Abstract
Background & aims
Maternal gestational infection is a well-characterized risk factor for offsprings’ development of mental disorders including schizophrenia, autism, and attention deficit disorder. The inflammatory response elicited by the infection is partly directed against the placenta and fetus and is the putative pathogenic mechanism for fetal brain developmental abnormalities. Fetal brain abnormalities are generally irreversible after birth and increase risk for later mental disorders. Maternal immune activation in animals models this pathophysiology. SARS-CoV-2 produces maternal inflammatory responses during pregnancy similar to previously studied common respiratory viruses.
Method
Choline, folic acid, Vitamin D, and n-3 polyunsaturated fatty acids are among the nutrients that have been studied as possible mitigating factors for effects of maternal infection and inflammation on fetal development. Clinical and animal studies relevant to their use in pregnant women who have been infected are reviewed.
Results
Higher maternal choline levels have positive effects on the development of brain function for infants of mothers who experienced viral infections in early pregnancy. No other nutrient has been studied in the context of viral inflammation. Vitamin D reduces pro-inflammatory cytokines in some, but not all, studies. Active folic acid metabolites decrease anti-inflammatory cytokines. N-3 polyunsaturated fatty acids have no effect.
Conclusions
Vitamin D and folic acid are already supplemented in food additives and in prenatal vitamins. Despite recommendations by several public health agencies and medical societies, choline intake is often inadequate in early gestation when the brain is forming. A public health initiative for choline supplements during the pandemic could be helpful for women planning or already pregnant who also become exposed or infected with SARS-CoV-2.
Keywords: Coronavirus, Choline, Pregnancy, Fetal development, Vaccine, COVID-19
1. Introduction
Offspring of women pregnant during previous influenza pandemics have been found to have a higher incidence of mental illnesses including schizophrenia, autism spectrum disorders, and attention deficit disorders [1,2]. A study of 57 Chinese infants whose mothers had COVID-19 infections, 53 in the third trimester, found that the offspring at 3 months of age had decreased motor, communication, and social development [3]. Women infected earlier in pregnancy had elective abortions. A study of 464 U.S. women with common viral and bacterial upper respiratory infections in the second trimester found the relative risk for schizophrenia spectrum disorders was >2 in the offspring [4]. There was no increase in risk with first or third trimester infections. For 8700 Norwegian women with fever in the second trimester, the relative risk for autism spectrum disorder was 1.4 in the offspring [5]. For 4300 Danish women with fevers between 9 and 16 weeks gestation, the relative risk for attention deficit disorder was 1.3 in the offspring [6]. A major period of brain development occurs from 9 to 16 weeks. Family history, indicative of presumptive genetic risk, interacts synergistically with the risk conveyed by maternal gestational infection [7]. Assessment of banked gestational serum samples and blood spots from pregnant women whose offspring later developed schizophrenia or autism demonstrate increased concentration of cytokines and C-reactive protein (CRP), which are biochemical indicators of the maternal inflammatory response [[8], [9], [10], [11], [12]].
Maternal immune activation is a widely used animal model for the pathophysiology of maternal infection during pregnancy. To mimic viral infection without otherwise sickening the dam, polyinosinic:polycytidylic acid (poly I:C), a double-stranded RNA similar to the double-stranded RNA produced during viral infections, is injected into the dam. To similarly mimic bacterial infection, lipopolysaccharide, the endotoxin of gram-negative bacteria, is injected. Poly I:C and lipopolysaccharide activate Toll-Like-Receptors on macrophages and other maternal cells [13,14]. Both exposures result in increased plasma concentrations of Interleukin-6 (IL-6) and other pro-inflammatory cytokines [15,16]. The placental chorionic villi's fetal Hofbauer macrophages are activated [17]. The damage to fetal brain depends on the timing of the exposure during gestation. For example, embryonic day 10–12 exposure of mice results in damage to the developing basal ganglia. The offspring have decreased prepulse inhibition and latent inhibition defects similar to those seen in autism and schizophrenia [15]. Offspring of dams with null mutation of the IL-6 gene do not exhibit behavioral changes from maternal immune activation [18].
The effect of the SARS-CoV-2 infection on fetal development was initially predicted by the U.S. Centers for Diseases Control (CDC) to be similar to the effect of other respiratory coronaviruses [19]. The possibility of placental transfer of the virus in severe cases has been raised; however this is unlikely for most women [20]. Maternal CRP plasma concentration is increased above reference levels in SARS-CoV-2 infected pregnant women, consistent with an inflammatory response (Table 1 ).
Table 1.
C-reactive Protein in pregnancy, SARS-CoV-2 infection, and offspring's risk for later mental illness.
| Country | N | C-reactive Protein (CRP) in mothers with SARS-CoV-2 (mg/L)a | Gestational Age at Birth | Citation |
|---|---|---|---|---|
| China | 60 cases 120 controls | 20.16 ± 41.96 infected versus 8.87 ± 20.4 uninfected | 17% < 37 weeks gestation compared to 7.5% of uninfected, P = 0.06 | Sun G, Zhang Y, Liao Q, Cheng Y. Blood test results of pregnant COVID-19 patients: an updated case–control study. Front Cell Infect Microbiol. 2020; 10:560,899. doi:10.3389/fcimb.2020.560899. |
| China | 106 cases | >10.0 71/106 cases and 8/8 severe cases |
Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020 18; 382(25):e100. doi:10.1056/NEJMc2009226. | |
| China | 8 cases | 6/8 cases >10.0 | Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395(10,226):809–15. doi:10.1016/S0140-6736(2030360-3). | |
| Spain | 8 case | 5.10 ± 7.64; severe cases 10.25,15.63 | 2 cases <34 weeks, 3 cases 34–37 weeks 3 cases >37 weeks |
Ortiz Molina E, Hernandez Pailos R, Pola Guillen M, Pascual Pedreno A, Rodriguez E, Hernandez Martinez A. COVID-19 infection in symptomatic pregnant women at the midpoint of the pandemic in Spain: a retrospective analysis. Ginekol Pol. 2020; 91:755–63. doi:10.5603/GP.a2020.0130. |
| Spain | 60 | 17.8 (range 0.8–147.4) | Pereira A, Cruz-Melguizo S, Adrien M, Fuentes L, Marin E, Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020; 99:839–47. https://doi.org/10.1111/aogs.13921. | |
| Israel | 11 | 20.6, range 5.62–41.01 | Mohr-Sasson A, Chayo J, Bart Y, Meyer R, Sivan E, Mazaki-Tovi S et al. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet. 2020; 302:629–34. doi:10.1007/s00404-020-05655-7. | |
| Comparison: CRP and offspring risk for mental illness | ||||
| Finland | 777 cases 777 controls | At 11 weeks gestation CRP ≥10 mg/L increased offspring schizophrenia odds ratio = 1.58, 95% CI = 1.04–2.40, P = 0.03. | Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014; 171(9):960–8. doi: 10.1176/appi.ajp.2014.13121579. | |
| Finland | 677 cases 677 controls |
At 11 weeks gestation CRP >9.55 mg/L increased offspring autism odds ratio = 1.80, 95% CI = 1.09–2.97, P = 0.02 | Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014; 19(2):259–64. doi:10.1038/mp.2012.197. | |
| U.S. | 500 cases 580 controls | At 16–19 weeks gestation CRP 4.391–10.25 increased offspring autism OR 1.33 (0.47–3.80) not significant |
Zerbo O, Traglia M, Yoshida C, Heuer LS, Ashwood P, Delorenze GN et al.. Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: the early markers for autism study. Transl Psychiatry. 2016 Apr 19; 6(4):e783. doi:10.1038/tp.2016.46. | |
Normal value cited by most studies <5 mg/L. Values > 10 mg/L are considered substantially elevated. Most SARS-CoV-2 infected women were sampled in the third trimester.
Effects of the inflammatory response from maternal SARS-CoV-2 infection on the brain development of fetuses that could predispose them to later mental illness will not be known for decades, but it seems likely that future epidemiologists will catalog the incidence of schizophrenia, autism, and attention deficit disorder in offspring. This paper reviews evidence for specific nutritional actions that families, their clinicians, and public health authorities can take to prevent the COVID-19 pandemic from becoming yet one more landmark study for the adverse effects of maternal inflammation on the fetus and increased risk for mental illness later in life.
2. Literature search
A Medline search for (Nutrients OR Vitamins OR Choline OR Folic Acid OR fatty acids, omega 3) AND pregnancy AND (coronavirus infection OR coronavirus) found only one study [21]. Searches in Clinical Nutrition, Clinical Nutrition ESPEN, and American Journal of Clinical Nutrition found no articles. A broader search for (Nutrients OR Vitamins OR Choline OR Folic Acid OR fatty acids, omega 3) AND pregnancy AND (Viral diseases OR Respiratory Tract Infections) found an additional 7 studies, all of which investigated the effects of prenatal Vitamin D, folic acid, or fish oil supplements on infant respiratory wheezing. In the animal model literature, a search for (Nutrients OR Vitamins OR Choline OR Folic Acid OR fatty acids, omega 3) AND the keyword Maternal Immune Activation found 3 studies. Others were found in a search for Maternal Immune Activation only. Table 2 is a selection of more recent relevant animal studies. Table 3 includes all human studies that were retrieved.
Table 2.
Maternal immune activation and nutrients in animal models.
| Nutrient | Inflammation | Findings | Reference |
|---|---|---|---|
| n-3 poly un-saturated fatty acid |
Lipopoly-saccharide inflammation with high ratio of linolenic acid to α-linolenic acid to produce n-3 fatty acid deficiency |
n-3 fatty acid deficiency exacerbated the inflammatory effect, with increased IL-6 levels in maternal plasma, placenta, and fetal brain and decreased Y-maze performance in offspring | Labrousse VF, Leyrolle Q, Amadieu C, Aubert A, Sere A, Coutureau E et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav Immun 2018; 73:427–40. doi:10.1016/j.bbi.2018.06.004. |
| n-3 poly un-saturated fatty acids |
Poly I:C inflammation with n-3 fatty acids (0.40% vs.1.43%) and n6:n3 ratio (20.77%vs.5.71%). | Higher n-3 fatty acid diet inhibited the maternal IL-6 response to poly I:C. It also prevented the adverse social behavior response after poly I:C. | Weiser MJ, Mucha B, Denheyer H, Atkinson D, Schanz N, Vassiliou E et al. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot Essent Fatty Acids. 2016; 106:27–37. doi:10.1016/j.plefa.2015.10.005. |
| n-3 poly un-saturated fatty acids |
Prenatal Poly I:C RNA inflammation with post weaning supplementation, standard n-6 versus enriched n-3 poly-unsaturated fatty acid diet | Postnatal supplement attenuated I:C RNA decrease in global gene methylation, but not for GABA transaminase and guanine nucleoside binding protein in GABAB receptors | Basil P, Li Q, Gui H, Hui TCK, Ling VHM et al. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by an n-3 polyunsaturated fatty acid diet. Transl Psychiatry. 2018; 8(1):125. doi:10.1038/41398-018-0167-x. |
| Vitamin D | Poly I:C RNA inflammation, pretreatment with Vitamin D 1–25,OH-D 400 ng/kg |
Vitamin D blocked inflammation decrease in social approach, abnormal digging, and tone conditioning. No effect of maternal or fetal proinflammatory cytokines. | Vuillermot S, Luan W, Meyer U, Darryl E. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism. 2017; 8:9. doi:10.1186/s13229-017-0125-0 |
| Vitamin D | Lipopolysaccharide inflammation in vivo and in placenta ex vivo. Vitamin D tested ex vivo. |
In placenta 25-hydroxyvitamin D3, suppressed lipopolysaccharide induced expression of IL-6 and the chemokine Ccl11. Increased IL-6 in Vitamin D Receptor VDR null mutant placentas. | Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011; 186(10):5968–74. doi:10.4049/jimmunol.1003332. |
| Folic acid | Lipopolysaccharide inflammation with folic acid 3 mg/kg 1 h before. | Folic acid diminished lipopolysaccharide decreased preterm delivery from 100% to 64% | Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H et al. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One. 2013; 8(12):e82713. doi:10.1371/journal.pone.0082713. |
| Choline | Poly I:C RNA inflammation with choline 1.1 g versus 5 g diets | Choline supplement decreased RNA-stimulated increase in fetal brain IL-6, offspring anxiety-like behavior in open field, and repetitive marble burying. Chrna7 null mutation increased fetal brain Il6 response to RNA and offspring behavioral deficits. | Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain BehavImmun. 2015; 46: 192–205. doi:10.1016/j.bbi.2015.02.005. |
| Choline | Lipopolysaccharide inflammation with choline 1.1 g versus 5 g diets | Higher choline attenuated inflammatory cytokines, decreased placental α7nAChR, lowered NF-κB signaling in placenta mononuclear cells, and inhibited placental AKT phosphorylation. | Zhang M, Han X, Bao J, Yang J, Shi SQ, Garfield RE et al. Choline supplementation during pregnancy protects against gestational lipopolysaccharide-induced inflammatory responses. Reprod Sci. 2018; 25(1):74–85. doi:10.1177/1933719117702247. |
Table 3.
Maternal infection and inflammation and nutrients in human gestation.
| Nutrient | Subjects | Findings | Reference |
|---|---|---|---|
| Choline | N = 43 women with 1st trimester viral infection, N = 53 no infection | For infants of mothers with choline levels ≥7.5 μM, there was no effect of viral infection on IBQ-R Regulation, compared to infants of mothers who were not infected. Physiological development of newborns' cerebral inhibition was adversely affected in mothers with higher CRP and IL-6 plasma levels at 16 wks gestation. Higher maternal choline levels diminished this effect. |
Freedman R, Hunter SK, Law AJ, D'Alessandro A, Noonan K, Wyrwa A, Camille Hoffman M. Maternal choline and respiratory coronavirus effects on fetal brain development. J Psychiatr Res 2020; 128:1–4. doi:10.1016/j.jpsychires.2020.05.019. Hunter SK, Hoffman MC, D'Alessandro A, Noonan K, Wyrwa A, Freedman R et al. Male fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychological Medicine 2019; doi:10.1017/S0033291719003313 [Epub ahead of print]. |
| Vitamin D | N = 2648 high risk women with placenta exami-nation | Pathological placenta changes in women with lowest quartile Vitamin D 13 wks gestation, compared to highest quartile. | Zhang Q, Chen H, Wang Y, Zhang C, Tang Z, Li H, Huang X, Ouyang F, Huang H, Liu Z. Severe vitamin D deficiency in the first trimester is associated with placental inflammation in high-risk singleton pregnancy. Clin Nutr 2019; 38:1921–1926. doi:10.1016/j.clnu.2018.06.978. |
| Vitamin D | N = 158 adolescents at 26 weeks gestation | Pregnant teens with Vitamin D < 30 ng/mL were more likely to test positive for candida and bacterial vaginosis. No significant effect on inflammatory cytokines. | Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O'Brien KO. Low Vitamin D is associated with infections and proinflammatory cytokines during pregnancy. Reprod Sci 2018; 25:414–423. doi:10.1177/1933719117715124. |
| Vitamin D | N = 178 women 21 weeks. gestation | Vitamin D serum concentration at 10 wks Inversely associated with IL-6 concentration | Bobbitt KR, Peters RM, Li J, Rao SD, Woodcroft KJ, Cassidy-Bushrow AE. Early pregnancy vitamin D and patterns of antenatal inflammation in African-American women. J Reprod Immunol 2015; 107:52–58. doi: 0.1016/j.jri.2014.09.054 |
| Omega-3 fatty acids | N = 51 women with n-3 fatty acid supplements at 20 weeks gestation | Placenta inflammatory cytokines not affected by supplement | Keelan JA, Mas E, D'Vaz N, Dunstan JA, Li S, Barden AE et al. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reprod 2015; 149:171–8. doi:10.1530/REP-14-0549. |
| Folate | N = 417 women 9.5 weeks gestation |
5-methyltetrahydrofolate, 65% of folate vitamers, had no relation to cervical fluid inflammatory cytokines and negative relation to anti-inflammatory cytokines | Simhan HN, Himes KP, Venkataramanan R, Bodnar LM. Maternal serum folate species in early pregnancy and lower genital tract inflammatory milieu. Am J Obstet Gynecol 2011; 205:61.e1-7. doi:10.1016/j.ajog.2011.03.039. |
| Multiple micro-nutrients | N = 740 women at 32 weeks gestation after folic acid, iron, zinc, and multivitamin supplements | Serum markers of inflammation, α1-acid glycoprotein and C-reactive protein, were not significantly decreased by any of the supplements. | Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr 2006; 83:788–94. doi: 10.1093/ajcn/83.4.788. |
3. Results
3.1. Maternal gestational COVID-19 as a fetal inflammatory challenge
The inflammatory response induced by SARS-CoV-2 infection can be measured as elevation above normal values in maternal plasma or serum CRP concentration [[22], [23], [24], [25], [26], [27]]. CRP concentrations in pregnant women with symptomatic SARS-CoV-2 infections have been reported for 174 women in China and 79 in Spain and Israel (Table 1). All studies found a significant increase in CRP in women with SARS-CoV-2 infection, compared to women in the same clinical setting who were not infected. The samples were generally taken in the third trimester. CRP concentrations over 10 mg/L are considered pathological [23]. Mean values in SARS-CoV-2 infected women across the studies ranged from 5.1 to 20.6 mg/L.
Vaccination produces an inflammatory reaction that is smaller than viral infection, but nonetheless measurable. Twelve participants in the Phase 2 trial of BNT162b2, the current Pfizer vaccine, had CRP levels measured before, one day after, and 8 days after their immunization at the dose approved for public emergency use. CRP levels rose for all participants after vaccination, generally by 3 mg/L. Of the 12 participants, 3 had levels exceeding 5 mg/L, the upper boundary of the reference level, with the highest value 23 mg/L. All CRP levels returned to pre-vaccination levels at 8 days. Levels were not reported after the 21-day booster immunization [28]. The Moderna Phase 2 vaccine trial did not report CRP responses.
3.2. Effects of nutrients in maternal immune activation animal models
Gestational n-3 polyunsaturated fatty acids deficiency was associated with higher IL-6 levels in maternal plasma, placenta, and fetal brain and decreased Y-maze performance, a memory test, in offspring [29]. Supplementation of dietary docosahexaenoic acid to three times control levels in another study decreased the maternal IL-6 response and prevented the adverse effect of gestational Poly I:C on offspring social behavior with other mice [30]. Postnatal supplementation with an enriched n-3 polyunsaturated fatty acid diet decreased global gene methylation, but not for genes targeted for their importance in brain function, a GABA transaminase and guanine nucleoside binding protein in GABAB receptors [31].
Vitamin D pretreatment blocked adverse effects of Poly I:C on social behavior, anxiety behaviors such as abnormal digging, and tone conditioning, a memory function [32]. There was no effect on maternal or fetal pro-inflammatory cytokines, however. Decreased IL-6 response to lipopolysaccharide is reported in isolated placenta ex vivo after Vitamin D treatment [33].
Folic acid administered before lipopolysaccharide inflammation moderately decreased preterm delivery; no other effects were reported [34].
Dietary choline supplementation, 5 times control levels, decreased Poly I:C-stimulated increase in fetal IL-6. The offspring had fewer anxiety-related behaviors. Null mutation of Chrna7, the gene for the α7-nicotinic acetylcholine receptor subunit that is activated by choline, prevented response to choline [35]. A similar study found that choline supplementation attenuates the cytokine response to lipopolysaccharide inflammation and inhibits NF-κβ signaling in placenta mononuclear cells and AKT phosphorylation. Choline decreased α7-nicotinic acetylcholine receptors in placenta, which are predominantly expressed in macrophages [36].
3.3. Interacting effects of maternal gestational infection and nutrition on offspring outcome
Human trials of choline and Vitamin D supplementation are ongoing during the COVID-19 pandemic, but most children of women infected in the first year of the pandemic have not yet reached the age at which they can be initially assessed [37,38]. Published data are available from children whose mothers experienced common viral respiratory infections, many of which are also coronaviruses (Table 3). The children in these studies are too young to have developed diagnosable mental illnesses. However, their parents can rate their child's attention, social behavior, and other aspects of behavior and temperament using rating scales that identify childhood precursors of later problems in school and psychopathology, including schizophrenia [[39], [40], [41]].
In the only study of choline and folic acid in mothers with viral respiratory infection, 43 women reported viral infection [21]. A comparison group of 53 women had neither viral nor bacterial infections. Viral infection was associated with increased CRP, 11.0 mg/L (SEM 1.4) in infected women versus 7.5 mg/L (SEM 1.2) in uninfected women, P = 0.047 [42]. Women were provided nutritional information and encouraged to eat foods with higher choline content but were not given choline supplements or monitored for dietary intake; 88% of the women were using prenatal vitamins with folic acid. When the infants reached 3 months of age, mothers completed the Infant Behavior Questionnaire-Revised Short Form (IBQ-R), a parent-report measure of infant behaviors indicative of temperamental reactivity and self-regulation [43]. The IBQ-R Orienting/Regulation Index includes duration of attention, enjoyment of quiet play, cuddliness and engagement with parents, and soothability.
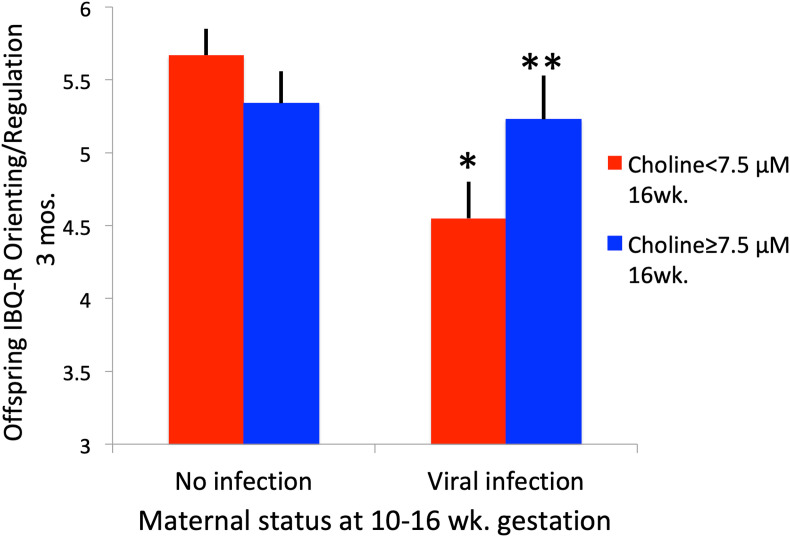
Infants of mothers who contracted viral infections and had choline levels ≥7.5 μM, the highest 20%ile of choline levels, had significantly higher (better) 3-month IBQ-R scores on the Regulation dimension and specifically the duration of attention scale in the Regulation dimension, compared to infants of mothers who had viral infections and had choline levels <7.5 μM (Fig. 1 ).
Fig. 1.
Offspring IBQ-R Orienting/Regulation at 3 months of age in relation to maternal viral infection and plasma choline concentration during early gestation, marginal means and standard errors21. ∗P = 0.005 for viral infection versus no infection with maternal choline <7.5 μM; ∗∗P = 0.002 for maternal choline ≥7.5 μM versus <7.5 μM with maternal viral infection. Maternal viral infection∗choline Fdf1,73 = 13.22, P = 0.001.
Thirty of the children whose mothers had viral or bacterial infections were re-assessed at 4 years of age using the Child Behavior Checklist 1½-5 year version (CBCL1½-5) [44]. Problems in Attention rated on CBCL1½-5 were significantly decreased for the children of mothers who also had higher gestational choline concentrations at 16 weeks gestation. Ratings ≥92nd percentile on the Attention Deficit Hyperactivity Disorder (ADHD) scale occurred in 8 of the 83 children between ages 40 and 48 months. Ratings at this level are generally associated with children referred for clinical evaluations [45]. Seven of these 8 children were from mothers who had gestational choline concentrations <7.5 μM CBCL1½-5 ratings were also acquired for children whose mothers participated in a randomized, placebo-controlled trial of phosphatidylcholine supplementation 6300 mg daily, equivalent to 900 mg choline. Among the 9 children whose mothers had gestational infections, Attention and Aggression problems were significantly lower if the mothers had received the phosphatidylcholine supplement [46]. Similar severity of childhood problems in Attention were rated retrospectively on the CBCL1½-5 by parents of offspring who developed schizophrenia in later life [41]. Choline levels ≥7.5 μM are within 1 standard deviation of mean reached with 6300 mg supplementation.
None of the other maternal nutrients have been specifically assessed in the context of the effects of maternal viral or bacterial infection on offspring's outcome. Investigations have primarily focused on the maternal inflammatory milieu, independent of infection. Effects of Vitamin D deficiency on inflammation have been investigated. Increased placenta pathology and increased rates of bacterial vaginosis have been found [47,48]. However, higher levels of Vitamin D have also been associated with increased IL-6 plasma concentrations [49]. n-3 polyunsaturated fatty acid supplements did not affect placental inflammatory cytokines [50]. Folic acid metabolites, specifically 5-methyltetrahydrofolate, did not affect pro-inflammatory cytokines, but did decrease concentrations of anti-inflammatory cytokines [51]. Multiple micronutrients did not affect serum markers of inflammation, including CRP [52].
4. Discussion
Research into the pathogenic effects of SARS-CoV-2 for fetal brain development and its possible interaction with maternal nutrition will not be conclusive until long after the pandemic is over. The research reviewed in this paper suggests that higher levels of maternal choline, together with other nutrients, might mitigate some of the effects of infection on fetal brain development, based on experience with other respiratory viruses and animal models. Otherwise, based on historical experience with previous pandemics, SARS-CoV-2 infection of the mother can be predicted to significantly affect the offspring's later cognitive and behavioral development and eventually the risk for mental illness. Several individuals who received a vaccine had substantial CRP elevation, with levels as high as those observed in infected women. Vaccination protocols for pregnant women might include provisions to encourage maternal choline and other prenatal vitamins' adequacy. Vitamin D and folic acid are already in optimal amounts in standard prenatal vitamin formulations.
Maternal plasma concentrations of choline ≥7.5 μM, associated with better development of attention and other orienting/regulation capabilities in early childhood, are within one standard deviation of the mean concentration in women receiving phosphatidylcholine supplementation. Over 80% of supplemented women would thus be expected to have this range of concentrations, but it was obtained by only 20% of women from diet alone [21]. The European Food Safety Authority (EFSA) and U.S. Food and Drug Administration (FDA) recommend daily choline intake, 480 and 550 mg respectively during pregnancy (Table 4 ). Recent FDA guidance explicitly makes no recommendation for or against supplements [53]. Higher choline intake is also recommended during lactation. Calf's liver, beef, egg yolks, salmon and soybeans are potential sources of choline, but dietary surveys in many countries find that women fail to eat sufficient amounts [[54], [55], [56], [57], [58], [59]]. For example, a recent survey of 90 US. women estimated dietary intake 318 ± 68 mg, with no woman reaching the recommended amount [60]. Thus for many pregnant women, increased choline intake from diet or supplements would not only benefit fetal development, but also overcome deficiency that is associated with increased incidence of non-alcoholic fatty liver and muscle damage during pregnancy [61].
Table 4.
Current daily recommended values of selected nutrients for pregnant women.
| European Food Safety Authority [73] | U.S. Food and Drug Administration53 | World Health Organization [74,75],b | |
|---|---|---|---|
| Folate | 600 μg dietary food equivalents or 400 μg folic acid supplement | 600 μg dietary food equivalents or 400 μg folic acid supplement | 400 μg folic acid supplement |
| Vitamin D | 15 μg | 15 μg | 5 μg if deficiency suspected, e.g., low sunlight exposure |
| Choline | 480 mg | 550 mg | Not considered |
| Alpha Linoleic Acida | 1.4 g | 1.6 g | Not considered |
| Linoleic Acida | 13 g | 17 g | Not considered |
General recommendations, not specifically for pregnant women.
WHO recommends daily iron 30–60 mg supplementation for all pregnant women. European Food Safety Authority and U.S. Food and Drug Administration recommend supplemental iron only for women with evidence for iron deficiency.
Moderate increases of choline in diets do not raise choline levels, but supplements raise levels and can overcome effects of polymorphisms in PEMT, the gene for phosphatidylethanolamine transferase, that otherwise lower choline levels [[62], [63], [64]]. Choline and phosphatidylcholine, or lecithin, are widely available as health supplements without prescription. Citicoline, an inosine–choline combination, is also available. A comparison of choline levels reached with supplements studied in clinical trials suggests that any of the formulations studied can facilitate adequate levels for many or most women (Table 5 ) [[65], [66], [67]].
Table 5.
Effects of phosphatidylcholine and choline supplements on plasma choline concentration in pregnant women.
| Supplement and gestational week initiated | Choline μM first post supplement concentration, mean ± std. deviation | % Predicted plasma concentration >7.5 μMa | Reference |
|---|---|---|---|
| Phosphatidylcholine 5400 mg at 18 weeks | 13.7 ± 4.2 | 93 | Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J et al. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr. 2010; 92(2):336–46. doi: 10.3945/ajcn.2010.29459. |
| Phosphatidyl-choline 6300 mg at 17 weeks | 15.2 ± 8.1 | 83 | Ross RG, Hunter SK, Hoffman MC et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry. 2016; 173:509–516. doi:10.1176/appi.ajp.2015.15091188 |
| Choline bitartrate 2.5 g at 23 weeks | 10.7 ± 4.2 | 78 | Jacobson SW, Carter RC, Molteno CD, Meintjes EM, Senekal MS, Lindinger NM et al. Feasibility and acceptability of maternal choline supplementation in heavy drinking pregnant women: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018; 42(7):1315–26. doi: 10.1111/acer.13768. |
| Choline chloride 550 mg at 27 weeks | 8.2 ± 1.2 | 72 | Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012; 95(5):1060–71. doi:10.3945/ajcn.111.022772. |
| No supplement, 16 weeks concentration | 7.1 ± 1.9 | 42 | Wu BTF, Dyer RA, King DJJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE. 2012; 7:e43448. doi:10.1371/journal.pone.0043448. |
Estimated % is based on the assumption of a normal distribution.
The issue of whether increasing choline intake through diet or supplements can cause atherogenesis by increasing concentrations of potentially toxic trimethylamine oxide (TMAO) is controversial [68]. Choline is metabolized by some gut microbacteria to trimethylamine, the precursor of TMAO. Most dietary choline is in the form of phosphatidylcholine, which is relatively impervious to bacterial decomposition. Three eggs per day for 4 weeks in 38 young adults raised fasting plasma choline concentration to over 8 μM and did not raise TMAO levels [69]. Higher choline-containing diets >486 mg/day were not associated with increased risk of cardiovascular disease in 14,430 middle aged men and women in the 14-year Atherosclerosis Risk in Communities study [70]. Phosphatidylcholine supplements containing 600 mg choline in 37 healthy men did not increase plasma or urinary TMAO, whereas choline bitartrate containing 600 mg raised plasma TMAO 3-fold [67]. A pharmaceutical company is developing an inhibitor of bacterial production of trimethylamine and commissioned a systematic review of effects of choline and choline and phosphatidylcholine supplements in fetal development [71,72].
The nutritional needs and metabolic physiology of human pregnancy are different from the nutritional needs and metabolic physiology of middle-aged men and women. Current evidence supports phosphatidylcholine from diet or supplements as a safe, effective nutrient to support fetal development.
5. Conclusions
Experience from other pandemics predicts that the COVID-19 pandemic is likely to increase the risk for schizophrenia, autism, attention deficit disorder, and other behavioral and cognitive problems in offspring whose mothers are infected during gestation. Higher maternal choline plasma levels are associated with more normal fetal development in all mothers and, in particular, mothers who are infected, but most women have lower choline plasma levels and dietary intake below currently recommended amounts. More widespread availability of phosphatidylcholine supplements for pregnant women may help mitigate the risks from SARS-CoV-2 infection to their fetuses. The time course of fetal and child development prohibits assessment of effectiveness of nutrient interventions until after the pandemic has subsided, but by then the opportunity to positively impact fetal brain development will have passed. The cost of providing prenatal choline or phosphatidylcholine supplements to women is quite low compared to the lifetime cost of caring for a child who later develops mental illness.
Statement of authorship
All authors planned and discussed the results of this study. All authors have approved the final article.
Funding
National Institute of Child Health and Human Development (K12HD001271-11 to M.C.H.); National Center for Advancing Translational Sciences (UL1 TR001082); The Institute for Children's Mental Disorders; The Anschutz Foundation.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgement
The late Randal G. Ross conceptualized the study of prenatal choline supplementation in infection and other conditions.
Footnotes
Submitted in response to the European Society for Clinical Nutrition and Metabolism (ESPEN) and World Health Organization Regional Office for Europe (WHO/Europe) call for papers on nutritional status and nutritional care in COVID-19.
References
- 1.Mednick S.A., Machon R.A., Huttunen M.O., Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatr. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 2.O'Callaghan E., Sham P., Takei N., Glover G., Murray R.M. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337:1248–1250. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Chen L., Wu T., Shi H., Li Q., Jiang H., et al. Impact of Covid-19 in pregnancy on mother's psychological status and infant's neurobehavioral development: a longitudinal cohort study in China. BMC Med. 2020;18(1):347. doi: 10.1186/s12916-020-01825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown A.S., Schaefer C.A., Wyatt R.J., Goetz R., Begg M.D., Gorman J.M., et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 5.Hornig M., Bresnahan M.A., Che X., Schultz A.F., Ukaigwe J.E., Eddy M.L., et al. Prenatal fever and autism risk. Mol Psychiatr. 2018;23:759–766. doi: 10.1038/mp.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werenberg D.J., Nybo-Andersen A.M., Hvolby A., Garne E., Kragh-Andersen P., Berg-Beckhoff G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. JCPP (J Child Psychol Psychiatry) 2016;57:540–548. doi: 10.1111/jcpp.12480. [DOI] [PubMed] [Google Scholar]
- 7.Clarke M.C., Tanskanen A., Huttunen M., Whittaker J.C., Cannon C. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatr. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein J.M., Cherkerzian S., Seidman L.J., Donatelli J.A., Remington A.G., Tsuang M.T., et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med. 2014;44:3249–3261. doi: 10.1017/S0033291714000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbe O., et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatr. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canetta S., Sourander A., Surcel H.M., Hinkka-Yli-Salomaki S., Levisika J., Kellendonk C., et al. Maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatr. 2014;171:960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown A.S., Sourander A., Hinkka-Yli-Salomäki S., McKeague I.W., Sundvall J., Surcel H.M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatr. 2014;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerbo O., Traglia M., Yoshida C., Heuer L.S., Ashwood P., Delorenze G.N., et al. Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: the early markers for autism study. Transl Psychiatry. 2016 Apr 19;6(4):e783. doi: 10.1038/tp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 14.Park B., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson P.H. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao E.Y., Patterson P.H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezouar S., Katsogiannou M., Ben Amara A., Bretelle F., Mege J.L. Placental macrophages: origin, heterogeneity, function and role in pregnancy-associated infections. Placenta. 2020;103:94–103. doi: 10.1016/j.placenta.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . 2020. Coronavirus disease 2019 (COVID-19) and pregnancy.https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html [Google Scholar]
- 20.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection be acquired in utero? more definitive evidence is needed. J Am Med Assoc. 2020;323:1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 21.Freedman R., Hunter S.K., Law A.J., D'Alessandro A., Noonan K., Wyrwa A., et al. Maternal choline and respiratory coronavirus effects on fetal brain development. J Psychiatr Res. 2020;128:1–4. doi: 10.1016/j.jpsychires.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G., Zhang Y., Liao Q., Cheng Y. Blood test results of pregnant COVID-19 patients: an updated case-control study. Front Cell Infect Microbiol. 2020;10:560899. doi: 10.3389/fcimb.2020.560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L., et al. Clinical characteristics of pregnant women with covid-19 in wuhan, China. N Engl J Med. 2020 18;382(25) doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 Mar 7;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz Molina E., Hernandez Pailos R., Pola Guillen M., Pascual Pedreno A., Rodriguez Rodriguez E., Hernandez Martinez A. COVID-19 infection in symptomatic pregnant women at the midpoint of the pandemic in Spain: a retrospective analysis. Ginekol Pol. 2020;91:755–763. doi: 10.5603/GP.a2020.0130. [DOI] [PubMed] [Google Scholar]
- 26.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohr-Sasson A., Chayo J., Bart Y., Meyer R., Sivan E., Mazaki-Tovi S., et al. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet. 2020 Sep;302(3):629–634. doi: 10.1007/s00404-020-05655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 29.Labrousse V.F., Leyrolle Q., Amadieu C., Aubert A., Sere A., Coutureau E., et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav Immun. 2018;73:427–440. doi: 10.1016/j.bbi.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Weiser M.J., Mucha B., Denheyer H., Atkinson D., Schanz N., Vassiliou E., et al. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot Essent Fatty Acids. 2016;106:27–37. doi: 10.1016/j.plefa.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Basil P., Li Q., Gui H., Hui T.C.K., Ling V.H.M., Wong C.C.Y., et al. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by an n-3 polyunsaturated fatty acid diet. Transl Psychiatry Psychiatry. 2018;8:125. doi: 10.1038/41398-018-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vuillermot S., Luan W., Meyer U., Eyles D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism. 2017;8:9. doi: 10.1186/s13229-017-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N.Q., Kaplan A.T., Lagishetty V., Ouyang Y.B., Ouyang Y., Simmons C.F., et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186:5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M., Chen Y.H., Dong X.T., Zhou J., Chen X., Wang H., et al. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PloS One. 2013 Dec 6;8(12) doi: 10.1371/journal.pone.0082713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W.L., Adams C.E., Stevens K.E., Chow K.H., Freedman R., Patterson P.H. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun. 2015;46:192–202. doi: 10.1016/j.bbi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Han X., Bao J., Yang J., Shi S.Q., Garfield R., Liu H. Choline supplementation during pregnancy protects against gestational lipopolysaccharide-induced inflammatory responses. Reprod Sci. 2018;25:74–85. doi: 10.1177/1933719117702247. [DOI] [PubMed] [Google Scholar]
- 37.University of Colorado, Denver Choline supplementation during pregnancy: impact on attention and social withdrawal. ClinicalTrials.gov Identifier: NCT03028857. Camille Hoffman, MD, Investigator. https://clinicaltrials.gov/ct2/show/NCT03028857
- 38.University of Aarrhus Vitamin D in pregnancy (GRAVITD). ClinicalTrials.gov Identifier: NCT04291313 Anna Louise Vestergaard, MD, Investigator. https://clinicaltrials.gov/ct2/show/NCT04291313
- 39.Gartstein M.A., Putnam S.P., Kliewer R. Do infant temperament characteristics predict core academic abilities in preschool-aged children? Learn Indiv Differ. 2016;45:299–306. doi: 10.1016/j.lindif.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slobodskaya H.R., Kozlova E.A. Early temperament as a predictor of later personality. Pers Indiv Differ. 2016;99:127–132. [Google Scholar]
- 41.Rossi A., Pollice R., Daneluzzo E., Marinangeli M.G., Stratt P. Behavioral neurodevelopmental abnormalities and schizophrenic disorder: a retrospective evaluation with the Child Behavior Checklist (CBCL) Schizophr Res. 2000;44:121–128. doi: 10.1016/S0920-9964(99)00223-6. [DOI] [PubMed] [Google Scholar]
- 42.Hunter S.K., Hoffman M.C., D'Alessandro A., Noonan K., Wyrwa A., Freedman R., et al. Male fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychol Med. 2021 Feb;51(3):450–459. doi: 10.1017/S0033291719003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gartstein M.A., Rothbart M.K. Studying infant temperament via the revised infant behavior Questionnaire. Infant Behav Dev. 2004;26:64–86. [Google Scholar]
- 44.Hunter S.K., Hoffman M.C., D'Alessandro A., Wyrwa A., Noonan K., Zeisel S.H., et al. Prenatal choline, cannabis, and infection, and their association with offspring development of attention and social problems through 4 years of age. Psychol Med. 2021:1–10. doi: 10.1017/S0033291720005061. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Achenbach T.M., Recorla L. ASEBA; Burlington, VT: 1991. Manual for the ASEBA preschool forms and profiles: an integrated system of multi-informant assessment. [Google Scholar]
- 46.Ross R.G., Hunter S.K., Hoffman M.C., McCarthy L., Chambers B.M., Law A.J., et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatr. 2016;173:509–516. doi: 10.1176/appi.ajp.2015.15091188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akoh C.C., Pressman E.K., Cooper E., Queenan R.A., Pillittere J., O'Brien K.O. Low Vitamin D is associated with infections and proinflammatory cytokines during pregnancy. Reprod Sci. 2018;25:414–423. doi: 10.1177/1933719117715124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q., Chen H., Wang Y., Zhang C., Tang Z., Li H., et al. Severe vitamin D deficiency in the first trimester is associated with placental inflammation in high-risk singleton pregnancy. Clin Nutr. 2019;38:1921–1926. doi: 10.1016/j.clnu.2018.06.978. [DOI] [PubMed] [Google Scholar]
- 49.Bobbitt K.R., Peters R.M., Li J., Rao S.D., Woodcroft K.J., Cassidy-Bushrow A.E. Early pregnancy vitamin D and patterns of antenatal inflammation in African-American women. J Reprod Immunol. 2015;107:52–58. doi: 10.1016/j.jri.2014.09.054. doi: 0.1016/j.jri.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 50.Keelan J.A., Mas E., D'Vaz N., Dunstan J.A., Li S., Barden A.E., et al. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction. 2015;149:171–178. doi: 10.1530/REP-14-0549. [DOI] [PubMed] [Google Scholar]
- 51.Simhan H.N., Himes K.P., Venkataramanan R., Bodnar L.M. Maternal serum folate species in early pregnancy and lower genital tract inflammatory milieu. Am J Obstet Gynecol. 2011;205 doi: 10.1016/j.ajog.2011.03.039. 61.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christian P., Jiang T., Khatry S.K., LeClerq S.C., Shrestha S.R., West K.P., Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr. 2006;83:788–794. doi: 10.1093/ajcn/83.4.788. [DOI] [PubMed] [Google Scholar]
- 53.Food and Drug Administration Nutrition labeling requirements. https://www.fda.gov/media/99069/download
- 54.Wu B.T.F., Dyer R.A., King D.J.J., Richardson K.J., Innis S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PloS One. 2012;7 doi: 10.1371/journal.pone.0043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu D.M., Wahlqvist M.L., Chang H.Y., Yeh N.H., Lee M.S. Choline and betaine food sources and intakes in Taiwanese. Asia Pac J Clin Nutr. 2012;21:547–557. [PubMed] [Google Scholar]
- 56.Mygind V.L., Evans S.E., Peddie M.C., Miller J.C., Houghton L.A. Estimation of usual intake and food sources of choline and betaine in New Zealand reproductive age women. Asia Pac J Clin Nutr. 2013;22:319–324. doi: 10.6133/apjcn.2013.22.2.19. [DOI] [PubMed] [Google Scholar]
- 57.Brunst K.J., Wright R.O., DiGioia K., Enlow M.B., Fernandez H., Wright R.J., et al. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Publ Health Nutr. 2014;17:1960–1970. doi: 10.1017/S1368980013003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis E.D., Subhan F.B., Bell R.C., McCargar L.J., Curtis J.M., Jacobs R.L., et al. APrON team Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr. 2014;112:112–121. doi: 10.1017/S0007114514000555. [DOI] [PubMed] [Google Scholar]
- 59.Vennemann F.B., Ioannidou S., Valsta L.M., Dumas C., Ocké M.C., Mensink G.B., et al. Dietary intake and food sources of choline in European populations. Br J Nutr. 2015;114:2046–2055. doi: 10.1017/S0007114515003700. [DOI] [PubMed] [Google Scholar]
- 60.Groth S.W., Stewart P.A., Ossip D.J., Block R.C., Wixom N., Fernandez I.D. Micronutrient intake is inadequate for a sample of pregnant African American women. J Acad Nutr Diet. 2017;117:589598. doi: 10.1016/j.jand.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisel S.H. 2006. Choline: critical role during fetal development and dietary requirements in adults. Annu RevNutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeisel S.H., Growdon J.H., Wurtman R.J., Magil S.G., Logue M. Normal plasma choline responses to ingested lecithin. Neurology. 1980;30:1226–1229. doi: 10.1212/wnl.30.11.1226. [DOI] [PubMed] [Google Scholar]
- 63.Abratte C.M., Wang W., Li R., Jaxume J., Moriarty D.J., Caudill M.A. Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. J Nutr Biochem. 2009;20:62–69. doi: 10.1016/j.jnutbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Fischer L.M., da Costa K.A., Galanko J., Sha W., Stephenson B., Vick J., et al. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr. 2010;92:336–346. doi: 10.3945/ajcn.2010.29459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobson S.W., Carter R.C., Molteno C.D., Meintjes E.M., Senekal M.S., Lindinger N.M., et al. Feasibility and acceptability of maternal choline supplementation in heavy drinking pregnant women: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018;42:1315–1326. doi: 10.1111/acer.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan J., Jiang X., West A.A., Perry C.A., Malysheva O.V., Devapatla S., et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- 67.Cho C., Aardema N.D.J., Bunnell M.L., SS, Bergeson J.R., Malysheva O.V., et al. Free choline, but not phosphatidylcholine, elevates circulating trimethylamine-N-oxide and this response is modified by the gut microbiota composition in healthy men. Curr Dev Nutr. 2020 June;4(Suppl 2):379. doi: 10.1093/cdn/nzaa045_012. [DOI] [Google Scholar]
- 68.Zeisel S.H., Warrier M. Trimethylamine n-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37:157–181. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- 69.DiMarco D.M., Missimer A., Murillo A.G., Lemos B.S., Malysheva O.V., Caudill M.A., et al. Intake of up to 3 eggs/day increases HDL cholesterol and plasma choline while plasma trimethylamine-N-oxide is unchanged in a healthy population. Lipids. 2017;52:255–263. doi: 10.1007/s11745-017-4230-9. [DOI] [PubMed] [Google Scholar]
- 70.Bidulescu A., Chambless L.E., Siega-Riz A.M., Zeisel S.H., Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta N., Buffa J.A., Roberts A.B., Sangwan N., Skye S.M., Li L., et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler Thromb Vasc Biol. 2020;40:1239–1255. doi: 10.1161/ATVBAHA.120.314139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derbyshire E., Obeid R. Choline, neurological development and brain function: a systematic review focusing on the first 1000 Days. Nutrients. 2020;12:1731. doi: 10.3390/nu12061731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.EFSA (European Food Safety Authority) 2017. Dietary reference values for nutrients. Summary Report. Amended September. 2019;23 doi: 10.2903/sp.efsa.2017.e15121. https://efsa.onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121 [DOI] [Google Scholar]
- 74.World Health Organization . WHO Press; Geneva: 2016. WHO recommendations on antenatal care for a positive pregnancy experience. [PubMed] [Google Scholar]
- 75.World Health Organization . WHO Press; Geneva: 2020. WHO recommendations on antenatal care for a positive pregnancy experience. Nutritional interventions update: vitamin D supplements during pregnancy. [PubMed] [Google Scholar]