Abstract
Cardiotoxicity in the late phase after anthracycline drugs administration remains to be defined. Of the 44 patients who received anthracycline treatment, 7 were found to have cancer therapeutics–related cardiac dysfunction (CTRCD). The global longitudinal strain determined by echocardiography and myocardial extracellular volume fraction (ECV) determined by cardiac computed tomography (CCT) of the CTRCD(+) group were significantly higher than those of the control group and CTRCD(-) group, whereas there were no significant differences between the control and CTRCD(-) groups. Our findings indicated that CCT may be a tool comparable to echocardiography, indicating the effective evaluation of CTRCD by CCT.
Keywords: Cardio-oncology, Cardiotoxicity, Anthracycline, Cardiac computed tomography
1. Introduction
The development and progress of cancer chemotherapeutic agents has been remarkable, and with the advent of molecular-targeted agents and immune checkpoint inhibitors and the diversification of treatment regimens, cancer survival time has been extended, and cancer survivors are on the rise. Cancer therapeutics–related cardiac dysfunction (CTRCD) is the most important consideration in the prognosis of cancer patients and is a new clinical issue. In particular, the onset of heart failure is a serious complication that causes a great loss in life prognosis and quality of life after the completion of cancer treatment. Therefore, early detection of cardiotoxicity induced by anticancer agents and monitoring are important. Cardiac magnetic resonance (CMR) can evaluate myocardial properties (myocardial tissue characterization) that cannot be evaluated by echocardiography. In the evaluation of late contrast enhancement after administration of gadolinium contrast medium, myocardial damage, namely, myocardial fibrosis, can be visualized as a high signal region. Recent advances in CMR permit the noninvasive assessment of myocardial extracellular volume fraction (ECV), a measure that captures myocardial fibrosis, which have been shown to be a surrogate marker for a cardiac tissue component affected in CTRCD [1]. Therefore, CMR is regarded as “the gold standard” diagnostic imaging method for detecting cardiotoxicity caused by anticancer drug use, and its use is recommended in many guidelines [2], [3]. However, the long examination times and medical costs and the limited number of facilities that can accommodate this method limit the widespread use of CMR. We have shown the usefulness of cardiac computed tomography (CCT), including for assessing myocardial ECV, in CTRCD patients treated with anthracycline (AC) anticancer agents [4], [5], and clinical applications of CCT in the cardio-oncology area are expected in the coming years [6].
Therefore, we investigated the use of CCT to characterize myocardial tissue in the late phase after AC anticancer drug use in Japanese breast cancer patients.
2. Methods
2.1. Ethical consideration
All procedures were conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the Institutional Review Board of Kumamoto University (Approval No. Rinri 1933), and written informed consent was obtained from each patient or the family of the patient. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000047554).
2.2. Study design and participant enrollment
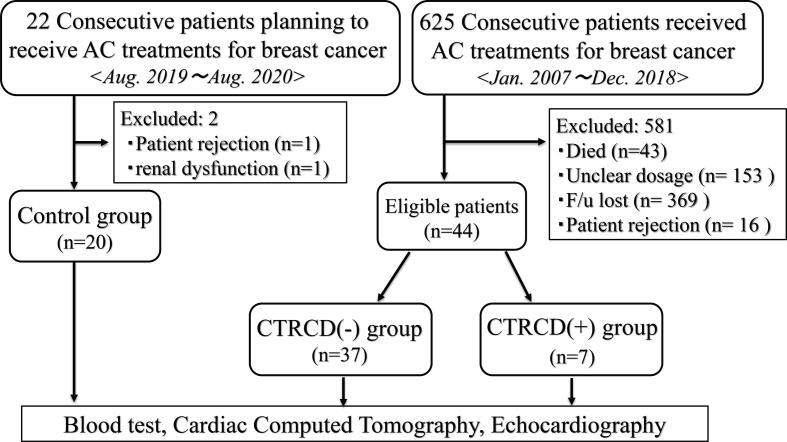
We prospectively investigated 625 consecutive patients who were diagnosed with breast cancer and received AC therapy at Kumamoto University Hospital between January 2007 and December 2018. We recorded each patient’s medical history and relevant clinical characteristics. We excluded 581 patients because of missing data, and the remaining 44 eligible patients were enrolled in this study. As a control group, patients planning to receive AC treatment for breast cancer (2019 August ~ 2020 August) were recruited. We excluded 2 patients (1 patient rejection and 1 renal dysfunction), and the remaining 20 control patients were enrolled. 2016 ESC Position Paper described “The cardiotoxicity of ACs may be acute, early or late. Early effects occur within the first year of treatment, while late effects manifest themselves after several years [2].” According to this Position Paper, we defined “late” AC-induced cardiotoxicity as patients more than 1 year after AC administration. The study flowchart is shown in Fig. 1.
Fig. 1.
Study Flowchart. AC: anthracycline, CTRCD: cancer therapeutics–related cardiac dysfunction.
2.3. Definition of CTRCD
CCT [5] and global longitudinal strain (GLS) determined by echocardiography [7] were performed as described in detail.
A decrease in the left ventricular (LV) ejection fraction (EF) > 10 percentage points and to a value <53% was defined as CTRCD, according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging expert consensus [8]. The utility of echocardiography in detection of CTRCD was described in the expert consensus above [8].
2.4. Statistical analysis
Continuous variables are expressed as the means ± standard deviations (SDs), and differences between groups were evaluated using an unpaired Student's t-test. Categorical variables are expressed as frequencies or percentages and were compared using the Chi-squared test. The statistical significance of differences was assessed by analysis of variance (ANOVA) followed by Fisher’s least squared distance test for multiple comparisons. In all tests, differences were considered statistically significant at P < 0.05. SPSS ver. 23.0 (IBM Institute, Armonk, NY, USA) was used for all statistical analyses
3. Results
The patient characteristics are shown in Table 1. The ages of the patients in the CTRCD(−) and CTRCD(+) groups were significantly lower than that of the control group patients, whereas there was no significant difference in ages between the CTRCD(−) and CTRCD(+) groups. The estimated glomerular filtration rate (eGFR) levels of the CTRCD(−) group and CTRCD(+) group were significantly higher than that of the control group, whereas there was no significant difference in eGFR levels between the CTRCD(-) and CTRCD(+) groups. The LVEF values determined by echocardiography of the CTRCD(−) and CTRCD(+) groups were significantly lower than that of control group, and that of the CTRCD(+) group was significantly lower than that of the CTRCD(−) group. The end-diastolic volume (EDV), end-systolic volume (ESV), and GLS determined by echocardiography and ECV determined by CCT of the CTRCD(+) group were significantly lower than those of the control group and the CTRCD(−) group, whereas there were no significant differences between the control and CTRCD(-) groups. CCT-derived ECV images of representative cases were described in Supplemental Fig. 1.
Table 1.
Patient characteristic.
| Control (n = 20) | Post AC (n = 44) |
P value by ANOVA | ||
|---|---|---|---|---|
| CTRCD (−) (n = 37) | CTRCD (+) (n = 7) | |||
| Age at cancer diagnosis, years old | 61.6 ± 11.2 | 52.9 ± 10.6** | 50.9 ± 11.4* | 0.02 |
| Dox-converted dose, mg/m2 | – | 254.8 ± 35.9 | 302.3 ± 35.0 | – |
| -Doxorubicin, n (%) | – | 4 (11) | 6 (86)‡ | – |
| -Epirubicin, n (%) | – | 33 (89) | 0 (0)‡ | – |
| -Dox plus epirubicin, n (%) | – | 0 (0) | 1 (14)† | – |
| Elapsed time from AC treatment, Months | – | 80.8 ± 47.1 | 52.6 ± 30.8 | – |
| BMI§, kg/m2 | 23.2 ± 4.6 | 23.3 ± 3.6 | 22.7 ± 1.9 | 0.92 |
| BSA§, m2 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 0.77 |
| Hypertension, n (%) | 6 (30) | 12 (32) | 4 (57) | 0.40 |
| Dyslipidemia, n (%) | 11 (55) | 1 (3)** | 1 (14) | <0.01 |
| Diabetes mellitus, n (%) | 2 (10) | 12 (32) | 1 (14) | 0.13 |
| eGFR§, mL/min/1.73 m2 | 73.6 ± 14.3 | 85.2 ± 18.1* | 90.6 ± 13.2* | 0.02 |
| BNP§, pg/mL | 15.6 ± 8.5 | 22.5 ± 19.8 | 22.7 ± 18.9 | 0.32 |
| Hs-cTnT§, ng/mL | 0.006 ± 0.003 | 0.005 ± 0.003 | 0.005 ± 0.003 | 0.87 |
| LVEF by UCG§, % | 66.6 ± 3.2 | 63.2 ± 3.3* | 38.9 ± 14.9**‡ | <0.01 |
| EDV by UCG§, mL | 60.0 ± 15.3 | 64.5 ± 14.6 | 102.5 ± 31.7**‡ | <0.01 |
| ESV by UCG§, mL | 20.1 ± 5.3 | 23.7 ± 5.8 | 66.6 ± 35.6**‡ | <0.01 |
| GLS by UCG§, % | −18.7 ± 2.4 | −17.5 ± 2.8 | −10.7 ± 5.5**‡ | <0.01 |
| ECV by CCT§, %# | 26.2 ± 2.5 | 27.5 ± 3.1 | 30.3 ± 4.8**† | 0.02 |
AC: anthracycline, CTRCD: cancer therapeutics–related cardiac dysfunction, ANOVA: analysis of variance, Dox: doxorubicin, BMI: body mass index, BSA: body surface area, eGFR: estimated glomerular filtration rate, BNP: B-type natriuretic peptide, Hs-cTnT: high-sensitivity cardiac Troponin T, LVEF: left ventricular ejection fraction, UCG: echocardiography, EDV: end-diastolic volume, ESV: end-systolic volume, GLS: global longitudinal strain, ECV: extracellular volume fraction, CCT: cardiac computed tomography.
‡:P < 0.01 vs CTRCD(-), §:at investigation.
#: normal reference value is 23–28%.
:P < 0.05 vs control, **:P < 0,01 vs control, †:P < 0.05 vs CTRCD(−).
4. Discussion
The cardiotoxicity of ACs may be detected in acute, early (within the first year of treatment) or late (after several years) phases [2]. Early effects occur within the first year of treatment, while late effects appear years later (median 7 years after treatment [9]). In a study by Cardinale et al., of 2,625 patients (mean follow-up 5.2 years), the overall incidence of cardiotoxicity after AC treatment was 9%, and in 98% of these cases, cardiotoxicity first occurred within a year and was asymptomatic [10]. The necessity and frequency of follow-up for CTRCD after cancer treatment is individually determined by the type and treatment of the cancer, prognosis, the patient's own cardiovascular risk, and cardiovascular events during cancer treatment. There is a lack of evidence regarding the prevention and management of CTRCD risk [11]; in particular, no clinical data have been obtained, indicating the need for long-term cardiovascular risk management.
Echocardiography, like other cardiac imaging methods (nuclear cardiac imaging (multigated radionuclide angiography) and CMR) and cardiac biomarkers (troponin I, high-sensitivity troponin I, B-type natriuretic peptide (BNP), and NT-ProBNP), is listed as one of the proposed diagnostic tools for screening and detecting cardiotoxicity [2], and detection of cardiotoxicity by assessing left atrial function is also promising [7]. Echocardiography has advantages such as wide availability, lack of radiation, assessment of hemodynamics and other cardiac structures; however, its major limitations include interobserver variability, image quality, intervendor variability, and technical requirements [2].
In animal experiments, the mechanisms for ECV changes by AC in CTRCD group were assumed to be myocardial edema in the early stage [1] and myocardial fibrosis in the late stage [12]. In the present study, we demonstrated that CCT may be a tool comparable to echocardiography because ECV determined by CCT behaved the same as LVEF (EDV and ESV) and GLS determined by echocardiography. We have shown the usefulness of CCT in the diagnosis of CTRCD. If the problems of radiation exposure and contrast medium use (renal function + allergy) are overcome, it will be an extremely useful tool in the cardio-oncology field. Moreover, we have already reported consistency between CCT and CMR in CTRCD cases [4], [5]. Our result clearly demonstrated that in patients with significant LV dysfunction detected by echocardiography after AC therapy, CCT could detect changes of myocardial tissue characterization. If CCT can be as useful as CMR in detecting myocardial damage in AC induced cardiotoxicity, CCT would be a promising modality in cardio-oncology.
To the best of our knowledge, this study is the first to evaluate myocardial impairment utilizing ECV determined by CCT in the late phase after AC administration. The method of CCT is not only simple and easy in clinical practice but also well validated and inexpensive, which indicates that the method can be widely applied. Moreover, this novel CT imaging method can be performed in conjunction with standard coronary artery evaluation and cancer follow-up, possibly contributing to a cost reduction of examination. ECV evaluation by CCT involves an increase in radiation dose, and it has little functional information over echocardiography in spite of increased radiation exposure. It can be important to effectively identify patients with suspected CTRCD by echocardiographic functional evaluation and then assess the myocardial tissue characterization by CCT. Hence, CCT may also become a gold standard for detecting cardiotoxicity in the future. Furthermore, it is highly likely that combining CCT and echocardiography will result in improved evaluation of myocardial properties as well as diagnostic ability. Importantly, large-scale clinical trials are essential to examine the usefulness of CCT and to show that the role of CT-ECV in clinical studies in cardio-oncology field is considered to be utilization as a quantitative and objective parameter for “detection of myocardial damage”, “severity evaluation”, and “risk stratification”.
5. Study limitations
The present study has several limitations. First, this is very preliminary data in a very small single center, highly selected patient population with one class of chemotherapeutic agents, with an incomplete follow-up. Thus, selection bias was inevitable. In fact, patients in the CTRCD (+) group showed low LVEF of less than 40%, which is not in agreement with previous studies in patients with AC induced cardiotoxicity. Moreover, the possibility that group differences in numbers have affected the results could not be ruled out. Therefore, a larger multiracial and multicenter study is required. Second, all patients within CTRCD(+) group had AC-induced cardiotoxicity. Thus, verification of CTRCD by using other drug classes, such as trastuzumab, is mandatory. Moreover, the effects of concomitant agents must be verified. Furthermore, it is unclear which factors contribute to the development of CTRCD and the prognosis of CTRCD. Hence, further pathophysiological and molecular physiological studies, including animal experiments, are warranted. Additional detailed, large-scale clinical studies may be required to verify our theories.
6. Conclusions
Despite these limitations, we have clearly, and for the first time, demonstrated that cardiac functions in the late phase after AC anticancer agent use that were significantly impaired could be identified with CCT. CCT was found to have the potential to be comparable to echocardiography and could therefore become a gold standard for detecting cardiotoxicity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank all the paramedical staff and clinical secretaries for their kind support during this work.
Sources of Funding
This study was supported, in part, by a Grant-in-Aid for Scientific Research (#20K17087) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100797.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hong Y.J., Kim T.K., Hong D., Park C.H., Yoo S.J., Wickum M.E. Myocardial characterization using dual-energy CT in doxorubicin-induced DCM: comparison with CMR T1-mapping and histology in a rabbit model. JACC Cardiovasc. Imaging. 2016;9:836–845. doi: 10.1016/j.jcmg.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Zamorano J.L., Lancellotti P., Rodriguez Munoz D., Aboyans V., Asteggiano R., Galderisi M. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 3.Curigliano G., Lenihan D., Fradley M., Ganatra S., Barac A., Blaes A. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sueta D., Kidoh M., Oda S., Tsujita K. Novel assessment of cancer therapy-related cardiac dysfunction by cardiac computed tomography: a case report. Eur. Heart J. Case Rep. 2020;4:1–2. doi: 10.1093/ehjcr/ytaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sueta D., Kidoh M., Oda S., Egashira K., Yamamoto E., Kaikita K. Usefulness of cardiac computed tomography in the diagnosis of anti-cancer therapy-related cardiac dysfunction- consistency with magnetic resonance imaging. Circ. J. 2021;85:393–396. doi: 10.1253/circj.CJ-20-1288. [DOI] [PubMed] [Google Scholar]
- 6.Rosmini S., Aggarwal A., Chen D.H., Conibear J., Davies C.L., Dey A.K. Cardiac computed tomography in cardio-oncology: an update on recent clinical applications. Eur Heart J Cardiovasc Imaging. 2021;22:397–405. doi: 10.1093/ehjci/jeaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sueta D., Usuku H., Kinoshita Y., Tsujita K. Left atrial function assessed by speckle tracking echocardiography in anthracycline-induced cardiotoxicity: a case report. Eur. Heart J. Case Rep. 2020;4:1–5. doi: 10.1093/ehjcr/ytaa355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plana J.C., Galderisi M., Barac A., Ewer M.S., Ky B., Scherrer-Crosbie M. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Steinherz L.J., Steinherz P.G., Tan C.T., Heller G., Murphy M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 10.Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C.A., Veglia F. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 11.Strongman H., Gadd S., Matthews A., Mansfield K.E., Stanway S., Lyon A.R. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galan-Arriola C., Lobo M., Vilchez-Tschischke J.P., Lopez G.J., de Molina-Iracheta A., Perez-Martinez C. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 2019;73:779–791. doi: 10.1016/j.jacc.2018.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.