Abstract
Candida albicans mannan consists of a large repertoire of oligomannosides with different types of mannose linkages and chain lengths, which act as individual epitopes with more or less overlapping antibody specificities. Although anti-C. albicans mannan antibody levels are monitored for diagnostic purposes nothing is known about the qualitative distribution of these antibodies in terms of epitope specificity. We addressed this question using a bank of previously synthesized biotin sulfone tagged oligomannosides (BSTOs) of α and β anomery complemented with a synthetic β-mannotriose described as a protective epitope. The reactivity of these BSTOs was analyzed with IgM isotype monoclonal antibodies (MAbs) of known specificity, polyclonal sera from patients colonized or infected with C. albicans, and mannose binding lectin (MBL). Surface plasmon resonance (SPR) and multiple analyte profiling (MAP) were used. Both methods confirmed the usual reactivity of MAbs against either α or β linkages, excepted for MAb B6.1 (protective epitope) reacting with β-Man whereas the corresponding BSTO reacted with anti-α-Man. These results were confirmed in western blots with native C. albicans antigens. Using patients’ sera in MAP, a significant correlation was observed between the detection of anti-mannan antibodies recognizing β- and α-Man epitopes and detection of antibodies against β-linked mannotriose suggesting that this epitope also reacts with human polyclonal antibodies of both specificities. By contrast, the reactivity of human sera with other α- and β-linked BSTOs clearly differed according to their colonized or infected status. In these cases, the establishment of an α/β ratio was extremely discriminant. Finally SPR with MBL, an important lectin of innate immunity to C. albicans, classically known to interact with α-mannose, also interacted in an unexpected way with the protective epitope. These cumulative data suggest that structure/activity investigations of the finely tuned C. albicans anti-mannose immune response are worthwhile to increase our basic knowledge and for translation in medicine.
Subject terms: Biochemistry, Chemical biology, Immunology, Microbiology
Introduction
Many studies have demonstrated that molecules essential for normal cell physiology are usually glycosylated and variations in their glycosylation patterns often induce changes in their function. These changes have been reported in both prokaryotic and eukaryotic organisms1. In fungi, oligosaccharides are important molecules playing a crucial role in fungal cell–cell communication and host–pathogen interactions involving innate immunity receptors. Conjugated to proteins or lipids, oligosaccharides elicit strong antibody responses as a result of fungal tissue invasion. From a more general point of view, as they have a high level of specificity, anti-oligosaccharide antibodies are widely used as a tool for blood grouping, control of vaccination status, or serotyping of viral and bacterial agents2, 3.
The C. albicans cell wall is a multilayered structure composed mainly of sugars (> 80%) and to a lesser extent proteins and lipids. The surface is covered by phosphopeptidomannan (commonly named mannan), a high molecular weight matricial component, expressing a large repertoire of α- and β-oligomannosides. PPM is interwoven with numerous mannoproteins (MPs) emerging at the cell surface and covalently bound to the inner layer composed of glucans. These mannoproteins are distributed in GPI anchor or PIR proteins depending on their type of linkage to glucans. Of note, these MPs display the same oligomannose repertoire as PPM4. The cell wall surface is also covered by a glycolipid with β-mannose epitopes known as phospholipomannan (PLM). The intermediate layer of the cell wall is composed of a polysaccharide polymer backbone consisting of β-glucans (both β-1,3, and β-1,6 D-glucopyrannosyls) while a chitin matrix participates in the hydrophobic layer of the cell membrane5. From an immunological point of view, mannans and β-glucans are differently recognized by host pattern recognition receptors (PRRs). β-1,3-glucans are recognized by dectin-1, α-mannan is recognized by the mannose receptor, dectin-2, DC-SIGN, mannose-binding lectin (MBL), and langerin, whereas β-mannans are specifically recognized by galectin-36.
Anti-glycan/oligosaccharide antibodies are also used as biomarkers for the diagnosis of human invasive7 or allergic8 fungal diseases. Anti-oligosaccharide antibody specificity is conferred by the nature of the constitutive sugar sequences, the type of linkage and the length of the oligosaccharide chain9. For immunoassays, these carbohydrate antigens need to be immobilized on the surface of microtiter plates or magnetic microspheres, which represents a challenging task. This pitfall has been overcome by using synthetic oligosaccharides, which can be prepared with a versatile anchor group. Biotin is the most widely used of these as it selectively binds to a large number of surfaces. Such tagged oligosaccharides are strongly recognized by avidin and are successfully used in immunoassays10.
For experimental purposes, surface plasmon resonance (SPR) has been used to decipher the interactions between oligosaccharides and proteins or peptides. Such investigations were performed to characterize lectin-microbial interactions. MBL is a multimer of polypeptide chains 32 kDa in size. Three polypeptide chains make up a triple helix with a collagenous region11, which is the basic circulating subunit of MBL. In serum, MBL is composed of oligomers of subunits from dimers to hexamers, which are effector forms of the lectins for glycan or pathogen interactions12, 13. MBL interacts with terminal-D-mannose residues, L-fucose, and GlcNAc14, 15 but also with bacteria, virus, molds, yeasts, and parasites. It is known that MBL can recognize C. albicans16 but the minimal epitope necessary for this recognition is unknown.
The main objectives of the present study were to use synthetic oligosaccharides to carry out epitope mapping of the main monoclonal antibodies (MAbs) directed against the cell wall of C. albicans, to identify the oligomannoside epitopes recognized by MBL through SPR, and to dissect the anti-mannan antibody response in the serum of patients with invasive candidiasis using multi-analyte profiling (MAP) technology10. To reach these objectives a bank of previously synthesized biotin sulfone tagged oligomannosides (BSTOs) of α and β anomery10 was complemented with the synthetic β-mannotriose described as a protective epitope. This revealed unexpected results regarding previous paradigms of C. albicans mannose epitope recognition.
Results
MAP analysis of BTSO reactivity with anti-carbohydrate MAbs
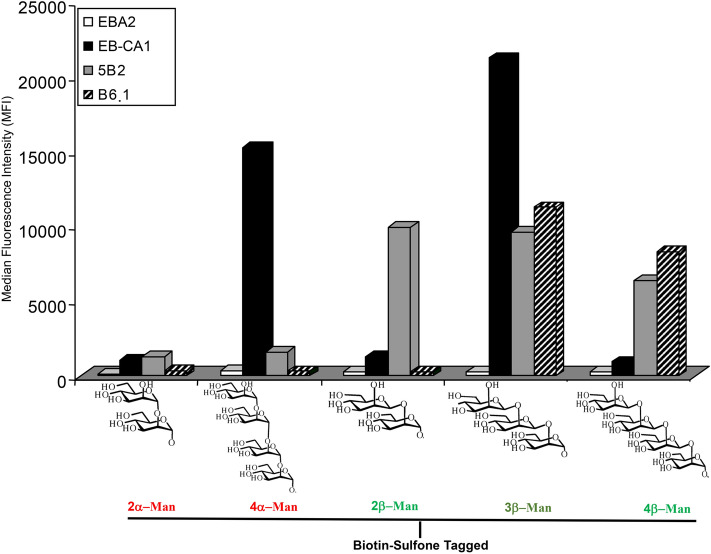
Five BSTO epitopes were individually coupled to different batches of microsphere beads and tested with the panel of anti-C. albicans MAbs. As shown in Fig. 1, the anti-galactofuran MAb (EB-A2) did not generate any signal with any of the mannose residues presented as BSTOs. As expected, MAb 5B2 bound selectively to members of the β-mannose family (β-Mans) with reactivity starting from mannobiose. MAb B6.1 reacted strongly with its specific β-mannotriose epitope and to a lesser extent with neighboring 3β-Man. MAb EB-CA1, described as reacting with 5α-Man, bound to 4α-Man, which was the closest epitope in our series of BSTOs, but did not react with 2β-Man or 4β-Man. Unexpectedly, MAb EB-CA1 displayed high reactivity with 3β-Man providing the first exception, so far, to the α/β Man dichotomy of anti-C. albicans antibody reactivity.
Figure 1.
Multi-analyte profiling (MAP) analysis of biotin sulfone tagged synthetic oligomannoside (BTSO) reactivity with anti-carbohydrate monoclonal antibodies (MAbs). Results are shown as the mean fluorescence intensity obtained with MAbs (EB-CA1: black bars; 5B2: grey bars; and B6.1: hatched bars), or EB-A2 (white bars) for each microbead set coated with different BSTOs including α-1,2-mannobioside (2α-Man), α-1,2-mannotetraose (4α-Man), β-1,2-mannobioside (2β-Man), β-1,2-mannotriose (3β-Man), and β-1,2-mannotetraose (4β-Man).
SPR analysis of BSTO reactivity with anti-carbohydrate MAbs
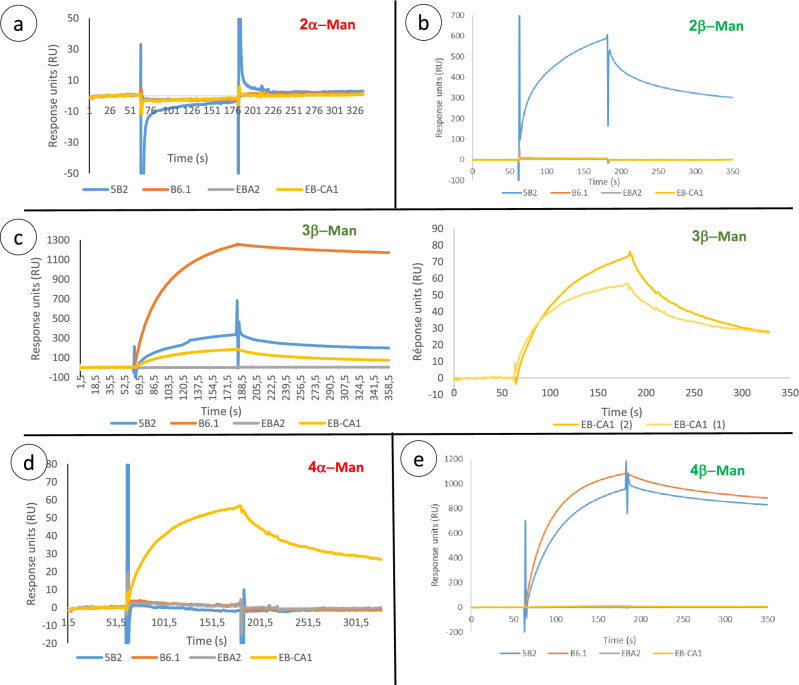
The interactions between ligand BSTOs and MAbs are shown in Fig. 2. Figure 2a shows an example of the absence of interaction concerning 2α-Man where changes to baseline are only due to variations in the injected buffer and the signal is stable. The functionality of the 2α-Man sensor was confirmed by reactivity with ConA (250 nM) (data not shown). Variations of about 5 RU for MAb 5B2 during the injection step are negligible and modifications of sensorgram profiles can only be attributed to variations in buffer composition. A comparison of relative affinity was performed at 250 nM for each antibody, and in the presence of binding two steps can be observed. During the injection step, the association can be seen as an exponential phase. Typical results of the interaction between BSTOs and antibodies are shown in Fig. 2b–e.
Figure 2.
Surface plasmon resonance analysis of biotin sulfone tagged synthetic oligomannoside (BSTO) reactivity with anti-carbohydrate monoclonal antibodies (MAbs). MAbs B6.1 (orange curve), 5B2 (blue curve), EB-CA1 (yellow curve), and control (EB-A2, grey curve) were successively tested with 2α-Man (a), 2β-Man (b), 3β-Man (c), 4α-Man (d), and 4β-Man (e).
MAb EB-CA1 weakly interacted with 4α-Man (~ 60 RU) and presented a typical profile of a sensorgram with association and dissociation curves (beginning after 60 s and 180 s, respectively). Surprisingly, EB-CA1 showed specific interaction with 3β-Man (Fig. 2c) and the amplitude of the signal was similar to that obtained with 4α-Man (Fig. 2d). The unexpected reactivity of EB-CA1 with 3β-Man was confirmed by replications of injections (3c, right panel). EB-A2, B6.1, and 5B2 MAbs did not show any interactions with 4α-Man.
Interactions of antibodies with β-Mans were more informative. Only MAb 5B2 interacted with 2β-Man (~ 600 RU) and 4β-Man (~ 800 RU) (Fig. 2b, e). These results can also be observed for 3β-Man in a less important manner (~ 300 RU) (Fig. 2c left panel). There was no interaction with 2α-Man and 4α-Man (Fig. 2a, 3d). MAb B6.1 showed strong binding to 3β-Man and 4β-Man. The absence of response with EB-A2 injections used as a control on each sensorgram confirms the specificity of the binding with the other antibodies. Saturability of the interactions was not performed because of the IgM nature of the antibodies which have 10 binding sites per molecule.
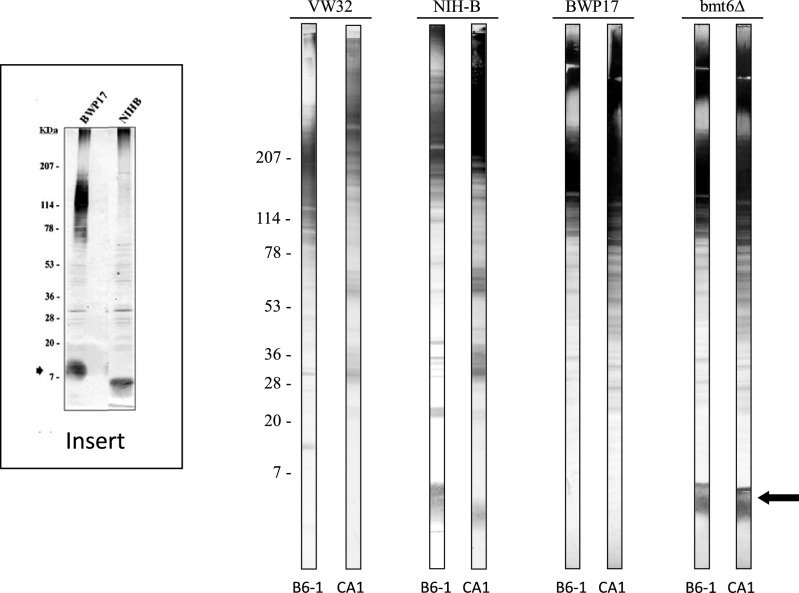
Figure 3.
Western blots of whole cell extracts from C. albicans serotype A (VW32, BWP17 and bmt6Δ) and serotype B (NIH B-792) strains probed with MAbs B6.1 and EB-CA1. The insert shows a serotype A strain (BWP17) and serotype B strain (NIHB) probed with MAb 5B2 showing the shift in rMW previously characterized structurally, and resulting from shortening of β-Man chain to 3 mannose residues in serotype B strains34.
Data obtained by SPR confirmed those observed with MAP in terms of differential binding conditioned by anomery and oligomannoside chain length. The only discrepancy concerned the amplitude of signals between the two technologies. Overall, the MAP and SPR results confirmed the reactivity of MAb EB-CA1 with 3β-Man as the first real exception observed so far to the specificity of anti-mannoside antibodies conferred by their anomeric configuration. It is not the specificity of the “protective” antibody that is questionable (B6.1), but the ability of the “protective epitope” to react with both anti-β-mannoside antibodies and anti-α-mannoside antibodies. We then aimed to confirm these data using native antigens from C. albicans known to express the protective epitope.
Western blot analysis of EB-CA1 and B6.1 MAb reactivities against mannoglycoconjugates from C. albicans serotype A and B strains
In order to confirm the reactivity of the 3β-Man "protective epitope" with an anti-α-Man antibody western blot experiments were performed involving an extensively characterized molecule, PLM18. PLM is a member of the manno-inositol phosphoceramide (MIPC) family with an apparent rMW of 14–18 kDa in western blots, and with a polysaccharide moiety composed of long linear chains of β-Man in C. albicans serotype A strains19 (Fig. 3 insert, lane BWP17). Later, it was discovered that this cell wall surface molecule differed in C. albicans serotype B strains20 with a rMW of approximately 7 kDa (Fig. 3 insert Lane NIH B). Structural studies established that this shift in rMW was related to a truncated glycan moiety that corresponded to a trimannoside. This truncation was reproduced by inactivation of β-mannosyl transferase 6 (BMT6), responsible for the addition of the third β-mannose in serotype A21. Natural and genetically constructed variants of PLM therefore seemed particularly well adapted to confirm the unexpected reactivity of the protective epitope with MAb EB-CA1 (Fig. 3). When these extracts were probed with B6.1 and EB-CA1 MAbs, reactivity was observed with numerous of bands of rMW > 40 kDa in accordance with previous data that showed that both epitopes are distributed over a large number of mannoproteins that display a polydisperse character in the gel as a function of the increased molecular weight and increased size of their reactive polysaccharide moiety. Western blots performed with control MAb EB-A2 did not show any reactivity. Among the C. albicans glycoconjugates, we focused our analysis on PLM (Fig. 3, arrow). PLM of C. albicans serotype A VW32 and BWP17 strains had no reactivity with MAb B6.1 in contrast to C. albicans serotype B NIH B-792 and C. albicans serotype A bmt6Δ strain where deletion of both alleles of BMT6 resulted in a truncated PLM with an accumulation of 3β-Man. Interestingly, MAb EB-CA1, specific for 4α-Man, displayed the same reactivity as MAb B6.1 with 3β-Man. Additional information concerning the technical procedure of WB used for generating Fig. 3 is available in the supplementary information section with specific references.
Analysis of human antibody response with anti-mannan ELISA test and MAP involving different BSTOs for their differential ability to discriminate between controls, colonized, and Candida-infected patients
Systemic Candida infection is generally associated with a sharp rise in anti-C. albicans mannan antibodies leading to titers rarely encountered in non-infected patients. In parallel, mannanemia, (i.e. the release of mannan into patients’ sera resulting from host tissue invasion by Candida) may also be detected. On this basis, it has been proposed to combine the detection of anti-C. albicans mannan antibodies and mannanemia as a diagnostic strategy to compensate for the poor sensitivity of blood cultures.
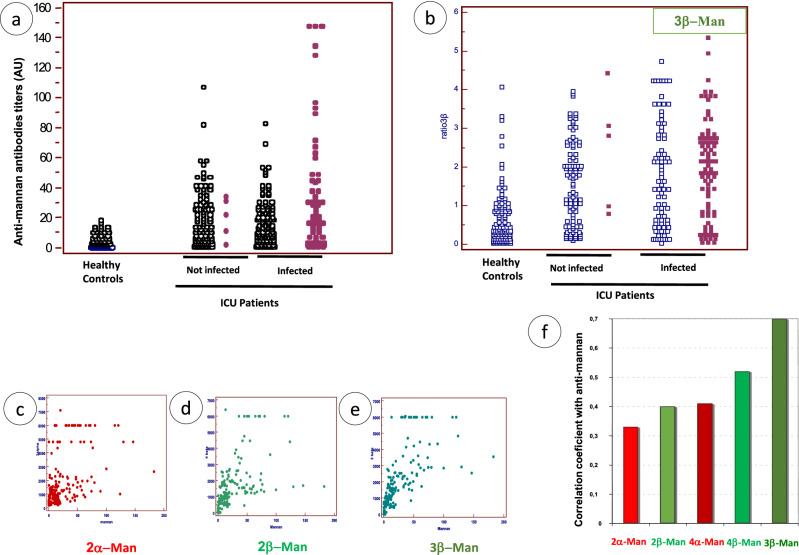
The results of the anti-C. albicans mannan antibody tests are plotted on Fig. 4a for each of the 181 sera taken from 30 infected intensive care unit (ICU) patients as well as the associated values for mannanemia. When considering the values for the same tests in non-infected ICU patients, a larger proportion than healthy controls also had high anti-mannan antibody titers in relation to the heavy colonization observed for most patients during hospitalization. A similar distribution was observed when considering the reactivity of human sera against 3β-Man (Fig. 4b).
Figure 4.
Reactivity of human sera against C. albicans mannan and BSTO. Reactivity against C. albicans mannan (a) as detected by ELISA (Platelia Candida Ab test), and against BST-3β-Man (b), as detected by multi-analyte profiling (MAP) analysis. Human sera consisted of sera from healthy controls (blood donors presenting no disease), and ICU patients either not-infected or infected with C. albicans. Anti-C. albicans mannan antibody response is shown for infected patients according to antigenemia status (negative: open symbols; positive: closed symbols) (b). The correlation between anti-mannan and 2α-Man, 2β-Man, 3β-Man antibodies is shown in (c, d, e) respectively. The values of Spearman rank correlation coefficients for the different BSTOs are given in f.
Although different results could have been expected using 3β-Man as a single epitope, the results observed with infected patients' sera correlated with those obtained with mannan (Fig. 4e). When performing the same analysis with other BSTOs the results were heterogeneous by comparison to anti-mannan reactivity. Examples are given for the distribution of 2α-Man and 2β-Man in Fig. 4c, d, respectively, and the Spearman's rank correlation coefficients with mannan reactivity are shown as a function of the BSTO in Fig. 4f. Altogether these findings revealed the property of 3β-Man to mimic the mannan repertoire of β- and α-mannoside epitopes, and confirms its unique ability to bind both anti-β and anti-α mannose antibodies in human sera, as shown previously with MAbs in MAP and SPR.
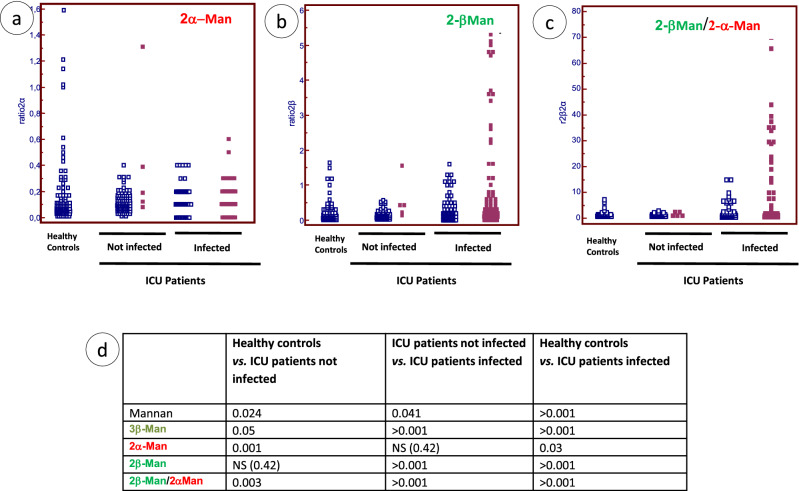
We then investigated whether other BSTOs would discriminate infected patients from other ICU patients (Fig. 5). Surprisingly, when using 2α-Man, reactivity decreased from controls to colonized and infected patients (Fig. 5a) (i.e. the converse of what would have been expected for identifying infected patients). By contrast, 2β-Man reactivity increased markedly in infected patients (Fig. 5b). Although these results were discriminatory per se we decided to determine the α/β reactivity ratio. As shown in Fig. 5c, more discrimination was observed between patients in relation to C. albicans saprophyte/pathogen transition.
Figure 5.
Reactivity of human sera against 2α-Man, 2-β-Man, and establishment of 2β-Man/2α-Man ratio for differentiation between uninfected and infected ICU patients. (a) 2α-Man, (b) 2β-Man and (c) 2βMan/2α-Man ratio (open squares: negative mannanemia; closed squares positive mannanemia). (d) Statistical analysis of the ability of different antigens to discriminate between study groups.
The results of the statistical analysis of the ability of antigens to discriminate the different study groups are shown in Fig. 5d. The p values are reported for each comparison. 3β-Man and 2β-Man/2α-Man ratio provided the best discrimination whatever the categories compared. However major differences were observed depending on the groups under comparison with some antigens even giving non-significant results. This reveals the complex framework of C. albicans mannose repertoire recognition depending on the patient’s status.
SPR analysis of BSTO-MBL interaction
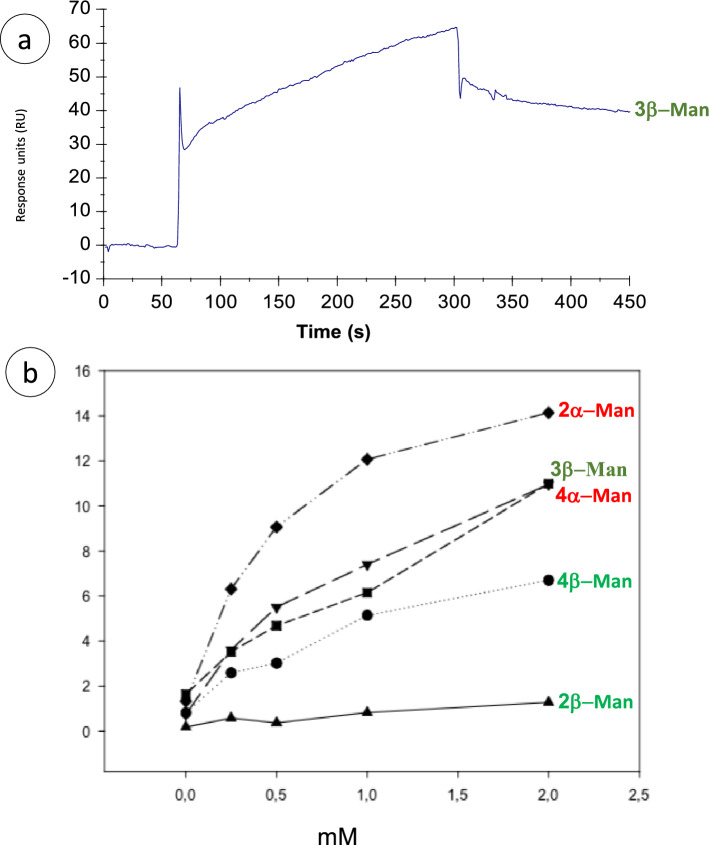
To further establish the interaction between oligomannosides and recombinant (r)MBL, SPR experiments were carried out using BIAcore 3000. An example of a typical sensorgram of the interaction is shown in Fig. 6a with 3β-Man. To characterize how the oligomannose anomery and degree of polymerization may influence binding to rMBL, the different oligomannosides were screened at 0, 0.25, 0.5, 1, and 2 mM (Fig. 6b). Although the interaction was characterized by low signals (< 100 RU), SPR sensograms clearly demonstrated differential interactions of BSTOs with rMBL.
Figure 6.
Affinity curves for recombinant mannose-binding lectin (rMBL) binding to oligomannosides. (a) Typical sensorgram of the interaction of MBL with 3β-Man (2 mM). (b) Normalized affinity curves of increasing mM of the different oligomannosides with MBL.
As expected, for one MBL ligand already identified, specific binding occurred with 2α-Man. 4α-Man, which presents the same structure at its terminal non-reducing end, also displayed strong binding. Among the β-1,2 linked oligomannosides, 2β-Man displayed very low affinity, and although the 4β-Man signal was better, it was still weak and was not saturable even at high concentrations. Interestingly, the affinity curves for 3β-Man and 4α-Man were almost superimposable. These results were confirmed by regression curves displaying a correlation coefficient of > 0.9 and similar slopes (data not shown). Thus, these results obtained with a lectin of innate immunity sensing C. albicans PAMPs confirm the unique behavior of the 3β-Man "protective epitope" among the β-Man series observed with monoclonal and polyclonal antibodies.
Discussion
The cell wall of C. albicans is a complex stratified and dynamic structure responsible for cell integrity and plays a pivotal role in host-yeast interactions. The cell wall is involved in adhesion, invasion, and is the target of innate and adaptive immune responses6, 22.
Among the cell wall polysaccharides, mannan is widely recognized to have an immunomodulatory effect23–25. Its structure has been well established by an impressive series of structural and serological studies from Suzuki's group26, 27. These authors established that C. albicans mannan is similar to the mannan of the closely related species S. cerevisiae, extensively studied as a model of glycosylation, with a comb-shaped structure composed of α-1,2, α-1,3, and α-1,6 mannose residues, but that C. albicans, C. tropicalis and C. glabrata, now reported as responsible for 90% of human Candida infections28, also have the ability to synthesize β1,2 mannose4, 21, 29, 30.
Simultaneously, at a time when yeast species identification was a lengthy procedure, Fukazawa's group31 developed an efficient Candida species identification kit (Candida Check; Iatron Laboratories, Tokyo, Japan). Cross-absorbed polyclonal rabbit antibodies were used for the identification of the main pathogenic Candida species by agglutination. The relevant antigen has been characterized as PPM and a series of seminal works about structure/activity relationships involving inhibition of agglutination together with nuclear magnetic resonance (NMR) and gas chromatography–mass spectrometry provided evidence that the antigenic determinants recognized by rabbit antisera are based on anomery of linkages (α-1,2, α-1,3, α-1,6, and β-1,2), and the degree of polymerization of mannose residues32, 33.
With the advent of hybridoma technology a relatively large number of anti-C. albicans MAbs have been produced which react against C. albicans mannan/mannoproteins34–37. Elucidation of their epitopes confirmed that they reacted with either anti-α-Man or anti-β-Man antibodies fitting the Iatron serological classification. Some of these MAbs were included in this study together with their BSTO epitopes. Due to interest in the protective effect of MAb B6.1 its epitope was specially constructed as a BSTO for this study.
In the present study, the specificity of these MAbs and polyclonal antibodies (human sera collected during Candida infection) was dissected using BSTOs in a qualitative and quantitative manner by SPR and MAP technology. We confirmed the specificity of 5B2 for 2β-Man34, B6.1 for 3β-Man35, and EB-CA1 for 4α-Man38. Unexpectedly, we observed that EB-CA1 also reacted with the 3β-Man epitope, which therefore represents the first exception to the dichotomous reactivity of mannan epitopes with either anti-α-Man or anti-β-Man antibodies established through a large number studies. This surprising observation, obtained by MAP and SPR, was confirmed by more conventional western blot analysis using C. albicans serotype B PLM and a serotype A deleted strain as both structurally defined antigens expressing the 3β-Man epitope. Studies by Cutler et al. emphasized the protective role of the humoral response directed against this epitope in both systemic and mucosal models of Candida infection39. A subsequent study showed that a monoclonal IgG3, called MAb C3.1, obtained from rabbits immunized with a liposome-mannan vaccine had the same specificity for β-mannotriose as MAb B6.1. Moreover, MAb C3.1 enhanced the resistance of mice to disseminated candidiasis40. Lipinski et al.41 developed a vaccine with 3β-Man and showed that it induced a strong secondary antibody response in rabbits. In addition, 3β-Man vaccination resulted in a reduction of the fungal burden in tissues of immunocompromised rabbits after challenge with live fungal cells. Another study combining NMR, chemical mapping, and computer simulation showed the importance of 3β-Man as the optimal oligosaccharide for the development of a vaccine against C. albicans42. In this study, importance was given to the anomery of linkage of the mannose at the reducing end, which is the case in our study for both the synthetic 3β-Man BSTO and the native 3β-Man-IPC from serotype B PLM. Despite experimental findings concerning the protection obtained with 3β-Man no translation was made in the analysis of the human response looking for protective antibodies targeting mannan epitopes. When BSTOs were used to dissect patient responses against individual epitopes in healthy individuals, colonized, or infected patients variable discrimination could be observed between these groups. For example, the 2α-Man response was higher in healthy controls than in patients. Conversely, 2β-Man antibodies were higher in infected patients, therefore representing a better diagnostic tool which was increased further by establishing the 2βMan/2α-Man ratio. These preliminary findings revealing the complex framework of C. albicans mannose repertoire recognition depending on patient status may have interesting clinical applications in terms of diagnosis and prediction of outcome. They certainly deserve further larger prospective studies combining MAP analysis and bioinformatic algorithms for the survey of hospitalized at-risk patients, particularly given that a recent study showed that patients infected with Candida strains displaying high levels of β1,2-mannosyl residues43 have a poor prognosis. With regard to 3β-Man, the significance of this response against a protective epitope cannot be evaluated in the absence of information on patient outcome. However, it had diagnostic value for discriminating colonized from infected patients. This property was shared with mannan as reflected by the Spearman correlation coefficient among the BSTOs. Thus, in coherence with results obtained with MAbs these findings revealed the property of 3β-Man to mimic the mannan repertoire of β- and α-mannoside epitopes.
MBL is a collectin with a molecular weight of 32 kDa, produced mainly by hepatocytes, that circulates in multimeric form with a predominance of the quaternary structure44. MBL forms a complex with three MBL-associated serine proteases45. MBL activates the lectin complement pathway after recognition of microorganisms through the carbohydrate-recognition domain. In vitro experiments using MBL purified from serum showed an interaction with the GlcNAc pentamer that activated the complement pathway. This interaction could be inhibited by mannose and N-acetylglucosamine. The carbohydrate-recognition domain of MBL senses polysaccharide patterns such as D-mannose, L-fucose, and GlcNAc on several clinically relevant pathogens including C. albicans. Lillegard et al.16 showed that depending on the growth conditions, C. albicans cells could fail to bind to MBL even though its cell wall contains mannose residues. They also reported variable patterns of MBL binding to yeast cells that are consistent with patterns of binding of anti-mannan MAbs to C. albicans. Epitopes recognized by some mannan specific MAbs are diffusely and continuously expressed on the surface of yeast cells (e.g. MAb EB-CA1 2α-Man46). In contrast, epitopes recognized by other MAbs are expressed as discontinuous patchy patterns (e.g. Mab B6.1 3β-Man35). It has been shown that the expression pattern of the ligand recognized by MBL is most similar to the expression of epitopes recognized by MAb B6.116.
We cannot currently explain why the B6.1 3β-Man epitope, which drew attention as a protective epitope, has the unique ability to be recognized by MAb EB-CA1 and MBL, molecules known to interact with mannose residues linked in α anomery. However, this dichotomy suggests that such an epitope could be involved in the recognition of yeasts by soluble and membrane receptors, stimulation of the adaptive immune response, and immunomodulation of mucosal and systemic defenses.
Our study suggests that an accumulation of basic and clinical information from the dissection of the anti-mannose immune response is worthwhile for a better understanding of Candida pathogenesis and better patient care through the development of vaccines.
Materials and methods
All methods were carried out in accordance with relevant guidelines and regulations.
Monoclonal antibodies and antigens
IgM isotype MAbs were selected according to their specificity. MAb EB-CA1, a rat IgM (Bio-Rad Laboratories, France), reacts with an α-(1,2)-mannopentaose as the minimal epitope36. Mab 5B2, a rat-mouse hybrid IgM, reacts with β-(1,2)-mannosides with a mannobiose as the minimal epitope41, 56. Mab B6.1, a mouse IgM, has been described as specific for a β-(1,2)-mannotriose42, 57. Mab EB-A2 (Bio-Rad), reacting with a galactofuranose epitope, was used as the control49. Tables 1 and 2 show the MAbs and antigens used in the study.
Table 1.
Information and references for the antigens used in the study and the technology involved in their analysis.
| Antigens/Epitopes | References | Main characteristics | Analytical methods | ||
|---|---|---|---|---|---|
| Synthetic | Multianalysis profiling | Surface plasmon resonance | Western Blotting/ELISA*** | ||
| Biotin Sulfone Tagged Oligomannosides (BSTOs), schematic representation of the epitope | |||||
| Manα-1,2Man | X | X | X | ||
| Manα-1,2Manα-1,2Manα-1,2Man | X | ||||
| Manβ-1,2Man | X | ||||
| Manβ-1,2Manβ-1,2Man | Mat & Meths | Synthesized for the present study | X | ||
| Manβ-1,2Manβ-1,2Manβ-1,2Man | X | X | X | ||
| Native | |||||
|
Phosphopeptidomannan (mannan) From C. albicans VW32 (Serotype A) |
27, 31–33, 55 | Complex cell wall surface polymer. Structure determined by NMR and sequential depolymerization of constitutive oligomannosides used for diagnostic purposes | *** | ||
| Phospholipomannan | 18, 56, 57 |
Glycoplipid of the mannoinositolphospho ceramide family Present at cell wall surface Shed in contact with host cells and has immunomodulatory properties |
X | ||
| From C. albicans VW 32 (serotype A) | 19 | Polysaccharide moiety consisting of β-mannosides up to 14 residues | X | ||
| From C. albicans NIH-B (serotype B) | 20 | Polysaccharide moiety consisting of β-1,2 mannotriose | X | ||
| From C. albicans BPW17 (serotype A) | 58 | PLM identical to that of VW32. Used for genetic manipulations | X | ||
| From C. albicans BPW17 depleted in bmt6 | 21 | Polysaccharide moiety truncated with a β-mannotriose | X | ||
Table 2.
Information about antibodies/probes used in the study and technology involved in their analysis.
| Probes | Analytical method | References | ||
|---|---|---|---|---|
| Multiple analysis profiling | Surface plasmon resonance | Western blotting | ||
| Monoclonal antibodies | ||||
| 5B2 | X | X | X | 34 |
| EB-CA1 | X | X | X | 36 |
| B6.1 | X | X | X | 35 |
| EB-A2 | X | X | X | 59 |
| Human polyclonal antibodies | ||||
| Polyclonal antibodies from healthy individuals | X | 55, 60, 61 | ||
| Polyclonal antibodies from colonized patients | X | |||
| Polyclonal antibodies from infected patients | X | |||
| Mannose binding lectin | X | |||
Human sera
Human antibodies against BSTOs were characterized in sera taken from three groups of individuals: (1) The first group represents 51 control sera collected from 51 blood donors presenting no disease; (2) The second group consisted of sera collected from 30 ICU patients with systemic C. albicans infection, proven by the isolation of C. albicans from blood cultures. In this group, sera were taken during the period of serological monitoring from 68 days before to 67 days after C. albicans was isolated from the blood; 181 sera were obtained (mean of 6.03 sera per patient); (3) The third group represents 119 sera from 30 patients hospitalized in ICU ward in Besançon University Hospital where colonization by C. albicans is followed bi-weekly in order to establish the colonization index (CI). The 30 patients had been hospitalized for > 10 days (mean duration 22 days, maximum 73 days). At admission, all had a corrected colonization index (CCI) of < 0.25. Eight patients were constantly negative for Candida colonization, 22 had a CCI ≤ 0.4, and five evolved towards a CCI of > 0.4. A total of 450 mycological samples were examined and the rates of positive isolation of Candida were: oropharynx 61%, gastric 30%, tracheal 22%, rectum 17%, and urine 6%. These colonized patients did not have firm clinical evidence of Candida infection.
Ethics statement
All sera used in this study were obtained from patients monitored at Lille University Hospital. No additional sampling was necessary. As sera were taken from a registered biological collection, patient consent was not required according to French law. Agreement for the establishment of a biological collection of invasive fungal infection samples was obtained from the French Ministry of Education and Research under reference DC2008-642. Institutional review board approval was given by the Comité de Protection des Personnes Nord-Ouest IV, the ethical committee of our institution. Sera from colonized patients were collected during a prospective study conducted by one of us which received institutional review approval from the local ethical committee50.
C. albicans strains
C. albicans serotype A strains consisted of our laboratory reference strain VW3251 and BWP17 strain, which was derived from SC5314, the C. albicans strain used for sequencing the whole genome52. The serotype B strain consisted of NIH B-79220. We included a bmt6Δ strain, which was constructed from BWP17 by deletion of both alleles for the BMT6 gene encoding the mannosyltransferase responsible for the addition of the third β-1,2 linked mannose to PLM21.
Western blotting
Strains were grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) at 37 °C for 16 h and were extracted using alkaline extraction under reducing conditions34. Briefly, cells were incubated on ice in 1.85 M NaOH and 5% β-mercaptoethanol. Proteins and glycoconjugates were then extracted in SDS for 5 min at 100 °C. Extracts were adjusted to the same protein concentration and analyzed by SDS-PAGE on a 5–20% acrylamide gel slab. Membranes were probed with MAbs B6.1 diluted 1:1000, CA1 diluted 1:1000, and 5B2 diluted 1:1000, and then incubated with a 1:1000 dilution of AP-conjugated anti-mouse IgM (B6.1), or anti-rat IgM (CA1 and 5B2).
Mannan extraction from C. albicans
Mannan was prepared from C. albicans VW32 grown in bioreactors under standard conditions used for the chemical and immunochemical analysis of this molecule53. Quantification of mannan was performed by the sulfuric phenol colorimetric method with a range of standard sucrose solutions. Optical density was measured at 492/620 nm.
Detection of human antibodies against C. albicans mannan
Antibodies to C. albicans mannan were detected using the commercially available Platelia Candida Ab kit (Bio-Rad, France). The antigen used to coat the ELISA plates is cell wall mannan extracted from C. albicans serotype A, strain VW32 (see above), extracted by the method of Kocourek and Ballou54. Detection of antibodies was performed according to manufacturer’s instructions.
Preparation of BSTOs
The synthesis of oligosaccharides and their biotinylation has been described previously10, except for mannotriose. The strategy using for the synthesis of biotinylated β (1 → 2) mannotriose is shown in Fig. 7.
Figure 7.
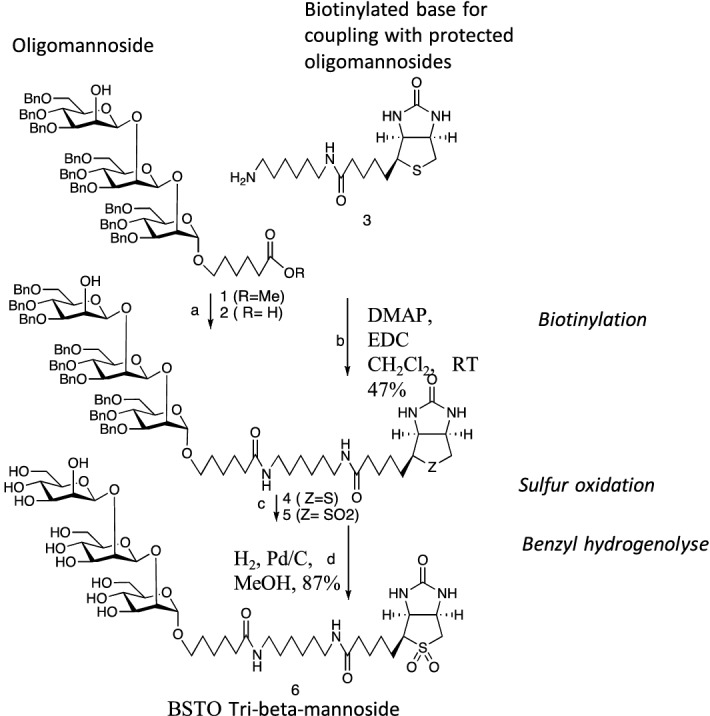
Synthesis of biotinylated β (1 → 2) mannotriose. Compound 6 (tribeta) was prepared in four steps from 110 using coupling and deprotection protocols employed for related di- and tetramannosides62. Reagents and conditions: (a) NaOH, THF, 60 °C, 98%; (b) DMAP, EDC CH2Cl2, RT 47%; (c) meta chloro perbenzoic acid, CH2Cl2, room temperature, 87%; (d) H2, Pd/C, MeOH, 87%. In the following Figs. 2, 3, 5, 6 and 7 oligomannosides are designated by acronyms referring to the number of residues and the anomery (i.e. 3β-Man for β-mannotriose) and presented with the color codes already used in studies on oligomannose synthesis (i.e. red for α anomery and green for β anomery17), and the main topic of this study, 3β-Man, is represented in darker green.
Coupling of BSTOs to fluorescent magnetic beads
Polystyrene fluromagnetic beads were prepared via a four-step procedure as previously described10. Briefly, carboxyl functionalized fluromagnetic beads were first activated with N-hydroxysulfosuccinimide and ethylcarbodiimide (Pierce Chemicals Co. Rockford, Ill, USA) to form activated esters. After washing in 50 mM phosphate buffered saline (PBS), pH 7.4, these esters were incubated with avidin (Sigma, Saint Quentin Fallavier, France) for 3 h RT. The beads are then blocked with 50 mM PBS containing 250 mM NH2OH for 30 min. After wash in PBS, the beads were coupled with BSTO for 1 h RT and then incubated overnight with 50 mM PBS containing 10% bovine serum albumin. After washing in PBS, the beads were kept at 4° C.
Quality control of BSTO coupling
For each set of experiments and after coupling BSTOs to magnetic beads, the reactivity of the BSTOs was controlled with a dilution range (1:2000–1:32,000) of biotinylated Galanthus nivalis lectin reacting with α-(1,3)-mannose residues or with a panel of polyclonal and monoclonal antibodies followed by detection with streptavidin–phycoerythrin (2 µg/mL). Mixed suspensions of microspheres coated with different BSTOs were incubated with PBS diluted antibodies for 30 min at 37 °C under agitation. After three washes in PBS-1% Tween 20 (PBST) the microspheres were incubated with appropriate anti-immunoglobulins coupled to phycoerythrin (Southern Biotech, USA).
MAP analysis of BTSO reactivity with sera from patients with invasive candidiasis, ICU colonized patients and controls
Sera were diluted 1:4800 in PBS and incubated for 30 min at 37 °C. After three washes in PBST, the microspheres were incubated with goat anti-human IgG coupled with phycoerythrin (1 µg/mL) (Southern Biotech, USA).
For both procedures, after three washes in PBST the microspheres were re-suspended in sheath fluid in a test tube and the reaction was monitored on a Luminex Lab MAP system 100 (Luminex USA) at 532 nm. The results are expressed as mean fluorescence intensity determined for 100 microspheres of each BSTO identified by its microsphere spectral signature. For human sera, the results were then treated with the "ETALONNAGE" software (University of Lille) and converted into arbitrary units (AU) from calibration curves. Pooled human serum was used as the control standard.
SPR analysis of BSTO reactivity with anti-carbohydrate MAbs
BIAcore 3000 instrument, BIAevaluation software 3.0, and sensor chip SA (streptavidin) were obtained from BIAcore (GE Healthcare). 5 nM of BSTOs were fixed onto the sensorchip in HBS (HEPES buffered saline) buffer according to the manufacturer’s instructions using NHS/EDC. The immobilization rate was approximately 25–30 response units (RU). 5B2, EB-CA1, B6.1 and EB-A2 MAbs were injected at 250 nM in HBS to 30 μL/min over a 2 min period. The regeneration of sensorship was performed with a 250 mM NaCl/10 mM NaOH buffer. A reference flow cell (i.e. flow cell without BSTOs) was used for each used BSTOs. Quantification of specific binding was obtained from the difference between the ligand and reference response as previously described10.
SPR analysis of BSTO-MBL interactions
With the aim of avoiding non-specific binding induced by non-carbohydrate structures, non-biotinylated oligomannosides were used. rMBL was immobilized onto a CM5 sensorchip (GE Healthcare) in HBS-EP 1X buffer to reach 4000 RU. BSTOs at different concentrations (0, 0.25, 0.5, 1, and 2 mg/mL) were diluted in running buffer composed of HBS-P, CaCl2 5 mM, and surfactant 0.05% and injected in BIAcore 3000 instrument with a flow at 20 µL/min during 4 min. The sensorship was regenerated with 0.25 M EDTA buffer. For each sample, the results are expressed as the difference between the tested flow cell and reference flow cell (flow cell without immobilized rMBL) as previously described12. Normalization of results was performed according to the molecular mass of each BSTO.
Statistical analysis
All statistical analyses were performed using SAS and Med Calc software. Spearman correlation coefficients were calculated to determine the relationship between the reactivity of sera against mannan from C. albicans and different BSTOs. The Mann–Whitney U test was used for comparison of antibody levels between different patient groups.
Supplementary Information
Acknowledgements
We thank the European Union for funding through the INTERREG V France—Wallonie—Vlaanderen program—SmartBioControl-BIOSENS project and the Programme Hospitalier de Recherche Clinique, CandiGene, 2011–1918.
Author contributions
S.D., A.-S.D., M.C., K.L., and C.F. performed the experiments. B.S., P.-M.D., D.P., S.D., and C.F. analyzed the data. K.L., B.S., and D.P. interpreted the results of the experiments. F.D., J.de R., S.J., F.G., J.-P.V., J.-M.M., and M.C. contributed by providing reagents/synthetic sugars/analysis tools. B.S., D.P., and J.-M.M. designed the experiments and drafted the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Boualem Sendid, Email: boualem.sendid@univ-lille.fr.
Daniel Poulain, Email: daniel.poulain@univ-lille.fr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90402-4.
References
- 1.Axford J. The impact of glycobiology on medicine. Trends Immunol. 2001;22:237–239. doi: 10.1016/S1471-4906(01)01890-7. [DOI] [PubMed] [Google Scholar]
- 2.Kappler K, Hennet T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun. 2020;21:224–239. doi: 10.1038/s41435-020-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azurmendi HF, et al. Chemical structure and genetic organization of the E. coli O6:K15 capsular polysaccharide. Sci. Rep. 2020;10:12608. doi: 10.1038/s41598-020-69476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fradin C, et al. Beta-1,2 oligomannose adhesin epitopes are widely distributed over the different families of Candida albicans cell wall mannoproteins and are associated through both N- and O-glycosylation processes. Infect. Immun. 2008;76:4509–4517. doi: 10.1128/IAI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gow NAR, van de Veerdonk FL, Brown AJP, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Netea MG, Joosten LAB, van der Meer JWM, Kullberg B-J, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015;15:630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 7.Sendid B, et al. Antibodies against glucan, chitin, and Saccharomyces cerevisiae mannan as new biomarkers of Candida albicans infection that complement tests based on C. albicans mannan. Clin. Vaccine Immunol. 2008;15:1868–1877. doi: 10.1128/CVI.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collot M, Wilson IBH, Bublin M, Hoffmann-Sommergruber K, Mallet J-M. Synthesis of cross-reactive carbohydrate determinants fragments as tools for in vitro allergy diagnosis. Bioorg. Med. Chem. 2011;19:1306–1320. doi: 10.1016/j.bmc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, et al. Identification of the antigenic determinants of factors 8, 9, and 34 of genus Candida. FEBS Lett. 1996;395:109–112. doi: 10.1016/0014-5793(96)01013-7. [DOI] [PubMed] [Google Scholar]
- 10.Collot M, et al. Biotin sulfone as a new tool for synthetic oligosaccharide immobilization: application to multiple analysis profiling and surface plasmonic analysis of anti-Candida albicans antibody reactivity against α and β (1→2) oligomannosides†. J. Med. Chem. 2008;51:6201–6210. doi: 10.1021/jm800099g. [DOI] [PubMed] [Google Scholar]
- 11.Sheriff S, Chang CY, Ezekowitz RAB. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple a-helical coiled-coil. Nat. Struct. Biol. 1994;1:6. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- 12.Damiens S, et al. Characterization of the recognition of Candida species by mannose-binding lectin using surface plasmon resonance. Analyst. 2013;138:2477–2482. doi: 10.1039/c3an36670g. [DOI] [PubMed] [Google Scholar]
- 13.Yokota Y, Arai T, Kawasaki T. Oligomeric structures required for complement activation of serum mannan-binding proteins1. J. Biochem. 1995;117:414–419. doi: 10.1093/jb/117.2.414. [DOI] [PubMed] [Google Scholar]
- 14.Choteau L, et al. Role of mannose-binding lectin in intestinal homeostasis and fungal elimination. Mucosal Immunol. 2015;9:767. doi: 10.1038/mi.2015.100. [DOI] [PubMed] [Google Scholar]
- 15.Weis WI. Structure of a C-type mannose-binding protein complexed with an oligosaccharide . Nature. 1992;360:8. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 16.Lillegard JB, Sim RB, Thorkildson P, Gates MA, Kozel TR. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J. Infect. Dis. 2006;193:1589–1597. doi: 10.1086/503804. [DOI] [PubMed] [Google Scholar]
- 17.Courjol F, et al. β-1,2-Mannosyltransferases 1 and 3 participate in yeast and hyphae O- and N-linked mannosylation and alter Candida albicans fitness during infection. Open Forum Infect. Dis. 2015;2:116. doi: 10.1093/ofid/ofv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinel P-A, et al. Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family. J. Biol. Chem. 2002;277:37260–37271. doi: 10.1074/jbc.M202295200. [DOI] [PubMed] [Google Scholar]
- 19.Trinel PA, Lepage G, Jouault T, Strecker G, Poulain D. Definitive chemical evidence for the constitutive ability of Candida albicans serotype A strains to synthesize beta-1,2 linked oligomannosides containing up to 14 mannose residues. FEBS Lett. 1997;416:203–206. doi: 10.1016/S0014-5793(97)01205-2. [DOI] [PubMed] [Google Scholar]
- 20.Trinel P-A, et al. Candida albicans serotype B strains synthesize a serotype-specific phospholipomannan overexpressing a β-1,2-linked mannotriose. Mol. Microbiol. 2005;58:984–998. doi: 10.1111/j.1365-2958.2005.04890.x. [DOI] [PubMed] [Google Scholar]
- 21.Mille C, et al. Members 5 and 6 of the Candida albicans BMT family encode enzymes acting specifically on β-mannosylation of the phospholipomannan cell-wall glycosphingolipid. Glycobiology. 2012;22:1332–1342. doi: 10.1093/glycob/cws097. [DOI] [PubMed] [Google Scholar]
- 22.Poulain D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015;41:208–217. doi: 10.3109/1040841X.2013.813904. [DOI] [PubMed] [Google Scholar]
- 23.Domer JE, Garner RE. Immunomodulation in response to Candida. Immunol. Ser. 1989;47:293–317. [PubMed] [Google Scholar]
- 24.Wang Y, Li SP, Moser SA, Bost KL, Domer JE. Cytokine involvement in immunomodulatory activity affected by Candida albicans mannan. Infect. Immun. 1998;66:1384–1391. doi: 10.1128/IAI.66.4.1384-1391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gow NAR, Latge J-P, Munro CA. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017;5:1–25. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 1997;8:57–70. [PubMed] [Google Scholar]
- 27.Shibata N, Kobayashi H, Suzuki S. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012;88:250–265. doi: 10.2183/pjab.88.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N. Engl. J. Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 29.Jouault T, Delaunoy C, Sendid B, Ajana F, Poulain D. Differential humoral response against alpha- and beta-linked mannose residues associated with tissue invasion by Candida albicans. Clin. Diagn. Lab. Immunol. 1997;4:328–333. doi: 10.1128/CDLI.4.3.328-333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno M, et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukazawa Y. Antigenic structure of Candida albicans. Immunochemical basis of the serologic specificity of the mannans in yeasts. Immunol Ser. 1989;47:37–62. [PubMed] [Google Scholar]
- 32.Faille C, et al. 1H-NMR spectroscopy of manno-oligosaccharides of the beta-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW-32 (serotype A) Biochem. Biophys. Res. Commun. 1991;181:1251–1258. doi: 10.1016/0006-291X(91)92073-S. [DOI] [PubMed] [Google Scholar]
- 33.Faille C, Wieruszeski JM, Michalski JC, Poulain D, Strecker G. Complete 1H- and 13C-resonance assignments for D-mannooligosaccharides of the beta-D-(1–>2)-linked series released from the phosphopeptidomannan of Candida albicans VW.32 (serotype A) Carbohydr. Res. 1992;236:17–27. doi: 10.1016/0008-6215(92)85004-J. [DOI] [PubMed] [Google Scholar]
- 34.Trinel PA, Faille C, Jacquinot PM, Cailliez JC, Poulain D. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect. Immun. 1992;60:3845–3851. doi: 10.1128/IAI.60.9.3845-3851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y, Kanbe T, Cherniak R, Cutler JE. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 1997;65:4100–4107. doi: 10.1128/IAI.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquinot PM, Plancke Y, Sendid B, Strecker G, Poulain D. Nature of Candida albicans -derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 1998;169:131–138. doi: 10.1111/j.1574-6968.1998.tb13309.x. [DOI] [PubMed] [Google Scholar]
- 37.Ponton J, et al. Characterization of Candida albicans cell wall antigens with monoclonal antibodies. Infect. Immun. 1993;61:4842–4847. doi: 10.1128/IAI.61.11.4842-4847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulain D, et al. Clearances of Candida albicans-derived alpha- and beta-linked mannose residues in sera from patients with candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:16–20. doi: 10.1007/BF01575114. [DOI] [PubMed] [Google Scholar]
- 39.Cutler JE, Deepe GS, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev.. Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han Y, Riesselman MH, Cutler JE. Protection against Candidiasis by an Immunoglobulin G3 (IgG3) Monoclonal Antibody Specific for the Same Mannotriose as an IgM Protective Antibody. Infect. Immun. 2000;68:1649–1654. doi: 10.1128/IAI.68.3.1649-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipinski T, et al. A β-mannan trisaccharide conjugate vaccine aids clearance of Candida albicans in immunocompromised rabbits. Vaccine. 2012;30:6263–6269. doi: 10.1016/j.vaccine.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MA, Cartmell J, Weisser NE, Woods RJ, Bundle DR. Molecular recognition of Candida albicans (1->2)-β-mannan oligosaccharides by a protective monoclonal antibody reveals the immunodominance of internal saccharide residues. J Biol Chem. 2012;287:18078–18090. doi: 10.1074/jbc.M112.355578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangneux J-P, et al. Clinical impact of antifungal susceptibility, biofilm formation and mannoside expression of Candida yeasts on the outcome of invasive Candidiasis in ICU: An ancillary study on the prospective AmarCAND2 cohort. Front. Microbiol. 2018;9:2907. doi: 10.3389/fmicb.2018.02907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensenius H, et al. Mannan-binding lectin: Structure, oligomerization, and flexibility studied by atomic force microscopy. J. Mol. Biol. 2009;391:246–259. doi: 10.1016/j.jmb.2009.05.083. [DOI] [PubMed] [Google Scholar]
- 45.Thiel S, et al. Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1), a serine protease associated with humoral pattern-recognition molecules: Normal and acute-phase levels in serum and stoichiometry of lectin pathway components: Serum concentrations of MASP-1. Clin. Exp. Immunol. 2012;169:38–48. doi: 10.1111/j.1365-2249.2012.04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinel PA, Cantelli C, Bernigaud A, Jouault T, Poulain D. Evidence for different mannosylation processes involved in the association of beta-1,2-linked oligomannosidic epitopes in Candida albicans mannan and phospholipomannan. Microbiology (Reading) 1996;142(Pt 8):2263–2270. doi: 10.1099/13500872-142-8-2263. [DOI] [PubMed] [Google Scholar]
- 47.Hopwood V, Poulain D, Fortier B, Evans G, Vernes A. A monoclonal antibody to a cell wall component of Candida albicans. Infect. Immun. 1986;54:222–227. doi: 10.1128/IAI.54.1.222-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y, Cutler JE. Antibody response that protects against disseminated candidiasis. Infect. Immun. 1995;63:2714–2719. doi: 10.1128/IAI.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stynen D, et al. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 1992;60:2237–2245. doi: 10.1128/IAI.60.6.2237-2245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piarroux R, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit. Care Med. 2004;32:2443–2449. doi: 10.1097/01.CCM.0000147726.62304.7F. [DOI] [PubMed] [Google Scholar]
- 51.Faille C, et al. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect. Immun. 1990;58:3537–3544. doi: 10.1128/IAI.58.11.3537-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with candida albicans through gene disruption with short homology regions. J. Bacteriol. 1999;181:7. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faille C, Mackenzie DWR, Michalski JC, Poulain D. Evaluation of an enzyme immunoassay using neoglycolipids constructed from Candida albicans oligomannosides to define the specificity of anti-mannan antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 1992;11:438–446. doi: 10.1007/BF01961859. [DOI] [PubMed] [Google Scholar]
- 54.Kocourek J, Ballou CE. Method for fingerprinting yeast cell wall mannans. J. Bacteriol. 1969;100:1175–1181. doi: 10.1128/JB.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yera H, Sendid B, Francois N, Camus D, Poulain D. Contribution of serological tests and blood culture to the early diagnosis of systemic candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:864–870. doi: 10.1007/s100960100629. [DOI] [PubMed] [Google Scholar]
- 56.Jouault T, Bernigaud A, Lepage G, Trinel PA, Poulain D. The Candida albicans phospholipomannan induces in vitro production of tumour necrosis factor-alpha from human and murine macrophages. Immunology. 1994;83:268–273. [PMC free article] [PubMed] [Google Scholar]
- 57.Poulain D, Slomianny C, Jouault T, Gomez JM, Trinel PA. Contribution of phospholipomannan to the surface expression of beta-1,2-oligomannosides in Candida albicans and its presence in cell wall extracts. Infect. Immun. 2002;70:4323–4328. doi: 10.1128/IAI.70.8.4323-4328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mille C, et al. Identification of a new family of genes involved in beta-1,2-mannosylation of glycans in Pichia pastoris and Candida albicans. J. Biol. Chem. 2008;283:9724–9736. doi: 10.1074/jbc.M708825200. [DOI] [PubMed] [Google Scholar]
- 59.Cattiaux L, et al. Synthetic biotinylated tetra β(1→5) galactofuranoside for in vitro aspergillosis diagnosis. Bioorg. Med. Chem. 2011;19:547–555. doi: 10.1016/j.bmc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 60.Sendid B, et al. New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J. Clin. Microbiol. 1999;37:1510–1517. doi: 10.1128/JCM.37.5.1510-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sendid B, et al. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J. Med. Microbiol. 2002;51:433–442. doi: 10.1099/0022-1317-51-5-433. [DOI] [PubMed] [Google Scholar]
- 62.Mikata Y, et al. Sugar-pendant diamines. J. Org. Chem. 2001;66:3783–3789. doi: 10.1021/jo001702+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.