Abstract
Immature citrus with peel was enzymatically treated for production of a hydrolysate with enriched bioactive components and higher antioxidant activity. The effects of reaction factors, including enzyme concentration, reaction time, and temperature on hesperetin and naringenin contents, total phenolic content (TPC), and antioxidant activity were investigated using response surface methodology. The models were adequate, and the enzyme concentration, temperature, and time positively affected hesperetin and naringenin contents and TPC, but negatively affected DPPH radical scavenging capacity. The reaction conditions for maximizing hesperetin, naringenin, and total phenol production and ferric reducing antioxidant power were optimized with the combination of enzyme concentration at 4%, 51 °C and 18 h. The hydrolysate at the optimized conditions contained higher hesperetin and naringenin contents and TPC compared with those before hydrolysis, by 251.7-, 45.5-, and 2.6-fold, respectively. This hydrolysate can be utilized in the production of functional beverages with high added values.
Keywords: Antioxidant activity, Enzymatic hydrolysis, Hesperetin, Immature citrus, Response surface methodology
Introduction
Citrus (Citrus unshiu Marcov.) flavonoids exist in the form of glycosides or aglycone, and the major flavonoid glycosides contain glucose, arabinose, rhamnose, and galactose that are mostly bonded to aglycone via α-1,4 or β-1,4 linkage (Tripoli et al., 2007). Citrus flavonoids have been associated with anti-oxidant, anti-inflammatory, anti-microbial, anti-carcinogenic, and cardioprotective activities (Hyun et al., 2015; Kawaii et al., 2012; Yao et al., 2012). These flavonoids are more abundant in the peel than in the pulp, and the contents of glycosides are higher in immature citrus than in mature citrus (Song et al., 1998). Hesperidin has rutinose attached to hesperetin, and naringin has two rhamnoses attached to naringenin, in which hesperidin and naringin show lower antioxidant activity than hesperetin and naringenin because the sugar moiety causes a structurally steric hindrance of scavenging group (Tripoli et al., 2007). The attached sugar moiety is the major determinant of absorption of dietary flavonoids, affecting the bioavailability of flavonoids, and flavonoid glycosides attached with rhamnose are poorly absorbed, compared with their glucosides or aglycone (Hollman et al., 1999). Conversion of flavonoid glycosides into aglycones would improve their absorption and bioactivity in the body.
The insoluble components in citrus account for sensory characteristics such as taste, aroma, and color, and cause turbidity in the juice. Commercial enzymes in single or mixed form of carbohydrases are used to improve the manufacturing process and quality of citrus beverages for clarification and reduced viscosity by hydrolysis of cell wall polysaccharides (Ribeiro et al., 2010). Cell wall-hydrolyzing enzymes (cellulase, hemicellulase, β-glucosidase, xylanase, β-glucanase, and pectinase) help to degrade the cell wall structure by depolymerizing cell wall polysaccharides, resulting in the release of the linked compounds (Gil-Chávez et al., 2013), and additionally convert flavonoid glycosides into aglycones (Ahn et al., 2005).
Response surface methodology (RSM) is a statistical method that analyzes the interactive relationship of independent variables on one or several dependent variables, and it has been widely applied in development of product in the food industry. Extraction method (ultrasonic or enzyme treated extraction), solvent (ethanol or methanol concentration), and conditions (temperature, time, and pH) have been optimized to obtain highly active antioxidative extracts with higher contents of phenolic compounds from citrus fruits or their by-products (Assefa et al., 2017; Iglesias-Carres et al., 2019; Ma et al., 2008; Montero-Calderon et al., 2019; Nishad et al., 2019). Furthermore, RSM verified that the extract of citrus peel, obtained using enzyme-assisted methods with Viscozyme® L, contained higher total phenolic and flavonoid contents, and had excellent antioxidant activity compared with that obtained using the ultrasonic-assisted method (Nishad et al., 2019).
Citrus is the most produced and consumed fruit in Korea. However, the citrus industry is in decline because of overproduction, consumption reduction, and market opening. To overcome this, an interest in the high value-added processed products using immature citrus with high functional substances has increased. In this study, immature citrus with peel was treated with commercial carbohydrase Viscozyme® L, and the effects of the enzyme concentration, reaction temperature, and time on the production of bioactive compounds (hesperetin, naringenin, and total phenols) and antioxidant activities were investigated using RSM. The reaction condition was optimized to obtain an enzymatic hydrolysate with high levels of bioactive compounds and antioxidant activity. Further, the enzymatic immature citrus hydrolysate was evaluated for potential usage in the production of functional beverages with high added values.
Materials and methods
Materials
Immature citrus cultivated in Jeju, Korea in 2018 was provided from by Fresh Bell Co., Ltd (Gyeongsan, Korea). The citrus was crushed with both peel and pulp using a blender. The standards hesperidin, hesperetin, naringin, naringenin, gallic acid, and L(+)-ascorbic acid were purchased from Sigma Chemical Co. Ltd (St. Louis, MO, USA). Acetic acid and HPLC grade methanol and water were purchased from J. T. Baker (Center Valley, PA, USA). Viscozyme® L was obtained from Novozymes (Bagsværd, Denmark). Folin-Ciocalteu phenol reagent, iron (III) sulfate hexahydrate, DPPH, and TPTZ were purchased from Sigma-Aldrich Co., Ltd.
Experimental design for RSM
A previous study has reported the suitable type of enzyme and the pH of reaction buffer for the conversion of hesperidin and naringin into hesperetin and naringenin, respectively (Shin et al., 2020). Based on the results, Viscozyme® L (100 Fungal beta-Glucanase Units/g) and a reaction buffer of pH 5.0 were used in this study.
The enzymatic hydrolysis reaction of immature citrus was optimized using RSM. A central composite design with three factors and three levels comprising 17 randomized runs in triplicate was selected using Modde® Pro version 12.1 (Umeritics, Umeå, Sweden). The independent variables (reaction factors) were enzyme concentration (X1), 1-4% (w/w) of citrus; reaction temperature (X2), 40–60 °C; and reaction time (X3), 2–24 h. The dependent variables (responses) were hesperetin content (Y1, mg/g), naringenin content (Y2, mg/g), TPC (Y3, GAE mg/100 g), DPPH radical scavenging capacity (RSC) (Y4, %), and FRAP (Y5, AAE mg/100 g). The second-order polynomial equation used for the optimization of the enzymatic reaction condition follows
Where Y is the response, and Xi and Xj are the reaction factors. β0 represents the constant coefficient, and βi, βii, and βij represent the linear, quadratic and interaction regression coefficients, respectively. The three reaction factors with three levels and their experimental response results are presented in Table 1.
Table 1.
Three factor and three-level central composite face design and the observed responses
| Experiment number | Independent variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme concentration (%) | Temperature (°C) | Time (h) | Hesperetin (mg/g) | Naringenin (mg/g) | TPC (mg GAE/100g) | DPPH RSC (%) | FRAP (mg AAE/100g) | |
| 1 | 1 (−1) | 40 (−1) | 2 (−1) | 0.139 | 0.185 | 304.58 | 40.22 | 174.88 |
| 2 | 1 (−1) | 60 (1) | 2 (−1) | 0.470 | 0.248 | 292.63 | 35.33 | 138.84 |
| 3 | 4 (1) | 40 (−1) | 2 (−1) | 0.521 | 0.316 | 334.72 | 34.64 | 190.15 |
| 4 | 4 (1) | 60 (1) | 2 (−1) | 1.554 | 0.436 | 347.56 | 28.75 | 176.23 |
| 5 | 1 (−1) | 40 (−1) | 24 (1) | 1.033 | 0.338 | 331.30 | 34.62 | 162.19 |
| 6 | 1 (−1) | 60 (1) | 24 (1) | 1.403 | 0.318 | 288.72 | 25.11 | 122.09 |
| 7 | 4 (1) | 40 (−1) | 24 (1) | 2.002 | 0.482 | 382.86 | 33.68 | 184.53 |
| 8 | 4 (1) | 60 (1) | 24 (1) | 2.567 | 0.509 | 375.30 | 28.31 | 150.07 |
| 9 | 2.5 (0) | 40 (−1) | 13 (0) | 1.195 | 0.402 | 369.57 | 34.41 | 191.98 |
| 10 | 2.5 (0) | 60 (1) | 13 (0) | 2.326 | 0.499 | 327.84 | 28.68 | 156.54 |
| 11 | 1 (−1) | 50 (0) | 13 (0) | 1.234 | 0.350 | 360.19 | 35.17 | 174.88 |
| 12 | 4 (1) | 50 (0) | 13 (0) | 2.460 | 0.542 | 392.49 | 33.00 | 172.86 |
| 13 | 2.5 (0) | 50 (0) | 2 (−1) | 0.737 | 0.355 | 338.62 | 34.98 | 190.29 |
| 14 | 2.5 (0) | 50 (0) | 24 (1) | 2.462 | 0.535 | 398.22 | 30.62 | 168.92 |
| 15 | 2.5 (0) | 50 (0) | 13 (0) | 1.946 | 0.495 | 385.84 | 32.89 | 178.90 |
| 16 | 2.5 (0) | 50 (0) | 13 (0) | 2.080 | 0.512 | 409.91 | 34.03 | 183.82 |
| 17 | 2.5 (0) | 50 (0) | 13 (0) | 2.096 | 0.511 | 377.59 | 33.66 | 172.15 |
TPC total phenolic compounds, DPPH RSC 2,2-diphenyl-1-picrylhydrazyl radical’s scavenging capacity, FRAP ferric reducing antioxidant power, GAE gallic acid equivalent, QE quercetin equivalent, AAE ascorbic acid equivalent.
Enzymatic hydrolysis of immature citrus
The crushed immature citrus with its peel was diluted with sodium acetate buffer and titrated to pH 5.0. Viscozyme® L (1–4%, w/w) was added, and incubated in a shaking water bath (40–60 °C) at 150 rpm for 2–24 h. The hydrolyzed immature citrus was boiled to deactivate the enzyme in a 90–100 °C water bath for 20 min, and freeze-dried (PVTFD20R, Il shin Bio Base, Dongducheon, Korea). After extracted in methanol (40 mL) by sonication (2 h) and centrifugation at 1763 × g for 15 min, the obtained supernatant was filtered through a syringe filter, and used for further analysis.
Quantification of flavonoids
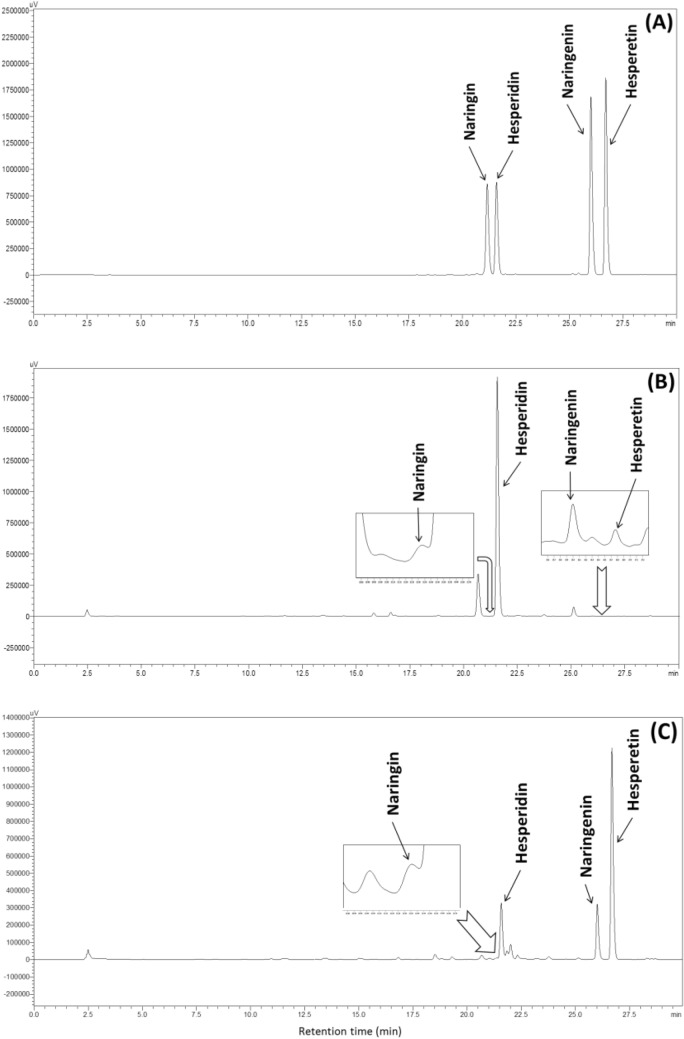
Quantitative analysis was performed by an HPLC (LC-20AD, Shimadzu Corp., Kyoto, Japan) with a Zorbax Eclipse XDB-C18 Column (4.6 × 250 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA), and a UV detection at 280 nm. The mobile phase solvents, methanol (A) and 0.5% acetic acid (B) were used. A gradient elution was performed: the ratio of solvent A and B was maintained at 15:85 (v/v) for 3 min, 85:15 (v/v) over 30 min and maintained for 2 min, and then 15:85 (v/v) over 3 min and maintained for 10 min. The injection volume was 10 μL, and column temperature and flow rate were set at 31 °C, and 1.0 mL/min, respectively. After obtaining standard curves, the contents of hesperidin, hesperetin, naringin, and naringenin were quantified as mg/g in hydrolyzed immature citrus. The HPLC chromatograms of standards, immature citrus, and the hydrolyzed immature citrus were presented in Fig.1.
Fig. 1.
The HPLC chromatograms of standards (A), immature citrus (B), and the hydrolyzed immature citrus (C) at 51°C, for 18 h, and 4% (w/w) of Viscozyme® L.
TPC
Methanol extract (0.5 mL), distilled water (4.5 mL), and Folin-Ciocalteu reagent (0.5 mL) were mixed, and left to stand for 3 min. One milliliter of 1 N Na2CO3 was added and placed in the dark for 1 h, and the absorbance was measured at 725 nm using a micro plate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA). A calibration curve of gallic acid was obtained; TPC was calculated and expressed as mg gallic acid equivalent (GAE)/100 g hydrolyzed immature citrus.
Analysis of antioxidant activities
DPPH RSC was measured using the method of Lee and Cho (2014). A 5-fold diluted methanol extract (0.2 mL), methanol (1.8 mL), and 0.15 mM DPPH reagent (2.5 mL) were mixed and placed in the dark for 30 min. The absorbance was measured at 517 nm with a micro plate reader, and DPPH RSC was calculated using the following equation.
Where AB is the absorbance of blank and AS is the absorbance of sample.
Ferric reducing antioxidant power (FRAP) was measured using the method of Lee and Cho (2014). A FRAP reagent was prepared by mixing 300 mM acetate buffer (250 mL, pH 3.6), 10 mM TPTZ solution, and 20 mM FeCl3·6H2O solution (50 mL). In a test tube, the diluted methanol extract (150 μL) and FRAP reagent (4.5 mL) were added, then the mixture was left to react at 37 °C for 4 min. The absorbance was measured at 593 nm using a micro plate reader. A calibration curve with ascorbic acid was obtained, and the FRAP value was calculated and expressed as mg AAE/g hydrolyzed immature citrus.
Statistical analysis
The RSM experimental design, the analysis of variance (ANOVA), regression coefficients, and prediction and response surface plots were performed using Modde® Pro version 12.1. The statistical difference between predicted and experimental values were determined using t-test at p < 0.05 with the Statistical Analysis System 9.2 (SAS Institute Inc., Cary, NC, USA).
Results and discussion
Model fitting
The effects of enzyme concentration, reaction temperature, and time on hesperetin and naringenin contents, TPC, and antioxidant activities (DPPH RSC and FRAP) of immature citrus were evaluated during the reaction. The observed responses generated using the RSM experimental design and ANOVA for the second-order polynomial regression model are presented in Tables 1 and 2. The lack of fit was not significant for the models of hesperetin, naringenin, TPC, DPPH RSC, and FRAP (p > 0.05), which indicates that the all models were adequately fit. For the models, the goodness of fit was evaluated with the coefficient of determination (R2), and the R2 shows that the models fit (Table 2). The adjusted coefficient (R2adj) indicated good agreement between actual and observed data for the models of hesperetin, naringenin, and DPPH RSC, because the R2adj, close to the R2, insures a satisfactory adjustment of the quadratic models to the experimental data (Trinh and Kang, 2010).
Table 2.
Analysis of variance of the second order polynomial models for the responses
| Source | df | Sum of Squares | Mean Square | F-value | p-value |
|---|---|---|---|---|---|
| Hesperetin | |||||
| Regression | 9 | 9.24 | 1.03 | 18.40 | 0.0004 |
| Lack of Fit | 5 | 0.38 | 0.08 | 11.11 | 0.0847 |
| Pure error | 2 | 0.01 | 0.01 | ||
| R2 | 0.96 | R2adj | 0.91 | ||
| Naringenin | |||||
| Regression | 9 | 0.17 | 0.02 | 43.11 | 0.0000 |
| Lack of Fit | 5 | 0.00 | 0.00 | 7.01 | 0.1295 |
| Pure error | 2 | 0.00 | 0.00 | ||
| R2 | 0.98 | R2adj | 0.96 | ||
| TPC | |||||
| Regression | 9 | 20119.83 | 2235.54 | 8.24 | 0.0055 |
| Lack of Fit | 5 | 1335.92 | 267.189 | 0.975 | 0.5854 |
| Pure error | 2 | 564.00 | 282.00 | ||
| R2 | 0.91 | R2adj | 0.80 | ||
| DPPH RSC | |||||
| Regression | 9 | 196.00 | 21.78 | 27.18 | 0.0001 |
| Lack of Fit | 5 | 4.93 | 0.99 | 2.92 | 0.2748 |
| Pure error | 2 | 0.68 | 0.34 | ||
| R2 | 0.97 | R2adj | 0.94 | ||
| FRAP | |||||
| Regression | 9 | 5322.80 | 591.42 | 8.90 | 0.0044 |
| Lack of Fit | 5 | 396.77 | 79.35 | 2.31 | 0.3290 |
| Pure error | 2 | 68.65 | 34.33 | ||
| R2 | 0.92 | R2adj | 0.82 | ||
R2, the coefficient of determination; R2adj, the adjusted coefficient
TPC total phenolic compounds, DPPH RSC 2,2-diphenyl-1-picrylhydrazyl radical’s scavenging capacity, FRAP ferric reducing antioxidant power, GAE gallic acid equivalent, QE quercetin equivalent, AAE ascorbic acid equivalent.
Effect of reaction factors on hesperetin, naringenin, and TPC of hydrolyzed immature citrus
The regression coefficients of the second order response models were determined to predict polynomial models for hesperetin, naringenin, and TPC; their second-order polynomial equations are:
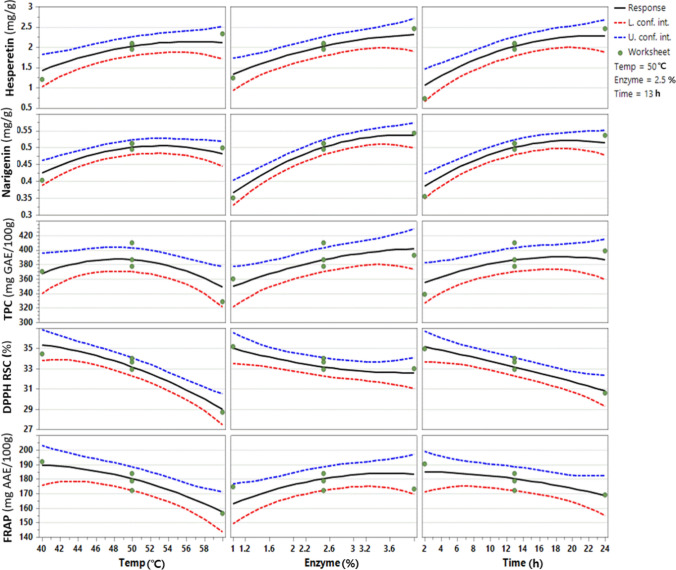
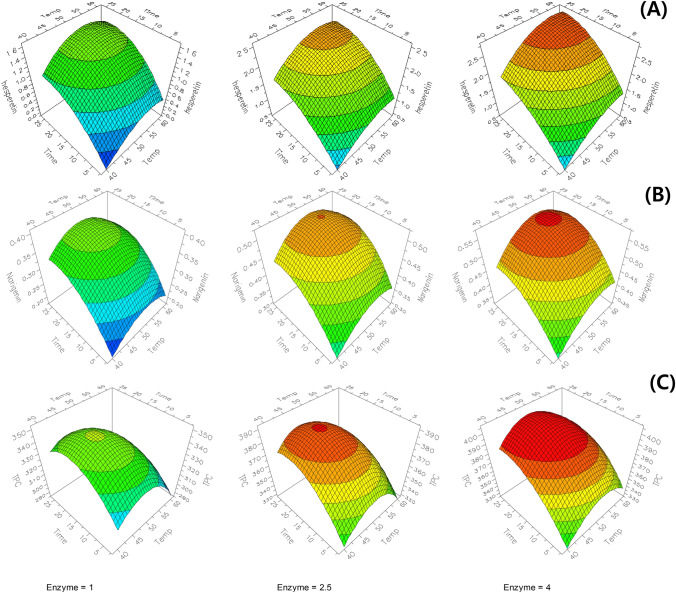
The enzyme concentration, reaction temperature, and time significantly affected the hesperetin content in the hydrolyzed immature citrus (p < 0.01). The hesperetin content observed was the lowest (0.139 mg/g) at 40 °C, 2 h, and 1% enzyme, and the content increased to 2.567 mg/g by increasing the conditions to 60 °C, 24 h, 4% enzyme (Table 1). The prediction plot presented the single effect of reaction factors by showing the predicted low, center, and high values with all observed values were within 95% confidence interval (Fig. 2). The response surface plots displayed the predicted response values by combination of reaction temperature and time at enzyme concentration of 1, 2.5, and 4%, and showed that the increase of reaction temperature, time, and enzyme concentration led to the increased hesperetin content (Fig. 3). The maximum hesperertin content was predicted as 2.825 mg/g at the reaction condition of 57 °C, 4% enzyme, and 24 h by the RSM.
Fig. 2.
Prediction plots for hesperetin, naringenin, TPC, DPPH RSC, and FRAP of the hydrolyzed immature citrus using Viscozyme® L. Average, minimum, and maximum predicted values (95% of confidence interval) represented by the black, red, and blue lines, respectively.
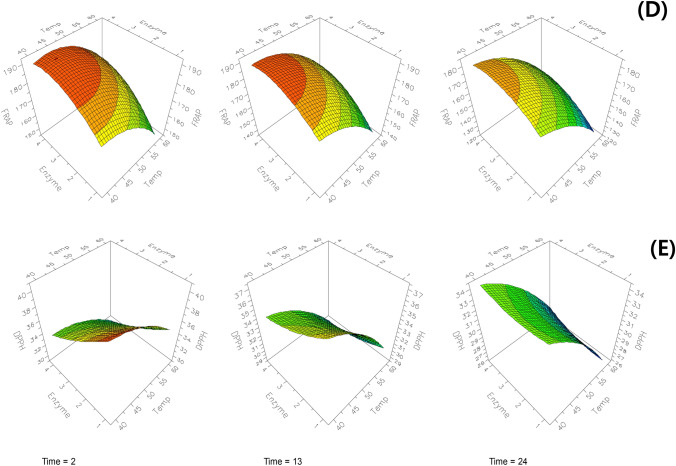
Fig. 3.
Response surface plots for hesperetin (A), naringenin (B), TPC (C), DPPH RSC (D), and FRAP (E) of the hydrolyzed immature citrus using Viscozyme® L.
The linear and quadratic terms of naringenin content had significant effects (p < 0.05), and the content significantly increased with an increased reaction time (p < 0.001), temperature (p = 0.004), and enzyme concentration (p = 0.001). The interaction between the enzyme concentration and temperature was also significant (p < 0.05). The lowest content was observed at 40 °C, 13 h, and 4% enzyme, whereas the highest content was observed at 50 °C, 13 h, and 4%. In the prediction and response surface plots, naringenin content increased with the increased enzyme concentration and time, respectively, and the content increased with temperature from 40 to 50 °C, and then stabilized or reduced at 60 °C. The maximum naringenin content (0.566 mg/g) was predicted at 53.3 °C, 4% enzyme, 19.9 h of reaction optimized by the RSM, and the minimum naringenin content (0.182 mg/g) was expected at 40 °C, 1% enzyme, and 2 h of reaction.
The TPC was significantly positively affected by enzyme concentration (p = 0.002) and reaction time (p = 0.017), but not affected by temperature (p = 0.118) and by interaction and quadratic terms (p > 0.05). The TPC was observed the lowest (288.72 mg GAE/100g) at 60 °C, 24 h, and 1% enzyme, and the highest (409.91 mg GAE/100g) at 50 °C, 13 h, and 2.5%. The TPC showed an increasing trend with an increase in enzyme concentration and time, but with a decrease in reaction temperature (Figs. 2 and 3). The maximum TPC (407.4 mg GAE/100 g) was predicted at the 49.1°C, 4% enzyme, and 20 h of reaction by the RSM, and the minimum TPC (287.0 mg GAE/100g) was predicted at 60 °C, 1% enzyme, and 24 h of reaction. This is consistent with the regression coefficient of −7.4 for reaction temperature, indicating a negative effect. In the prediction plot, several observed values were higher than the upper confidence interval (at 95% confidence level). R2 and R2adj of the model were 0.914 and 0.803, respectively, suggesting a lower agreement between the predicted and experimental values (Fig. 2).
Plant cell-walls comprise polysaccharides of cellulose, hemicellulose, and pectins, which function as barriers to the release of intracellular substances. Phenolic compounds are bound to polysaccharides in the cell-wall or exist as “non-cell-wall phenolics” inside vacuoles (Pinelo et al., 2006). Flavonoids are naturally located within the cells and are present in various forms while interacting with cell-wall components. Thus, the decomposition of the cell-wall structure is a crucial fundamental step to improve the release of phenolic compounds and flavonoids from the cell-wall.
Viscozyme® L (by Aspergillus aculeatus) is a multi-enzyme complex with the activities of β-1,3(4)-glucanase, arabinase, cellulase, hemicellulase, and xylanase as given by the supplier. Aspergillus aculeatus has both endo-β-1,4-glucanase and β-glucosidase activities, whose activities were optimal at pH 5 (Sharma et al., 1991). Endo-β-1,3(4)-glucanase acts on degradation of cellulose by randomly cleaving glucosidic bonds of (1,3)- or (1,4)-linkage, and release of glucose and cello-oligosaccharides. β-1,4-glucosidase acts on hydrolysis of β-1,4-linkage in 1,4-β-D-glucans and related oligosaccharides, removing successive glucose unit, thus, cleaves polysaccharides cross-linked with phenolic compounds (Yang et al., 2010). β-glucosidase also hydrolyzes the β-1,4 glucosidic linkages in glucosides (flavonoids in conjunction with glucose) (Yang et al., 2010).
Using Viscozyme® L-mediated hydrolysis, TPC of the hydrolyzed immature citrus significantly increased than before hydrolysis (176.56 mg GAE/100 g), with the increase in extracted phenolic compounds from degradation of the cell-wall structure through hydrolysis of the glycosidic bonds between the cell-wall components and phenolic compounds (Table 1 and 3). Nishad et al. (2019) reported that the TPC of Viscozyme® L-hydrolysate of citrus peel was 2216.4 mg GAE/100 g at 0.7% and 4 h, increased to 2766.25 mg GAE/100 g by increasing the enzyme concentration (0.9%), and to 3266.95 mg GAE/100 g by increasing the reaction time (5 h); which were consistent with the results in this study.
Table 3.
Predicted and experimental values of the responses at the optimized reaction condition
| Before enzymatic hydrolysisa | Enzymatic hydrolysisb | |||
|---|---|---|---|---|
| Predicted value | Experimental value | P-value (t-test) | ||
| Hesperidin (mg/g) | 7.838 ± 0.034 | –c | 1.387 ± 0.062 | – |
| Naringin (mg/g) | 0.004 ± 0.000 | – | 0.035 ± 0.001 | – |
| Hesperetin (mg/g) | 0.010 ± 0.000 | 2.599 | 2.517 ± 0.043 | p = 0.081 |
| Naringenin (mg/g) | 0.013 ± 0.001 | 0.562 | 0.592 ± 0.005 | p = 0.010 |
| Naringenin + Hesperetin (mg/g) | 0.023 ± 0.001 | 3.161 | 3.109 ± 0.327 | p = 0.174 |
| TPC (mg GAE/100 g) | 176.56 ± 1.55 | 405.99 | 467.81 ± 4.57 | p = 0.002 |
| FRAP (mg AAE/100 g) | 159.53 ± 7.76 | 177.44 | 185.35 ± 4.85 | p = 0.106 |
| DPPH RSC (%) | 26.95 ± 1.10 | 32.04 | 34.05 ± 1.30 | p = 0.115 |
aReaction at 51°C, 18 h without Viscozyme® L, and boiled
bReaction at 51°C, 18 h with 4% (w/w) of Viscozyme® L, and boiled for inactivation of enzyme
cNot available
TPC total phenolic compounds, DPPH RSC 2,2-diphenyl-1-picrylhydrazyl radical’s scavenging capacity, FRAP ferric reducing antioxidant power
Concentration of Viscozyme® L, reaction time, and temperature showed positive linear effects on the production of hesperetin and naringenin. This implies that Viscozyme® L led to the release of flavonoid glycosides, through cell-wall polysaccharide degradation, and the released hesperidin and naringin were converted to hesperetin and naringenin by decomposition of the bonds attaching rutinose or rhamnose, respectively. The deglycosylation capacity of Viscozyme® L was demonstrated in cauliflower leaves in which kaempferol-3-feruloyltriglucoside-7-glucoside and kaempferol-3-diglucoside were converted into kaempferol-3-feruloyldiglucoside and kaempferol-3-glucoside by removing one and two glucose molecules, respectively (Huynh et al., 2014). Furthermore, Ahn et al. (2005) and Lee et al. (2007) reported that naringenin and hesperetin contents in hydrolyzed citrus peel and mature citrus increased as the concentration of Viscozyme® L and reaction time increased.
Effect of reaction factors on DPPH RSC and FRAP of the hydrolyzed immature citrus
The regression coefficients and the second order polynomial equations of reaction factors for DPPH RSC and FRAP of the hydrolyzed immature citrus are as follows;
DPPH RSC was most negatively affected by the reaction temperature, followed by reaction time, and enzyme concentration (p < 0.01), and significantly affected by the interaction of the enzyme concentration and reaction time (p < 0.001). DPPH RSC was observed the lowest (25.11%) at 60 °C, 24 h, and 1% enzyme, and the highest (40.22%) at 40 °C, 2 h, and 1% enzyme (Table 1), and DPPH RSC was predicted as minimum values (25.8%) and maximum (40.8%), respectively, at these reaction conditions. The prediction and surface response plots showed that DPPH RSC decreased with an increase in reaction temperature, time, and enzyme concentration, which contrasted with the positive effect of reaction factors on the prediction of hesperetin and naringenin contents (Figs. 2 and 3).
The FRAP value was positively affected by enzyme concentration (p < 0.05), whereas the reaction temperature and time had a negative effect (p < 0.05). The FRAP value was observed the lowest (122.09 mg AAE/100 g) at 60 °C, 24 h, and 1%, and the highest (191.98 mg AAE/100 g) at 40 °C, 13 h, and 2.5%. The prediction and response surface plots showed the FRAP value decreased with higher reaction temperature and longer reaction time but increased with higher enzyme concentration (Figs. 2 and 3), and predicted the maximum FRAP (192.5 mg AAE/100g) at 43°C, 3.6 h, and 3.5% of reaction, and the minimum FRAP (122.4 mg AAE/100g) at 60°C, 24 h, and 1.1% enzyme.
Antioxidative activity of citrus corresponds to antioxidant substances including phenols, flavonoids, carotenoids, and vitamin C (Song et al., 1998). During Viscozyme® L-mediated hydrolysis, immature citrus undergoes the degradation of cell-wall polysaccharides that results in release of phenolic compounds, and the decomposition of the sugar in flavonoid glycoside converts it into aglycone compounds, hesperetin, and naringenin, leading to increased antioxidant activity. Alternatively, the antioxidant activity of the hydrolysate can be reduced by the destruction or loss of the antioxidants during enzymatic reaction with higher temperature and longer time, or during the boiling treatment for termination of enzyme activity after the reaction. The DPPH RSCs of immature citrus and boiled immature citrus were 35.54 ± 0.45% and 31.04 ± 0.33%, respectively (Data not shown), whereas those of Viscozyme® L-added and Viscozyme® L-free immature citrus at 51°C and 18 h were 34.05 ± 1.30% and 26.95 ± 1.10%, respectively (Table 3). This indicated that DPPH RSC of immature citrus was decreased by the boiling process, and can be affected by enzyme concentration, temperature, and time during hydrolysis.
Thermal processing of citrus products provides an acceptable shelf-life by inhibiting microbial spoilage and inactivating degrading enzymes, however it causes detrimental influences on the nutritional properties. Pasteurization treatment (75–95 °C) causes a degradation of vitamin C, carotenoids, and anthocyanins, and as the pasteurization temperature increases, the negative effect increases, resulting in reduced antioxidant contents and activities. However, flavanones (hesperetin and naringenin) are highly stable to thermal treatment, and their contents remain unaffected (Galaverna and Dall’Asta, 2014), which corresponds to the result observed in this study (Table 3).
Optimization of enzymatic hydrolysis conditions and model validation
The major flavonoid in immature citrus before hydrolysis was hesperidin (7.838 mg/g) with a trace amount of naringin, hesperetin, and naringenin; its TPC was 176.56 mg GAE/100 g, and the DPPH RSC and FRAP were 26.95% and 159.53 mg AAE/100 g, respectively (Table 3). To optimize the combination of hydrolysis reaction conditions for simultaneously predicting maximum values of flavonoids, total phenolics, and antioxidative capacities in the immature citrus hydrolysate, the responses of hesperetin and naringenin, TPC, and FRAP were applied with the RSM since the DPHP RSC was rather expected to be a minimum within the reaction range of the predicting maximum hesperetin, naringenin, and total phenolics contents.
The optimal reaction conditions were predicted as the combination of Viscozyme® L of 4%, reaction temperature of 51 °C, and time of 18 h. The predicted responses of hesperetin, naringenin, TPC, DPPH RSC, and FRAP were 2.599 mg/g, 0.562 mg/g, 405.99 g GAE/100 g, 32.04%, and 177.44 mg AAE/100 g, respectively (Table 3). To verify the predicted responses, the hydrolysis reaction was performed at the optimized conditions, and the response of hesperetin, naringenin, TPC, DPPH’s RSC, and FRAP were 2.517 mg/g, 0.592 mg/g, 467.81 mg GAE/100 g, 32.04% and 177.44 mg AAE/100 g, respectively (Table 3).
The t-test showed no significant difference between the predicted and actual experimental values for hesperetin content, DPPH RSC, and FRAP (p > 0.05), confirming that the validity and adequacy of predicted values, and response models were acceptable. For naringenin content, the predicted values were valid at a 90% confidence interval (p = 0.01). There was no significant difference between the predicted and actual value for naringenin + hesperetin content (p = 0.174). Whereas the TPC showed a significant difference between the predicted and actual value (p = 0.002), which appeared consistent with low R2adj value (0.803) of ANOVA results.
In this study, the Viscozyme® L-hydrolysis reaction condition for higher flavonoid production with higher antioxidant activity of immature citrus was optimized using RSM. The models are adequate, and the reaction factors significantly affected hesperetin and naringenin, TPC, DPPH RSC, and FRAP. The enzymatic hydrolysis with Viscozyme® L could clarify and reduced the viscosity of citrus juice by acting on degradation of cell-wall components. In addition, it can improve the functionality of citrus products by converting flavonoid glycosides to aglycones, and by releasing phenolic compounds leading to higher antioxidative activities. The obtained model can be used as the basis for operating a hydrolysis reaction with commercial carbohydrase for higher production of flavonoids and phenolic compounds and higher antioxidative capacities of immature citrus.
Acknowledgments
This work (Grants No S2602087) was supported by project for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Ministry of SMEs and Startups in 2018.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kwang-Seup Shin, Email: botomak2@naver.com.
Jeung-Hee Lee, Email: jeunghlee@daegu.ac.kr.
References
- Ahn SC, Kim MS, Lee SY, Kang JH, Kim BH, Oh WK, Ahn JS. Increase of bioactive flavonoid aglycone extractable from Korean citrus peel by carbohydrate-hydrolysing enzymes. Microbiology and Biotechnology Letters. 33: 288-295 (2005)
- Assefa AD, Saini RK, Keum YS. Extraction of antioxidants and flavonoids from yuzu (Citrus junos Sieb ex Tanaka) peels: a response surface methodology study. Journal of Food Measurement and Characterization. 11: 364-379 (2017)
- Galaverna G, Dall'Asta C. Production processes of orange juice and effects on antioxidant components. In: Processing and Impact on Antioxidants in Beverages, Preedy V (ed), Academic Press, Cambridge, MA, USA. pp. 203-214 (2014)
- Gil-Chávez G, Villa JA, Ayala-Zavala JF, Heredia JB, Sepulveda D, Yahia EM, González-Aguilar GA. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Comprehensive Reviews in Food Science and Food Safety. 12: 5-23 (2013)
- Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radical Research. 31: 569-573 (1999) [DOI] [PubMed]
- Huynh NT, Smagghe G, Gonzales GB, Camp JV, Raes K. Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica oleracea L. var. botrytis) outer leaves. Journal of Agricultural and Food Chemistry. 62: 7468-7476 (2014) [DOI] [PubMed]
- Hyun JM, Park KJ, Kim SS, Park SM. Lee YJ, An HJ. Antioxidant and anti-inflammatory effects of solvent fractions from the peel of the native Jeju citrus ‘Hongkyool’ and ‘Pyunkyool’. Journal of Life Science. 25: 1132-1138 (2015)
- Iglesias-Carres L, Mas-Capdevila A, Bravo FI, Aragonav G, Muguerza B, Arola-Arnal A. Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS One. 14: e0211267 (2019) [DOI] [PMC free article] [PubMed]
- Kawaii S, Ikuina T, Hikima T, Tokiwano T, Yoshizawa Y. Relationship between structure and antiproliferative activity of polymethoxyflavones towards HL60 cells. Anticancer Research. 32: 5239-5244 (2012) [PubMed]
- Lee JH, Cho BK. Effects of red grape, wild grape and black raspberry wines on ground pork during refrigerated storage. Acta Alimentaria. 43: 553-563 (2014)
- Lee MH, Huh D, Jo DJ, Lee GD, Yoon SR. Flavonoids components and functional properties of citrus peel hydrolysate. Journal of the Korean Society of Food Science and Nutrition. 36: 1358-1364 (2007)
- Ma YQ, Chen JC, Liu DH, Ye XQ. Effect of ultrasonic treatment on the total phenolic and antioxidant activity of extracts from citrus peel. Journal of Food Science. 73: T115-T120 (2008) [DOI] [PubMed]
- Montero-Calderon A, Cortes C, Zulueta A, Foglia A, Esteve MJ. Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peel compounds. Scientific Reports. 9: 16120 (2019) [DOI] [PMC free article] [PubMed]
- Nishad J, Saha S, Kaur C. Enzyme‐and ultrasound‐assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. Journal of Food Processing and Preservation. 43: e14046 (2019)
- Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends in Food Science & Technology. 17: 579-590 (2006)
- Ribeiro DS, Henrique SMB, Oliveira LS, Macedo GA, Fleuri LF. Enzymes in juice processing: a review. International Journal of Food Science & Technology. 45: 635-641 (2010)
- Sharma S, Sandhu DK, Bagga PS. Physical characterization of isozymes of endo-β,1,4-glucanase and β-1,4-glucosidase from Aspergillus species. FEMS Microbiology Letters. 79: 99-104 (1991) [DOI] [PubMed]
- Shin KS, Chang HJ, Lee JH. Effect of commercial carbohydrases on the hesperetin and narigenin contents of citrus fruits. Korean Journal of Food Preservation. 27: 446-456 (2020)
- Song EY, Choi YH, Kang KH, Koh JS. Free sugar, organic acid, hesperidin, naringenin and inorganic elements changes of Cheju citrus fruits according to harvest date. Korean Journal of Food Science and Technology. 30: 306-312 (1998)
- Trinh TK, Kang LS. Application of response surface method as an experimental design to optimize coagulation tests. Environmental Engineering Research. 15: 63-70 (2010)
- Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chemistry. 104: 466-479 (2007)
- Yang YC, Li J, Zu YG, Fu YJ, Luo M, Wu N, Liu XL. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chemistry. 122: 373-380 (2010)
- Yao X, Zhu X, Pan S, Fang Y, Jiang F, Phillips GO, Xu X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chemistry. 132: 1883-1890 (2012)