Abstract
Background
Secondary plasma cell leukaemia (sPCL) is an aggressive form of multiple myeloma (MM), but the mechanism underlying MM progresses into PCL remains unknown.
Methods
Gene expression profiling of MM patients and PCL patients was analysed to identify the molecular differences between the two diseases. Cox survival regression and Kaplan–Meier analysis were performed to illustrate the impact of integrin subunit alpha 6 (ITGA6) on prognosis of MM. Invasion assays were performed to assess whether ITGA6 regulated the progression of MM to PCL.
Results
Gene expression profiling analyses showed that cell metastasis pathways were enriched in PCL and ITGA6 was differentially expressed between PCL and MM. ITGA6 expression was an independent prognostic factor for event-free survival (EFS) and overall survival (OS) of MM patients. Moreover, the stratification ability of the International Staging System (ISS) of MM was improved when including ITGA6 expression. Functional studies uncovered that increased ITGA6 reduced the myeloma cell invasion. Additionally, low expression of ITGA6 resulted from epigenetic downregulating of its anti-sense non-coding RNA, ITGA6-AS1.
Conclusion
Our data reveal that ITGA6 gradually decreases during plasma cell dyscrasias progression and low expression of ITGA6 contributes to myeloma metastasis. Moreover, ITGA6 abundance might help develop MM prognostic stratification.
Subject terms: Prognostic markers, Myeloma
Background
Multiple myeloma is a bone-marrow-based malignancy characterised by proliferation of clonal plasma cells.1 A characteristic of MM is its main localisation in the bone marrow and its tendency to progress from the original bone position to the new bone position in local and distal bones.1,2 During the progression of MM, cancer cells develop the ability to proliferate at sites outside of the bone marrow, manifesting as plasma cell leukemia3. Plasma cell leukaemia can be divided into two types according to the source. One is primary plasma cell leukaemia (pPCL) in a newly diagnosed setting, and the other is secondary plasma cell leukaemia (sPCL) in a relapsed MM setting.3
Genomic differences between PCL and MM have been studied.3 Hypodiploidy, 1q gains, 13q deletion and p53 deletion partially defined the progressive the plasma cell (PC) disease.3 However, genetic characteristics could not completely explain PC disease progression.4 Thus, the comprehensive assessment of transcriptional characterisation might provide fresh information about biological differences between MM and PCL.
A large part of the human genome is transcribed into lncRNAs longer than 200 nt that have no protein coding potential.5,6 Recent studies indicate that lncRNAs have abundant functions in multiple biological processes.7–9 In addition, emerging evidences support the notion that anti-sense lncRNAs play key roles in their associated protein coding genes and particularly affecting the latter expression.10–12
Current prognosis markers for MM is International Staging System (ISS), which may not reflect the molecular characteristics of the myeloma clone.13 It is therefore essential to develop biomarkers that are specific to define the biological aspect of MM.
Herein, we analysed the gene expression profiling of MM patients and PCL patients using GEO databases and identified an integrin, ITGA6, whose expression was decreased (>threefold) in PCL samples versus its expression in MM samples in two datasets (GSE2113 and GSE39925). We also performed survival analysis to determine the impact of ITGA6 on patient outcomes and noticed that high level of ITGA6 was related to increased EFS and OS compared with lower expression levels of ITGA6 in MM. Meanwhile, functional studies revealed that the overexpression of ITGA6 reduced invasion in MM cell lines. Furthermore, we discovered that a lncRNA, ITGA6-AS1 formed an RNA duplex with ITGA6 pre-mRNA, which increased the level of ITGA6 pre-mRNA stability and reduced the invasion of MM cells. Therefore, our data indicate that downregulation of ITGA6 confers to the invasion of multiple myeloma, further suggesting that a potential therapy targeted ITGA6 might inhibit myeloma cell migration associated with progression of MM to PCL.
Methods
Collection of patient samples
All samples were collected with ethical permission from the Second Affiliated Hospital of Soochow University (characteristics of patients in Table S1). The study was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from each subject or each subject’s guardian.
RNA extraction and real-time PCR Analysis
Total RNA was isolated using TRIZOL (Invitrogen, USA) according to the manufacturer’s instruction. QPCR performed with the Real-Time PCR System (7500). The expression of indicated genes was normalised to endogenous reference control (GAPDH) by using 2−ΔΔCt method (primer sequences in Table S2).
Cell proliferation
The cell proliferation rate was determined using Cell Counting Kit-8 (CCK-8, Dojindo Corporation) according to the instructions. Assays were performed at 24, 48 and 72 h, and each sample was assayed in triplicate.
Transwell assay
The invasion ability of the MM cells was assessed using the transwell assays by means of 8-μm pore-size Matrigel-coated transwell chambers (Corning). After starvation in serum-free RPMI 1640 medium for 12 h, the cells (2 × 105) were placed on Matrigel (BD Biosciences)-coated transwell chambers (Corning). After 24 h incubation in an incubator at 37 °C and 5% CO2, non-invasive cells were removed from the upper surface. The cells invading through the Matrigel were fixed using 4% paraformaldehyde, stained by haematoxylin, imaged using the microscope and counted in the upper surface and the lower medium. These experiments were performed in triplicate for each condition.
Statistical analysis
Survival analysis was performed to investigate the associations between the censored outcomes, the clinical variables and the expression of ITGA6 using the R package survival. All clinical variables and the expression of ITGA6 were numeric and standardised to facilitate the comparison of hazards ratios (HR).
We first used univariate Cox survival regression to separately analyse predictor variables. To illustrate the impact of ITGA6, we partitioned the patients into two groups based on the medians of ITGA6 expression, estimated the survival curves of these two groups of patients using the Kaplan–Meier method and compared their differences using the log-rank test. We then used multivariate Cox survival regression to jointly fit all the clinical variables with ITGA6, which was adjusted for the potential confounding effects of the clinical variables on ITGA6. By fitting these Cox models, we obtained estimates of the hazards ratios (HR), their 95% confidence intervals (CIs), corresponding p-values, and Harrell’s concordance indices (C-indices). We also validated the prognostic values of these models using leave-one-out cross-validation.
All results were listed as the mean ± SD (standard deviation). Student’s t-test was used when the variance between groups was similar. The difference in mean values between two groups was analysed by Student’s t-test. Pearson correlation analysis was used as mentioned above. A value of P < 0.05 indicated significance.
Other methods are detailed in supporting information.
Results
ITGA6 may regulate the progression of MM to PCL
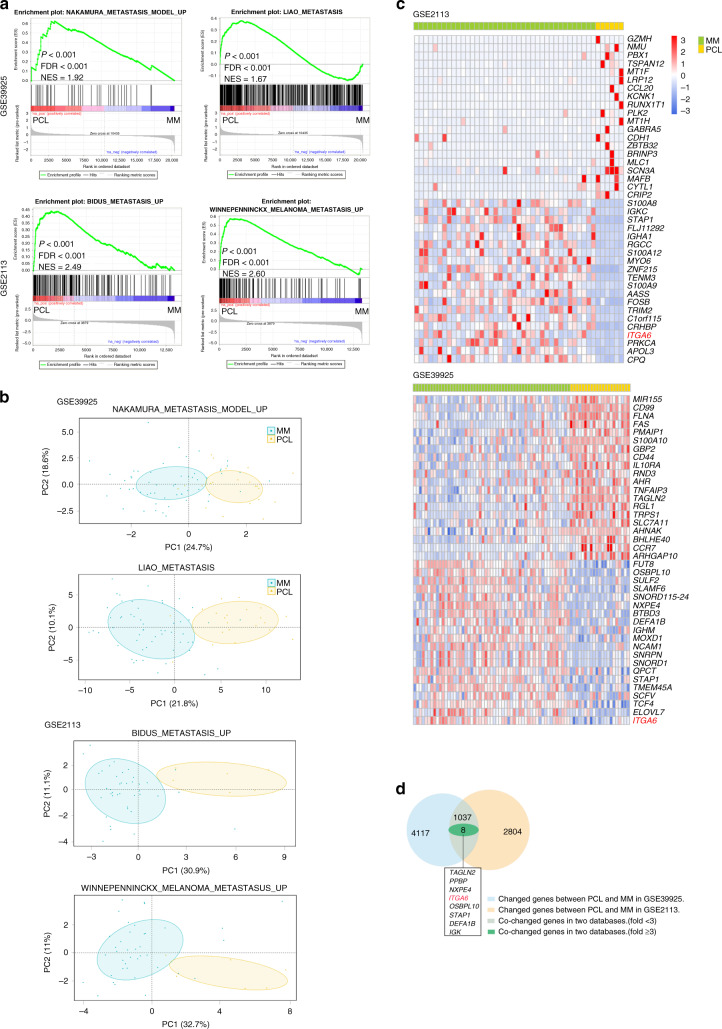
To clarify the underlying mechanisms that lead to extramedullary myeloma (PCL) from MM, the gene expression profiling of MM patients and PCL patients was analysed using GEO datasets (GSE39925 and GSE2113). GSEA indicated that gene signatures for the positive regulation of cell metastasis (the gene sets: LIAO_METASTASIS, NAKAMURA_METASTASIS_MODEL_UP, BIDUS_METASTASIS_UP and WINNEPENNINCKX_MELANOMA_METASTASIS_UP) were significantly enriched in PCL samples (Fig. 1a). Furthermore, the distribution of principal component analysis (PCA) showed a clear distinction between MM samples and PCL samples in the four pathways (Fig. 1b). To identify the specific mRNAs involved in cell metastasis, the differentially expressed genes between PCL and MM were analysed (Fig. 1c). Both datasets (GSE39925 and GSE2113) identified the same seven genes which were increased or decreased (>threefold) in PCL versus MM, probably reflecting their distinct oncogenic programs and genetic profiling (Fig. 1d). Among them, we noted integrin subunit alpha 6 (ITGA6, GSE39925: fold change = 4.88, P = 0.01, GSE2113: fold change = 3.51, P = 0.004), which was shown to be responsible for cell adhesion and invasion.14 Integrins are a class of glycoproteins that are widely found in many cell types, and have pivotal roles in regulating cell adhesion, migration, homing, invasion and drug resistance.15–17 A wide range of integrins is also detected in MM cells including α4, α5, β1, β2, β3 and β7.18–21 ITGA6 is a member of the integrin alpha chain family of proteins, which are responsible for mediating epithelial-basement membrane interactions.14 Recent evidence suggests that signalling events mediated by the α6 subunit also regulate the processes involved in tumorigenesis, including proliferation and metastasis.22–27 It is not yet clear what role ITGA6 plays in the progression from MM to PCL and whether its functions are essential for the invasion of myeloma extramedullary sites.
Fig. 1. ITGA6 may regulate the progression of MM to PCL.
a Specific gene expression signatures were associated with cell metastasis. Representative GSEA plots illustrating the enrichment of genes involved in positive regulation in PCL samples. FDR false discovery rate, NES normalised enrichment score. GSE2113 (MM n = 39, PCL n = 6), GSE39925 (MM n = 55, PCL n = 21). b Principal component analysis (PCA) plot showed unsupervised clustering, demonstrating a clear distinction between MM and PCL samples in each pathway. c The 30 up- and downregulated genes in PCL versus MM were presented in heat maps. d Venn diagram showing the overlapping differentially expressed genes identified in the two groups.
ITGA6 could be used as a biomarker of prognosis in MM patients
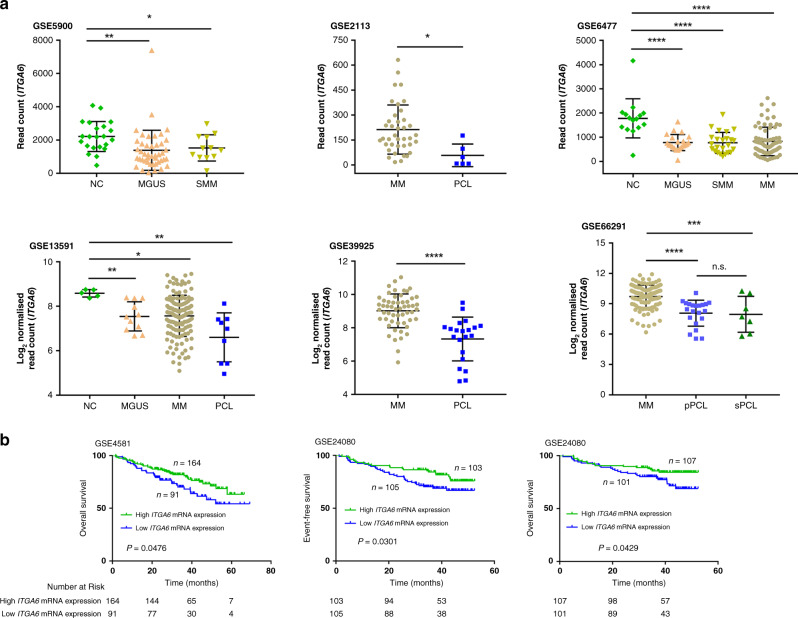
To further explore the role of ITGA6 in plasma cell tumour transformation, we analysed the ITGA6 expression in patients with plasma cell disease at different stages (MGUS, SMM, MM and PCL) and PCs of healthy donor (NC). As shown in Fig. 2a, the expression level of ITGA6 in patients with MGUS, SMM and MM was lower than that of healthy donors, but there was no significant difference among MGUS, SMM and MM. And ITGA6 level in PCL was lower than that of MM patients. Together, ITGA6 expression was reduced by the progression of plasma cell dyscrasias. We subsequently sought to explore the differences in ITGA6 expression between pPCL and sPCL samples. Furthermore, comparison of pPCL samples to sPCL samples showed that the expression of ITGA6 was no significant difference (Fig. 2a GSE66291). To investigate whether ITGA6 expression was related to the prognosis of MM, we analysed two datasets obtained from the GEO databases (GSE24080 and GSE4581). The effect of ITGA6 on the EFS and OS was illustrated by Kaplan–Meier curves dichotomised expression of ITGA6 at the median. We observed that reduced expression of ITGA6 consistently associated with poor survival (Fig. 2b). Consistent with this, univariate and multivariate analyses were performed using the Cox risk proportion model and ITGA6 was significantly associated with OS (cut-off = 10.8116) (Table 1, GSE24080). To further assess the prognostic value of ITGA6, we tested the c-indices of the models fit with ISS alone and ISS with ITGA6. Leave-one-out cross-validation was performed to validate the prognostic value of ITGA6. The c-indices of model fit with the combination of ISS and ITGA6 were higher than those of models fit without ITGA6, suggesting that ITGA6 improved the prognostic stratification of patients with MM (Table 2). To further assess the prognostic significance for ITGA6, we validated this risk stratification in another cohort of patients. All samples were collected from the Second Affiliated Hospital of Soochow University. The detailed clinical features of these patients were described in our previous publication.28 In the univariate (HR = 0.2534, 95% CI: 0.0963–0.6666, P = 0.0054) and multivariate (HR = 0.3033, 95% CI: 0.1002–0.9183, P = 0.0348) Cox analyses, ITGA6 was an independent predictor for OS (cut-off = 1.3600) (Table S3). Together, the above results suggested that ITGA6 probably has an important effect on the progression from MM to PCL.
Fig. 2. ITGA6 could be used as a biomarker of prognosis in MM patients.
a The expression of ITGA6 in plasma cell dyscrasias (MGUS, SMM, MM and PCL) and PCs of healthy donor (NC). The expression level of ITGA6 in patients with MGUS, SMM and MM was lower than that of healthy donors. ITGA6 level in PCL was lower than that of MM patients. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns not significant versus NC. b Kaplan–Meier plots indicating the prognostic relevance of ITGA6 expression in the event-free and overall survival of MM patients (GSE24080 and GSE4581).
Table 1.
Summary for the results of univariate and multivariable analysis.
| Factors | OS (overall survival) | EFS (event-free survival) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Patients | Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR | p-value | HR | 95% CI | p-value | HR | p-value | HR | 95% CI | p-value | ||
| Age | 345 | 1.0309 | 0.1081 | 1.0385 | 0.9960–1.0828 | 0.0764 | 1.0245 | 0.1421 | 1.0380 | 1.0008–1.0767 | 0.0453 |
| Beta-2 microglobulin | 345 | 1.0819 | 0.0000 | 1.0734 | 1.0212–1.1282 | 0.0053 | 1.0770 | 0.0000 | 1.0530 | 1.0055–1.1027 | 0.0283 |
| C-reactive protein | 345 | 1.0041 | 0.4113 | 0.9882 | 0.9710–1.0057 | 0.1841 | 1.0096 | 0.0045 | 1.0013 | 0.9917–1.0109 | 0.7958 |
| Creatinine | 345 | 1.2250 | 0.0035 | 1.0848 | 0.8580–1.3717 | 0.4964 | 1.2504 | 0.0001 | 1.1106 | 0.9120–1.3523 | 0.2967 |
| Lactate dehydrogenase | 345 | 1.0083 | 0.0000 | 1.0103 | 1.0057–1.0150 | 0.0000 | 1.0081 | 0.0000 | 1.0077 | 1.0037–1.0118 | 0.0002 |
| Albumin | 345 | 0.5633 | 0.0005 | 0.6611 | 0.4469–0.9780 | 0.0383 | 0.5826 | 0.0003 | 0.7157 | 0.5016–1.0211 | 0.0651 |
| Haemoglobin | 345 | 0.8082 | 0.0216 | 0.9508 | 0.7418–1.2187 | 0.6906 | 0.7604 | 0.0013 | 0.8868 | 0.7130–1.1030 | 0.2807 |
| Aspirate plasma cells | 345 | 1.0069 | 0.2831 | 1.0050 | 0.9896–1.0206 | 0.5272 | 1.0079 | 0.1653 | 1.0081 | 0.9947–1.0217 | 0.2361 |
| Bone marrow biopsy plasma cells | 345 | 1.0126 | 0.0366 | 1.0138 | 0.9979–1.0300 | 0.0894 | 1.0131 | 0.0141 | 1.0083 | 0.9947–1.0221 | 0.2342 |
| Number of foal lesions under MRI | 345 | 1.0191 | 0.0247 | 1.0238 | 1.0042–1.0438 | 0.0173 | 1.0146 | 0.0669 | 1.0171 | 0.9995–1.0351 | 0.0570 |
| ITGA6 | 345 | 0.7652 | 0.0057 | 0.7270 | 0.5829–0.9068 | 0.0047 | 0.8005 | 0.0136 | 0.7946 | 0.6507–0.9703 | 0.0241 |
| ISS | 1.6141 | 0.0099 | 0.5505 | 0.2998–1.0109 | 0.0542 | 1.6166 | 0.0092 | 0.6503 | 0.3905–1.0828 | 0.0981 | |
| ISS II | 272 | ||||||||||
| ISS III | 73 | ||||||||||
MRI magnetic resonance imaging, EFS the length of time after primary treatment for a cancer ends that the patient remains free of certain complications or events that the treatment was intended to prevent or delay.
Table 2.
C-index of ISS alone, ISS with ITGA6 (GSE24080).
| OS | EFS | |||
|---|---|---|---|---|
| C-index | C-index (cross-validated) | C-index | C-index (cross-validated) | |
| ISS | 0.7588 | 0.6940 | 0.7515 | 0.6858 |
| ISS+ITGA6 | 0.7647 | 0.7396 | 0.7524 | 0.6972 |
ITGA6 mediated MM cell invasion
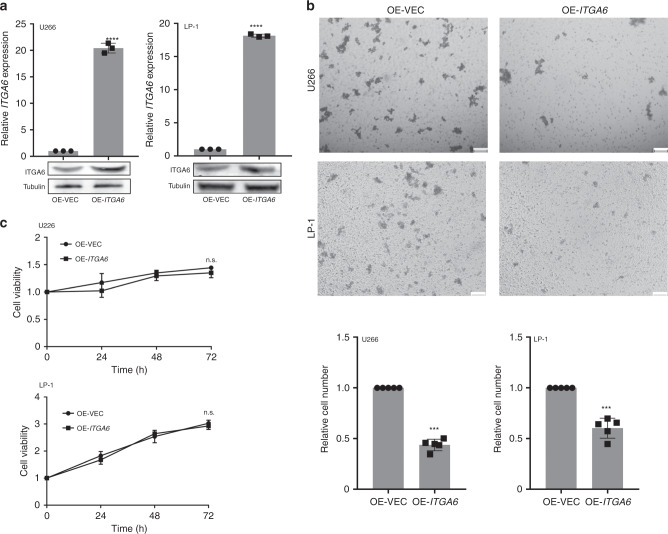
To confirm this hypothesis, we transfected the ITGA6 expression vector (pcDNA3.1-ITGA6) or negative control (pcDNA3.1) into the MM cell lines to enhance the expression of ITGA6 (Fig. 3a). To investigate whether ITGA6 altered cell invasion, transwell assays were performed. As shown in Fig. 3b, the overexpression of ITGA6 greatly reduced the invasion of U266 and LP-1 cells compared to the control. Moreover, we performed Cell Counting Kit-8 (CCK-8) assays to further elucidate the effects of ITGA6 expression on tumour cell proliferation. However, there were no differences in cell proliferation between the two groups (Fig. 3c). Overall, these data indicated that ITGA6 could regulate the invasion of MM cells, suggesting a potential role of ITGA6 in the progression of MM to PCL.
Fig. 3. ITGA6 mediated MM cell invasion.
a MM cells were transiently transfected with ITGA6 overexpression vector or empty vector. The levels of ITGA6 were analysed by qPCR and western blot. OE-VEC overexpression empty vector, OE-ITGA6, overexpression of ITGA6. qPCR results are shown as the means ± SDs from three independent experiments (n = 3). b Upper: Transwell assays were performed to evaluate the invasion of U266 and LP-1 cells overexpressing ITGA6. Scale bar = 100 μm. Lower: statistical graph indicating the number of cells averaged from 5 random high-power fields. The results are presented as the mean ± SD from three independent experiments (n = 3). c Cell proliferation in U266 and LP-1 cells overexpressing ITGA6 was measured using CCK-8 assays. **P < 0.01, ***P < 0.001, ****P < 0.0001, ns not significant versus control cells (n = 3).
Characterisation of the lncRNA ITGA6-AS1
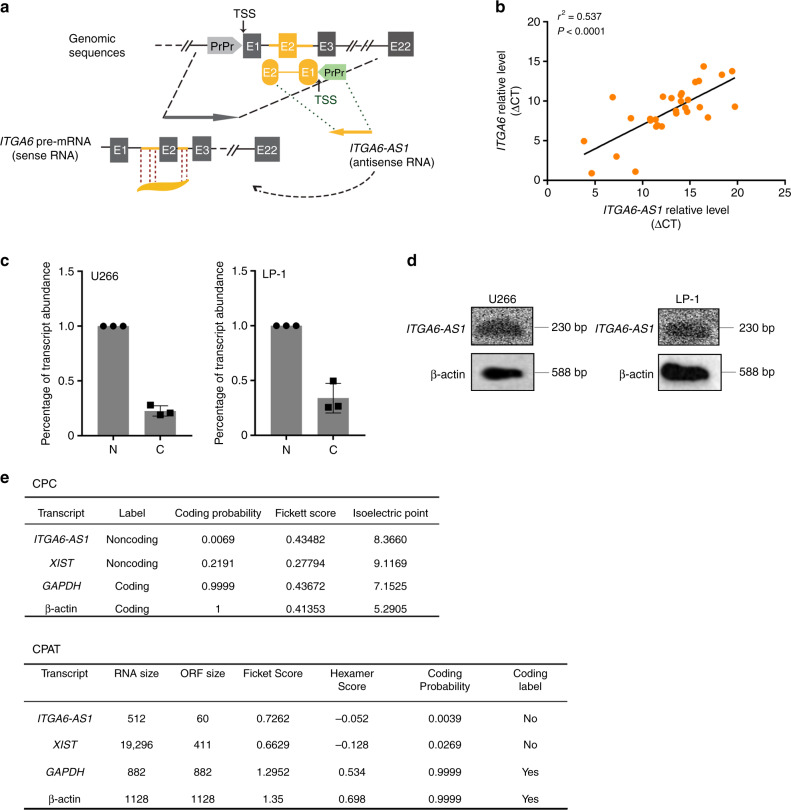
To further elucidate the underlying mechanisms of invasion in MM, we sought to identify the elements that might contribute to the preferential regulation of ITGA6. Considering that lncRNAs regulate the expression of neighbouring genes by diverse mechanisms,29 we concentrated on intergenic lncRNAs located near the ITGA6 gene. To this end, we focused on a long non-coding RNA, ITGA6-AS1, located on chromosome 2 (chr2: 172464262-172466022, GRCh38.p13). ITGA6-AS1 is transcribed in the opposite direction as ITGA6 and contains 521 nucleotides complementary to the ITGA6 pre-mRNA (Fig. 4a). Based on the sequence, we speculated that overlapped region was likely to mediate the interaction between ITGA6-AS1 and ITGA6. We further examined the expression levels of ITGA6 and ITGA6-AS1 in CD138+ MM cells from 30 MM subjects by qPCR. We found that ITGA6-AS1 expression was positively correlated with the expression of ITGA6 (Pearson r2 = 0.537, P < 0.0001) (Fig. 4b). To gain insights into the lncRNA ITGA6-AS1, we characterised it. ITGA6-AS1 was detected in both the cytoplasm and the nucleus by qPCR (Fig. 4c). Northern blot was performed to demonstrate the presence of ITGA6-AS1 in U266 and LP-1 cells (Fig. 4d). Based on these results, only one transcript variant of ITGA6-AS1 existed in MM cells. Furthermore, Coding Potential Calculator (CPC)30 and Coding Potential Assessment Tool (CPAT)31 analyses displayed that ITGA6-AS1 had no coding potentiality (Fig. 4e). Collectively, these data indicated that ITGA6-AS1, as an lncRNA, was present in MM cells and positively correlated with its anti-sense coding RNA, ITGA6.
Fig. 4. Characterisation of the lncRNA ITGA6-AS1.
a Genomic organisation of ITGA6 and ITGA6-AS1. ITGA6 and ITGA6-AS1 are transcribed from opposite strands of the same region on chromosome 2. b The expression levels of ITGA6 and ITGA6-AS1 were detected in 30 MM samples and subjected to correlation analysis. Data were normalised to GAPDH expression and were shown as ▵CT (threshold cycle). c qPCR analysis of ITGA6-AS1 purified from nuclear and cytoplasmic compartments in U266 and LP-1 cells. Data are shown as the means ± SDs. d Northern blot analysis with in vitro transcribed strand-specific RNA probes for ITGA6-AS1 was performed with U266 and LP-1 cells. β-Actin served as a loading control. e In silico analysis of ITGA6-AS1 coding potential using the Coding Potential Calculator (CPC) and the Coding Potential Assessment Tool (CPAT). As shown by the CPC Coding probability, the score indicates the quality of a predicted ORF, and the higher the score is, the higher the quality is. In the CPAT, analysis was performed using the default settings for human sequences and coding probabilities below 0.364 were considered to indicate non-coding sequences.
ITGA6-AS1 and ITGA6 pre-mRNA may form an RNA duplex
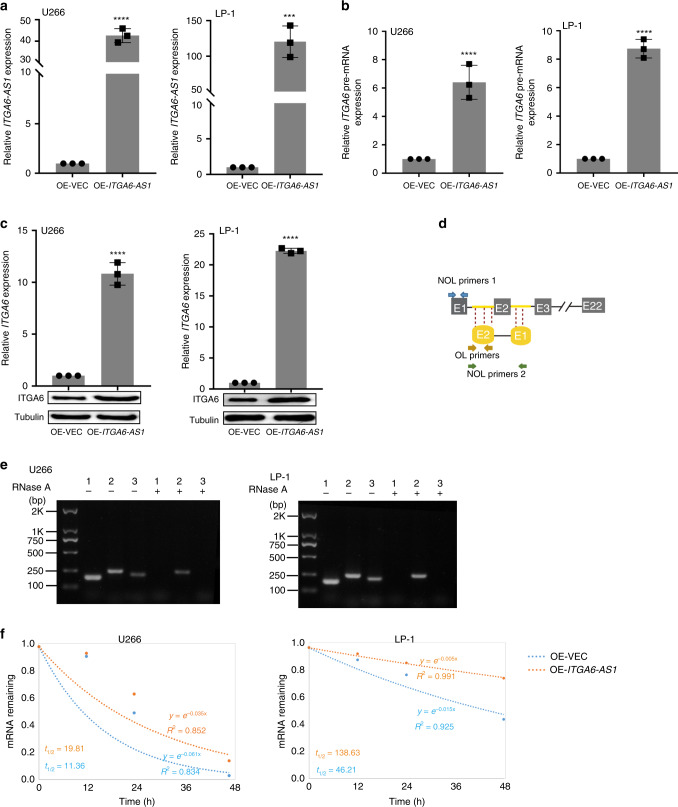
To investigate whether ITGA6-AS1 regulate ITGA6, ITGA6-AS1 expression vector (OE-ITGA6-AS1) were transfected into U266 and LP-1 cells (Fig. 5a). Notably, the upregulation of ITGA6-AS1 led to a significant increase in ITGA6 pre-mRNA and ITGA6 at both the mRNA and protein levels (Fig. 5b, c). Based on their sequence complementarity, we hypothesised that ITGA6-AS1 might regulate ITGA6 expression through base-pairing interactions with single strands. To test the hypothesis, we used RNase protection assays to assess the interaction between ITGA6-AS1 and ITGA6. RNase A digested single-stranded RNA, and double-stranded RNA remained that was analysed by qRCR using specific primers (Fig. 5d). Notably, ITGA6 pre-mRNA and ITGA6-AS1 indeed formed an RNA duplex at the overlapping part of both transcripts that was protected from degradation (Fig. 5e). It has been reported that an RNA duplex could protect the RNA from RNase degradation and enhance RNA stability.32 Next, we set out to investigate whether ITGA6-AS1 affected the ITGA6 pre-mRNA stability. Our data demonstrated that overexpression of ITGA6-AS1 elongated the half-life of ITGA6 pre-mRNA, suggesting that ITGA6-AS1 is required for the regulation of ITGA6 pre-mRNA stability (Fig. 5f). These data suggest that an RNA duplex formed between ITGA6 pre-mRNA and ITGA6-AS1 affected on the ITGA6 transcript at the post-transcriptional level.
Fig. 5. ITGA6-AS1 and ITGA6 pre-mRNA may form an RNA duplex.
a Cells were treated with ITGA6-AS1 overexpression vector (OE-ITGA6-AS1) or empty vector, and then the expression of ITGA6-AS1 was analysed by qPCR. Data are shown as the means ± SD and normalised to GAPDH expression. b ITGA6 pre-mRNA expression was analysed by qPCR in cells treated with ITGA6-AS1 overexpression vector or empty vector. c The expression of ITGA6 was analysed by qPCR and western blotting in cells treated with ITGA6-AS1 overexpression vector or empty vector. d The primers used with RPA assays in RNA samples. NOL primers non-overlapping primers, OL primers overlapping primers. e The qPCR results from three sets of primers covering overlapping or non-overlapping regions of ITGA6 pre-mRNA and ITGA6-AS1 are shown. The overlapping regions of ITGA6 pre-mRNA and ITGA6-AS1 were protected from degradation by RNase A, suggesting that an RNA duplex was formed. (1) non-overlapping region in ITGA6 pre-mRNA; (2) overlapping region; (3) non-overlapping region in ITGA6-AS1. *P < 0.05, **P < 0.01, ***P < 0.001 versus control cells. f U266 or LP-1 cells was transiently transfected with vector control or overexpression of ITGA6-AS1. The stability of ITGA6 pre-mRNA was measured. t1/2, the half-life.
ITGA6-AS1 reduced invasion by regulating ITGA6 expression in MM cells
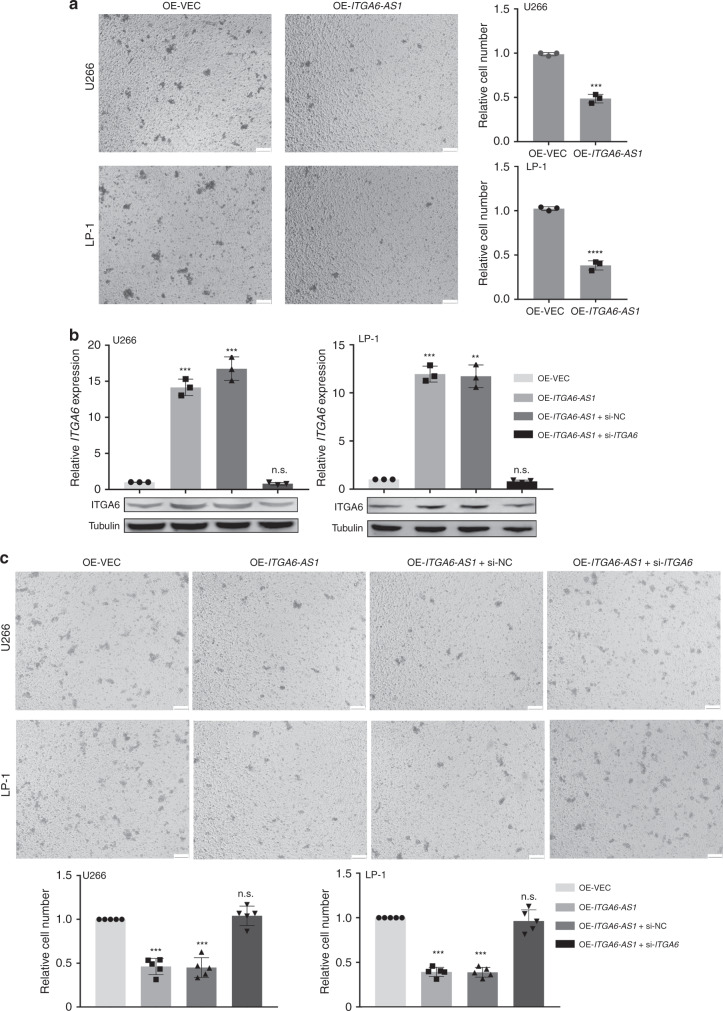
Having shown that ITGA6-AS1 specifically targets ITGA6 and leads to its increased expression, we decided to investigate the ability of ITGA6-AS1 to interfere with cell invasion. Towards this end, transwell invasion assays were performed. As expected, the overexpression of ITGA6-AS1 dramatically downregulated the invasion of U266 and LP-1 cells compared to that of control cells (Fig. 6a). To further evaluate the possibility that ITGA6-AS1 contributes to the invasion of MM cells by regulating ITGA6, we sought to determine whether the downregulation of ITGA6 reversed the biological activity altered by ITGA6-AS1. Our data demonstrated that the downregulation of ITGA6 in ITGA6-AS1 overexpressed cells significantly abrogated the impeded invasion of MM cells (Fig. 6b, c, Table S4). Taken together, these data demonstrated that ITGA6-AS1 affects invasion by regulating ITGA6 expression in MM cells.
Fig. 6. ITGA6-AS1 reduced invasion by regulating ITGA6 expression in MM cells.
a The invasion of cells in transiently transfected with vector control or overexpression of ITGA6-AS1 was detected by transwell invasion assays. Representative images are shown, and invasive cells were counted. Scale bar, 100 μm. The results are presented as the mean ± SD from three independent experiments (n = 3). b The expression of ITGA6 was detected in each treatment group by qPCR and western blot. The results are presented as the mean ± SD from three independent experiments (n = 3). c The changes in invasion following transfection with ITGA6-AS1 overexpression vector alone or in combination with ITGA6 siRNA were investigated through transwell assays. Cells were counted, and representative images was shown (n = 3). Scale bar, 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns not significant versus control cells.
Discussion
Multiple myeloma is a neoplasia of plasma cells, hallmarked by clonal proliferation and immunoglobulin overproduction. It may progress from an intramedullary form to PCL.33 Whole-genome sequencing of multiple myeloma and plasma cell leukaemia patients reveals that seven single-nucleotide variants (SNVs) unique to sPCL samples may contribute to the leukemic transformation from myeloma to sPCL.34 Global miRNA expression profiling shows significantly different miRNA expression patterns between pPCL and multiple myeloma.35 Little, however, has been verified about the unique biological roles of the differentially expressed genes between the two diseases. We collected PC data from multicentre, descriptive, cross-sectional studies and conducted the gene expression difference analysis. Particularly, our study aims to identify critical genes that may mediate the MM migration, and further progression to PCL.
The interaction of plasma cells with bone marrow extracellular matrix (BM-ECM) components depends on adhesion molecules.23 As such, integrins play an important role in the development of malignant plasma cells, including their invasion, proliferation and survival.22,23,26,36 Accordingly, we focused on the integrin α6 subunit, which presented significant p-values and was among the top 8 candidate genes. ITGA6 forms α6β1 and α6β4 integrin complexes with other integrin subunits, and then mediate several biological activities such as embryogenesis, organogenesis, and the invasion of carcinoma cells.24,25,27,37 In previous studies on breast cancer, ITGA6 was necessary for cell tumourigenicity.14,26 A recent report indicated that adhesion molecules and extracellular matrix proteins became increasingly downregulated in pPCL compared to non-pPCL MM.38 Consistent with this research, we found that ITGA6 was expressed at high levels in MM samples and low levels in PCL samples. Our functional experiments demonstrated that ITGA6 acts as a pivotal player in the invasion of MM cells. We overexpressed ITGA6 and identified that it was sufficient to reduce MM cell invasion.
The International Staging System (ISS) are the most extensively used prognostic factors in MM.13 However, MM patients in the same prognostic group exhibited genetically heterogeneous outcomes. Therefore, combining ISS with molecular markers might develop the prognostic value for MM. Using the Kaplan–Meier analysis with the log-rank test of MM patients, high expression levels of ITGA6 were correlated with good outcomes in regards to EFS in univariate and multivariate analyses, suggesting that ITGA6 influenced MM progression. ITGA6 also predicted EFS in MM patients in an independent manner, improving the predictive value of the ISS for MM. Our study provides evidence of an association between ITGA6 and outcome in MM patients. Interestingly, patients with high ITGA6 expression in GSE4581 appears poorer prognosis than patients with low ITGA6 expression in GSE24080, suggesting that the prognostic effect can be partially abrogated by treatment. And according to our present study, ITGA6 should be dichotomised at the median based on a low-versus-high expression. The Cox model need to be employed to compute the cut-off values for ITGA6. Together, combined with GEO data and our clinical sample data, we identified the prognostic significance of ITGA6 in MM patients, which requires further validation in other independent prospective MM cohorts.
LncRNAs have been implicated in gene regulation and many cellular processes, such as transcriptional regulation, epigenetic modulation through chromatin modification, scaffolding of nuclear or cytoplasmic complexes, and pairing with other RNAs.6,39 Here we identified a 521bp lncRNA, ITGA6-AS1, which corresponds to the intron of the ITGA6 gene. Gene expression analysis by qPCR of 30 MM specimens showed a positive correlation between ITGA6 and ITGA6-AS1 expression. Here, we hypothesised that ITGA6-AS1 affected ITGA6 expression through a direct cis-mediated mechanism. The localisation of ITGA6-AS1 transcripts in both the cytoplasm and the nucleus, where this lncRNA can affect transcription and pre-mRNA processing, lend support to this hypothesis. Remarkably, our data indicated that ITGA6-AS1 formed an RNA duplex with ITGA6 pre-mRNA, which affected the ITGA6 pre-mRNA stability and the invasion of MM cells.
Even though the overall expression of ITGA6 in MM is high, there are still 7.7% of MM patients (GSE2113) and 5.4% of MM patients (GSE39925) whose ITGA6 expression level is lower than the mean level of PCL. And this suggested that low ITGA6 expression might not be the unique factor contributing to PCL transformation. As shown in Fig. 1d, besides ITGA6, significant changes in TAGLN2, PPBP, NXPE4, OSBPL10, STAP1, DEFA1B, IGK level were identified. Further studies are needed to investigate the significance of those genes in the process of PCL transformation.
In summary, the findings from this study supported essential roles of the integrin, ITGA6 in mediating MM cell metastasis. Our study elucidated a new mechanism underlying the correlation between ITGA6 and ITGA6-AS1 that provided new insights into the efficient prevention of and therapeutic strategies for MM. More importantly, our results suggested that ITGA6 may improve survival prediction and enhance the risk factor-based stratification provided by ISS in MM patients.
Supplementary information
Acknowledgements
None.
Author contributions
S.S. planned experiments and performed experiments, J.Z. wrote the paper and performed experiments. Q.S., WeiminZ., Y.J., G.F. and C.Q. conceived the work that led to the submission and played an important role in its completion, drafted and revised the paper, approved the final version and agreed to be accountable for all aspects of the work. B.L. contributed reagents or other essential material and designed the work that led to the submission, acquired data and played an important role in interpreting the results. W.Z. contributed funding, conception and design.
Ethics approval and consent to participate
The collection of the samples for research have appropriate patient consent and ethical approval. All samples were collected with ethical permission from the Second Affiliated Hospital of Soochow University. The ethics committee’s reference number is JD-LK-2018-071-02. The study was conducted in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Data availability
The data supporting the findings of this study can be found in the article, Supplementary Information or available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
The research leading to these results has received funding from National Natural Science Foundation of China (grants 81673448 and 81670191), Jiangsu Social Development Project New Clinical Diagnosis and Treatment Technology (BE2019664), Clinical Key Diagnosis and Treatment Technologies in Suzhou City (LCZX201805), The Fifth Phase of "333 High-level Talents Training Project" in Jiangsu Province (BRA2018138) and Scientific and Technological Projects of People’s Livelihood in Suzhou City (SS201856).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sha Song, Ji Zhang
Contributor Information
Bingzong Li, Email: lbzwz0907@hotmail.com.
Wenzhuo Zhuang, Email: zhuangwenzhuo@suda.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01362-5.
References
- 1.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu. Rev. Pathol. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 2.Noll JE, Williams SA, Tong CM, Wang H, Quach JM, Purton LE, et al. Myeloma plasma cells alter the bone marrow microenvironment by stimulating the proliferation of mesenchymal stromal cells. Haematologica. 2014;99:163. doi: 10.3324/haematol.2013.090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albarracin F, Fonseca R. Plasma cell leukemia. Blood Rev. 2011;25:107–112. doi: 10.1016/j.blre.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas EA, Corchete LA, Mateos MV, Garcia-Sanz R, Misiewicz-Krzeminska I, Gutierrez NC. Transcriptome analysis reveals significant differences between primary plasma cell leukemia and multiple myeloma even when sharing a similar genetic background. Blood Cancer J. 2019;9:90. doi: 10.1038/s41408-019-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 6.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojic L, Niemczyk M, Orjalo A, Ito Y, Ruijter AE, Uribe-Lewis S, et al. Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions. Nat. Commun. 2016;7:10406. doi: 10.1038/ncomms10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchese FP, Grossi E, Marín-Béjar O, Bharti SK, Raimondi I, González J, et al. A long noncoding RNA regulates sister chromatid cohesion. Mol. Cell. 2016;63:397–407. doi: 10.1016/j.molcel.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009;10:637–643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 12.Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457. doi: 10.1038/nature11508. [DOI] [PubMed] [Google Scholar]
- 13.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 14.Primo, L., Seano, G., Roca, C., Maione, F., Gagliardi, P. A., Sessa, R. et al. Increased expression of 6 integrin in endothelial cells unveils a proangiogenic role for basement membrane. Cancer Res. 70, 5759–5769 (2010). [DOI] [PubMed]
- 15.Xu L, Mohammad KS, Wu H, Crean C, Poteat B, Cheng Y, et al. Cell adhesion molecule CD166 drives malignant progression and osteolytic disease in multiple myeloma. Cancer Res. 2016;76:6901–6910. doi: 10.1158/0008-5472.CAN-16-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlehurst LA, Landowski TH, Dalton WS. Role of the tumor microenvironment in mediating de novo resistance to drugs and physiological mediators of cell death. Oncogene. 2003;22:7396–7402. doi: 10.1038/sj.onc.1206943. [DOI] [PubMed] [Google Scholar]
- 17.Neri P, Ren L, Azab AK, Brentnall M, Gratton K, Klimowicz AC, et al. Integrin beta7-mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood. 2011;117:6202–6213. doi: 10.1182/blood-2010-06-292243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchiyama H, Barut BA, Chauhan D, Cannistra SA, Anderson KC. Characterization of adhesion molecules on human myeloma cell lines. Blood. 1992;80:2306. doi: 10.1182/blood.V80.9.2306.2306. [DOI] [PubMed] [Google Scholar]
- 19.Van DFA, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 20.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 1999;11:737–744. doi: 10.1016/S0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 21.Van RI, De WM, Remels L, Lacor P, Schots R, Van CB. Expression of cytoadhesion molecules (CD56, CD54, CD18 and CD29) by myeloma plasma cells. Br. J. Haematol. 2010;79:421–427. doi: 10.1111/j.1365-2141.1991.tb08050.x. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 23.Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr. 2013;7:251–261. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penfornis P, Cai DZ, Harris MR, Walker R, Licini D, Fernandes JD, et al. High CD49f expression is associated with osteosarcoma tumor progression: a study using patient-derived primary cell cultures. Cancer Med. 2014;3:796–811. doi: 10.1002/cam4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Dong P, Fu X, Li Q, Ma S, Wu D, et al. CD49f acts as an inflammation sensor to regulate differentiation, adhesion, and migration of human mesenchymal. Stem Cells Stem Cells. 2015;33:2798–2810. doi: 10.1002/stem.2063. [DOI] [PubMed] [Google Scholar]
- 26.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int. J. Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 27.Brooks DL, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol. Cancer. 2016;15:26. doi: 10.1186/s12943-016-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Han H, Song S, Yi N, Qian C, Qiu Y, et al. Exosome-transmitted PSMA3 and PSMA3-AS1 promote proteasome inhibitor resistance in multiple myeloma. Clin. Cancer Res. 2019;25:1923–1935. doi: 10.1158/1078-0432.CCR-18-2363. [DOI] [PubMed] [Google Scholar]
- 29.Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz C, et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16:439–447. doi: 10.1016/j.stem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–W349. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Park HJ, Dasari S, Wang S, Kocher JP, Li W. CPAT: Coding-potential assessment tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013;41:e74. doi: 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattioli M, Agnelli L, Fabris S, Baldini L, Morabito F, Bicciato S, et al. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct IGH translocations in multiple myeloma. Oncogene. 2005;24:2461–2473. doi: 10.1038/sj.onc.1208447. [DOI] [PubMed] [Google Scholar]
- 34.Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120:1060–1066. doi: 10.1182/blood-2012-01-405977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lionetti M, Musto P, Di Martino MT, Fabris S, Agnelli L, Todoerti K, et al. Biological and clinical relevance of miRNA expression signatures in primary plasma cell leukemia. Clin. Cancer Res. 2013;19:3130–3142. doi: 10.1158/1078-0432.CCR-12-2043. [DOI] [PubMed] [Google Scholar]
- 36.Groulx JF, Giroux V, Beausejour M, Boudjadi S, Basora N, Carrier JC, et al. Integrin alpha6A splice variant regulates proliferation and the Wnt/beta-catenin pathway in human colorectal cancer cells. Carcinogenesis. 2014;35:1217–1227. doi: 10.1093/carcin/bgu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin YP, Hu YP, Wu XS, Wu YS, Ye YY, Li HF, et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018;9:182. doi: 10.1038/s41419-017-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schinke C, Boyle EM, Ashby C, Wang Y, Lyzogubov V, Wardell C, et al. Genomic analysis of primary plasma cell leukemia reveals complex structural alterations and high-risk mutational patterns. Blood Cancer J. 2020;10:70. doi: 10.1038/s41408-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study can be found in the article, Supplementary Information or available from the corresponding author upon reasonable request.