 years later! What do we know about the effects on cognition? Neurosci Biobehav Rev.2014;45:63–71. doi: 10.1016/j.neubiorev.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Beste C, Steenbergen L, Sellaro R, et al. Effects of concomitant stimulation of the GABAergic and norepinephrine system on inhibitory control–a study using transcutaneous vagus nerve stimulation. Brain Stimulation. 2016;9(6):811–818. doi: 10.1016/j.brs.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Borges U, Knops L, Laborde S, Klatt S, Raab M. Transcutaneous Vagus Nerve Stimulation May Enhance Only Specific Aspects of the Core Executive Functions. A Randomized Crossover Trial. Frontiers in Neuroscience. 2020;14. doi: 10.3389/fnins.2020.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongkees BJ, Immink MA, Finisguerra A, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during sequential action. Front Psychol 2018;9:1159. doi: 10.3389/fpsyg.2018.01159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giraudier M, Ventura-Bort C, Weymar M. Transcutaneous Vagus Nerve Stimulation (tVNS) Improves High-Confidence Recognition Memory but Not Emotional Word Processing. Frontiers in Psychology. 2020;11. doi: 10.3389/fpsyg.2020.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens A, Naert L, Miatton M, et al. Transcutaneous Vagus Nerve Stimulation Does Not Affect Verbal Memory Performance in Healthy Volunteers. Frontiers in Psychology. 2020;11. doi: 10.3389/fpsyg.2020.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen N Memory Reinforcement and Attenuation by Activating the Human Locus Coeruleus via Transcutaneous Vagus Nerve Stimulation. Frontiers in Neuroscience. 2019;12:955. doi: 10.3389/fnins.2018.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellaro R, van Leusden JW, Tona K-D, Verkuil B, Nieuwenhuis S, Colzato LS. Transcutaneous vagus nerve stimulation enhances post-error slowing. Journal of cognitive neuroscience. 2015;27(11):2126–2132. doi: 10.1162/jocn_a_00851 [DOI] [PubMed] [Google Scholar]
- 20.Colzato LS, Ritter SM, Steenbergen L. Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia. 2018;111:72–76. doi 10.1016/j.neuropsychologia.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Tona K-D, Revers H, Verkuil B, Nieuwenhuis S. Noradrenergic Regulation of Cognitive Flexibility: No Effects of Stress, Transcutaneous Vagus Nerve Stimulation, and Atomoxetine on Task-switching in Humans. Journal of Cognitive Neuroscience. 2020:1–15. doi: 10.1162/jocn_a_01603 [DOI] [PubMed] [Google Scholar]
- 22.Verkuil B, Burger AM. Transcutaneous vagus nerve stimulation does not affect attention to fearful faces in high worriers. Behaviour research and therapy. 2019;113:25–31. doi: 10.1016/j.brat.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part II. Headache: The Journal of Head and Face Pain 2016;56(2):259–266. doi: 10.1111/head.12650 [DOI] [PubMed] [Google Scholar]
- 24.Ryan JJ, Carruthers CA, Miller LJ, et al. The WASI matrix reasoning subtest: performance in traumatic brain injury, stroke, and dementia. Int J Neurosci 2005;115(1):129–136. doi: 10.1080/00207450490512704 [DOI] [PubMed] [Google Scholar]
- 25.Stewart KJ, Gale SD, Diamond PT. Early assessment of post-stroke patients entering acute inpatient rehabilitation: utility of the WASI and HVLT-R. Am J Phys Med Rehabil 2002;81(3):223–228. doi: 10.1097/00002060-200203000-00011 [DOI] [PubMed] [Google Scholar]
- 26.Spencer RJ, Reckow J, Drag LL, Bieliauskas LA. Incidental learning: A brief, valid measure of memory based on the WAIS–IV vocabulary and similarities subtests. Cogn Behav Neurol 2016;29(4):206–211. doi: 10.1097/WNN.0000000000000108 [DOI] [PubMed] [Google Scholar]
- 27.Tierney MC, Black SE, Szalai JP, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol 2001;58(10):1654–1659. doi: 10.1001/archneur.58.10.1654 [DOI] [PubMed] [Google Scholar]
- 28.Narayanan J, Duncan R, Greene J, et al. Accelerated long-term forgetting in temporal lobe epilepsy: Verbal, nonverbal and autobiographical memory. Epilepsy Behav 2012;25(4):622–630. doi: 10.1016/j.yebeh.2012.06.038 [DOI] [PubMed] [Google Scholar]
- 29.Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. cortex. 2013;49(5):1195–1205. doi: 10.1016/j.cortex.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichenbaum H Prefrontal–hippocampal interactions in episodic memory. Nature Reviews Neuroscience. 2017;18(9):547–558. [DOI] [PubMed] [Google Scholar]
- 31.Lerman I, Davis B, Huang M, et al. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PloS One 2019;14(2). doi: 10.1371/journal.pone.0201212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borckardt JJ, Anderson B, Andrew Kozel F, et al. Acute and long-term VNS effects on pain perception in a case of treatment-resistant depression. Neurocase. 2006;12(4):216–220. doi: 10.1080/13554790600788094 [DOI] [PubMed] [Google Scholar]
- 33.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain stimulation. 2017;10(1):19–27. doi: 10.1016/j.brs.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 34.Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High‐resolution multi‐scale computational model for non‐invasive cervical vagus nerve stimulation. Neuromodulation: Technology at the Neural Interface. 2018;21(3):261–268. doi: 10.1111/ner.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 36.Fd Boisgueheneuc, Levy R, Volle E, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain 2006;129(12):3315–3328. doi: 10.1093/brain/awl244 [DOI] [PubMed] [Google Scholar]
- 37.Hu S, Ide JS, Zhang S, Chiang-shan RL. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J Neurosci 2016;36(50):12688–12696. doi: 10.1523/JNEUROSCI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP. FMRI of visual encoding: reproducibility of activation. Hum Brain Mapp 2000;9(3):156–164. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangun GR, Buonocore MH, Girelli M, Jha AP. ERP and fMRI measures of visual spatial selective attention. Hum Brain Mapp 1998;6(5‐6):383–389. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L, Peräkylä J, Holm K, et al. Vagus nerve stimulation improves working memory performance. J Clin Exp Neuropsychol 2017;39(10):954–964. doi: 10.1080/13803395.2017.1285869 [DOI] [PubMed] [Google Scholar]
- 41.Schevernels H, van Bochove ME, De Taeye L, et al. The effect of vagus nerve stimulation on response inhibition. Epilepsy & Behavior. 2016;64:171–179. doi: 10.1016/j.yebeh.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 42.Rizzo P, Beelke M, De Carli F, et al. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep 2003;26(5):607–611. Doi: 10.1093/sleep/26.5.607 [DOI] [PubMed] [Google Scholar]
- 43.Rufener KS, Geyer U, Janitzky K, Heinze HJ, Zaehle T. Modulating auditory selective attention by non‐invasive brain stimulation: Differential effects of transcutaneous vagal nerve stimulation and transcranial random noise stimulation. European Journal of Neuroscience. 2018;48(6):2301–2309. doi: 10.1111/ejn.14128 [DOI] [PubMed] [Google Scholar]
- 44.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 2012;76(1):130–141. doi: 10.1016/j.neuron.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 45.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain research. 2006;1119(1):124–132. doi: 10.1016/j.brainres.2006.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045 [DOI] [PubMed] [Google Scholar]
- 47.Hassert D, Miyashita T, Williams C. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 2004;118(1):79. doi: 10.1037/0735-7044.118.1.79 [DOI] [PubMed] [Google Scholar]
- 48.Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy research. 1995;20(3):221–227. doi: 10.1016/0920-1211(94)00083-9 [DOI] [PubMed] [Google Scholar]
- 49.Chamberlain SR, Del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biological psychiatry. 2007;62(9):977–984. doi: 10.1016/j.biopsych.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 50.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199(3):439–456. [DOI] [PubMed] [Google Scholar]
- 51.van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clinical EEG and neuroscience. 2006;37(4):330–335. doi: 10.1177/155005940603700411 [DOI] [PubMed] [Google Scholar]
- 52.Agneta H, Johanna L. Sex differences in cognitive functions. Acta Psychologica Sinica. 2009;41(11):1081–1090. [Google Scholar]
- 53.Castro-Alonso JC, Jansen P. Sex differences in visuospatial processing. In: Visuospatial processing for education in health and natural sciences. Springer; 2019:81–110. doi: 10.1007/978-3-030-20969-8_4 [DOI] [Google Scholar]
- 54.Clements A, Rimrodt S, Abel J, et al. Sex differences in cerebral laterality of language and visuospatial processing. Brain and language. 2006;98(2):150–158. doi: 10.1016/j.bandl.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 55.Gur RC, Alsop D, Glahn D, et al. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain and language. 2000;74(2):157–170. doi: 10.1006/brln.2000.2325 [DOI] [PubMed] [Google Scholar]
- 56.Kerchner GA, Racine CA, Hale S, et al. Cognitive processing speed in older adults: relationship with white matter integrity. PloS one. 2012;7(11):e50425. doi: 10.1371/journal.pone.0050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Frontiers in neuroscience. 2011;5:25. doi: 10.3389/fnins.2011.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


