Abstract
The low-affinity nerve growth factor receptor p75 is a stratified epithelial stem/progenitor marker of human epithelia. We found OM-1, a human squamous cell carcinoma (SCC) cell line, showed distinct cells with p75 cluster, especially located at the center of a growing colony in a monolayer culture. A cell with p75 cluster was surrounded by cytokeratin 14- and cytokeratin 13-expressing cells that settled at the outer margin of the colony. OM-1 cells were also capable of forming tumor spheres in a cell suspension culture, an ability which was attenuated by the inhibition of p75-signaling. Intriguingly, we also found a p75-negative cell population from a growing culture of OM-1 that re-committed to become p75-clustering cells. These results indicated the possibility that SCC with epithelial multi-layering capacity can exploit the p75-dependent stratified epithelial progenitor property for the cancer stemness.
Keywords: p75, Squamous cell carcinoma (SCC), Cancer stem cell (CSC)
Highlights
-
•
Squamous cell carcinoma cell line OM-1 maintained cell population with p75 cluster in the cell.
-
•
Single cell-derived OM-1 colonies with the cell at centre maintained hierarchical stratified epithelial linages.
-
•
OM-1 cells have p75-dependent epithelial progenitor and stemness properties.
1. Introduction
The stratified squamous epithelium, such as the skin, and the oral, esophageal, and cervical mucosa, is composed of keratinocytes. The stem/progenitor cells in the basal layer attached to the underlying basement membrane create multiple lineages of keratinocytes that proliferate or undergo differentiation to build the homeostatic architecture of the epithelium (basal, spinous, granular, and cornified layers). For the linage markers of stratified squamous epithelium, intermediate filament cytokeratins are useful to distinguish proliferative basal cells of squamous epithelium from the upper epithelium layers by exclusive expression of cytokeratin 14 and cytokeratin 13 in a cell, respectively [1].
Adult stem cells and cancer stem cells (CSCs) share common features, such as the ability of self-renewal and differentiation into multiple cell lineages and recent studies have demonstrated that the persistence of CSCs induces therapeutic resistance, clinical relapses, and metastasis [2].
The low-affinity nerve growth factor receptor p75 is a prospective stem cell marker that enriches stem/progenitor cell subsets in various tissue models. In human adults, the presence of p75 characterizes several stem/progenitor cell types, such as the bone marrow, epidermis, corneal epithelium, and the oral and esophageal squamous epithelia [3,4]. We previously reported that an oral SCC cell line, OM-1, exhibited CSC properties [5] and expressed an isoform of p63 [6,7], which is also known as a keratinocyte stem/progenitor marker [8]. Intriguingly, Huang et al. demonstrated that p63-expressing cells were confined to p75-expressing cells with CSC properties of esophageal squamous cell carcinomas [9], motivating us to investigate possible contribution of p75 in OM-1.
In the present study, we identified OM-1 cells were also able to build hierarchal squamous epithelium linages in a single cell-derived colony and CSC property as well, which depended on p75-signaling.
2. Materials and methods
2.1. Cell lines and cell culture
Human epidermoid carcinoma cell lines (A431, OM-1, HSC-2, HSC-3), an immortalized keratinocyte cell line (RT-7), and a fibroblast cell line (GT-1) have been described previously [[10], [11], [12]]. All cell lines were cultured at 37 °C in a humidified atmosphere of 5% CO2 in air and maintained in DMEM (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich) except for RT-7, which was grown in a KGM-Gold BulletKit (Lonza, Switzerland) culture medium.
2.2. RNA extraction, first-strand cDNA synthesis, and semi-quantitative RT-PCR
Total RNA was isolated from the cells using an RNeasy Mini Kit (Qiagen, USA) according to the manufacturer's protocol. First-strand synthesis was performed using a first-strand cDNA synthesis kit (Roche, Sweden) according to the manufacturer's protocol. Semi-quantitative PCR was performed using a Go Taq Green Master Mix(Promega, USA)for 30 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 1 min. The PCR products were analyzed via 1.8% agarose gel electrophoresis. The primers for p75, CK13, CK14, and G3PDH, respectively, are listed below.
5′-TCAGTGGCATGGCTCCAGTC-3′ and 5′-GCAGTATCCAGTCTCAGCCCAAG-3’.
5′-CCAACACTGCCATGATTCAG-3′ and 5′-CGTGTCTTGATGTCCAGCAG-3’.
5′-GGAGATGATTGGCAGCGTGGA-3′ and 5′-GGACCTGCTCGTGGGTGGACA-3’.
5′-ACCACAGCCATGCCATCAC-3′ and 5′-CAGCCCCAGCGTCGTCAAAGGTG-3’.
2.3. Reagents and antibodies
The antibodies and dilution ratios used in immunocytochemistry were as follows: anti-p75 mouse monoclonal antibody (cloneME20.4, 1:200, sc-13577, Santa Cruz Technology, USA), anti-cytokeratin 13 mouse monoclonal antibody (clone AE8, 1:200, ab16112, Abcam, UK), and anti-cytokeratin 14 rabbit monoclonal antibody (clone EPR17350, 1:200, ab181595, Abcam). The p75 signaling inhibitor TAT-Pep5 [13] was purchased from Merck KGaA (506181, Germany) and applied in culture at a concentration of 100 nM.
2.4. Immunocytochemistry
Cells were cultured on 24-well glass-bottom plates (IWAKI, Japan). The growth medium was then removed, and the cell monolayers were fixed with 4% paraformaldehyde for 15 min. Following blocking and permeabilization with 5% BSA and 0.1% Triton X-100 in PBS (all from Sigma-Aldrich), respectively, the cells were incubated with the antibody solutions diluted with PBS containing 1% BSA at 4 °C for 12 h. Antibody binding was visualized via incubation with Alexa Fluor 488 goat anti-rabbit IgG (A-11008, Molecular Probes, USA) and Alexa Fluor 568 goat anti-mouse IgG (A-11004, Molecular Probes), diluted 1:10000 in PBS. After mounting with DAPI containing VECTASHIELD (Vector Laboratories Inc., USA), images were analyzed with a BZ-9000 fluorescence microscope (KEYENCE, Japan).
2.5. Three-dimensional (3D) cultures
Cell cultures in 3D were grown according to our previous reports [14,15]. Briefly, immortalized fibroblasts GT-1 were suspended in a mixture of type I collagen (Koken, Japan) and DMEM containing 10% FBS and seeded in a 12-well culture plate. The final concentrations of collagen and immortalized fibroblasts were 1 mg/mL and 1 × 106 cells/mL, respectively. Collagen was allowed to solidify by incubating at 37 °C for 1 h. OM-1 cells or RT-7 cells (1 × 106) suspended in 1 mL of DMEM with 10% FBS were seeded on the collagen gel. After incubation at 37 °C for 1 h, the gels were detached from the wall to float into the medium. After a week, the floating contracted gel was placed on an inverted nylon mesh strainer (BD Biosciences, UK) to create an air-liquid interface culture. The culture medium was changed to cover the side wall of the disc gel every other day for an additional week. The gel was then fixed with Mildform (Wako, Japan) and embedded in paraffin. Paraffinized sections were stained with hematoxylin-eosin (Wako) and analyzed using a BZ-9000 fluorescence microscope (KEYENCE).
2.6. Flow cytometry
Cells were detached using Accutase (Nakarai Tesque, Inc., Japan) to create a single cell suspension in the culture medium, which was subsequently stained with PE-conjugated anti-p75 mouse monoclonal antibody (clone C40-1457, 1:200, 557196, BD Biosciences) and 7-AAD (1:1000, 559925, BD Biosciences). PE-conjugated IgG isotype control (555749, BD Biosciences) was also used as a negative control for the aliquoted cells. The cells were analyzed using a FACSCalibur with CellQuest software (BD Biosciences) or a FACSAria II with FACSDiva software (BD Biosciences) for subsequent cultures.
2.7. Sphere formation assay
Two hundred or 400 cells were plated in ultra-low attachment 24-well plates (CLS3437 Corning, USA), and the number of spheres (>70 μm in diameter) was counted on day 10 of culture [16]. Aliquots of 200 cells were also inoculated into a well of a 24-well glass-bottom plate (IWAKI) for the immunocytochemistry of colonies on day 7.
2.8. Statistical analysis
Data are expressed as mean ± SD from at least three independent experiments. Statistical comparisons were made using Student's t-test. Statistical significance was set at P < 0.05.
3. Results
3.1. Immortalized keratinocyte RT-7 possessed p75-clustering cells
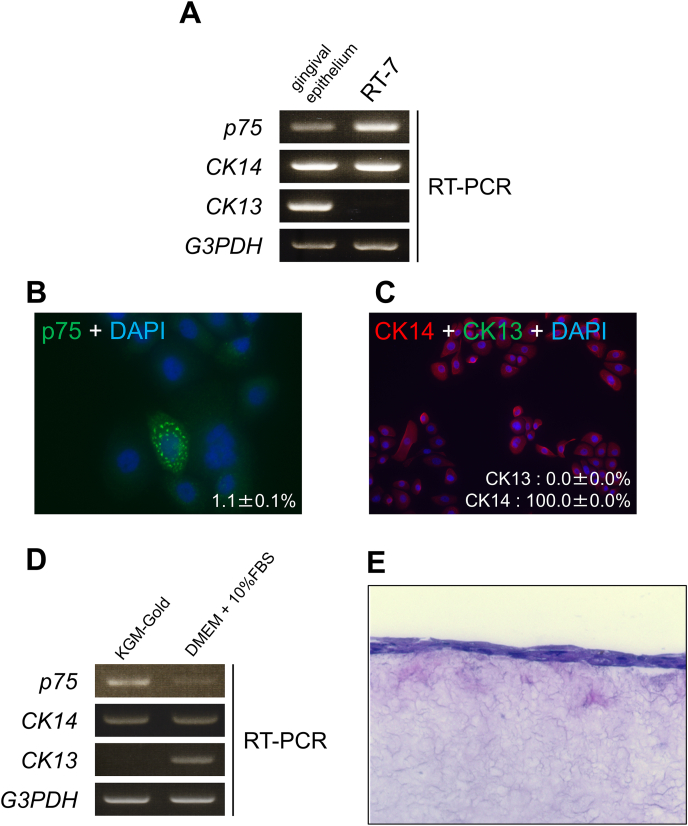
We first analyzed the immortalized human gingival keratinocyte cell line, RT-7, as a potential stratified epithelial stem/progenitor model based on the expression of p75 in keratinocytes grown in a low-calcium serum-free culture condition (e.g., KGM-Gold) (Fig. 1A). We found distinct cells in the growing monolayer culture (1.1% ± 0.1%, n = 12) which showed strong expression and clustering of p75 (Fig. 1B), while cytokeratin 14 (CK14) but not cytokeratin 13 (CK13), was constitutively expressed (Fig. 1A and C). Although RT-7 gained cytokeratin 13 with diminished p75 expression after differentiation with 10% FBS (Fig. 1D), multi-layering was impossible despite cornification upon in vitro 3D culture (Fig. 1E), indicating that the culture condition did not fully support possible stem/progenitor property (e.g., stratified epithelial structure) of RT-7 cells.
Fig. 1.
RT-7 cells expressed the possible epithelial stem/progenitor marker protein p75. (A) mRNA expression profile for p75, CK13, CK14, and G3PDH in gingival tissue and RT-7 cells. CK13 was undetectable in proliferating RT-7 cells grown in serum-free keratinocyte growth media while gingival tissue displayed CK14 and CK13 as lineage markers. (B) Immunostaining with anti-p75 antibody in growing RT-7 cells (green: p75, blue: DAPI). Distinct RT-7 cells expressed p75 with a characteristic clusters. (C) Double immunostaining with anti-CK14 antibody and anti-CK13 antibody in RT-7 cells (green: CK13, red: CK14). All cells expressed CK14 only; CK13 was undetectable. (D) RT-7 cells gained CK13 mRNA upon growth in DMEM with 10% FBS. (E) Histological analysis via HE staining of air-exposed 3D cultures of RT-7 grown in DMEM with 10% FBS. RT-7 cells failed to form a multi-layered stratified structure. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. The SCC cell line OM-1 maintained stratified epithelial cell lineage
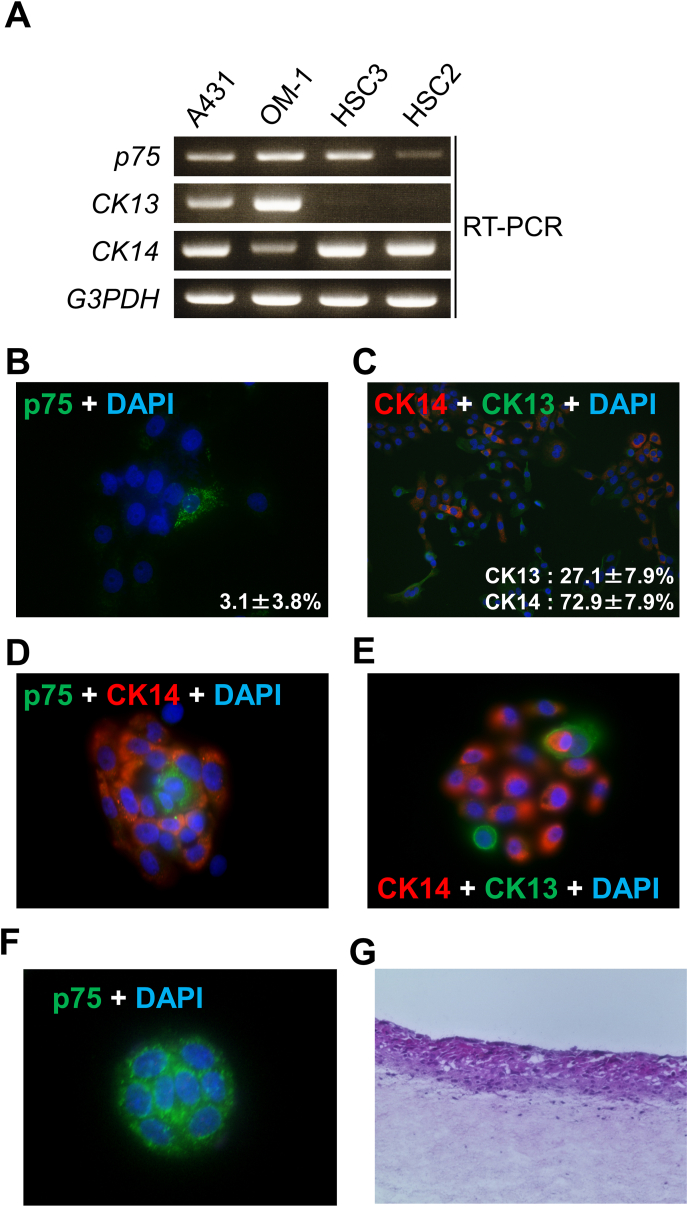
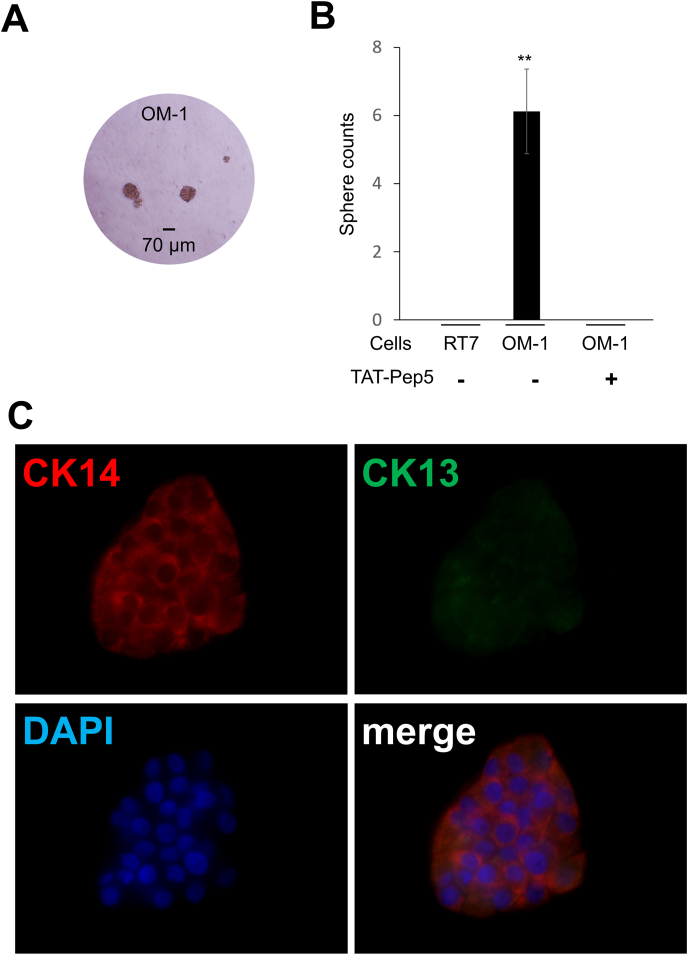
Next, we analyzed the mRNA expression of p75 in various SCC cell lines. OM-1 showed both CK13 and CK14 mRNA, as well as p75 (Fig. 2A). Fluorescence immunocytochemistry in a growing OM-1 monolayer showed distinct cells with p75 cluster (3.1% ± 3.8%, n = 12, Fig. 2B). The growing cells exclusively expressed either CK14 or CK13 in a cell (Fig. 2C). In the colony formation assay, 55.7 ± 3.8 colonies were formed from 200 OM-1 cells (n = 12), whereas RT-7 did not form any colonies (data not shown). The OM-1 cell colonies derived from single cells displayed p75 clustering cells at their centers (Fig. 2D). CK13-positive cells were observed only at the outer edges of the colonies, implying that they divided asymmetrically from CK14-positive cells (Fig. 2E). Although in low efficiency (0.9% ± 1.8%, n = 12), there were also small, distinct colonies comprised of only 8 cells all of which displayed p75 clustering (Fig. 2F), implying slow self-renewal of the stem cells [17]. Accordingly to the production of CK13-positive lineages from CK14-positive populations in a colony (Fig. 2E), OM-1 was capable of reconstructing stratified epithelial layers in in vitro 3D culture using conventional growth media (e.g., DMEM with 10% FBS) (Fig. 2G). We also confirmed OM-1 cells, but not RT-7 cells, were able to form spheres [5] under floating culture conditions (Fig. 3A and B) as a hallmark of CSC properties. These results indicated that the cancer cell line OM-1 maintained stratified epithelial progenitor properties to produce cell lineages for stratified epithelia while maintaining its CSC properties.
Fig. 2.
OM-1 cells maintained stratified epithelial progenitor and CSC properties. (A) The mRNA expression profiles of p75, CK13, CK14, andG3PDH in various squamous cell carcinoma cell lines are shown. A431 cells were used as the positive control for CK13 and CK14 expression [23]. (B) Immunocytochemistry using anti-p75 antibody in growing OM-1 cell cultures (green: p75, blue: DAPI). Some cells expressed p75 with the characteristic clusters. (C) Immunofluorescence of CK14 and CK13 in growing OM-1 cultures (green: CK13, red: CK14). CK14 and CK13 expression were exclusive in a given cell. (D, E) Immunofluorescence of a colony of OM-1 cells derived from a single cell. The cells with the characteristic p75 cluster were located at the center. CK14-negative cells can be found at the outer layer of the colony in D (green: p75, red: CK14, blue: DAPI). Outer cells displayed CK13 expression in E (green: CK13, red: CK14, blue: DAPI). (F) Distinct slow-growing small colonies with p75 clustering. (G) Histological analysis with HE staining of air-exposed 3D cultures of OM-1 cells on a dermis-mimic collagen gel. OM-1 cells built a multi-layered stratified epithelium. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Suppression of epithelial stem/progenitor and CSC properties of OM-1 by an intracellular p75 signal inhibitor TAT-Pep5. (A) OM-1 formed tumor spheres. (B) The average number of tumor spheres obtained from 200 cells with or without the p75 signal inhibitor TAT-Pep5. Double asterisks indicate a statistical difference (p < 0.01). (C) Immunofluorescences (green: CK13, red: CK14, blue: DAPI) of single cell-derived colonies formed under treatment with TAT-Pep5. Outer cells lacked CK14 failed to express CK13. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. A p75-signaling inhibitor suppressed tumor sphere formation and generation of CK13-positive cells in OM-1 cell colonies
To confirm whether the p75 signaling pathway induces the stratified epithelial progenitor and CSC properties in OM-1 cells, we added the p75 signaling inhibitor TAT-Pep5 [13] in the culture medium. TAT-Pep5 completely blocked tumor sphere formation in OM-1 cells under floating culture conditions (Fig. 3B), while the expression level of p75 was not altered (data not shown). It also prevented the generation of CK13-positive cells from the clonal growth of OM-1 cell colonies (Fig. 3C), which constantly expressed CK14 (Fig. 3B). Taken together, OM-1 maintained its CSC properties and stratified epithelial cell lineage via intracellular p75 signaling.
3.4. Re-emergence of p75-positive cells from p75-negative cells
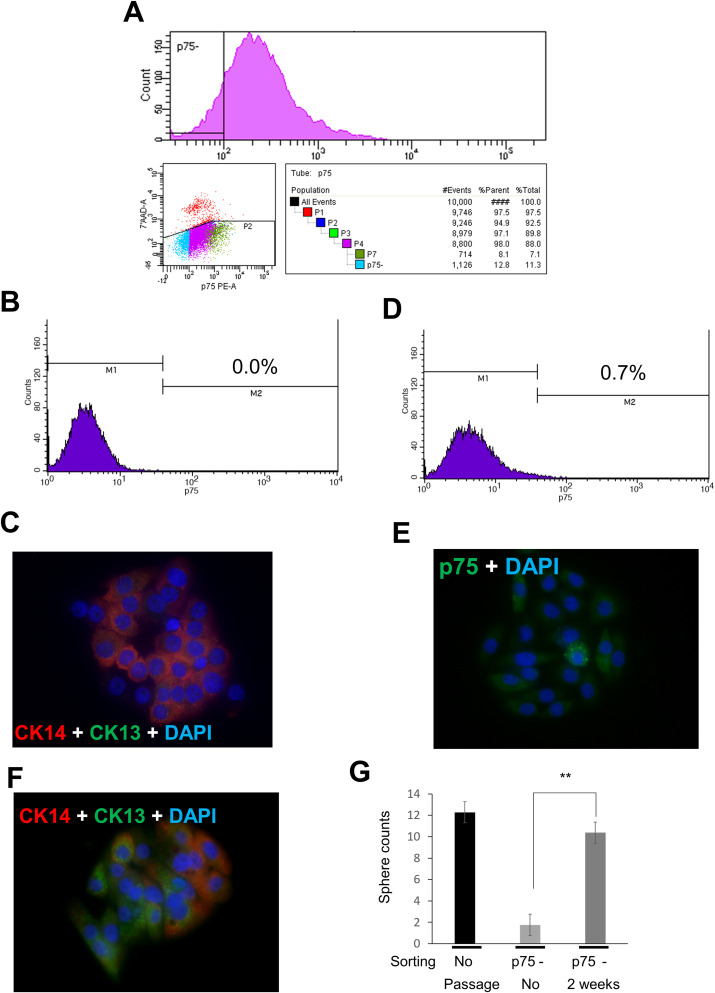
To further characterize the stem/progenitor property of OM-1 cells, we isolated p75-negative cells from a live culture (Fig. 4A and B). The isolated p75-negative OM-1 cells failed to generate CK13-expressing cells in a growing colony (Fig. 4C). After a week of culturing p75-negative cells, we found that some cells (0.7%) recovered p75 expression (Fig. 4D). Fluorescence immunocytochemistry further demonstrated the presence of p75-positive cells both with and without clusters (Fig. 4E). Moreover, CK13-positive cells in a growing colony were also generated in this culture (Fig. 4F). The tumor sphere formation assay also demonstrated that the p75-negative population of OM-1 cells initially lost their CSC properties but eventually regained them after several passages (Fig. 4G). Taken together, p75-negative OM-1 cells eventually regenerated into p75-positive cells and maintained p75-dependent stratified epithelial progenitor and CSC properties.
Fig. 4.
Re-emergence of p75-positive cells from p75-negative cells. (A) p75-negative cells were isolated by a Becton Dickenson FACSAria II flow cytometer as indicated. The sorted cells were recovered as a monolayer culture. (B) Flow cytometry confirmed that the aliquoted cells were p75-negative. (C) Immunofluorescences of a representative colony formed by p75-negative OM-1 cells (green: CK13, red: CK14, blue: DAPI). (D) Flow cytometry of cells after expansion for two weeks showed the re-emergence of a p75-positive population. (E), (F) Immunofluorescences of re-emerged p-75 positive cells in colony. Cells with and without clusters were in E (green: p75, blue: DAPI). In F, CK13-expressing cells in a colony were recovered (green: CK13, red: CK14, blue: DAPI), but lacking the layered distributions observed in Fig. 2 (G) The number of tumor spheres obtained from 400 cells of the indicated cell types. The initial p75-negative population in A turned to be comparable to the parental OM-1 cells. Double asterisks indicate statistical differences (p < 0.01). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Histologically, clinical SCC specimens retain varying degrees of differentiation. SCCs with a lower degree of differentiation tend to be at a higher risk of poor prognosis due to their invasiveness and metastatic ability. The most common features of secondary tumors, including those in recurrent local cancer and metastatic cancer, resemble the histology of primary cancer lesions [18]. Therefore, CSC-dependent secondary tumor formation from well-differentiated primary tumors and rebuilding the original tissue architecture should be considered separately to gain insight into the invasiveness and metastatic abilities of stem cells [19,20]. We found that both stratified epithelial progenitor and CSC properties re-emerged in accordance to the sporadic re-appearance of p75-clustering cells from p75-negative OM-1 cells during proliferation. The re-emerged p75-dependent architecture of CK14/13 in a colony lost its polarity (Fig. 4F), however, cell culture passages made these re-emergent p75-expressing cells indistinguishable from the parental cells not only in the distribution of CK14/13 cells in a colony (data not shown) but also in their tumor sphere-forming ability (Fig. 4G). In addition CK14/13 double staining revealed inability to express CK13 in CK14-negative peripheral cells in the colony (Fig. 4C) from the p75-negative population. Therefore, OM-1 cells acquired autonomous tuning machinery to maintain their p75-depedent CSC and epithelial progenitor properties. Since cells with p75 clusters in growing culture appeared sporadically in growing culture, p75 ligands (e.g., neurotrophins [21])-independent signaling might control asymmetric divisions to produce the CK13 layer from CK14 through lateral signaling [22], initiated by a cell with p75-cluster.
With these results, although we further need to determine how p75 forms cluster in a cell, how the sporadic emergence of p75-clastering cells is regulated, and how stem/progenitor properties couples CSC properties, our results suggested SCC in both primal and metastatic lesions might abuse progenitor properties to cancer stemness.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
We are always grateful to our dear Drs. Nobuyuki Kamata and Koichiro Higashikawa, who passed away after we had established a research group in Hiroshima. We would also like to thank our colleagues at the Department of Oral and Maxillofacial Surgery for encouragement in accomplishing the study. This work was carried out at the Analysis Center of Life Science, Hiroshima University. This work was supported by KAKENHI JP19K10309, JP19K10358, JP19K19161, JP18K17198, and JP20K10139 by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, and Technology.
References
- 1.Dowdall J.R., Sadow P.M., Hartnick C., Vinarsky V., Mou H., Zhao R., Song P.C., Franco R.A., Rajagopal J. Identification of distinct layers within the stratified squamous epithelium of the adult human true vocal fold. Laryngoscope. 2015;125:E313–E319. doi: 10.1002/lary.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Okumura T., Shimada Y., Imamura M., Yasumoto S. Neurotrophin receptor p75NTR characterizes human esophageal keratinocyte stem cells in vitro. Oncogene. 2003;22:4017–4026. doi: 10.1038/sj.onc.1206525. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T., Endo K., Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cell. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- 5.Seino S., Shigeishi H., Hashikata M., Higashikawa K., Tobiume K., Uetsuki R., Ishida Y., Sasaki K., Naruse T., Rahman M.Z., Ono S., Simasue H., Ohta K., Sugiyama M., Takechi M. CD44high/ALDH1high head and neck squamous cell carcinoma cells exhibit mesenchymal characteristics and GSK3β-dependent cancer stem cell properties. J. Oral Pathol. Med. 2016;45:180–188. doi: 10.1111/jop.12348. [DOI] [PubMed] [Google Scholar]
- 6.Higashikawa K., Yoneda S., Tobiume K., Taki M., Shigeishi H., Kamata N. Snail-induced down-regulation of deltaN p63α acquires invasive phenotype of human squamous cell carcinoma. Canc. Res. 2007;67:9207–9213. doi: 10.1158/0008-5472.CAN-07-0932. [DOI] [PubMed] [Google Scholar]
- 7.Higashikawa K., Yoneda S., Tobiume K., Saitoh M., Taki M., Mitani Y., Shigeishi H., Ono S., Kamata N. deltaNp63α-dependent expression of Id-3 distinctively suppresses the invasiveness of human squamous cell carcinoma. Int. J. Canc. 2009;124:2837–2844. doi: 10.1002/ijc.24280. [DOI] [PubMed] [Google Scholar]
- 8.Senoo M., Pinto F., Crum C.P., McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Huang S.D., Yuan Y., Liu X.H., Gong D.J., Bai C.G., Wang F., Luo J.H., Xu Z.Y. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Canc. 2009;9 doi: 10.1186/1471-2407-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto R., Kamata N., Yokoyama K., Taki M., Tomonari M., Tsutsumi S., Yamanouchi K., Nagayama M. Establishment of immortalized human oral keratinocytes by gene transfer of a telomerase component. J. Japan. Soc. Oral. Mucous Membr. 2002;8:1–8. doi: 10.6014/jjomm1995.8.1. [DOI] [Google Scholar]
- 11.Taki M., Kamata N., Yokoyama K., Fujimoto R., Tsutsumi S., Nagayama M. Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Canc. Sci. 2003;94:593–597. doi: 10.1111/j.1349-7006.2003.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta K., Shigeishi H., Taki M., Nishi H., Higashikawa K., Takechi M., Kamata N. Regulation of CXCL9/11 in oral keratinocytes and fibroblasts. J. Dent. Res. 2008 doi: 10.1177/154405910808701211. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita T., Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka F., Rizqiawan A., Higashikawa K., Tobiume K., Okui G., Shigeishi H., Ono S., Shimasue H., Kamata N. Snail promotes Cyr61 secretion to prime collective cell migration and form invasive tumor nests in squamous cell carcinoma. Canc. Lett. 2013;329:243–252. doi: 10.1016/j.canlet.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Rizqiawan A., Tobiume K., Okui G., Yamamoto K., Shigeishi H., Ono S., Shimasue H., Takechi M., Higashikawa K., Kamata N. Autocrine galectin-1 promotes collective cell migration of squamous cell carcinoma cells through up-regulation of distinct integrins. Biochem. Biophys. Res. Commun. 2013;441:904–910. doi: 10.1016/j.bbrc.2013.10.152. [DOI] [PubMed] [Google Scholar]
- 16.Shigeishi H., Biddle A., Gammon L., Emich H., Rodini C.O., Gemenetzidis E., Fazil B., Sugiyama M., Kamata N., MaCkenzie I.C. Maintenance of stem cell self-renewal in head and neck cancers requires actions of GSK3b influenced by CD44 and RHAMM. Stem Cell. 2013;31:2073–2083. doi: 10.1002/stem.1418. [DOI] [PubMed] [Google Scholar]
- 17.Biddle A., Liang X., Gammon L., Fazil B., Harper L.J., Emich H., Costea D.E., Mackenzie I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Canc. Res. 2011;71:5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 18.Wahi P.N., Cohen B., Luthra U.K., Torloni H., Organization W.H. 1971. Histological Typing of Oral and Oropharyngeal Tumours/P. N. Wahi , in Collaboration with B. Cohen, Usha K. Luthra, H. Torloni and Pathologists in Eleven Countries.https://apps.who.int/iris/handle/10665/41523 accessed. [Google Scholar]
- 19.Li F., Tiede B., Massagué J., Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 20.Dekoninck S., Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019;21:18–24. doi: 10.1038/s41556-018-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 22.Sancho R., Cremona C.A., Behrens A. Stem cell and progenitor fate in the mammalian intestine: notch and lateral inhibition in homeostasis and disease. EMBO Rep. 2015;16:571–581. doi: 10.15252/embr.201540188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortolomai I., Canevari S., Facetti I., De Cecco L., Castellano G., Zacchetti A., Alison M.R., Miotti S. Cell Cycle Tumor initiating cells: development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension. Cell Cycle. 2010;9:1194–1206. doi: 10.4161/cc.9.6.11108. [DOI] [PubMed] [Google Scholar]