Abstract
Purpose
To develop a nomogram for predicting the prognosis of T1 esophageal squamous cell carcinoma (ESCC) patients with positive lymph node.
Methods
T1 ESCC patients with lymph node metastasis diagnosed between 2010 and 2015 were selected from the Surveillance, Epidemiology, and Final Results (SEER) database. The entire cohort was randomly divided in the ratio of 7:3 into a training group (n=457) and validation group (n=192), respectively. Prognostic factors were identified by univariate and multivariate Cox regression models. Harrell's concordance index (C-index), receiver operating characteristic (ROC) curve, and calibration curve were used to evaluate the discrimination and calibration of the nomogram. The accuracy and clinical net benefit of the nomogram compared with the 7th AJCC staging system were evaluated using net reclassification improvement (NRI), integrated discrimination improvement (IDI), and decision curve analysis (DCA).
Results
The nomogram consisted of eight factors: insurance, T stage, summary stage, primary site, radiation code, chemotherapy, surgery, and radiation sequence with surgery. In the training and validation cohorts, the AUCs exceeded 0.700, and the C-index scores were 0.749 and 0.751, respectively, indicating that the nomogram had good discrimination. The consistency between the survival probability predicted by the nomogram and the actual observed probability was indicated by the calibration curve in the training and validation cohorts. For NRI>0 and IDI>0, the predictive power of the nomogram was more accurate than that of the 7th AJCC staging system. Furthermore, the DCA curve indicated that the nomogram achieved better clinical utility than the traditional system.
Conclusions
Unlike the 7th AJCC staging system, the developed and validated nomogram can help clinical staff to more accurately, personally and comprehensively predict the 1-year and 3-year OS probability of T1 ESCC patients with lymph node metastasis.
Keywords: Esophageal squamous cell carcinoma, Lymph node metastases, Overall survival, Prognosis, Nomogram
Introduction
Esophageal cancer (EC) is the 7th most common cancer and the 6th leading cause of cancer deaths worldwide [1]. Esophageal squamous cell carcinoma (ESCC) accounts for approximately 90% of the 456,000 cases of esophageal cancers per year [2], and its prognosis remains poor. The causes of poor prognosis might be related to the main characteristics of ESCC, including extensive lymph node networks, early regional tumor progression, and early regional lymph node metastases (LNM) [3], [4], [5]. For ESCC patients with LNM, the greater number the metastatic lymph nodes, the worse was the prognosis [6,7]. Cancer patients can show favorable survival performance if they are identified at an early stage; for example, the 5-year survival rate of non-small cell lung cancer patients with surgical resection at 0-I stage is 70%, and the 5-year survival rate for ovarian cancer patients diagnosed at stage I is 92% [8,9]. In recent decades, early esophageal cancer with LNM can be detected by endoscopy [10]. However, despite early detection and complete resection, the 5-year survival rate for patients is only 40% to 50%, and the prognosis remains unsatisfactory [11]. It is therefore important to find prognostic evidence for ESCC patients with LNM at the early stage.
The most common cancer-related prognosis assessment system is the American Joint Committee on Cancer (AJCC) staging system, which has been serving as a valuation system in the United States since 1959. This system classifies the extent of cancer based primarily on anatomical information of the extent of the primary tumor, regional lymph nodes, and distant metastases. However, it has disadvantages in predicting the prognosis of cancer because it does not consider certain personal factors such as demographics, number of tumors, and clinical treatments. Hence, even patients at the same cancer stage display different progress and survival times. Therefore, a comprehensive and personalized model for prediction should be developed for patients diagnosed with some cancers. For the past few years, nomograms have been applied to predict the prognosis of cancer [12]. Nomograms meet the requirements for an integrated model that includes clinical and demographical variables, and thus play an essential part in driving toward individualized- and comprehensive-related factor evaluation model [13,14]. To date, no nomogram has been constructed to predict risk factors related to prognosis in T1 ESCC patients with positive lymph nodes. In the present study, by using the ESCC cohort of the Surveillance, Epidemiology and End Results (SEER) database, we aimed to identify prognostic factors for patients with ESCC and construct a prognostic nomogram model for these targeted populations.
Materials and methods
Data source
The data were obtained from the SEER database (https://seer.cancer.gov), which is a comprehensive source of population-based cancer-related information from collaboration between the US Centers for Disease Control and Prevention, the National Cancer Institute, and regional and state cancer registries. The specific dataset with chemotherapy and radiotherapy applied in the present study was the "SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub 1975-2016". Study data was screened using SEER*Stat software (version 8.3.5) provided by the SEER database.
Patient selection
Patients diagnosed with EC were initially recovered from the SEER database for the period between January 1975 and November 2016. The data were then filtered using the Code of International Classification of Diseases, 3th edition (ICD-O-3) defined as 8070-8076; the stage of tumor infiltration was T1, the number of metastatic lymph nodes was greater than 0, and survival status as well as vital status were clear.
Variable selection
Variables for the nomogram, included demographic information, pathological features, and clinical treatments. For demographic data, age at diagnosis, race, sex, insurance, rural-urban continuum, marital status, and follow-up time were selected. For pathological features, primary sites, summary stage, AJCC stage, T stage, N stage, M stage, grade, CS extension, number of malignant tumors as well as benign tumors were included. For clinical treatments, surgery status, radiotherapy status, and chemotherapy status were included in this study. The primary outcomes were the vital status and survival months.
According to the 7th AJCC staging system, seven variables are used for the evaluation of EC and include the depth of infiltration of the tumor (T), number of metastatic lymph nodes (N), distant metastases (M), histopathological type, biological activity of the tumor (histological grade), histological stage (AJCC Stage), and location. In the present study, only one subtype of EC-ESCC patient was used for the study population, the histopathological type was not entered into the AJCC staging system as a variable. The final variables that were applied for the AJCC staging system were TNM stage, histological grade, AJCC stage, and location.
Statistical analysis
All patients were randomly divided into a training and validation group. Frequencies and percentages were described as class variables. A chi-square test was used to compare categorical variables and Student's t-test to continuous variables. Cox regression analyses were used to identify factors that may affect OS. A nomogram to predict 1- and 3-year overall survival (OS) probabilities was developed by applying variables from Cox multivariate regression analyses. After the model was established, several indicators were used to validate the model. The concordance index (C-index) and the receiver operating characteristic (ROC) curves with area under the curve (AUC) were used to evaluate the discrimination capability of model. C-index and AUC ranged from 0.5 to 1.0. Basically, C-index and AUC over 0.7 indicate that the nomogram provides a reasonable estimation. Calibration plots were applied to assess the uniformity between the actual outcomes and the predicted probabilities. The net reclassification index (NRI) quantifies how well a new model reclassifies subjects as compared to an old model, while the integrated discrimination improvement index (IDI) considers different tangent lines that can be used to assess the overall improvement of the model. These two indicators can be used to evaluate the improved or decreased accuracy of a new model compared to the 7th AJCC staging system. Formally, a NRI>0 indicates the new model is more accurate than the old model for prediction. To the same is true for IDI. Finally, decision-curve analysis (DCA) was used to evaluate the clinical validity of the nomogram by quantifying the net benefit compared to the 7th AJCC staging system.
Statistical analysis was conducted using R software (version 4.0.3, https://www.r-project.org/). In the present study, the R packages used in the study included doBy, plotrix, stringi, stringr, nnet, car, mgcv, gdata, caret, rms, survival, rms, foreign, survival, ROC, and nricens packages. p-values were derived from two-tailed tests, and a p < 0.05 was considered statistically significant.
Results
Baseline characteristics of patients
Data on 649 T1 ESCC patients diagnosed with positive lymph nodes from 2010 to 2015 were extracted from the SEER database and then randomly divided into a training set and validation set in a 7:3 ratio. The average age of the patients was 68.17±11.25 years. The majority of patients were ethnically white (61.48%), male (66.72%), living in the counties (86.59%), married (46.84%), and insured (74.73%). The most common TNM stages were T1NOS (74.42%), N1 (73.96%), and M0 (65.95%), and the predominant AJCC stage was IIB (47.46%). For clinical treatments, only a few patients received primary site surgery and radiotherapy, while over half had chemotherapy. Almost the entire cohort (70.72%) had one malignant tumor. The average follow-up time was 15.01 months. Demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of T1 ESCC patients with LNM.
| Variable | Whole cohort [n = 649] | Training cohort [n = 457] | Validation cohort [n = 192] | p-value |
|---|---|---|---|---|
| Age, n (%) | 0.977 | |||
| 25-44 years | 13 (2.00%) | 9 (1.97%) | 4 (2.08%) | |
| 45-64 years | 233 (35.90%) | 166 (36.32%) | 67 (34.90%) | |
| 65-84 years | 357 (55.01%) | 249 (54.49%) | 108 (56.25%) | |
| ≥85 years | 46 (7.09%) | 33 (7.22%) | 13 (6.77%) | |
| Median, years, (IQR) | 68.17±11.25 | 67.19±11.44 | 70.49±10.45 | 0.201 |
| Follow-up time (months), mean±SD | 15.01±0.65 | 14.66±0.64 | 14.47±1.34 | 0.149 |
| Race, n (%) | 0.778 | |||
| White | 399 (61.48%) | 282 (61.71%) | 117 (60.94%) | |
| Black | 172 (26.50%) | 123 (26.91%) | 49 (25.52%) | |
| American Indian/Alaska Native | 5 (0.77%) | 4 (0.88%) | 1 (0.52%) | |
| Asian/Pacific Islander | 73 (11.25%) | 48 (10.50%) | 25 (13.02%) | |
| Sex, n (%) | 0.869 | |||
| Female | 216 (33.28%) | 153 (33.48%) | 63 (32.81%) | |
| Male | 433 (66.72%) | 304 (66.52%) | 129 (67.19%) | |
| Rural and Urban, n (%) | 0.215 | |||
| Comp rural | 12 (1.85%) | 10 (2.19%) | 2 (1.04%) | |
| Counties | 562 (86.59%) | 389 (85.12%) | 173 (90.10%) | |
| Urban | 75 (11.56%) | 58 (12.69%) | 17 (8.85%) | |
| Marital Status, n (%) | 0.505 | |||
| Married | 304 (46.84%) | 208 (45.51%) | 96 (50.00%) | |
| Divorced/Separated | 85 (13.10%) | 65 (14.22%) | 20 (10.42%) | |
| Unmarried/Single | 129 (19.88%) | 92 (20.13%) | 37 (19.27%) | |
| Widowed | 92 (14.18%) | 62 (13.57%) | 30 (15.62%) | |
| Unknown | 39 (6.01%) | 30 (6.56%) | 9 (4.69%) | |
| Insurance Recode, n (%) | 0.241 | |||
| Insured | 485 (74.73%) | 350 (76.59%) | 135 (70.31%) | |
| Any Medicaid | 122 (18.80%) | 80 (17.51%) | 42 (21.88%) | |
| Uninsured | 42 (6.47%) | 27 (5.91%) | 15 (7.81%) | |
| Primary Site – labeled, n (%) | 0.431 | |||
| C15.0-Cervical esophagus | 40 (6.16%) | 23 (5.03%) | 17 (8.85%) | |
| C15.1-Thoracic esophagus | 47 (7.24%) | 31 (6.78%) | 16 (8.33%) | |
| C15.3-Upper third esophagus | 81 (12.48%) | 58 (12.69%) | 23 (11.98%) | |
| C15.4-Middle third esophagus | 211 (32.51%) | 154 (33.70%) | 57 (29.69%) | |
| C15.5-Lower third esophagus | 183 (28.20%) | 127 (27.79%) | 56 (29.17%) | |
| C15.8/9-Overlapping lesion/NOS | 87 (13.41%) | 64 (14.00%) | 23 (11.98%) | |
| Grade, n (%) | 0.242 | |||
| Grade I | 22 (3.39%) | 17 (3.72%) | 5 (2.60%) | |
| Grade II | 240 (36.98%) | 175 (38.29%) | 65 (33.85%) | |
| Grade III | 243 (37.44%) | 160 (35.01%) | 83 (43.23%) | |
| Grade IV | 9 (1.39%) | 5 (1.09%) | 4 (2.08%) | |
| Unknown | 135 (20.80%) | 100 (21.88%) | 35 (18.23%) | |
| AJCC Stage, n (%) | 0.815 | |||
| IIB | 308 (47.46%) | 217 (47.48%) | 91 (47.40%) | |
| IIIA | 40 (6.16%) | 31 (6.78%) | 9 (4.69%) | |
| IIIC | 13 (2.00%) | 8 (1.75%) | 5 (2.60%) | |
| IV | 221 (34.05%) | 155 (33.92%) | 66 (34.38%) | |
| Unknown | 67 (10.32%) | 46 (10.07%) | 21 (10.94%) | |
| T Stage, n (%) | 0.729 | |||
| T1a | 99 (15.25%) | 68 (14.88%) | 31 (16.15%) | |
| T1b | 67 (10.32%) | 45 (9.85%) | 22 (11.46%) | |
| T1NOS | 483 (74.42%) | 344 (75.27%) | 139 (72.40%) | |
| N Stage, n (%) | 0.690 | |||
| N1 | 480 (73.96%) | 338 (73.96%) | 142 (73.96%) | |
| N2 | 65 (10.02%) | 48 (10.50%) | 17 (8.85%) | |
| N3 | 87 (13.41%) | 61 (13.35%) | 26 (13.54%) | |
| NX | 17 (2.62%) | 10 (2.19%) | 7 (3.65%) | |
| M Stage, n (%) | 0.910 | |||
| M0 | 428 (65.95%) | 302 (66.08%) | 126 (65.62%) | |
| M1 | 221 (34.05%) | 155 (33.92%) | 66 (34.38%) | |
| Summary stage, n (%) | 0.552 | |||
| Localized | 67 (10.32%) | 46 (10.07%) | 21 (10.94%) | |
| Regional | 261 (40.22%) | 190 (41.58%) | 71 (36.98%) | |
| Distant | 321 (49.46%) | 221 (48.36%) | 100 (52.08%) | |
| Radiation with surgery, n (%) | 0.279 | |||
| None | 575 (88.60%) | 398 (87.09%) | 177 (92.19%) | |
| Before surgery | 40 (6.16%) | 31 (6.78%) | 9 (4.69%) | |
| After surgery | 33 (5.08%) | 27 (5.91%) | 6 (3.12%) | |
| Before and after surgery | 1 (0.15%) | 1 (0.22%) | 0 (0.00%) | |
| Surg Prim Site, n (%) | 0.314 | |||
| None | 596 (91.83%) | 417 (91.25%) | 179 (93.23%) | |
| Endoscopic therapy | 2 (0.31%) | 2 (0.44%) | 0 (0.00%) | |
| Partial esophagectomy | 9 (1.39%) | 9 (1.97%) | 0 (0.00%) | |
| Total esophagectomy | 9 (1.39%) | 6 (1.31%) | 3 (1.56%) | |
| Esophagectomy, NOS | 33 (5.08%) | 23 (5.03%) | 10 (5.21%) | |
| Radiation recode, n (%) | 0.257 | |||
| Beam radiation | 391 (60.25%) | 283 (61.93%) | 108 (56.25%) | |
| Radiation, NOS | 6 (0.92%) | 3 (0.66%) | 3 (1.56%) | |
| None/Unknown | 252 (38.83%) | 171 (37.42%) | 81 (42.19%) | |
| Chemotherapy, n (%) | 0.927 | |||
| Yes | 404 (62.25%) | 285 (62.36%) | 119 (61.98%) | |
| None/Unknown | 245 (37.75%) | 172 (37.64%) | 73 (38.02%) | |
| CS extension, n (%) | 0.662 | |||
| 100 mm | 74 (11.40%) | 51 (11.16%) | 23 (11.98%) | |
| 110-170 mm | 123 (18.95%) | 83 (18.16%) | 40 (20.83%) | |
| 210-300 mm | 452 (69.65%) | 323 (70.68%) | 129 (67.19%) | |
| No. of malignant tumors, n (%) | 0.033 | |||
| 1 | 459 (70.72%) | 337 (73.74%) | 122 (63.54%) | |
| 2 | 151 (23.27%) | 95 (20.79%) | 56 (29.17%) | |
| 3 | 39 (6.01%) | 25 (5.47%) | 14 (7.29%) | |
| No. of benign tumors, n (%) | 0.359 | |||
| 0 | 647 (99.69%) | 455 (99.56%) | 192 (100.00%) | |
| 1 | 2 (0.31%) | 2 (0.44%) | 0 (0.00%) |
LNM: lymph node metastases; NOS: Not specific.
SEER data represents a collaboration between the US Centers for Disease Control and Prevention, the National Cancer Institute, and regional and state cancer registries from 18 states of United States.
Identifying variables for the nomogram
In the Cox univariate regression analysis, 14 variables were identified that were associated with OS (p < 0.05). In the Cox multivariate analysis, these factors were integrated, and eight of them were identified as independent prognostic factors related to OS (p < 0.05). These factors were insurance, T stage, summary stage, primary site, radiation code, chemotherapy, surgery, and radiation sequence with surgery. The Cox multivariate analysis showed that uninsured status, lower third of the esophagus, regional esophagus, radiation before surgery, no radiation therapy, and chemotherapy were related to the deterioration of OS. For the T1b stage, esophagectomy served as a protective factor for OS. The Cox analyses for screening factors related to OS are listed in Table 2.
Table 2.
Univariate and multivariate Cox analyses for screening factors related to overall survival of T1 ESCC patients with LNM
| Variable | Univariate analysis | p-value | Multivariate analysis | p- value | ||
|---|---|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |||
| Age | ||||||
| 25–44 years | 1.000 | - | ||||
| 45–64 years | 1.110 | 0.604–2.038 | 0.737 | - | - | - |
| 65–84 years | 1.117 | 0.611–2.039 | 0.720 | - | - | - |
| ≥85years | 1.879 | 0.969–3.646 | 0.062 | - | - | - |
| Race | ||||||
| White | 1.000 | - | ||||
| Black | 1.160 | 0.955–1.409 | 0.136 | - | - | - |
| Ameri Indian/Alaska Native | 1.325 | 0.547–3.208 | 0.533 | - | - | - |
| Asian/Pacific Islander | 1.013 | 0.767–1.336 | 0.929 | - | - | - |
| Sex | ||||||
| Female | 1.000 | 1.000 | ||||
| Male | 1.208 | 1.008–1.448 | 0.041 | 1.085 | 0.887–1.327 | 0.427 |
| Rural and Urban | ||||||
| Comp rural | 1.000 | - | ||||
| Counties | 0.826 | 0.465–1.465 | 0.512 | - | - | - |
| Urban | 0.998 | 0.540–1.846 | 0.996 | - | - | - |
| Marital Status | ||||||
| Married | 1.000 | - | ||||
| Divorced/ Separated | 1.263 | 0.974–1.638 | 0.079 | 1.243 | 0.948–1.631 | 0.115 |
| Unmarried/Single | 1.208 | 0.965–1.513 | 0.100 | 1.164 | 0.907–1.496 | 0.233 |
| Widowed | 1.283 | 0.992–1.660 | 0.058 | 1.075 | 0.803–1.439 | 0.629 |
| Unknown | 1.184 | 0.827–1.696 | 0.356 | 0.917 | 0.627–1.340 | 0.653 |
| Insurance Recode | ||||||
| Insured | 1.000 | 1.000 | ||||
| Any Medicaid | 1.061 | 0.854–1.319 | 0.594 | 0.971 | 0.766–1.231 | 0.807 |
| Uninsured | 1.653 | 1.178–2.321 | 0.004 | 1.574 | 1.075–2.302 | 0.020 |
| Primary Site – labeled | ||||||
| C15.0-Cervical esophagus | 1.000 | 1.000 | ||||
| C15.1-Thoracic esophagus | 1.481 | 0.916–2.396 | 0.109 | 1.029 | 0.622–1.702 | 0.913 |
| C15.3-Upper third esophagus | 1.205 | 0.772–1.881 | 0.412 | 1.185 | 0.750–1.875 | 0.467 |
| C15.4-Middle third esophagus | 1.350 | 0.905–2.013 | 0.141 | 1.358 | 0.893–2.063 | 0.152 |
| C15.5-Lower third esophagus | 1.652 | 1.104–2.471 | 0.015 | 1.461 | 0.955–2.235 | 0.080 |
| C15.8/9-Overlappinglesion/NOS | 1.892 | 1.228–2.915 | 0.004 | 1.401 | 0.892–2.202 | 0.143 |
| Grade | ||||||
| Grade I | 1.000 | - | ||||
| Grade II | 1.156 | 0.694–1.924 | 0.578 | - | - | - |
| Grade III | 1.389 | 0.835–2.311 | 0.206 | - | - | - |
| Grade IV | 0.823 | 0.322–2.105 | 0.684 | - | - | - |
| Unknown | 1.305 | 0.773–2.203 | 0.319 | - | - | - |
| AJCC Stage | ||||||
| IIB | 1.000 | 1.000 | ||||
| IIIA | 1.123 | 0.775–1.625 | 0.540 | 0.873 | 0.479–1.591 | 0.657 |
| IIIC | 2.221 | 1.208–4.084 | 0.010 | 1.379 | 0.410–4.637 | 0.604 |
| IV | 2.191 | 1.809–2.653 | <0.001 | 1.213 | 0.906–1.626 | 0.195 |
| Unknown | 1.969 | 1.478–2.624 | <0.001 | 0.765 | 0.425–1.375 | 0.370 |
| T Stage | ||||||
| T1a | 1.000 | 1.000 | ||||
| T1b | 0.442 | 0.301–0.648 | <0.001 | 0.554 | 0.311–0.989 | 0.045 |
| T1NOS | 1.217 | 0.960–1.542 | 0.105 | 1.088 | 0.583–2.029 | 0.791 |
| N Stage | ||||||
| N1 | 1.000 | 1.000 | ||||
| N2 | 1.089 | 0.821–1.444 | 0.556 | 1.309 | 0.830–2.066 | 0.247 |
| N3 | 1.632 | 1.279–2.083 | <0.001 | 1.376 | 0.839–2.256 | 0.206 |
| NX | 1.543 | 0.920–2.589 | 0.100 | 0.894 | 0.326–2.449 | 0.827 |
| M Stage | ||||||
| M0 | 1.000 | 1.000 | ||||
| M1 | 1.922 | 1.611–2.294 | <0.001 | NA | NA | NA |
| Summary stage | ||||||
| Localized | 1.000 | 1.000 | ||||
| Regional | 0.981 | 0.741–1.299 | 0.894 | 1.364 | 1.040–1.789 | 0.025 |
| Distant | 0.463 | 0.345–0.621 | <0.001 | NA | NA | NA |
| Radiation with surgery | ||||||
| None | 1.000 | 1.000 | ||||
| Before surgery | 0.398 | 0.262–0.606 | <0.001 | 5.282 | 2.579– 10.819 | <0.001 |
| After surgery | 0.427 | 0.276–0.661 | <0.001 | 1.029 | 0.607–1.745 | 0.916 |
| Before and after surgery | 0.000 | 0 | 0.989 | 0.000 | 0 | 0.992 |
| Surg Prim Site | ||||||
| None | 1.000 | 1.000 | ||||
| Endoscopic therapy | 1.481 | 0.369–5.945 | 0.579 | 1.393 | 0.336–5.769 | 0.647 |
| Partial esophagectomy | 0.205 | 0.066–0.637 | 0.006 | 0.098 | 0.026–0.375 | <0.001 |
| Total esophagectomy | 0.173 | 0.055–0.537 | 0.002 | 0.170 | 0.047–0.615 | 0.007 |
| Esophagectomy, NOS | 0.273 | 0.166–0.450 | <0.001 | 0.196 | 0.091–0.422 | <0.001 |
| Radiation recode | ||||||
| Beam radiation | 1.000 | 1.000 | ||||
| Radiation, NOS | 1.127 | 0.466–2.729 | 0.790 | 0.947 | 0.359–2.495 | 0.912 |
| None/Unknown | 2.265 | 1.905–2.693 | <0.001 | 1.585 | 1.288–1.951 | <0.001 |
| Chemotherapy | ||||||
| Yes | 1.000 | 1.000 | ||||
| None/Unknown | 2.786 | 2.340–3.317 | <0.001 | 3.020 | 2.432–3.751 | <0.001 |
| CS extension | ||||||
| 100 mm | 1.000 | 1.000 | ||||
| 110–170 mm | 0.584 | 0.421–0.811 | 0.001 | 1.021 | 0.591–1.764 | 0.940 |
| 210–300 mm | 1.127 | 0.862–1.474 | 0.381 | 0.866 | 0.439–1.710 | 0.679 |
| No. of malignant tumors | ||||||
| 1 | 1.000 | 1.000 | ||||
| 2 | 0.762 | 0.620–0.935 | <0.001 | 0.898 | 0.723–1.117 | 0.335 |
| 3 | 0.734 | 0.509–1.059 | 0.098 | 0.832 | 0.559–1.239 | 0.365 |
| No. of benign tumors | ||||||
| 0 | 1.000 | – | ||||
| 1 | 0.302 | 0.042–2.150 | 0.232 | – | – | – |
LNM: lymph node metastases; NOS: Not specific; HR: hazard ratio.
Establishment of the nomogram
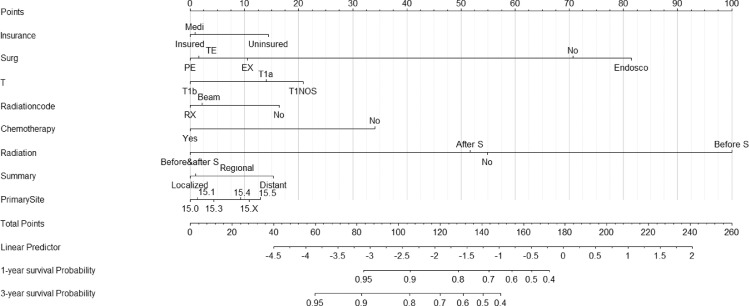
The eight factors that influenced prognosis of ESCC were identified from Cox multivariate analysis and entered into the present nomogram model. Among these eight factors, radiation sequence with surgery was the most influential for the forecasting of OS, followed by surgery type, chemotherapy status, and T stage. The relative results are shown in Fig. 1.
Fig. 1.
Nomogram to predict 1- and 3-year survival probability for T1 lymph node-positive ESCC patients. Medi: Any Medicaid; Surg=surgery status, PE: Partial esophagectomy, TE: Total esophagectomy, EX: Esophagectomy, not specific, Endosco: Endoscopic therapy; RX: Radiation, not specific; Beam: Beam radiation; Before & after S: Radiation before and after surgery, After S: Radiation after surgery, Before S: Radiation before surgery; 15.0: Cervical esophagus, 15.1: Thoracic esophagus, 15.3: Upper third esophagus, 15.4: Middle third esophagus, 15.5: Lower third esophagus, 15.X: Overlapping lesion/not specific.
Nomogram validation
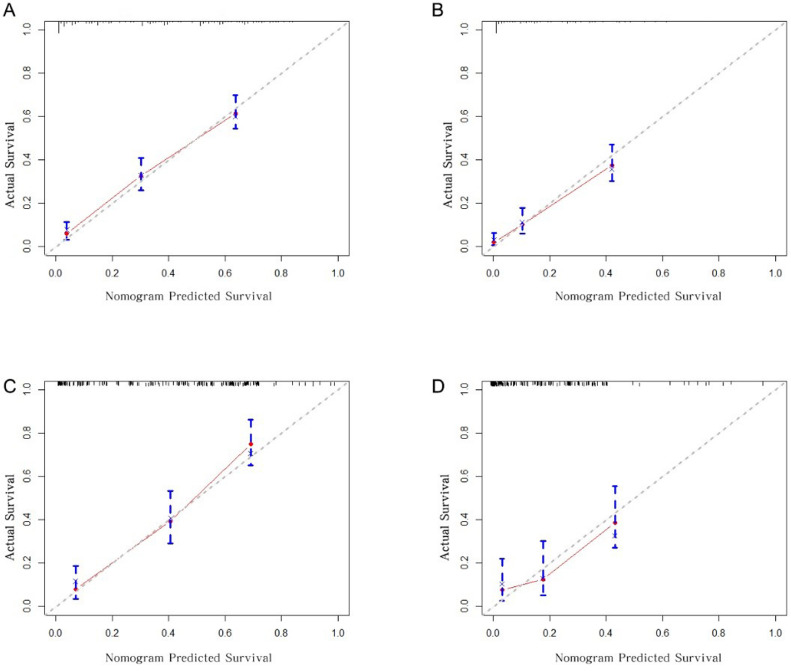
The discrimination ability of the nomogram was evaluated using C-index and ROC-related AUC. The prediction model's C-index was 0.749 and 0.751 in the training and validation cohort, respectively; both scores were significantly higher than those of the 7th AJCC stage (p < 0.001), which achieved a score of 0.640 and 0.638, respectively (Table 3). Similarly, for the AUCs, the nomogram outperformed the 7th AJCC stage in the training (1-year AUC: 0.799 vs. 0.738; 3-year AUC: 0.810 vs. 0.735, Fig. 2A and 2B) and validation (1-year AUC: 0.817 vs. 0.716; 3-year AUC: 0.756 vs. 0.721, Fig. 2C and 2D) groups. The nomogram had superior discrimination ability compared to that of the 7th AJCC staging system. The calibration curves of the nomogram for 1-year and 3-year OS indicated that the predicted survival probability and the actual observed ones in both training and validation groups matched each other closely (Fig. 3).
Table 3.
The validation of the nomogram versus the 7th AJCC staging system by C-index, NRI, and IDI
| Index | Training cohort | Validation cohort | ||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | P- value | Estimate | 95%CI | P- value | |
| NRI versus AJCC Staging system | ||||||
| For 1-year OS | 0.313 | 0.182–0.408 | <0.001 | 0.364 | 0.129–0.526 | <0.001 |
| For 3-year OS | 0.483 | 0.198–0.776 | <0.001 | 0.325 | 0.017–0.557 | 0.040 |
| IDI versus AJCC Staging system | ||||||
| For 1-year OS | 0.132 | 0.085–0.184 | <0.001 | 0.171 | 0.078–0.257 | <0.001 |
| For 3-year OS | 0.110 | 0.038–0.204 | <0.001 | 0.161 | 0.042–0.275 | <0.001 |
| C-index | ||||||
| Nomogram | 0.749 | 0.723–0.774 | <0.001 | 0.751 | 0.712–0.790 | <0.001 |
| AJCC Staging system | 0.640 | 0.522–0.671 | 0.638 | 0.471–0.805 | ||
Fig. 2.
ROC Curves of nomogram and the 7th AJCC staging system to predict 1-year and 3-year OS for T1 lymph node-positive ESCC patients in the training cohort (A-B) and in the validation cohort (C-D). The blue curve was for nomogram and the red one was for the AJCC staging system. The larger the AUC, the better was the discrimination of the model.
Fig. 3.
Calibration curves for predicting 1-year and 3-year OS for T1 lymph node-positive ESCC patients in the training cohort (A-B) and in the validation cohort (C-D). The red dots represent the accuracy of the nomogram prediction. The closer the red line to the gray line, the greater is the accuracy of the nomogram.
From the accuracy analysis, the NRI values for the 1-year and 3-year OS probabilities were 0.313 (95% CI: 0.182–0.408) and 0.483 (95% CI: 0.198–0.776) in the training cohort and 0.364 (95% CI: 0.129–0.526) and 0.325 (95% CI: 0.017–0.557) in the validation cohort (Table 3). In addition, the IDI values for the 1-year and 3-year OS probabilities were 0.132 (95% CI: 0.085–0.184) and 0.110 (95% CI: 0.038-0.204) respectively, in the training dataset and 0.171 (95% CI: 0.078–0.257) and 0.161 (95% CI: 0.042–0.275), respectively, in the validation dataset (Table 3). All these results indicated that the present nomogram had improved accuracy compared to the 7th AJCC staging system in predicting OS.
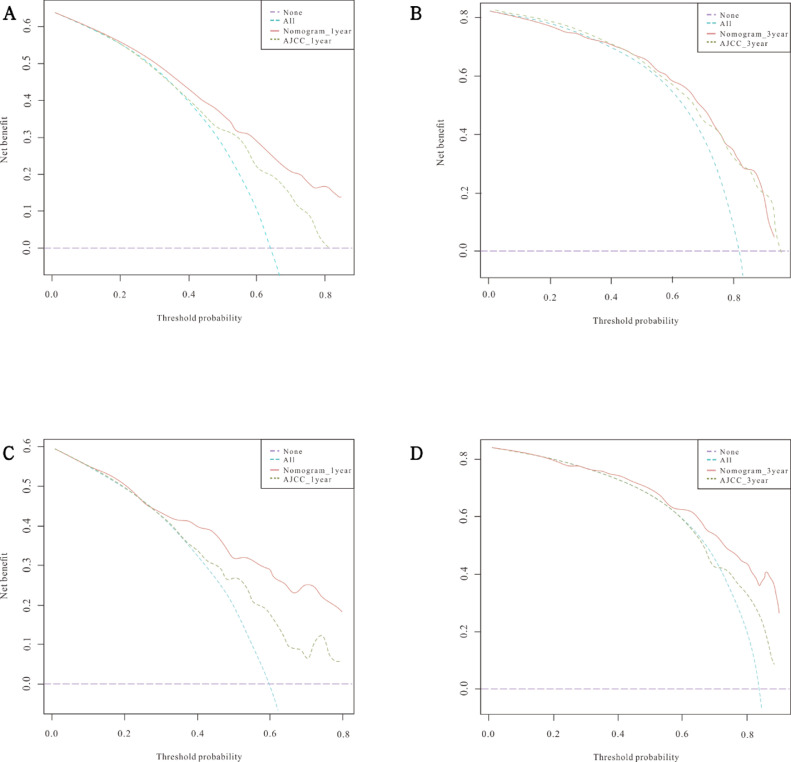
DCA was performed to compare the clinical validity of the nomogram to the 7th AJCC staging system. As shown in Fig. 4A-D, the nomogram achieved greater net clinical benefits across a wide range of threshold probabilities versus the 7th AJCC stage system in both the training cohort and the validation cohort.
Fig. 4.
Decision curve analysis (DCA) for the nomogram model and the AJCC staging system in the training cohort (A-B) and validation cohort (C-D) for predicting 1- and 3-year OS. The red solid lines represent the DCA of the nomogram, and the green dashed lines represent the AJCC staging system. The turquoise dashed line assumes that all patients were alive, while the purple dashed line assumes that all patients were dead.
Discussion
No comprehensive and personalized prediction model currently exists to evaluate the prognosis for early ESCC patients with lymph nodes positive despite the unfavorable OS [15]. We developed and validated a nomogram that integrates various variables to predict the risk factors related to 1- and 3-year OS for T1 ESCC patients who had positive lymph nodes. NRI, IDI and ROC curve analyses indicated the nomogram showed possessed favorable discrimination, calibration, and clinical usage.
The nomograms that have been developed for EC patients mainly focus on the whole stage and type. Thus far, there are only five papers for early EC patients, and none of the nomograms targeted early ESCC with lymph node metastases [16], [17], [18], [19], [20]. Li et al. have published a study on early EC patients who are nonoperative [17]. In this study, the OS of patients who had undergone chemoradiotherapy (CRT) was initially compared with that of patients who had undergone radiotherapy (RT). In this study, subgroup analysis was introduced to make the comparison more reliable, and the identified prognostic factors to build the nomogram were evaluated. Eight variables were fitted into the model, in which the type of therapy was recognized as the most significant contributor to the prognosis, followed by histology, T stage, race, N stage, age, sex, and site. The discrimination of the nomogram model was poor. The C-index value was 0.595 in the training dataset and 0.587 in the validation dataset, and the AUC values of the 3-year survival rate were 0.642 and 0.642 in the training and validation cohorts, respectively. Moreover, the accuracy and clinical value for the nomogram were not discussed. In the present study, the discrimination and calibration as well as the accuracy and clinical utility of the nomogram were analyzed, and compared with the 7th AJCC staging system by applying C-index, calibration plots, NRI, IDI, and DCA. Our nomogram C-index was 0.750 vs. 0.645 for the 7th AJCC. From the calibration plots, good calibration was observed. As indicated by the positive NRI and IDI, the present nomogram is more accurate in predicting OS than the 7th AJCC system. Moreover, based on DCA, the nomogram built in this study achieved better clinical benefits than the conventional staging system. In the present nomogram, in addition to the pathological features (e.g., summary stage, primary site) which are included in the 7th AJCC evaluated system, the clinical treatments such as radiation, chemotherapy, and surgery, which influence the prognosis significantly, and demographic factors were entered into the nomogram. Overall, the present nomogram can be more comprehensive and personalized to evaluate the prognosis of T1 ESCC patients with lymph node metastases.
For the most significant factor to predict the OS for the nomogram, radiation sequence with surgery was considered for patients who underwent radiation before and/or after surgery/Endoscopic therapy (ET). ET or Surgery followed by Radiotherapy (RT) have been recommended for T1 ESCC patients at risk of lymph node invasion [21] In this study, patients who underwent radiation after ET or surgery indeed showed prolonged OS than those who had radiation before ET or surgery, in agreement with existing findings [22,23]. However, Zheng wang. et al. demonstrated that neoadjuvant therapy with surgery would be the current optimal treatment for thoracic esophageal cancer (EC) patients with the potential risk of metastatic lymph node [24]. Regardless of the sequence with surgery/ET, patients with chemotherapy or radiation therapy exhibited better OS performance than those without such a therapy. In this study, esophagectomy had better performance than endoscopic therapies (e.g., photodynamic therapy, electrocautery, cryosurgery, and laser esophagectomy), which was similar to findings in other studies [25], [26], [27]. However, it should be noted that as adverse events, the mortality rate and morbidity rate accompanied with esophagectomy is about 10% and 50%, respectively. The 5-year survival rate after receiving treatment is stable at 20%. [28]. For the summary stage, in agreement with the findings of Kim et al. [29], patients with localized ESCC outperformed those with regional and distanced ones. Localized patients would have fewer lymph node metastases than the other two types would, which might contribute to this result.
Uninsured status, as a financial factor, was identified as a risk factor for this group of patients. This conclusion is consistent with that of Grant et al. [30] and Saraiya et al. [31], who found that the patients without insurance experienced unfavorable cancer outcomes. This can be explained by several reasons. First, an uninsured population is less likely to undergo cancer screening than an insured one [32]. Second, an uninsured population is more prone to have poor financial conditions and thus have reduced access to health services [33]. Lastly, an uninsured group tends to have poorer living habits (e.g., smoking, alcohol abuse, and poor nutrition), which are risk factors for ESCC [34]. The different effects of insured and uninsured care on the prognosis of ESCC observed here indicate the need for health care reform.
In this study, surprisingly, T1a patients exhibited worse outcomes than T1b patients. Normally, patients in the T1b stage have worse prognoses than those in the T1a stage [35], [36], [37], [38]. However, several studies have reported that the Tn stages have better outcomes than the Tn-1 stage (e.g., T2 over T1 [26,39], T3/4 over T1/T2 [40,41] as well as, T4 over T3 [42]), and is primarily associated with lymph node invasion. The prognosis of advanced cancers (Tn) with a few positive nodes may be similar to or better than that of less advanced cancers (Tn-1) with more positive nodes. Moreover, as compared to T1a patients (except for T1a involving the muscularis mucosa), T1b patients with positive lymph nodes are more likely to receive adjuvant therapy after surgery, leading to better clinical results [43]. It is noteworthy that the prognosis of patients cannot be evaluated merely based on the T stage.
There are several limitations of this study. First, this cohort was based on the American population, which may not fit the population of other countries and limit the prediction ability of the nomogram. Furthermore, some demographic factors related to ESCC prognosis (e.g., smoking, drinking and dietary habits) were not considered in this nomogram. Third, this study lacks external validation. For subsequent research, more data regarding valuable prognosis risk characteristics should be included, and an external validation should be conducted to testify the prediction ability of the nomogram.
Conclusion
Eight variables, comprising insurance, T stage, summary stage, primary site, radiation code, chemotherapy, surgery, and radiation sequence with surgery, were integrated into a nomogram. Among these OS-related factors, the radiation sequence with surgery was identified as the most significant predictive variable, followed by the type of surgery and chemotherapy. For the T stage, patients at T1a stage showed more disappointing performance, based on OS, as compared to patients at T1b stage. The present nomogram with internal validation showed its advantage in predicting 1-year and 3-year OS compared to the 7th AJCC staging system and may provide more optimal performance for the prediction of survival time and the provision of suitable treatment recommendations for patients.
Author contribution
Jia Yu: Conceptualization, Methodology, Software, Validation, Resources, Formal analysis, Data Curation, Writing - Original Draft.
Wenyu Hu: Resources, Validation, Data Curation.
Nan Yao: Resources, Data Curation.
Mengzi Sun: Resources, Data Curation.
Xiaotong Li: Data Curation.
Ling Wang: Data Curation.
Yixue Yang: Data Curation.
Bo Li: Writing - Review & Editing, Supervision.
Funding
There was no funding from the public, commercial, or not-for-profit sectors for the present study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Jianjun Zhang and Yutong Sui for assistance with data analysis and comments that improved the manuscript substantially.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101127.
Appendix. Supplementary materials
References
- 1.Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology. 2018;154:390–405. doi: 10.1053/j.gastro. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Li B, Gong H. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: a report of 1077 cases. Radiotherapy and oncology. J. Eur. Soc. Therap. Radiol. Oncol. 2010;95:229–233. doi: 10.1016/j.radonc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Akutsu Y, Murakami K, Kano M. The concentration of programmed cell death-ligand 1 in the peripheral blood is a useful biomarker for esophageal squamous cell carcinoma. Esophagus: Off. J. Jpn. Esophageal Soc. 2018;15:103–108. doi: 10.1007/s10388-018-0604-1. [DOI] [PubMed] [Google Scholar]
- 5.Aikou T, Kitagawa Y, Kitajima M. Sentinel lymph node mapping with GI cancer. Cancer Metastasis Rev. 2006;25:269–277. doi: 10.1007/s10555-006-8507-3. [DOI] [PubMed] [Google Scholar]
- 6.Cho JW. The role of endosonography in the staging of gastrointestinal cancers. Clin. Endosc. 2015;48:297–301. doi: 10.5946/ce.2015.48.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SG, Sun JY, Yang LC. Prognosis of patients with esophageal squamous cell carcinoma after esophagectomy using the log odds of positive lymph nodes. Oncotarget. 2015;6:36911–36922. doi: 10.18632/oncotarget.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blandin S, Crosbie PA, Balata H. Progress and prospects of early detection in lung cancer. Open Biol. 2017;33:121–128. doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner AB, Runowicz CD. Ovarian cancer screening and early detection. Womens Health. 2009;5:693–699. doi: 10.2217/whe.09.65. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Sharma G, Sanaka MR. Role of endoscopic therapy in early esophageal cancer. World J. Gastroenterol. 2018;24:3965–3973. doi: 10.3748/wjg.v24.i35.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altorki NK. Three-field lymphadenectomy for esophageal cancer. Chest Surg. Clin. N. Am. 2000;10:553–560. doi: 10.1016/j.jss.2020.05.057. [DOI] [PubMed] [Google Scholar]
- 12.Balachandran VP, Gonen M, Smith JJ. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:173–180. doi: 10.1016/s1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins GS. How can I validate a nomogram? Show me the model. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2015;26:1034–1035. doi: 10.1093/annonc/mdv069. [DOI] [PubMed] [Google Scholar]
- 14.Park SY. Nomogram: An analogue tool to deliver digital knowledge. J. Thorac. Cardiovasc. Surg. 2018;155:1793. doi: 10.1016/j.jtcvs.2017.12.107. [DOI] [PubMed] [Google Scholar]
- 15.Codipilly DC, Qin Y, Dawsey SM. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest. Endosc. 2018;88:413–426. doi: 10.1016/j.gie.2018.04.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan XF, Tang P, Shang XB. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: a retrospective study of 143 cases. Surg. Oncol. 2018;27:1–6. doi: 10.1016/j.suronc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Jia Y, Cheng Y. Chemoradiotherapy vs radiotherapy for nonoperative early stage esophageal cancer: a seer data analysis. Cancer Med. 2020;9:5025–5034. doi: 10.1002/cam4.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, Tang JW, Cao ZR. Nomograms for predicting survival in early-onset esophageal cancer. Expert Rev. Gastroenterol. Hepatol. 2020;15:1–10. doi: 10.1080/17474124.2021.1842194. [DOI] [PubMed] [Google Scholar]
- 19.Tian D, Jiang KY, Huang H. Clinical nomogram for lymph node metastasis in pathological T1 esophageal squamous cell carcinoma: a multicenter retrospective study. Ann. Transl. Med. 2020;8:292–300. doi: 10.21037/atm.2020.02.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue LY, Qin XM, Liu Y. Clinicopathological parameters predicting recurrence of pT1N0 esophageal squamous cell carcinoma. World J. Gastroenterol. 2018;24:5154–5166. doi: 10.3748/wjg.v24.i45.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt A, Kamath S, Murthy SC. Multidisciplinary evaluation and management of early stage esophageal cancer. Surg. Oncol. Clin. N. Am. 2020;29:613–630. doi: 10.1016/j.soc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Lyu X, Huang J, Mao Y. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J. Surg. Oncol. 2014;110:864–868. doi: 10.1002/jso.23716. [DOI] [PubMed] [Google Scholar]
- 23.Qin RQ, Wen YS, Wang WP. The role of postoperative adjuvant chemotherapy for lymph node-positive esophageal squamous cell carcinoma: a propensity score matching analysis. Med. Oncol. 2016;31:1–10. doi: 10.1007/s12032-016-0746-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Mao Y, Gao S. Lymph node dissection and recurrent laryngeal nerve protection in minimally invasive esophagectomy. Ann. N. Y. Acad. Sci. 2020;148:20–29. doi: 10.1111/nyas.14427. [DOI] [PubMed] [Google Scholar]
- 25.Stahl M, Mariette C, Haustermans K. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 2013;24:51–56. doi: 10.1093/annonc/mdt342. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann. Thorac. Surg. 2011;91:1494–1500. doi: 10.1016/j.athoracsur.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Chen H, Xiang J. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2013;146:1198–1203. doi: 10.1016/j.jtcvs.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Linden PA, Sugarbaker DJ. Section V: techniques of esophageal resection. Semin. Thorac. Cardiovasc. Surg. 2003;15:197–209. doi: 10.1016/S1043-0679(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 29.Kim A, Ashman P, Ward-Peterson M. Racial disparitie s in cancer-related survival in patients with squamous cell carcinoma of the esophagus in the US between 1973 and 2013. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0183782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant SR, Walker GV, Guadagnolo BA. A brighter future? The impact of insurance and socioeconomic status on cancer outcomes in the USA: a review. Future Oncol. 2016;12:1507–1515. doi: 10.2217/fon-2015-0028. [DOI] [PubMed] [Google Scholar]
- 31.Saraiya M, Cheung LC, Soman A. Risk of cervical precancer and cancer among uninsured and underserved women from 2009 to 2017. Am. J. Obstet. Gynecol. 2020;1:11–32. doi: 10.1016/j.ajog.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G, Okoro CA, Li J. Health insurance status and clinical cancer screenings among U.S. adults. Am. J. Prev. Med. 2018;54:11–19. doi: 10.1016/j.amepre.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo CG, Zhao DB, Liu Q. A nomogram to predict lymph node metastasis in patients with early gastric cancer. Oncotarget. 2017;8:12203–12210. doi: 10.18632/oncotarget.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohlfing ML, Mays AC, Isom S. Insurance status as a predictor of mortality in patients undergoing head and neck cancer surgery. Laryngoscope. 2017;127:2784–2789. doi: 10.1002/lary.26713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo CG, Zhao DB, Liu Q. A nomogram to predict lymph node metastasis in patients with early gastric cancer. Oncotarget. 2017;8:12203–12210. doi: 10.18632/oncotarget.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo CG, Chen YJ, Ren H. A nomogram for predicting the likelihood of lymph node metastasis in early gastric signet ring cell carcinoma: A single center retrospective analysis with external validation. Medicine. 2016;95:1–6. doi: 10.1097/MD.0000000000005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin H, Feng Y, Guo K. Prognostic nomograms for predicting overall survival and cancer-specific survival of patients with early onset colon adenocarcinoma. Front. Oncol. 2020;10:1–11. doi: 10.3389/fonc.2020.595354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu J, Jia Z, Yao W. Predicting lymph node metastasis in early gastric cancer patients: development and validation of a model. Future Oncol. 2019;15:3609–3617. doi: 10.2217/fon-2019-0377. [DOI] [PubMed] [Google Scholar]
- 39.Yin XY, Pang T, Liu Y. Development and validation of a nomogram for preoperative prediction of lymph node metastasis in early gastric cancer. World J. Surg. Oncol. 2020;18:1–10. doi: 10.1186/s12957-019-1778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Jia Y, Cheng Y. Chemoradiotherapy vs radiotherapy for nonoperative early stage esophageal cancer: a seer data analysis. Cancer Med. 2020;9:5025–5034. doi: 10.1002/cam4.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Xu G, Wu H. The nomogram for early death in patients with bone and soft tissue tumors. J. Cancer. 2020;11:5359–5370. doi: 10.7150/jca.46152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu J, Wu L, Jiang M. Clinical nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kam TY, Kountouri M, Roth A. Endoscopic resection with adjuvant chemo-radiotherapy for superficial esophageal squamous cell carcinoma: a critical review. Crit. Rev. Oncol. Hematol. 2018;124:61–65. doi: 10.1016/j.critrevonc.2018.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.