Abstract
D-pantothenic acid (D-PA), as a crucial vitamin, is widely used in food, animal feed, cosmetics, and pharmaceutical industries. In our previous work, recombinant Escherichia coli W3110 for production of D-PA was constructed through metabolic pathway modification. In this study, to enhance D-PA production, statistical optimization techniques including Plackett–Burman (PB) design and Box-Behnken design (BBD) first were adopted to optimize the culture condition. The results showed that the glucose, β-alanine and (NH4)2SO4 have the most significant effects on D-PA biosynthesis. The response surface model based on BBD predicted that the optimal concentration is glucose 56.0 g/L, β-alanine 2.25 g/L and (NH4)2SO4 11.8 g/L, the D-PA titer increases from 3.2 g/L to 6.73 g/L shake flask fermentation. For the fed-batch fermentation in 5 L fermenter, the isoleucine feeding strategy greatly increased the titer and productivity of D-PA. As a result, titer (31.6 g/L) and productivity (13.2 g/L·d) of D-PA were achieved, they increased by 4.66 times and 2.65 times, respectively, compared with batch culture. At the same time, the accumulation of acetate reduced from 29.79 g/L to 8.55 g/L in the fed-batch fermentation. These results demonstrated that the optimization of medium composition and the cell growth rate are important to increase the concentration of D-PA for microbial fermentation. This work laid the foundation for further research on the application of D-PA microbial synthesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02773-0.
Keywords: D-pantothenic acidm, Escherichia coli, Fed-batch fermentation, Optimization
Introduction
D-pantothenic acid (D-PA) is an essential precursor for the biosynthesis of an acyl carrier protein (ACP) and coenzyme A (CoA), and can maintain the normal physiological function of the organism (Haughey et al. 2012; Yao et al. 2018). D-PA is catalyzed by pantothenate kinase and pyruvate dehydrogenase to convert into acetyl-CoA, which provides an important substrate for the TCA cycle of all cells (Xu et al. 2020). It also plays a central role in the acetylation of histones and the anabolic and catabolic pathways of many substances such as carbohydrates and fatty acids (Xu et al. 2020). As an essential vitamin for the human body, D-PA is widely used in food, animal feed, chemical, and pharmaceutical industries (Haughey et al. 2012; Yao et al. 2018)
To date, the commercial production of D-PA mainly relies on chemical or chemical-enzymatic synthesis (Bozell and Petersen 2010; Liu and Sun 2004; Liu et al. 2006, 2018). However, these routes for D-PA synthesis requires the use of nonrenewable and highly toxic feedstocks, such as hydrocyanic acid and sodium cyanide, and often lead to serious environmental pollution (Eggersdorfer et al. 2012). In addition, the optical resolution of chiral molecules involved in chemical synthesis also have a negative impact on the large-scale production of D-PA. Therefore, it is urgent to develop an environmentally friendly and sustainable method to synthesize D-PA. Microbial fermentation to directly synthesize D-PA from renewable resources has broad prospects for the industrial-scale production of D-PA, and has attracted more and more attention in recent years.
Recently, modifying the metabolic pathways of microorganisms by metabolic engineering makes it possible to synthesize D-PA from microorganisms, such as Escherichia coli or Corynebacterium glutamicum (Elischewski et al. 1999; Huser et al. 2005; Sahm and Eggeling 1999; Vyazmensky et al. 2009; Zhang et al. 2019). Although various efforts have been made to construct microbial cell factories of D-PA, there are still many challenges in biosynthesis of D-PA that need to be overcome, such as low yield of D-PA and industrial application has not yet been developed (Chen et al. 2012; Jing et al. 2018; Long et al. 2018; Wang et al. 2015; Zhou et al. 2019; Zhu et al. 2015). The main reason is that its complex regulatory network and critical importance in many cellular reactions, making it is difficult to obtain a large amount of D-PA production from natural microorganisms (Webb et al. 2004). Therefore, in order to strengthen the accumulation of D-PA, it is necessary to first adopt reasonable metabolic engineering strategies to improve its metabolic pathways and regulate feedback inhibition to establish new D-PA producers. In previous reports, a series of D-PA producing strains (E. coli W3110 derivatives) were constructed based on the comprehensive analysis of the limiting factors of D-PA biosynthesis. And the accumulation of D-PA was significantly improved (Zhang et al. 2019).
Apart from the strain improvement, optimization of culture medium and fermentation conditions is essential to enhance the output of the target product (Xu et al. 2018). The optimization methods of recombinant E. coli culture medium mainly include single factor experiment, Plackett–Burman (PB) design and response surface methodology (RSM) (Long et al. 2018). Because multiple components are involved, how to screen out the main factors quickly and effectively and optimize them is one of the key issues in the research field. The PB design method is an economical and effective secondary experimental design method, which can use the least number of trials to effectively determine the main factors from the numerous investigation factors (Wu et al. 2018). The RSM has the advantages of short test cycle, high precision of the regression equation, and the ability to study the interaction between several factors. It is an effective method to optimize reaction conditions and process parameters (Ghaderi et al. 2018). Compared with the conventional one-factor at-a-time (OFAT) strategy, PB design and RSM have more advantages in multivariable systems (Dayana Priyadharshini and Bakthavatsalam 2016). In recent years, this method has been successfully applied to optimize biochemical processes (Wu et al. 2018; Xu et al. 2018; Zhou et al. 2019). In addition, it is necessary to feed auxotrophic amino acid during the fed-batch fermentation process for auxotrophic strains to maintain cell growth. The feeding of auxotrophic amino acid will significantly affect cell growth and target product accumulation (Zhang et al. 2019). So, the exploration of feeding mode is necessary for the fed-batch fermentation of auxotrophic strains, but there are few relevant research reports at present.
In this study, to achieve high concentration production of D-PA from recombinant E. coli W3110, the fermentation medium was first screened in shake flask fermentation. After determining the basic medium according to the accumulation of D-PA, the different medium composition and culture conditions that may affect D-PA biosynthesis were studied through PB design, and the key factors were initially screened for further optimization using RSM based on Box-Behnken design (BBD). Then, fed-batch fermentation based on auxotrophic amino acid (isoleucine) limited feed was carried out in a 5 L fermenter to develop an effective fermentation process to increase the production of D-PA.
Materials and methods
Strain and plasmid
A recombinant E. coli W3110 harboring a plasmid pTrc99A-panB-panC, previously constructed and preserved in our laboratory (Hangzhou, China) was used as D-PA producing strain in this study. In plasmid pTrc99A-panB-panC, the genes panB (encoding 3-methyl-2-oxobutanoate hydroxy methyltransferase), and panC (encoding pantoate-beta-alanine ligase) were cloned under the control of the trc promoter in plasmid pTrc99A with Kanamycin (Kan) resistance to enhance expression of key genes involved in D-PA biosynthetic pathway (Zhang et al. 2019). The gene panB and panC was cloned from C. glutamicum.
Medium
The seed medium was Luria–Bertani medium (LB) (/L): tryptone 10 g, yeast extract 5 g, and NaCl 10 g.
The fermentation medium included SF (g/L) [glucose 10, Na(CH3COO)·3H2O 2.3, Na2HPO4·7H2O 5.66, KH2PO4 1.5, NaCl 0.25, NH4Cl 0.5, MgSO4·7H2O 0.1, CaCl2·2H2O 0.013, β-alanine 1.5], SMAC (g/L) [glucose 20, Na2HPO4·12H2O 15.12, KH2PO4 0.5, NaCl 3.0, MgSO4·7H2O 0.5, CaCl2 0.011, NH4Cl 1.0], HM (g/L) [glucose 10, tryptone10, K2HPO4 2.63, KH2PO4 1.38, (NH4)2SO4 3, citricacid-H2O 2.19, yeast extract 5, MgSO4·7H2O 0.82, β-alanine 1.5], NBS (g/L) [glucose 10, NH4Cl 1.0, betaine 0.117, β-alanine 1.5], Tarof and Hobbs (TB) (g/L) [tryptone 12, yeast extract 24, glycerol 4, K2HPO4 12.54, β-alanine 1.5, KH2PO4 2.31], Luria–Bertani (LB) (g/L) [tryptone 10, yeast extract 5, β-alanine 1.5, NaCl 10], M-1 (g/L) [glucose 20, (NH4)2SO4 5, yeast extract 2, KH2PO4 0.8, MgSO4 0.5, β-alanine 1.5], M-9 (g/L) [CaCl2 0.01, MgSO4 0.24, Na2HPO4·7H2O 12.8, KH2PO4 3, NaCl 0.5, NH4Cl 1, sucrose, glycerin or maltose 20].
Microelement mix (/L): NiCl2·7H2O 0.02 g, CuCl2 10 g, FeSO4·7H2O 10 g, ZnSO4·7H2O 10 g, CuSO4 0.2 g.
The feeding medium (/L): glucose 500 g, MgSO4 8 g, (NH4)2SO4 10 g, KH2PO4 14 g, yeast extract 2 g, β-alanine 40 g, VB12 4 mg, VB1 10 mg, Kan 150 mg, isopropyl β-D-thioacetamide (IPTG) 0.2 mM and microelement mix 2 mL.
Culture conditions
The seed preparation, the recombinant E. coli W3110 were cultured in Luria Bertani (LB) medium containing 75 mg/L Kan. The growth condition was maintained at 37 °C and 180 rpm for 12 h.
For shake flask fermentations for D-PA production, the recombinant E. coli W3110 were cultured in 250 mL Erlenmeyer flasks containing 20 mL fermentation medium, the medium was cultivated at 180 rpm and 30 °C for 48 h in a constant temperature shaking incubator shaker BPMJ-70F (Shanghai Yuefeng instrument Co., Ltd, Shanghai, China). The inoculation volume is 200 μL (1%) of seed. Besides, sterilized 15 g/L CaCO3 was added to maintain pH and 0.2 mM IPTG was added to induce plasmid expression.
For fed-batch fermentations for D-PA production, a colony was cultured in 10 mL LB medium at 37 ℃ and 180 rpm for 12 h to obtain first-degree seed. 1 mL (10%) first- degree seed was inoculated to three 500 mL flasks containing 100 mL of LB medium and pre-cultured at 37 °C and 150 rpm for 10 h to obtain second-degree seed. 75 mg/L of Kan and 0.2 mM of IPTG were added into 300 mL of seed culture. Finally, seed culture was inoculated into 2 L of fermentation medium in a 5 L bioreactor (Biotech5BJ; Shanghai BaoXing Bio-Engineering Equipment Engineering Co. Ltd, Shanghai, China). The pH was maintained in the 6.80 by sterile aqueous ammonia (45%). The fermentation temperature was maintained at 30 °C. The flow feeding method was selected to maintain low concentration of glucose in the medium, and isoleucine (40 g/L) was added after 14–15 h.
Plackett–Burman design and data analysis
The PB design experiment mainly analyzes two levels of each factor, and determines the significance of the factor by comparing the difference between the two levels of each factor and the overall difference (Bezerra et al. 2008). To define the influences of the main composition of culture condition on D-PA production by recombinant E. coli W3110, PB design was used to evaluate the effects of 11 variables (glucose, (NH4)2SO4, yeast extract, KH2PO4, MgSO4, salt solution, temperature, CaCO3, Inoculum, β-alanine, and IPTG) on D-PA production (Suppl. Table S1). Twelve experimental runs were conducted to screen important components that affect D-PA biosynthesis. Among them, the high level is "1" and the low level is " − 1". All experiments were performed in triplicate. For the results, the effects of each factor were calculated separately, and the t test was performed on the effects of each factor. The theory selects factors with a confidence > 95% as significant factors for further investigation. The software Minitab Release 16 (Minitab Inc., State College, PA, USA) was used for statistical analysis of the experimental data.
Path of the steepest ascent experiment
After determining the most important variables through PB design, the steepest ascent experiment was conducted to determine the central point of the RSM. Only in the adjacent area under investigation can the real situation be better reflected. Therefore, the maximum D-PA production area should be approached before establishing an effective fitting equation. According to the PB design results, a factor of P < 0.05 was selected, showing a positive effect. If the factor has a positive effect, its high-level value was selected and increased appropriately. If the factor has a negative effect, its low-level value was selected and reduced appropriately. When the maximum value was obtained, this point can be regarded as the center point of the BBD. Suppl. Table S2 summarized the experimental design, variables, and their values.
Box–Behnken design and statistical analysis
In the present study, determining the optimal level of the most significant factors by employing BBD-RSM. The effects of the three most significant variables (glucose, (NH4)2SO4, β-alanine) and three levels ( − 1, 0, and 1) on the response (Y) (D-PA titer) were explored to determine the best conditions for D-PA production (Suppl. Table S3). Fitting the test results to establish a polynomial regression model describing the relationship between response (D-PA titer) and independent variables (significant factors affecting D-PA titer). Then we conducted a standardized analysis of the fitting equation to find the stable point of the regression model, and obtain the optimal level of the significant factors for the maximum D-PA titer, Finally, it was tested and verified. BBD experiment content and experiment results are shown in Suppl. Table S3. The BBD comprised a set of 12 experiment runs and five central point replications. The experiment data was analyzed by Design Expert 8.0.6 software, and the quadratic regression model was established relating the response to the variables, as described in the following equation.
| 1 |
where Y is the measured response variable, Xi and Xj for the independent variables of the coded value; β0 is intercept; βi is the linear coefficient; βii is square coefficient; βij (i ≠ j) is interaction coefficient.
Isoleucine feeding
The current research assumes that adding enough isoleucine to the system can facilitate the production of D-PA and improve the glucose conversion efficiency. Therefore, in this work, the isoleucine solution (40 g/L) was flowed into the fermenter at a constant rate (5 mL/h, 10 mL/h, 15 mL/h), and fully explored its function.
Analytical methods
10 mL of fermentation broth was pipetted and centrifuged at 12,000 rpm for one minute, and took the supernatant for the detection of D-PA, acetic acid, and residual glucose concentration. Dry cell weight (DCW) was determined using a calibration Eq. OD600 = 0.31 g/L DCW. OD600 was measured based on the optical density at 600 nm (OD600) with an Eppendorf Bio Photometer (Eppendorf AG, Hamburg, Germany) (Zhou et al. 2019). The glucose concentration was detected using the DNS assay (Lee et al. 2017; Zhou et al. 2019). The concentration of D-PA was determined by HPLC system equipped with C18 column (250 × 4.6 mm, 5 μm, Agilent Technologies, Santa Clara, USA) and UV detector set at a wavelength of 200 nm. The mobile phase was composed of water, acetonitrile, and phosphoric acid with a ratio of 949:50:1 at the flow rate of 0.9 mL/min. The column temperature was 30 °C (Zhang et al. 2019).
Results and discussions
Screening the basic medium and carbon and nitrogen sources by single-factor analysis
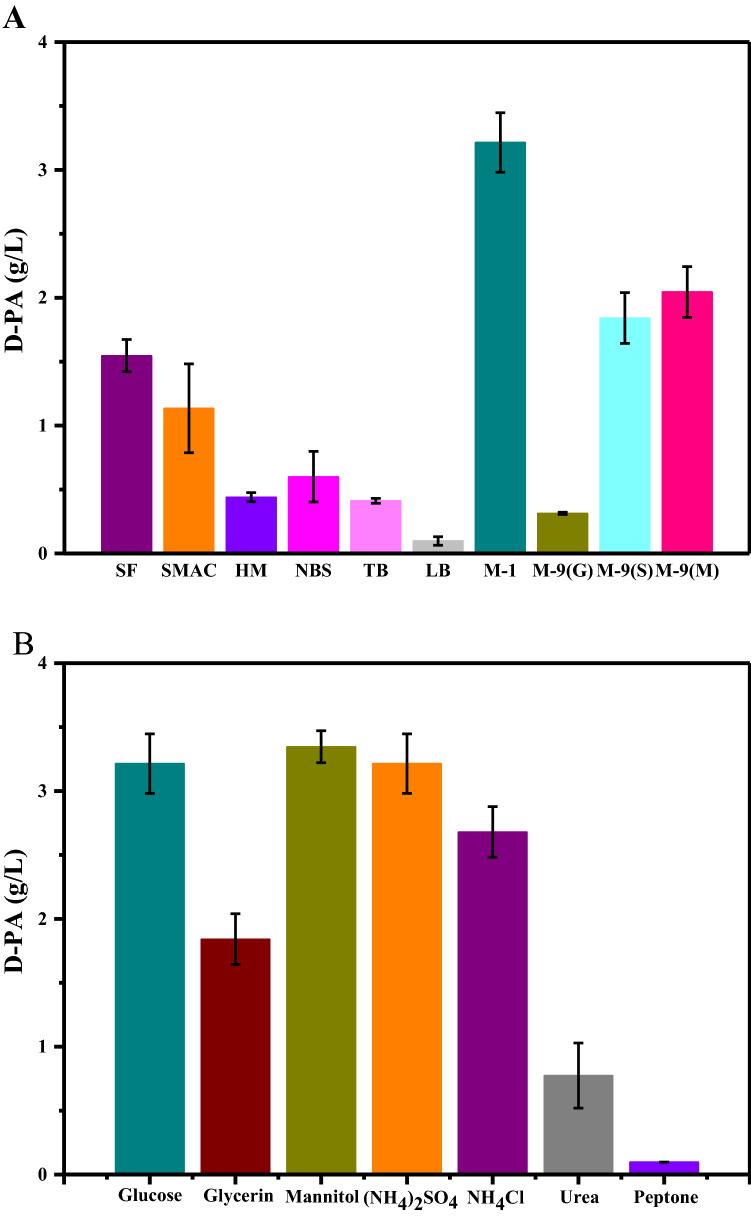
Ten basic media, i.e., SF, SMAC, HM, NBS, TB, LB, M-1, M-9 (S), M-9 (G) and M-9 (M), were used to produce D-PA by the recombinant E. coli W3110. As show in Fig. 1a, M-1 medicum was the most advantageous to produce D-PA induced by IPTG and 3.21 g/L of D-PA concentration was obtained. Similar results have been reported by Zhang et al. for D-PA production from MS medium (Zhang et al. 2019). This highest D-PA production obtained from M-1 medium may be attributed to the presence β-alanine, which is the precursor of D-PA biosynthesis. Dusch et al. discussed the effect of enhancing expression of the panD gene encoding L-Aspartate-α-decarboxylase in E. coli on yield of D-PA, which also showed that the high yield of D-PA depended on the exogenous addition of β-alanine (Dusch et al. 1999). López-Sámano et al. also reported that β‐alanine is an indispensable component of D-PA (López-Sámano et al. 2020).
Fig. 1.
The effect of different types of basic media on the synthesis of D-PA. a Evaluation of the optimal basal culture medium among SF, SMAC, HM, NBS, TB, LB, M-1, M-9 (S), M-9 (G) and M-9 (M) in shaking flask culture. b Selection of optimal carbon and nitrogen sources in shaking flask culture. Data are expressed as the mean ± standard deviation (SD) of 3 independent experiments
Meanwhile, it is found that organic carbon sources (such as glucose and mannitol) and inorganic nitrogen sources (such as (NH4)2SO4 and NH4Cl) are more conducive to the production of D-PA than inorganic carbon sources and organic nitrogen sources respectively, comparing with the fermentation results of the basic medium. Performing a single factor analysis can initially determine the preference of the bacteria for nutritional components (Couto et al. 2017; Long et al. 2018; Trchounian et al. 2016). Therefore, the subsequent single-factor screening of carbon and nitrogen sources mainly focuses on common organic carbon sources and inorganic nitrogen sources. As show in Fig. 1b, glucose, mannitol and (NH4)2SO4 can significantly improve the D-PA titer compared to other carbon and nitrogen sources. Since the price of mannitol is higher than glucose and the metabolic research is not as thorough as glucose. Considering the needs of industrial applications, subsequent studies use glucose as the sole carbon source. Our results are consistent with those of Chemler, who used a genome model to calculate the metabolic flux of E. coli and it indicated that glucose is the optimal carbon source for 796 metabolites of E. coli (Chemler et al. 2010).
PB design screening elucidated the critical media components for D-PA production
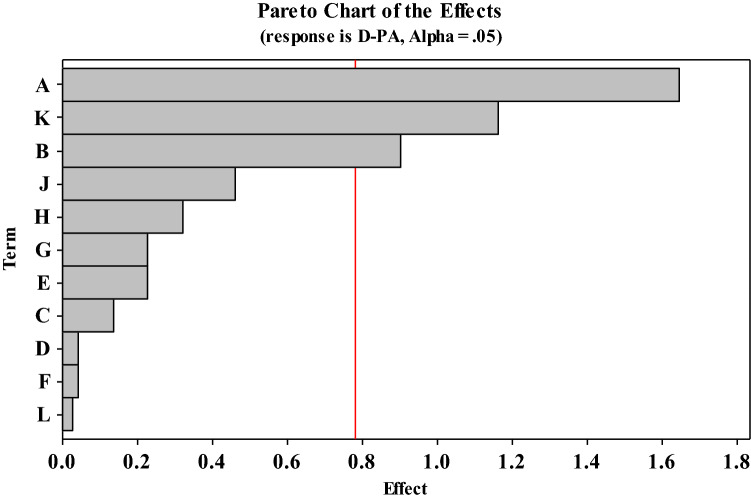
Generally, the composition and culture conditions of the culture medium are one of the main factors that affect cell growth and target metabolite accumulation during microbial fermentation (Zhou et al. 2019; Fan et al.2020). According to the basic medium obtained from the screening, in order to select the important factors affecting the biosynthesis of D-PA by recombinant E. coli W3110, we conducted the PB design for the medium composition and culture conditions as shown in Table S1. As shown in Fig. 2, Pareto Chart as an effective tool to determine important factors was used to show the normalization effect of independent variables and their interactions on the synthesis of D-PA (Yetilmezsoy et al. 2009). The length of each bar in the chart indicates the normalization effect of the factor on the response, and the factor whose length exceeds the red vertical line is a significant influencing factor (Fig. 2). Statistical analysis of the experimental data for PB design with 11 variables showed that glucose, (NH4)2SO4 and β-alanine were considered as the main influencing factors of this study (Table 1). The regression analysis of PB design in Table 1 illustrated the effectiveness of our model. The determination coefficient R2 is 0.9996, which shows the high quality of the model. The fitting model for the titer of D-PA was significant (p < 0.05). The F value and p value were selected for analysis of variance (ANOVA) of the model (Zhou et al. 2019). Among these variables, glucose, β-alanine and (NH4)2SO4 were determined to have the highest relative importance that influences D-PA production. Therefore, these three variables are selected for further optimization by BBD.
Fig. 2.
Pareto chart shows the standardization effect of independent variables and their interactions on the efficiency of D-PA production by recombinant E. coli W3110
Table 1.
The regression analysis of variance for Plackett–Burman design model for E. coli W3110
| Source | Factors | Low level ( − 1) | High level ( − 1) | Sum of Squares | Mean Square | F Value | P value |
|---|---|---|---|---|---|---|---|
| Model | 13.331 | 1.333 | 277.721 | 0.0467* | |||
| A | glucose | 30.00 | 50.00 | 10.01 | 10.01 | 2085.444 | 0.0139*** |
| B | (NH4)2SO4 | 7.50 | 12.50 | 1.658 | 1.658 | 345.340 | 0.0342** |
| C | Yeast extract | 1.00 | 3.00 | 0.156 | 0.156 | 15.365 | 0.2751 |
| D | KH2PO4 | 0.60 | 1.00 | 0.087 | 0.087 | 18.063 | 0.4471 |
| E | MgSO4 | 0.30 | 0.70 | 0.002 | 0.002 | 0.340 | 0.1458 |
| F | Salt solution | 0.75 | 1.25 | 0.006 | 0.006 | 1.174 | 0.4745 |
| G | Temperature | 30.00 | 37.00 | 0.002 | 0.002 | 0.340 | 0.1438 |
| H | CaCO3 | 0.30 | 0.40 | 0.626 | 0.626 | 130.340 | 0.0756 |
| J | Inoculum | 100.00 | 200.00 | 0.023 | 0.023 | 4.694 | 0.0553 |
| K | β-alanine | 1.00 | 3.00 | 0.897 | 0.897 | 186.778 | 0.0465* |
| L | IPTG | 1.00 | 3.00 | 0.023 | 0.023 | 4.694 | 0.5153 |
The regression analysis of variance for Plackett–Burman design model for E. coli W3110
Values of “Prob. > F” less than 0.0300, 0.0400, and 0.0500 indicate that model terms are extremely significant (***), highly significant (**), and significant (*), respectively
Before the BBD test, steepest ascent experiment is required, the purpose is to find the − 1 and + 1 points of the factor level to ensure the accuracy of the results (Fan et al. 2020). Since these three most important variables have a positive effect on the production of D-PA, its concentration should be increased in the steepest ascent direction to bring it closer to the best experimental area for maximum D-PA production. The steepest ascent experiment results show that when the glucose, (NH4)2SO4 and β-alanine were selected to be 40 g/L, 12 g/L and 2 g/L, respectively, the response value of D-PA titer reached its peak with the highest response of 4.25 g/L gave the maximum yield (Suppl. Table S2). If the three variables are further improved, the response value cannot be further increased, which suggested that it was proximal to the region of optimal response. Accordingly, these levels of the three variables in the third set were considered the center point of BBD.
Optimization of culture medium with BBD for D-PA production
Previous steepest ascent experiment has confirmed that glucose (40 g/L), β-alanine (2 g/L) and (NH4)2SO4 (12 g/L) were the center point of the three significant factors (Suppl. Table S2). In this experiment, a three-factor, three-level BBD was used to optimize the individual and interactive effects of the three factors on D-PA production precisely (Sibanda and Pretorius 2012; Zhou et al. 2019). Suppl. Table S3 shows the maximum and minimum levels of the variables selected for the BBD experiment, as well as the experimental responses of 17 experimental runs. The results show that D-PA concentration has a considerable change, which depended on different culture conditions (Fan et al. 2020). Test number 9 achieved the largest D-PA yield (6.36 g/L), while test number 1 observed the smallest D-PA yield (2.94 g/L). The center point in the design was repeated three times to estimate the error. Through multiple regression analysis of the experimental data, shown in Table 2 (Zhou et al. 2019). The multiple quadratic regression equation of D-PA production (Y) on glucose (X1), β-alanine (X2) and (NH4)2SO4 (X3):
| 2 |
Table 2.
The regression analysis of a full second-order polynomial model for the optimized titer of D-PA by the E. coli W3110.
| Source | Sum of Squares | df | Mean Square | F Value | P value |
|---|---|---|---|---|---|
| Model | 27.16 | 9 | 3.02 | 18.97 | 0.0004 |
| X1 (glucose) | 9.89 | 1 | 9.89 | 62.19 | < 0.001** |
| X2 (β-alanine) | 4.67 | 1 | 4.67 | 29.34 | 0.001** |
| X3 ((NH4)2SO4) | 0.06 | 1 | 0.06 | 0.4 | 0.5451 |
| X1X2 | 1.38 | 1 | 1.38 | 8.66 | 0.0216* |
| X1X3 | 0.03 | 1 | 0.03 | 0.19 | 0.6781 |
| X2X3 | 0.01 | 1 | 0.01 | 0.05 | 0.8318 |
| X1^2 | 3.26 | 1 | 3.26 | 20.48 | 0.0027* |
| X2^2 | 6.08 | 1 | 6.08 | 38.26 | 0.0005* |
| X3^2 | 0.8 | 1 | 0.8 | 5.01 | 0.0603 |
| Residual | 1.11 | 7 | 0.16 | ||
| Lack of Fit | 0.81 | 3 | 0.27 | 3.61 | 0.1236 |
| Pure Error | 0.3 | 4 | 0.08 | ||
| Cor Total | 28.27 | 16 |
aR2 = 0.9836, adj-R2 = 0.9626, pred-R2 = 0.9359, Adequate precision = 12.265, CV = 1.25%,
*significant (p-value < 0.05)
** highly significant (p-value < 0.01)
where Y is the predicted response of D-PA titer, X1, X2, and X3 are the coded values of the test variables, glucose, β-alanine, and (NH4)2SO4, respectively.
The statistical significance of the second-order model was checked by the F-test, and the analysis of variance (ANOVA) for response surface quadratic model is summarized in Table 2. A large model F value (F value = 18.97) indicates that most of the variation in the response can be explained by a given regression equation. The selected regression model has a P-value of 0.004 and a lower value of the coefficient of variation (CV = 1.91%), indicating that the overall model has a significant impact on the test results and has credibility. While the P-value of the lack-of-fit is 0.1236 (> 0.05), model selection is appropriate (Bezerra et al. 2008). The R2 value of the model was 0.9836, suggesting a high degree of correlation between the predicted values with BBD model and experimental values. In addition, the model “Pred R2” of 0.9359 was in reasonable agreement with the “Adj R2” of 0.9626. Thus, it could be applied to predict theoretical D-PA titer (Bezerra et al. 2008).
Meantime, the corresponding P value indicated that among the independent variables, X1 (glucose), X2 (β-alaine) and X3 ((NH4)2SO4) have a significant effect on D-PA yield. The linear coefficients X1 and X2, the interaction coefficient X1X2 as well as the quadratic coefficient X12 and X22 were all significant at a 5% significance level. Therefore, they were the most influential factors. The variables X3, X1X3, X2X3 and X32 were insignificant model terms (p > 0.05) (Bezerra et al. 2008). Equation (2) can be used to calculate the maximum estimated response value of D-PA titer under the optimal conditions for maximum D-PA production, which was 6.80 g/L.
From Fig. 3, we can see that some small variations in glucose, β-alanine concentrations could cause great changes on D-PA titer, indicating the variables of square interaction (X1, X2, and ) were all significant on the D-PA titer, which was consistent with the results of the ANOVA. The p value of each variable shown in Table 2 indicates that glucose is the most important factor affecting growth and D-PA production (p value < 0.0001). As shown in Fig. 3a, b, the production of D-PA increases with the increase of glucose. In E. coli, glucose is the carbon skeleton for D-PA synthesis. Compared to the low glucose concentration, the adequate glucose concentration satisfies both the growth of bacteria and the production of D-PA, which significantly mitigates the risk causing glucose starvation. The results also indicate that appropriate glucose and β-Alanine concentration enhanced the production of D-PA. Table S3, Fig. 3a, c show that β-Alanine concentration and the interaction between β-alanine and initial glucose has an important effect on the production of D-PA. Therefore, to obtain a large amount of D-PA, these factors must be kept at the appropriate level at the same time (Zhou et al. 2019). The feedback of Acetyl-Coenzyme A and Coenzyme A will inhibit the synthesis of β-alanine in the cell, which may lead to insufficient intracellular availability of β-alanine, it is necessary to add an appropriate amount of β-alanine to ensure an adequate supply of precursors (Zhang et al. 2019). However, the too high β-alanine concentration will cause waste of raw materials and inhibit the growth of bacteria. D-PA titer generally increased to a peak value with an increase in glucose or β-alanine concentration and then decreased when glucose or β-alanine concentration further increased beyond 56 g/L or 2.25 g/L, respectively.
Fig. 3.
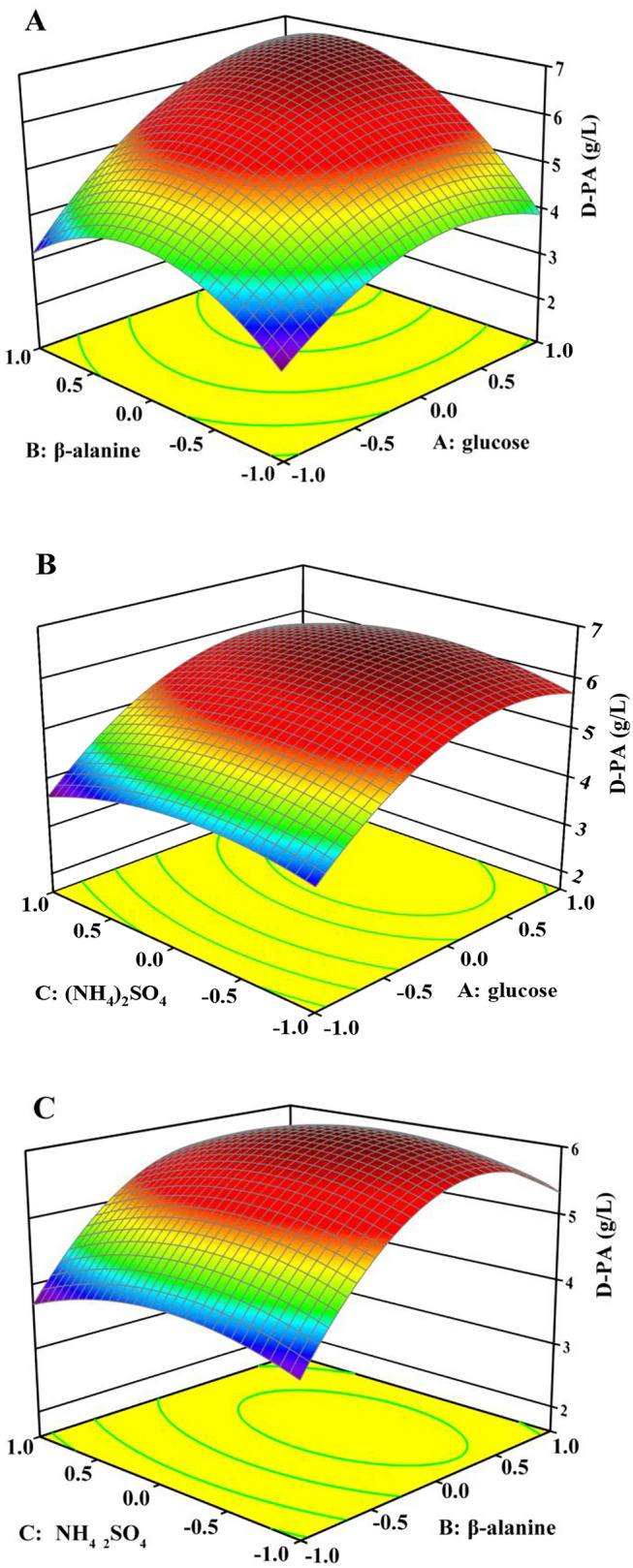
Response surface 3D model to assess the effects of the three variables (glucose, β-Alanine, (NH4)2SO4) on the titer of D-PA produced by E. coli W3110. a Effect of glucose and β-Alanine on the titer of D-PA; b Effect of glucose and (NH4)2SO4 on the titer of D-PA; c Effect of β-Alanine and (NH4)2SO4 on the titer of D-PA
Based on the numerical optimization of the software, the optimal response is found within the specified variable range in the fermentation optimization of D-PA production. The best values of the predicted variables are as follows: glucose, X1 = 56.0 g/L, β-Alanine, X2 = 2.25 g/L, and (NH4)2SO4, X3 = 11.8 g/L. Under optimal conditions, the maximum predicted titer of D-PA is 6.80 g/L. Three replicate experiments were carried out using the optimal conditions in shake flask culture to confirm the effectiveness of the model in predicting the maximum titer of D-PA. The D-PA titer obtained under the optimized condition was 6.73 g/L ± 0.12 g/L (N = 3), which is close to the predicted titer of 6.80 g/L.
Influence of fed-batch culture on D-PA production in 5-L fermenter
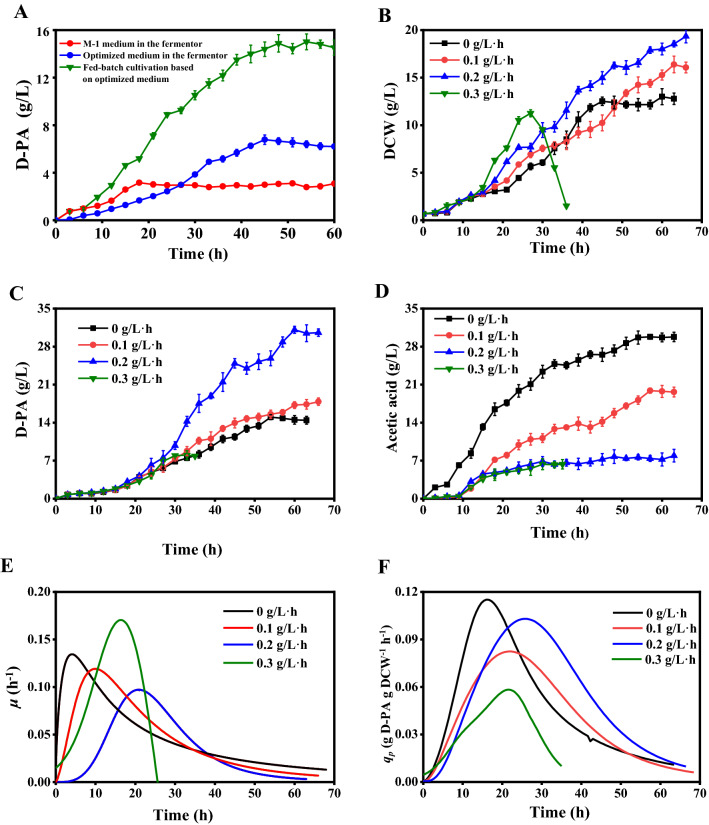
Figure 4a shows the effect of using the original and optimized medium on D-PA production in the 5 L fermenter. When using the optimized medium for batch fermentation, the D-PA concentration reached a maximum of 6.78 g/L, which is 2.15 times the value of the original medium (3.2 g/L). In order to further improve the titer of D-PA, try to carry out the research of fed-batch culture on the basis of optimized nutrition composition ratio (Cheng et al. 2012). When the glucose concentration was controlled at a low level during fermentation, the maximum D-PA concentration was 15.1 g/L (Fig. 4a), which was 2.53 times that of batch fermentation. The reason may be that the fed-batch fermentation removed the substrate limitation and improved the production efficiency of D-PA (Chen et al. 2012).
Fig. 4.
The effects of different fermentation conditions on D-PA production in 5 L fermenter by E. coli W3110. a Comparison of original fermentation medium and optimal medium; b Effects of different isoleucine feeding rates on D-PA biosynthesis; c Effects of different isoleucine feeding rates on DCW; d Effects of different isoleucine feeding rates on acetic acid accumulation; e Specific cell growth rate (μ) with different isoleucine feeding rates; f Specific D-PA yield on glucose (qp) with different isoleucine feeding rates
Figure 4b until f show the influence of constant-rate isoleucine feeding on D-PA production. As shown in Fig. 4b, the addition of isoleucine increased the maximum DCW (16.58 g/L) and the growth rate of the cells. At the same time, it delayed and prolonged the rapid growth period of the cell (Fig. 4e). The results show that the addition of exogenous amino acids maintained the normal growth of the auxotrophic strains. Our results are agreed with Cakar's reports (Cakar et al. 2000). Barth et al. reported auxotrophic strains require amino acids for growth and the increase in amino acid content plays an important role in the maintenance of nutrient-deficient strains (Barth and Pitt 1996). Marcos et al. also reported that leucine can extend the life of leucine-deficient strains (Ohtsuka et al. 2019). As the rate of isoleucine feeding increases, the specific growth rate (μ) of cell has been further extended (Fig. 4c, e). However, when the rate of isoleucine feeding was further increased to 0.3 g/L·h, the cell began to die prematurely after 27 h of fermentation. This may be due to the following two reasons: the first possibility is the serious consequences of the imbalance of essential amino acids on cell physiology, life span and resistance (Gomes et al. 2007). On the other hand, an excessively high cell growth rate may also result in an imbalance between the glucose uptake rate and the central metabolic capacity of the cell, resulting in a large amount of inhibitory metabolic by-products, resulting in incomplete fermentation (Brauer et al. 2008). Brauer et al. reported that many (but not all) genes related to stress response are closely related to growth rate (Brauer et al. 2008). This study can provide novel insights on the role of auxotrophic amino acids and their effects on cell growth, and prove that the nutritional status of the environment is an important factor in promoting cell growth. Besides, a warning was given to avoid waste of energy and imbalance of cell physiological state due to excessive nutrients (Fessler et al. 2020).
Meanwhile, the titer of D-PA with different isoleucine feeding rate of 0 mL/h, 5 mL/h, 10 mL/h and 15 mL/h were 15.01 g/L, 18.10 g/L, 31.61 g/L and 8.36 g/L, respectively (Table 3). These results revealed that the biosynthesis of D-PA has a strict correlation with cell growth. Our results are consistent with those of Zou, who reported that the biosynthesis of D-PA depends on biomass under different feeding strategies (Zou et al. 2020). Besides, when controlling the isoleucine feeding rate of 10 mL/h, the maximum 13.08 g/L·d of D-PA productivity and 1.63 g D-PA/g DCW of cell yield were obtained, which were 1.96 and 1.55 times that of the control group, respectively (Table 3). The main reason behind the high ability of cell yield for D-PA production may be that the stable cell metabolism environment makes cell growth and D-PA accumulation in a balanced state to relieve metabolic burden (Wang et al. 2019). Since the use of substrates to maintain cell growth will compete with product synthesis. Klamt et al. discussed the feasibility of the synthesis of growth coupling products in microbial strains based to the metabolic model, indicating which theoretical requirements must be met to ensure the maximum coupling yield (Klamt et al. 2015).
Table 3.
The fermentation parameters of D-PA production by E. coli W3110 under different isoleucine feeding rates
| Isoleucine feeding rate (mL/h) | DCW (g/L) | D-PA concentration (g/L) | acetic acid concentration (g/L) | D-PA productivity (g/L·d) | D-PA yield on glucose (mol/mol) | Yield of D-PA on cell (Y D-PA/X) |
|---|---|---|---|---|---|---|
| 0 | 13.00 | 15.01 | 29.79 | 6.67 | 0.09 | 1.15 |
| 5 | 16.58 | 18.10 | 19.90 | 6.39 | 0.10 | 1.09 |
| 10 | 19.35 | 31.61 | 8.55 | 13.08 | 0.14 | 1.63 |
| 15 | 11.25 | 8.36 | 6.35 | 6.47 | 0.27 | 0.74 |
Figure 4c, f show the time for obtaining the maximum value of the specific production rate was delayed and the specific growth rate was greatly improved in the late fermentation period with the addition of isoleucine. These results further prove the importance of balancing cell growth rate for D-PA biosynthesis. As far as we know, no such studies have been conducted so far, and there is no general standard for testing the coupling of cell growth and product synthesis. Compared with the control group, the consumption of glucose during fermentation increased while reducing the accumulation of acetic acid (Fig. 4d). In addition, when controlling the isoleucine feeding rate of 10 mL/h, the accumulation of acetic acid decreased from 29.79 g/L to 8.55 g/L, which reduced the accumulation of acetic acid by 71.3%. These results are consistent with previous reports (Zou et al. 2020). Meantime, increased glucose consumption increased the availability of intracellular pyruvate. As an important precursor of D-PA, pyruvate directly determines the experimental yield of D-PA (Leonardi et al. 2007). This shows that this fermentation process is more suitable to produce D-PA.
According to reports, the synthesis of D-PA and related compounds may also depend on the availability of intracellular precursors: 2 molecules of pyruvate, 2 molecules of NADPH and 1 molecule of β-alanine to form 1 molecule of D-PA (Huser et al. 2005). In addition, recent studies have found that increased production of branched chain amino acids is related to the intracellular uptake of NADPH (Wang et al. 2018). The catabolism of isoleucine bypasses the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase steps to provide acetyl-CoA and succinyl-CoA to the TCA cycle (Johansen et al. 2007; Ruklisha et al. 2007). The increase in TCA flux provides more ATP and NADPH for the metabolic needs of D-PA synthesis. So, adding isoleucine, the intracellular absorption of NADPH can be improved, thereby eliminating the important rate-limiting point in the D-PA pathway, and greatly improving the glucose conversion rate of D-PA (Huser et al. 2005). On the other hand, the addition of isoleucine increases the availability of intracellular CoA. CoA reacts with acetic acid to generate acetyl-CoA, which reduces the accumulation of acetic acid and further increases the effective TCA cycle. This indirectly promotes the absorption of the substrate glucose and increases the intracellular concentration of key intermediates pyruvate and NADPH. (Krige et al. 2015; Zou et al. 2020; Jing et al. 2018). Therefore, adding an appropriate amount of isoleucine to the medium can form a higher biomass and make the D-PA yield per unit glucose close to the maximum value.
The effect of isoleucine on the synthesis of D-PA may be attributed to the following aspects: On the one hand, the externally supplemented isoleucine is converted to acetyl-CoA and succinyl-CoA. The availability of intracellular acetyl-CoA affects the TCA cycle enzyme activity. The supplementation of isoleucine can be converted into succinyl-CoA, and then through a series of enzymatic reactions to generate oxaloacetate. This is extremely important for the growth of bacteria and the formation of target metabolites because the amount of oxaloacetate directly affects the speed of circulation (TCA cycle), so the constant addition of isoleucine supplementation is conducive to the smooth progress of the TCA cycle. At the same time, the isoleucine externally supplemented is converted to acetyl-CoA and succinyl-CoA to enter the TCA cycle as a source of ATP and NADPH, and thereby supporting metabolism required for biomass formation and D-PA production (Johansen et al. 2007; Murin et al. 2009). Successfully improved the intracellular utilization of NADPH, thereby upregulating the carbon metabolic flux in the D-PA pathway and greatly increasing the glucose conversion rate of D-PA (Holatko et al. 2009; Wang et al. 2018). In addition, due to the addition of isoleucine, more metabolic flux flows to the TCA cycle, and the D-PA pathway reduces the overflow of pyruvate to the acetic acid pathway (Jing et al. 2018). The balance of the glycolysis pathway and central metabolic capacity inhibits the formation of by-product acetic acid, which further improves the fermentation process of D-PA. The above analysis shows that maintaining the cell growth rate at an appropriate level through the feedback isoleucine feeding strategy is important for maintaining sufficient key enzyme activity and intracellular availability of NADPH in the cell, and successfully reduced the accumulation of acetic acid. Satisfactory D-PA synthesis was obtained by fermentation culture maintaining an optimal cell growth rate.
Conclusions
In this study, an optimization means of fermentation condition for recombinant E. coli W3110 was used to improve D-PA production from renewable resource. The highest D-PA concentration obtained was 6.73 g/L from optimal medium in shaking flasks fermentation, which was a greatly improvement of 1.1-fold compared with the results obtained using basic medium. It is widely used in the field of microbial fermentation by removing the substrate limitation through fed-batch fermentation. Therefore, a fed-batch fermentation process combining auxotrophic amino acid (isoleucine) feeding strategy was established in 5 L fermenter, the maximum D-PA concentration obtained was 31.6 g/L, which was 2.11-fold compared with the results obtained without isoleucine supplementation. Our results demonstrate that optimization of factors that have significant effects on D-PA biosynthesis and isoleucine feeding strategies are beneficial to D-PA microbial fermentation. The fermentation process established is expected to use low-cost raw materials to produce high-yield D-PA, and it provides a reference for future research on D-PA microbial fermentation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Program of the National Key Research and Development Project (2019YFA09005000) .
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Barth AL, Pitt TL. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudornonas aeruginosa. J Med Microbiol. 1996;45:110–119. doi: 10.1099/00222615-45-2-110. [DOI] [PubMed] [Google Scholar]
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010;12:539–554. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- Brauer MJ, et al. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol Biol Cell. 2008;19:352–367. doi: 10.1091/mbc.e07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakar ZP, Sauer U, Bailey JE, Muller M, Stolz M, Wallimann T, Schlattner U. Vacuolar morphology and cell cycle distribution are modified by leucine limitation in auxotrophic Saccharomyces cerevisiae. Biol Cell. 2000;92:629–637. doi: 10.1016/S0248-4900(01)01111-X. [DOI] [PubMed] [Google Scholar]
- Chemler JA, Fowler ZL, McHugh KP, Koffas MA. Improving NADPH availability for natural product biosynthesis in Escherichia coli by metabolic engineering. Metab Eng. 2010;12:96–104. doi: 10.1016/j.ymben.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu L, Li J, Liu J, Du G, Chen J. Optimization of glucose feeding approaches for enhanced glucosamine and N-acetylglucosamine production by an engineered Escherichia coli. J Ind Microbiol Biotechnol. 2012;39:359–365. doi: 10.1007/s10295-011-1046-0. [DOI] [PubMed] [Google Scholar]
- Cheng L-K, Wang J, Xu Q-Y, Xie X-X, Zhang Y-J, Zhao C-G, Chen N. Effect of feeding strategy on l-tryptophan production by recombinant Escherichia coli. Ann Microbiol. 2012;62:1625–1634. doi: 10.1007/s13213-012-0419-6. [DOI] [Google Scholar]
- Couto MR, Rodrigues JL, Rodrigues LR. Optimization of fermentation conditions for the production of curcumin by engineered Escherichia coli. J R Soc Interface. 2017;14:20170470. doi: 10.1098/rsif.2017.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayana Priyadharshini S, Bakthavatsalam AK. Optimization of phenol degradation by the microalga Chlorella pyrenoidosa using Plackett-Burman design and response surface methodology. Bioresour Technol. 2016;207:150–156. doi: 10.1016/j.biortech.2016.01.138. [DOI] [PubMed] [Google Scholar]
- Dusch N, Pühler A, Kalinowski J. Expression of the Corynebacterium glutamicum panD gene encoding L-aspartate-α-Decarboxylase leads to pantothenate overproduction in Escherichia coli. Appl Environ Microbiol. 1999;65:1530–1539. doi: 10.1128/AEM.65.4.1530-1539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggersdorfer M, Laudert D, Letinois U, McClymont T, Medlock J, Netscher T, Bonrath W. One hundred years of vitamins-a success story of the natural sciences. Angew Chem Int Ed Engl. 2012;51:12960–12990. doi: 10.1002/anie.201205886. [DOI] [PubMed] [Google Scholar]
- Elischewski F, Pühler A, Kalinowski J. Pantothenate production in Escherichia coli K12 by enhanced expression of the panE gene encoding ketopantoate reductase. J Biotechnol. 1999;75:135–146. doi: 10.1016/S0168-1656(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Fan G, Zhu Y, Fu Z, Sun B, Teng C, Yang R, Li X. Optimization of fermentation conditions for the production of recombinant feruloyl esterase from Burkholderia pyrrocinia B1213. 3 Biotech. 2020;10:216. doi: 10.1007/s13205-020-02198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler M, Gummesson B, Charbon G, Svenningsen SL, Sorensen MA. Short-term kinetics of rRNA degradation in Escherichia coli upon starvation for carbon, amino acid or phosphate. Mol Microbiol. 2020;113:951–963. doi: 10.1111/mmi.14462. [DOI] [PubMed] [Google Scholar]
- Ghaderi H, Arasteh J, Hesampour A. Using response surface methodology in combination with Plackett-Burman design for optimization of culture media and extracellular expression of Trichoderma reesei synthetic endoglucanase II in Escherichia coli. Mol Biol Rep. 2018;45:1197–1208. doi: 10.1007/s11033-018-4272-y. [DOI] [PubMed] [Google Scholar]
- Gomes P, Sampaio-Marques B, Ludovico P, Rodrigues F, Leao C. Low auxotrophy-complementing amino acid concentrations reduce yeast chronological life span. Mech Ageing Dev. 2007;128:383–391. doi: 10.1016/j.mad.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Haughey SA, Elliott CT, Oplatowska M, Stewart LD, Frizzell C, Connolly L. Production of a monoclonal antibody and its application in an optical biosensor based assay for the quantitative measurement of pantothenic acid (vitamin B5) in foodstuffs. Food Chem. 2012;134:540–545. doi: 10.1016/j.foodchem.2012.02.116. [DOI] [Google Scholar]
- Holatko J, Elisakova V, Prouza M, Sobotka M, Nesvera J, Patek M. Metabolic engineering of the L-valine biosynthesis pathway in Corynebacterium glutamicum using promoter activity modulation. J Biotechnol. 2009;139:203–210. doi: 10.1016/j.jbiotec.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Huser AT, et al. Rational design of a Corynebacterium glutamicum pantothenate production strain and its characterization by metabolic flux analysis and genome-wide transcriptional profiling. Appl Environ Microbiol. 2005;71:3255–3268. doi: 10.1128/AEM.71.6.3255-3268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing K, Tang Y, Yao C, Del Rio-Chanona EA, Ling X, Zhang D. Overproduction of L-tryptophan via simultaneous feed of glucose and anthranilic acid from recombinant Escherichia coli W3110: Kinetic modeling and process scale-up. Biotechnol Bioeng. 2018;115:371–381. doi: 10.1002/bit.26398. [DOI] [PubMed] [Google Scholar]
- Johansen ML. The metabolic role of isoleucine in detoxification of ammonia in cultured mouse neurons and astrocytes. Neurochem Int. 2007;50:1042–1051. doi: 10.1016/j.neuint.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Klamt S, Mahadevan R. On the feasibility of growth-coupled product synthesis in microbial strains. Metab Eng. 2015;30:166–178. doi: 10.1016/j.ymben.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Krige A, Nicol W. Continuous succinic acid fermentation by Escherichia coli KJ122 with cell recycle. Process Biochem. 2015;50:2004–2011. doi: 10.1016/j.procbio.2015.09.023. [DOI] [Google Scholar]
- Lee A, Choi K-H, Yoon D, Kim S, Cha J. Characterization of a thermostable glycoside hydrolase family 36 α-galactosidase from Caldicellulosiruptor bescii. J Biosci Bioeng. 2017;124:289–295. doi: 10.1016/j.jbiosc.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Jackowski S. Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus. 2007 doi: 10.1128/ecosalplus.3.6.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Sun Z. Cloning and expression of d -lactonohydrolase cDNA from Fusarium moniliforme in Saccharomyces cerevisiae. Biotech Lett. 2004;26:1861–1865. doi: 10.1007/s10529-004-5320-3. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun Z, Leng Y. Directed evolution and characterization of a novel d-pantonohydrolase from Fusarium moniliforme. J Agric Food Chem. 2006;54:5823–5830. doi: 10.1021/jf060794m. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Wu L, Zheng L, Wang WZ, Zhang XJ, Jin LQ, Zheng YG. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media. Bioresour Technol. 2018;249:161–167. doi: 10.1016/j.biortech.2017.09.204. [DOI] [PubMed] [Google Scholar]
- Long J, Zhao X, Liang F, Liu N, Sun YY, Xi YZ. Optimization of fermentation conditions for an Escherichia coli strain engineered using the response surface method to produce a novel therapeutic DNA vaccine for rheumatoid arthritis. J Biol Eng. 2018;12:22. doi: 10.1186/s13036-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Sámano M, Beltran LFL, Sanchez-Thomas R, Davalos A, Villasenor T, Garcia-Garcia JD, Garcia-de Los Santos A. A novel way to synthesize pantothenate in bacteria involves beta-alanine synthase present in uracil degradation pathway. Microbiologyopen. 2020 doi: 10.1002/mbo3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin R, Mohammadi G, Leibfritz D, Hamprecht B. Glial metabolism of isoleucine. Neurochem Res. 2009;34:194–204. doi: 10.1007/s11064-008-9840-4. [DOI] [PubMed] [Google Scholar]
- Ohtsuka H, Kato T, Sato T, Shimasaki T, Kojima T, Aiba H. Leucine depletion extends the lifespans of leucine-auxotrophic fission yeast by inducing Ecl1 family genes via the transcription factor Fil1. Mol Genet Genomics. 2019;294:1499–1509. doi: 10.1007/s00438-019-01592-6. [DOI] [PubMed] [Google Scholar]
- Ruklisha M, Paegle L, Denina I. l-Valine biosynthesis during batch and fed-batch cultivations of Corynebacterium glutamicum: Relationship between changes in bacterial growth rate and intracellular metabolism. Process Biochem. 2007;42:634–640. doi: 10.1016/j.procbio.2006.11.008. [DOI] [Google Scholar]
- Sahm H, Eggeling L. D-pantothenate synthesis in Corynebacterium glutamicum and use of panBC and genes encoding L-valine synthesis for D-pantothenate overproduction. Appl Environ Microbiol. 1999;65:1973–1979. doi: 10.1128/AEM.65.5.1973-1979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SibandaP P W. Comparative study of the application of box behnken design (BBD) and binary logistic regression (BLR) to study the effect of demographic Characteristics on HIV risk in South Africa. J Appl Med Sci. 2012;1:15–40. doi: 10.1007/s13721-013-0032-z. [DOI] [Google Scholar]
- Trchounian K, Poladyan A, Trchounian A. Optimizing strategy for Escherichia coli growth and hydrogen production during glycerol fermentation in batch culture: effects of some heavy metal ions and their mixtures. Appl Energy. 2016;177:335–340. doi: 10.1016/j.apenergy.2016.05.129. [DOI] [Google Scholar]
- Vyazmensky M, Zherdev Y, Slutzker A, Belenky I, Kryukov O, Barak Z, Chipman DM. Interactions between large and small subunits of different acetohydroxyacid synthase isozymes of Escherichia coli. Biochemistry. 2009;48:8731–8737. doi: 10.1021/bi9009488. [DOI] [PubMed] [Google Scholar]
- Wang J, Wen B, Xu Q, Xie X, Chen N. Optimization of carbon source and glucose feeding strategy for improvement of L-isoleucine production by Escherichia coli. Biotechnol Biotechnol Equip. 2015;29:374–380. doi: 10.1080/13102818.2015.1006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang H, Quinn PJ. Production of L-valine from metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2018;102:4319–4330. doi: 10.1007/s00253-018-8952-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang R, Zhang Y, Yang Y, Lin Y, Yan Y. Developing a pyruvate-driven metabolic scenario for growth-coupled microbial production. Metab Eng. 2019;55:191–200. doi: 10.1016/j.ymben.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb ME, Smith AG, Abell C. Biosynthesis of pantothenate. Nat Prod Rep. 2004;21:695–721. doi: 10.1039/b316419p. [DOI] [PubMed] [Google Scholar]
- Wu K, Ding LJ, Zhu P, Li S, He S. Application of the response surface methodology to optimize the fermentation parameters for enhanced docosahexaenoic acid (DHA) production by Thraustochytrium sp ATCC 26185. Molecules. 2018;23:974. doi: 10.3390/molecules23040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SK, Zhou Y, Li Z. Biocatalytic selective functionalisation of alkenes via single-step and one-pot multi-step reactions. Chem Commun. 2019;55:883–896. doi: 10.1039/c8cc07828a. [DOI] [PubMed] [Google Scholar]
- Xu J, et al. Cerebral deficiency of vitamin B5 (D-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer's disease. Biochem Biophys Res Commun. 2020;527:676–681. doi: 10.1016/j.bbrc.2020.05.015. [DOI] [PubMed] [Google Scholar]
- Xu FX, Cao H, Cui XW, Guo H, Han CC. Optimization of fermentation condition for echinacoside yield improvement with Penicillium sp. H1, an endophytic fungus isolated from Ligustrum lucidum ait using response surface methodology. Molecules. 2018 doi: 10.3390/molecules23102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Chou J, Wang T, Zhao H, Zhang B. Pantothenic acid, vitamin c, and biotin play important roles in the growth of Lactobacillus helveticus. Front Microbiol. 2018;9:1194. doi: 10.3389/fmicb.2018.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetilmezsoy K, Demirel S, Vanderbeic RJ. Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: Box-Behnken experimental design. J Hazard Mater. 2009;171:551–562. doi: 10.1016/j.jhazmat.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhang XM, Wang W, Liu ZQ, Zheng YG. Metabolic engineering of Escherichia coli for D-pantothenic acid production. Food Chem. 2019;294:267–275. doi: 10.1016/j.foodchem.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Wu WJ, Niu K, Xu YY, Liu ZQ, Zheng YG. Enhanced L-methionine production by genetically engineered Escherichia coli through fermentation optimization. 3 Biotech. 2019;9:96. doi: 10.1007/s13205-019-1609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu Y, Li J, Shin H-d, Du G, Liu L, Chen J. An optimal glucose feeding strategy integrated with step-wise regulation of the dissolved oxygen level improves N-acetylglucosamine production in recombinant Bacillus subtilis. Biores Technol. 2015;177:387–392. doi: 10.1016/j.biortech.2014.11.055. [DOI] [PubMed] [Google Scholar]
- Zou SP, Wang ZJ, Zhao K, Zhang B, Niu K, Liu ZQ, Zheng YG. High-level production of d-pantothenic acid from glucose by fed-batch cultivation of Escherichia coli. Biotechnol Appl Biochem. 2020 doi: 10.1002/bab.2044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.