Abstract
Though a plethora of functional magnetic resonance imaging (fMRI) studies explored the neurobiological underpinnings of borderline personality disorder (BPD), findings across different tasks were divergent. We conducted a systematic review and activation likelihood estimation (ALE) meta-analysis on the fMRI studies conducted in BPD patients compared to healthy controls (HC). We systematically searched PubMed and PsychINFO from inception until July 9th 2020 using combinations of database-specific terms like ‘fMRI’, ‘Neuroimaging’, ‘borderline’. Eligible studies employed task-based fMRI of the brain in participants of any age diagnosed with BPD compared to HC, during any behavioral task and providing a direct contrast between the groups. From 762 entries, we inspected 92 reports full-texts and included 52 studies (describing 54 experiments). Across all experiments, the HC > BPD and BPD > HC meta-analyses did not yield any cluster of significant convergence of differences. Analyses restricted to studies of emotion processing revealed two significant clusters of activation in the bilateral hippocampal/amygdala complex and anterior cingulate for the BPD > HC meta-analysis. Fail-safe N and single study sensitivity analysis suggested significant findings were not robust. For the subgroup of emotional processing experiments, on a restricted number of experiments providing results for each group separately, another meta-analysis method (difference of convergence) showed a significant cluster in the insula/inferior frontal gyrus for the HC > BPD contrast. No consistent pattern of alteration in brain activity for BPD was evidenced suggesting substantial heterogeneity of processes and populations studied. A pattern of amygdala dysfunction emerged across emotion processing tasks, indicating a potential pathophysiological mechanism that could be transdiagnostic.
Subject terms: Neuroscience, Human behaviour, Psychiatric disorders
Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM 5), Borderline personality disorder (BPD) is characterized by a pervasive pattern of instability referred to interpersonal relationship, self-image and affects together with marked impulsivity and emotional dysregulation1. The disorder has a considerable prevalence (5.9% lifetime2) and is associated with significant and widespread impairment of patients’ lives2–5. A plethora of neuroimaging studies6, most of which employed functional Magnetic Resonance Imaging (fMRI), attempted to delineate the neurobiological underpinnings of BPD. However, findings across different types of tasks were divergent. For example, some studies showed activation increased in the amygdala7,8, insula9, occipital, frontal and temporal areas10,11, while others reported decreased activation in both frontal and temporal regions12, cingulate cortex and nucleus accumbens13. Yet other studies found no significant differences in activation between BPD and healthy subjects (HC)14. These contradictory findings could be due to methodological aspects related to differences in the processes studied (i.e, emotion processing, theory of mind, cognitive functions), paradigms or stimuli used, but also use of small samples, Region of Interest (ROI) analysis or uncorrected statistics. Conversely, heterogeneous findings could be indicative of “real” heterogeneity among BPD patient populations.
Hence, we conducted a systematic review and activation likelihood estimation (ALE) meta-analysis on the fMRI studies conducted in BPD patients compared to healthy controls. The meta-analytical technique considers nuclei of activation reported in single experiments as spatial probability distributions centered at the coordinate itself. These distributions are then used for the generation of a brain map representing the likelihood of activation of each candidate location15. An earlier meta-analysis on 19 studies16 focused solely on the contrast between negative and neutral stimuli and found higher convergence of differences in BPD as compared to HC in the left amygdalae and in the posterior cingulate, along with a blunted response of the bilateral dorsolateral prefrontal cortex. Here, we implemented a broader approach to BPD as we hypothesized that despite heterogeneity, a consistent pattern of dysfunction in BPD would nonetheless emerge, with more specific patterns observed in homogeneous subgroups. In particular, network analysis highlighted17 difficulties in emotional regulation as central features of BPD, while several reports underscored overlapping brain networks related to negative emotion processes and working memory18 or even a casual effect of difficulties in emotional regulation on other cognitive functions such mentalizing19 and working memory20. Thus, we expected that associated neurobiological dysfunctions would also impact other mental functions and hence emerge consistently across studies, despite the use of different tasks or evaluation domains.
Methods
Study selection
The study was pre-registered on PROSPERO repository (CRD42019121856) and is reported following the PRISMA guidelines21 (see Supplementary Material for PRISMA checklist). We conducted systematic searches in PubMed and PsychINFO from inception until 9th of July 2020. We used combinations of database-specific terms as ‘fMRI’, ‘borderline’, ‘Neuroimaging’ (see Fig. 1 and Supplementary Material for the exact search string). Eligible studies (1) employed task-based fMRI of the brain in (2) participants of any age diagnosed with BPD according to the DSM IV, IV-TR or 5, based on diagnostic interviews, with or without comorbid disorders, (3) compared to a matched healthy control group (HC), during (4) any behavioral task using the same experimental paradigm was used for both BPD and HC, and had to include (5) a direct univariate comparison of brain activation between BPD and HC (i.e., BPD > BPD and/or BPD > HC), for which (6) 3D coordinates of peak activations in stereotactic space of the Montreal Neurological Institute (MNI) or Talairach were reported, and (7) whole-brain (i.e., not just Region of Interest/ROI) analysis were employed. There were no restrictions regarding receipt of any kind of treatment, past or current. For multiple reports on overlapping samples, only one (i.e., the one with the largest sample) was included. Reviews, meta-analyses and case-studies were excluded. Studies in English and Italian were considered eligible. Two authors (GD, CG) independently screened and selected studies.
Fig. 1. PRISMA diagram for the systematic research.
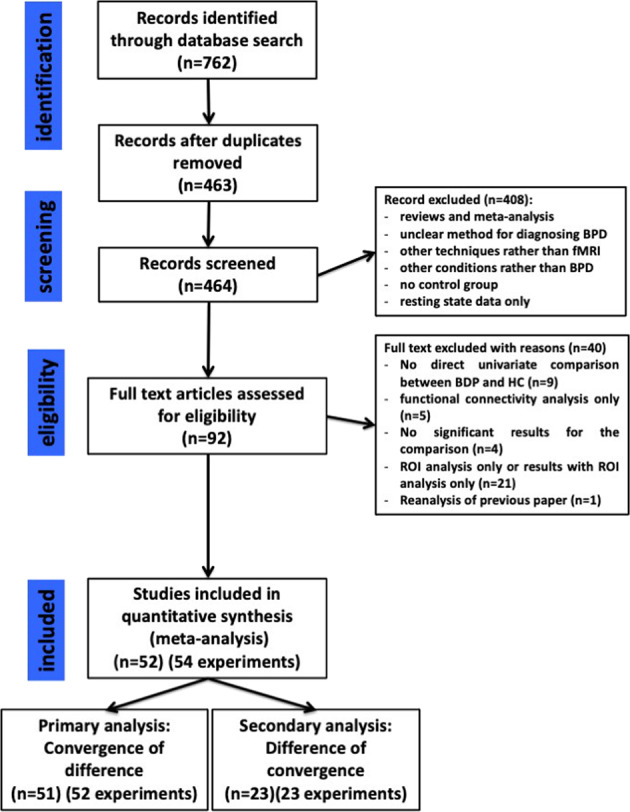
BPD borderline personality disorder, HC healthy controls, ROI region of Interest.
Data extraction
From each report, we extracted the following information: (1) participant mean age and gender (number of male and female participants); (2) comorbidities; (3) concurrent treatments; (5) type of task (e.g., passive or active tasks; task involving impulsivity control; emotional or cognitive tasks) and stimuli (e.g., faces, scripts, images, words); (6) coordinates for direct comparison of brain activation between BPD and HC; (7) where available, coordinates for the activations within each single group (BPD and HC). Data were extracted independently by two researchers (EdR, GD).
Risk of Bias
The Risk of Bias (RoB) of included studies were evaluated with a modified version of the Newcastle-Ottawa scale (NOS)22, (mNOS) adapted to fMRI data23 (See supplementary material for details).
ALE meta-analysis
Stereotactic coordinates for the ALE meta-analysis were extracted from the studies. The ALE algorithm was used as implemented in GingerALE 3.02 software24. We used the correction proposed by Turkeltaub and colleagues15, as implemented in GingerALE to control for multiple comparisons on the same dataset. Sample size for each experiment were used to calculate the Full‐Width Half‐Maximum (FWHM) of the Gaussian function used to blur the foci. Coordinates in the MNI 152 standard space were converted into the Talairach space using the GingerALE foci converter tool.
Two approaches can be employed in an ALE meta-analysis of two groups. The first (“convergence of activation differences”) uses coordinates from the contrast ‘patients vs. controls’ (i.e., patients > controls and controls > patients). The second (“differences in convergence”) pools the activation reported within each group separately, and subsequently computes a contrast between the resultant ALE-maps. We performed both for each of the two contrasts of interest (i.e., HC > BPD, BPD > HC) in each experiment.
We used convergence of activation differences as the primary analysis because it used data from all included studies. Statistical significance was assessed and corrected for multiple comparisons using a cluster-based method implemented in GingerALE: p < 0.001 cluster forming threshold, p < 0.05 cluster corrected FWE and N = 2000 permutations.
To check the robustness of the findings we performed a pooled analysis combining coordinates across BPD > HC and HC > BPD. This analysis reflects a more adequate summary of group differences because at the single study level, differences in analysis approaches and control conditions may have influenced the direction of reported group differences. To explore heterogeneity, we also conducted post-hoc sensitivity analyses by assembling more homogeneous subgroups of similar studies, based on extracted study characteristics such as type of task (e.g., passive or active tasks), type of stimuli (e.g., International Affective Picture System - IAPS) or type of domain evaluated (e.g., memory, impulsivity, emotion).
As additional robustness checks for analyses that produced significant findings, we conducted a “Fail-Safe N” analysis adapted for ALE meta-analysis25 to evaluate the potential publication bias, and a “leave-one-out” analysis to assess the impact of single studies on the results.
For the secondary analysis (differences in convergence), we computed separate meta-analyses for activations of controls and BPD and then contrasted them in a differences of convergence analysis. For the single group meta-analysis, we used the same parameters as in the primary analysis. To compute the differences of convergence, we used an uncorrected p value < 0.05, N = 10.000 permutations and a cluster threshold of 100 mm3.
Potential differences between the two methods could be attributed to the different number of included studies rather than to genuine discrepancies between methods. We tested for this hypothesis in sensitivity analyses, in which the primary and secondary methods were limited to the experiments reporting coordinates for the same type of contrasts for both single- and between groups results. (Fig. 1, Supplementary Methods).
Across all analyses, we set the minimum number of studies to 17. According to previous simulations, meta-analyses with less than 17 studies are likely to have insufficient power to detect smaller effects, increasing the risk that results are driven by single/few experiments26,27.
Results
Study selection
The search produced 762 entries (463 after duplicate removal), 371 of which were excluded based on the abstract, leaving 92 reports for full-text inspection. From these, 40 reports were further excluded due to (1) lack of direct univariate comparison between BPD and HC (n = 9); (2) comparison restricted to functional connectivity analysis (n = 5); (3) non-significant results for the comparison (n = 4); (4) ROI only reported (n = 21); (5) re-analyses of previous, already included, studies or paper reported no new results (n = 1). A total of 52 articles (Table 1 and Supplementary Table S1) were included in the meta-analysis, as described in the PRISMA flow diagram (Fig. 1). The 52 articles described 56 experiments and we further excluded two experiments for lack of significant results for the primary or secondary analysis.
Table 1.
Characteristics of the included studied including, number of participants, gender and mean age of participants, comorbidities, concurrent treatments, type of tasks and type of stimuli used.
| Study | Borderline personality disorder | Healthy controls | Task & stimuli | Type of stimuli | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M/F | Age (SD) | Comorbidity | Medication | N | M/F | Age (SD) | |||
| Aguilar-Ortiz, 2019 | 67 | 3/64 | 31.54 (7.13) | ? | AD, MS, AP | 67 | 3/64 | 32.5 (9.68) | N-back task | Letters |
| Beblo, 2006 | 20 | 0/20 | 31.3 (8) | AvPD, Depr PD, PPD, P-APD, OCPD, DPD, PTSD, MDD, PD, PH, BN, OCD, DYS, GAD, HYP | SSRI, TAD, AP, b-block, BDZ, acamprosate | 21 | 0/21 | 32.6 (7.8) | Recall of unresolved and resolved negative events | Individual cue words |
| Beeney, 2016 | 17 | 0/17 | 35.51 (10.84) | Aff D, Anx D, PTSD, AAD, SFD, ED | AD, AP, MS, BDZ | 21 | 0/21 | 33.33 (9.81) | Personality trait evaluation | |
| Bertsch, 2019 | 15 | 15/0 | 27.9 (9.1) | Aff D, Anx D, PTSD, SAD, ED, APD, Sub D, SFD, | – | 25 | 0/25 | 29.8 (5.6) | Social approach-avoidance task | Ekman and Friesen, KDEF, A&R, JACFEE |
| Brown, 2017 | 14 | 0/14 | 23.6 (4.1) | MDD, ED, Anx D, PTSD | AD, MS | 17 | 0/17 | 23.2 (4.4) | Cyberball game | Virtual ball-tossing game |
| Buchheim, 2008 | 11 | 0/11 | 27.8 (6.7) | MDD, Anx D, PD, SFD, DD, PH, DA, AAD | AP, SSRI, Li | 17 | 0/17 | 28.4 (7.5) | AAP | AAP |
| Cullen, 2016 | 12 | 0/12 | 25.17 (4.67) | ? | AEP | 12 | 0/12 | 24.17 (4.63) | Passive viewing of overt and covert emotional faces | Ekman and Friesen |
| Doell et al., 2020 | 21 | 0/21 | 27.43 (5.22) | MDD, BP, Anx D, PTSD, SAD, PD, GAD, ADHD; ED | SSRI, SNRI, AP, BDZ | 24 | 0/24 | 24.71 (5.50) | Modified version of the monetary and social incentive delay task | KDEF |
| Domsalla, 2014 | 20 | 0/20 | 29.2 (7.5) | PTSD, PD, SAD, Spec P, OCD, BN, AN, Sub D, MDD, BP | – | 20 | 0/20 | 28.7 (7.8) | Cyberball game | Virtual ball-tossing game |
| Dudas, 2017 | 14 | 0/14 | 36,3 | ? | PD | 14 | 0/14 | 29,6 | Emotion induction task | IAPS |
| Fertuck, 2019 | 16 | 0/16 | 25.94 (5.47) | PD, Spec P, GAD, SAD, PPD, OCPD, DPD, AvPD, ED, Sub D, MDD | – | 17 | 0/17 | 23.71 (3.35) | Trustworthiness-Fear facial appraisal task | NimStim |
| Frick, 2012 | 21 | 0/21 | 27.14 (7.48) | PD, Spec P, GAD, PTSD, SAD, MDD, ED, PPD, STPD, OCPD, APD, NPD, P-APD, Sub D | – | 20 | 0/20 | 24.80 (5.23) | Reading the mind in the eye’s test (RMET) | Original pictures of eye gazes |
| Gottlich et al., 2020 | 19 | 0/19 | 26.5 (5.8) | MDD, SAD, PD, PTSD, OCD, ADHD, ED, other personality disorders | SSRI, SNRI, TAI, AP, AEP, methylphenidate | 22 | 0/22 | 26.4 (4.6) | Script-driven-imagery paradigm | Standardized scripts |
| Guitart-Masip, 2009 | 10 | 5/5 | 31.3 (9.47) | ? | – | 10 | 5/5 | 31.2 (9.05) | Emotional discrimination task | Ekman and Friesen series |
| Hazlett, 2012 | 33 | 13/20 | 31.6 (9.1) | ? | – | 32 | 12/20 | 32.8 (9.7) | Emotion recognition task | IAPS |
| Herbort, 2016 | 21 | 0/21 | 25.67 (5.98) | MDD, ED, AAD, DA, PTSD, SAD, AvPD, HPD | – | 23 | 0/23 | 25.78 (5.75) | Categorial monetary incentive delay | |
| Herpertz, 2001 | 6 | 0/6 | 26.2 (8.1) | ? | – | 6 | 0/6 | 27.2 (4.5) | Passive viewing of emotion-inducing pictures | IAPS |
| Herpertz, 2017 (female sample) | 33 | 0/33 | 26.19 | Aff D, Anx D, PTSD, SFD, ED, AjD, APD, AvPD, Sub D | 30 | 0/30 | 27.69 (6.38) | Script-driven-imagery paradigm | Standardized scripts | |
| Herpertz, 2017 (male sample) | 23 | 23/0 | 30.65 (8.75) | Aff D, Anx D, PTSD, ED, AjD, APD, AvPD, Sub D | 26 | 26/0 | 31.09 (6.65) | Script-driven-imagery paradigm | Standardized scripts | |
| Holtmann, 2013 | 16 | 0/16 | 25.56 (4.70) | PTSD, Sub D, PD, SAD, OCD, BN, AvPD, DPD, OCPD, HPD, MDD | – | 24 | 0/24 | 26.83 (5.35) | Modified Eriksen Flanker task (emotional/neutral distractors) | Neutral or fearful faces |
| Homan, 2017 | 18 | 7/11 | 36.2 (11.1) | ? | ? | 17 | 7/10 | 37.4 (12.4) | Social judgment task | Behavioral ad situational scenarios |
| King-Casas, 2008 | 55 | 4/51 | 32.9 (8.5) | ? | ? | 38 | 1/37 | 31.3 (9.5) | Economic exchange game | Money exchange |
| Koenigsberg, 2009 (a) | 18 | 10/8 | 32.6 (10.4) | GAD, PTSD, PPD, APD, NPD, DPD, HPD, OCPD, STPD, MDD, PD, ED | – | 16 | 9/7 | 31.8 (7.7) | Distance from or look emotion-inducing pictures | IAPS |
| Koenigsberg, 2009 (b) | 19 | 7/12 | 34.9 (11.1) | BD-II, PTSD, PPD, AvPD, APD, STPD, OCPD, HPD, NPD, MDD | 17 | 8/9 | 31.2 (10.6) | Passive viewing of emotion-inducing pictures | IAPS | |
| Koenigsberg, 2014 | 19 | 11/8 | 31.9 (9.9) | NPD, PPD, OCPD, APD, DPD, GAD, BED, DYS, OCD, Spec P, MDD, Sub D | – | 25 | 13/12 | 28.1 (6.9) | Rating of novel and repeated emotion-inducing pictures | IAPS and EPS |
| Krauch, 2018 (adolescents) | 20 | 0/20 | 16.35 (0.88) | Aff D, Anx D, PTSD, ED, Sub D | SSRI, Atypical AD | 20 | 0/20 | 15.85 (0.81) | Script-driven imagery paradigm | |
| Kraus, 2010 | 11 | 0/11 | 25.64 (3.83) | DYS, PD, AP, SAD, PTSD, Spec P, AvPD, PPD, BD-II, MDD, AN, BN, AAD, Sub D | – | 10 | 0/10 | 25.6 (5.23) | Script-driven imagery | Standard script describing self-injury behavior |
| Krause-Utz, 2017 (dissociation) | 17 | 0/17 | 27.41 (6.20) | PTSD, MDD, DYS, PD, SAD, Spec P, OCD, ED, AAD, SD, DA | 18 | 0/18 | 29.61 (8.61) | EWMT | Uppercase letters and IAPS as distractors | |
| Krause-Utz, 2017 (neutral) | 12 | 0/12 | 25.17 (6.21) | PTSD, MDD, DYS, PD, SAD, Spec P, OCD, ED, AAD, SD, DA | – | 18 | 0/18 | 29.61 (8.61) | EWMT | |
| Lamers, 2019 | 20 | 0/20 | 25.95 (6.94) | PTSD, PTSD and SAD, SAD, SAD and AP, Spec P, OCD, BN | AD, AP | 20 | 0/20 | 26.90 (9.43) | Passive viewing of emotion-inducing stimuli | Movie clips of faces |
| Lang, 2012 | 14 | 0/14 | 27.21 (7.66) | PD, PH, GAD, SAD, DA, AAD, MDD, ED, PPD, STPD, SzPD, OCPD, APD, NPD | – | 15 | 0/14 | 24.73 (5.64) | Up- and down-regulation to negative scripts | |
| Malejko, 2018 (a) | 15 | 0/15 | 23.33 (1.07) | MDD, PTSD, DYS | AD (SSRI, SNRI, MAOIs) | 15 | 0/15 | 23.27 (1.11) | Somatosensory unpleasant stimulation | Electrical |
| Malejko, 2018 (b) | 15 | 0/15 | 23.3 (4.13) | PTSD, DYS, PTSD | AD (SSRI, SNRI, MAOIs) | 17 | 0/17 | 23.1 (4.26) | Cyberball paradigm | Virtual ball-tossing game |
| Mensebach, 2009 | 18 | 0/18 | 31.94 (8.13) | PTSD, MDD, DYS, PD, AP, OCD, AP with PD, PH, BN, SD | SSRI, TAD, AP, b-block, BDZ, acamprosate | 18 | 0/18 | 32.94 (8.33) | EMR/SMR | Words |
| Mier, 2013 | 13 | 9/4 | 28.15 (7.06) | MDD, Sub D, GAD, PTSD, AjD, MDCE | AD, AP, MS, BDZ | 13 | 8/5 | 30.46 (4.29) | Neutral face processing, Emotion recognition, Affective ToM | Emotional facial expression |
| Minzenberg, 2007 | 12 | 5/7 | 30.3 (8) | OCD, PTSD, PD, PPD, STPD, APD, NPD, HPD, AvPD | – | 12 | 6/6 | 30.7 (10) | Gender discrimination | Ekman and Friesen set (modified) |
| Mortensen, 2016 | 14 | 0/14 | 30.1 (6.7) | – | – | 14 | 0/14 | 28.3 (7.4) | Posner task | |
| Nicol, 2015 | 20 | 17/3 | 35.8 (8.6) | MDD, BP, ED, PTSD, OCD | AD, AP | 16 | 14/2 | 34.8 (9.6) | Gender recognition task | Ekman series of emotional faces |
| Niedtfeld, 2010 | 20 | 0/20 | 30.50 (8.30) | ? | – | 23 | 0/23 | 27.13 (8.26) | Picture and temperature stimulus presentation | IAPS and warm stimulus |
| Peters, 2018 | 13 | 0/13 | 21.23 (3.30) | ? | SSRI | 16 | 0/16 | 21.81 (4.02) | Provocation task, Directed rumination task | |
| Scherpiet, 2014 | 18 | 0/18 | 28.44 (8.50) | Sub D, ED, MDD, ADHD, PTSD | AD, AP, MS, BDZ, zolpidem | 18 | 0/18 | 28.89 (7.11) | Emotional anticipation task | IAPS |
| Scherpiet, 2015 | 19 | 0/19 | 31.11 (6.29) | MDD, MDD with Sub D, ADHD, ADHD with Sub D, Sub D, PTSD, ED | AD, AP, MS, BDZ, pramipexole | 19 | 0/19 | 29.37 (4.48) | Mindful introspection, Cognitive self-reflection | |
| Schmahl, 2006 | 12 | 0/12 | 28.67 (5.88) | MDD, DYS, PD, PD with AP, AP, PTSD, SAD, BDD, AN, OCD, SzPD, PPD, OCPD, AvPD, DA, Sub D | – | 12 | 0/12 | 27.67 (6.83) | Heat stimulation | Fixed temperature and individual temperature stimuli |
| Schnell, 2007 (a) | 6 | 0/6 | 23.7 (4.8) | BN, DYS | – | 6 | 0/6 | 23.4 (5.0) | Passive viewing of emotion-inducing pictures | IAPS and similar photographic images |
| Schnell, 2007 (b) | 14 | 0/14 | 28 (5.77) | ED, PG, PTSD, ADD | – | 14 | 0/14 | 28. 4 (5.21) | Passive viewing of emotion-inducing pictures | TAT and IAPS as control stimuli |
| Schulze, 2011 | 15 | 0/15 | 27.60 (7.85) | PTSD, OCD, PD with AP, AP, BN, DD, DYS, AAD, Sub D | – | 15 | 0/15 | 24.53 (2.85) | Delayed reappraisal paradigm | IAPS and supplementary similar pictures |
| Silbersweig, 2007 | 16 | 15/1 | 31.25 (range 19-50) | PD, SAD, Spec P, PTSD, OCD, GAD, SD, AAD, DA | AD, AP, MS, BDZ | 14 | 10/4 | 23.8 (18-31) | Emotional linguistic go/no-go task | Negative, positive and neutral words |
| Sosic-Vasic, 2019 | 20 | 0/20 | 24.9 (5.26) | MDD, DYS, PD, AP, SAD, GAD, PTSD, BN | AD (SSRI, SNRI, NaSSA, TAD) | 20 | 0/20 | 26.05 (5.96) | Passive viewing of emotion-inducing stimuli | Stylized scenes showing reaction to loss |
| van Schie, 2019 | 26 | 0/26 | 30.46 (9.2) | MDD, DYS, PD, AP, SAD, Spec P, GAD, PTSD, ADHD, AAD, D, APD, PPD | AD (SSRI, SNRI) | 32 | 0/32 | 28.12 (9.8) | Social feedback task | Evaluative words |
| van Zutphen, 2017 | 55 | 0/55 | 30.80 (8.78) | MDD, DYS, BP, GAD, PD, AP, PD with AP, SAD, Spec P, OCD, PTSD, SFD, ED, Sub D, IED, AvPD, DPD, OCPD, P-APD, Depr PD, PPD, STPD, SzPD | AD, AP, MS, BDZ | 42 | 0/42 | 28.33 (10.50) | Emotion regulation paradigm | IAPS and erotic pictures |
| van Zutphen, 2019 | 53 | 0/53 | 31.02 (8.77) | MDD, DYS, BP, GAD, PD, AP, PD with AP, SAD, Spec P, OCD, PTSD, SFD, ED, Sub D, IED, AvPD, DPD, OCPD, P-APD, Depr PD, PPD, STPD, SzPD | AD, AP, MS, BDZ | 34 | 0/34 | 29.44 (11.31) | Affective go/no-go task | IAPS and erotic picture |
| Wingenfeld, 2009 | 20 | 14/6 | 29.75 (13.2) | PTSD, MDD, DYS, BN, GAD, SPD | SSRI, MAOIs, AP | 20 | 14/6 | 29.45 (12.4) | Individual emotional Stroop task | General words and individual negative words from individual report |
| Winter, 2015 (neutral) | 19 | 0/19 | 28.05 (7.82) | MDD, DYS, PD, SAD, Spec P, OCD, PTSD | 19 | 0/19 | 28.74 (8.07) | Emotional stroop task | Aachener emotional word list | |
| Wrege, 2019 | 39 | 30/9 | 27.5 (8.2) | Depr PD, Anx D, SD, ED, SFD, PTSD | AD, MS, AP | 29 | 25/4 | 25.7 (6.0) | Cyberball paradigm | Virtual ball-tossing game |
Comorbidity? comorbidity was not declared, – comorbidity was an exclusion criteria, AAD alchool abuse disorder, ADD attention deficit disorder, ADHD attention deficit hyperactivity disorder, Aff D affective disorder, AjD adjustment disorder, AN anorexia nervosa, Anx D anxiety disorder, AP agoraphobia, APD antisocial personality disorder, AvPD avoidant personality disorder, BD bipolar disorder, BDD body dysmorphic disorder, BED binge eating disorder, BN bulimia nervosa, DA drug abuse, DD dissociative disorder, Depr PD depressive personality disorder, DPD dependent personality disorder, DYS dyshymia, ED eating disorder, GAD generalized anxiety disorder, HPD histrionic personality disorder, HYP hypochondria, IED intermitted explosive disorder, MDCE mixed disorder of conduct and emotion, MDD major depressive disorder, NPD narcisistic personality disorder, OCD obsessive compulsive disorder, OCPD obsessive compulsive personality disorder, P-APD passive-aggressive personality disorder, PD panic disorder, PG pathological gambling, PH phobia, PPD paranoid personality disorder, PTSD post-traumatic stress disorder, SAD social anxiety disorder, SD somatization disorder, SFD somatoform disorder, Spec P specific phobia, SPD somatization pain disorder, STPD schizotypal personality disorder, Sub D substance use disorder, SzPD schizoid personality disorder, Medication? the medication status of the subjects was not described, – ongoing medication was an exclusion criteria, AD antidepressants, AEP anti-epileptic drugs, AP antipsychotics, Atypical AD atypical antidepressants, b-block beta blockers, BDZ benzodiazepines, Li lithium, MAOIs monoamine oxidase inhibitors, MS mood stabilizers, NaSSA noradrenergic and specific serotonergic antidepressants, PD psychotropic drugs, SNRI serotonin-norepinephrine reuptake inhibitors, SSRI serotonin reuptake inhibitors, TAD tryciclic antidepressant, Task & stimuli type of stimuli, AAP the adult attachment projective, A&R the AR face database, Martinez and Benavente (1998), Affective ToM affective theory of mind, EMR/SMR episodic and semantic memory retrieval task, EPS empathy picture system, EWMT emotional working memory task, IAPS international affective picture system, JCFEE Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion, KDEF The Karolinska directed emotional faces, Lundqvist et al. (1998), NimStim NimStim face database, Tottenham et al. (2009), TAT thematic apperception test.
Characteristics of included studies
The 54 experiments included 2084 subjects (1104 BPD and 1100 HC). All studies performed whole-brain analyses. For the primary analysis we used 52 experiments: 24 reported both contrasts HC > BPD and BPD > HC, seven the HC > BPD contrast only, whereas 21 the BPD > HC contrast only. Twenty-three studies also reported single group analyses (Fig. 1 and Supplementary Table S1): 21 for both HC and BPD, one for HC only and two for BPD only. A complete description of the studies including type of task, stimuli, presence of comorbidity and medications status is presented in the Table 1). Based on these characteristics, we assembled more homogenous groups for sensitivity analyses. We conducted analyses restricted to studies (1) using only active or (2) passive tasks; (3) employing a task related to emotion processing (generation, recognition, regulation) (4) restricted to unmedicated patients.
Risk of bias
According to our mNOS scale scores no study presents a high risk of bias, eleven have low risk while the great majority (41) have an intermediate risk (Supplementary Table S2 and Supplementary Fig. S1). The full description of the study quality is described in the Supplementary Material.
Primary analysis: convergence of differences
For the voxel-wise whole-brain analysis 51 reports (52 experiments) were considered. For the HC > BPD meta-analysis we included 31 experiments and obtained a minimum cluster size of 624 (MCS) mm³, while for the BPD > HC meta-analysis we included 45 experiments and obtained an MCS of 752 mm³. Across both ALE meta-analyses, we did not find any cluster of significant convergence.
Sensitivity analyses for primary analysis
In analysis restricted to studies of emotion processing (42 experiments) for the contrast BPD > HC (34 experiments, MCS 656 mm3), we found significant clusters in the two amygdalae along with the anterior cingulate (ACC)/middle frontal gyrus (MFG) (Table 2 and Fig. 2). For the contrast HC > BPD (24 Experiments, MCS 680) we did not find any significant cluster.
Table 2.
Sensitivity analysis for the primary analysis (convergence of difference) including studies on emotions (without considering impulsivity and reward).
| Contrast | Hemisphere | Region | BA | Center of mass | Peak | Peak ALE p value | Volume (mm³) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||||
| BPD > HC | L | Amygdala | 29.9 | 2.1 | −17.8 | 30 | 0 | −20 | 0.023 | 784 | |
| R | Amygdala | −28 | 2.2 | −17.4 | −28 | −4 | −18 | 0.024 | 776 | ||
| L | MFG | −11.7 | 26 | 32 | −12 | 34 | 26 | 0.017 | 736 | ||
| MFG | −8 | 28 | 32 | 0.017 | |||||||
| ACC | −12 | 20 | 34 | 0.017 | |||||||
| ACC | −14 | 14 | 38 | 0.016 | |||||||
| MFG | −14 | 32 | 34 | 0.015 | |||||||
Results are cluster-wise corrected (uncorrected p value < 0.001, cluster-wise corrected p value < 0.05).
HC healthy controls, BPD Borderline Personality Disorders, MFG Middle Frontal Gyrus, ACC Anterior Cingulate Cortex.
Fig. 2. Sensitivity analysis for the primary analysis (convergence of difference) including studies with Emotional task (without studies on impulsivity) as stimuli for the BPD > HC meta-analysis.
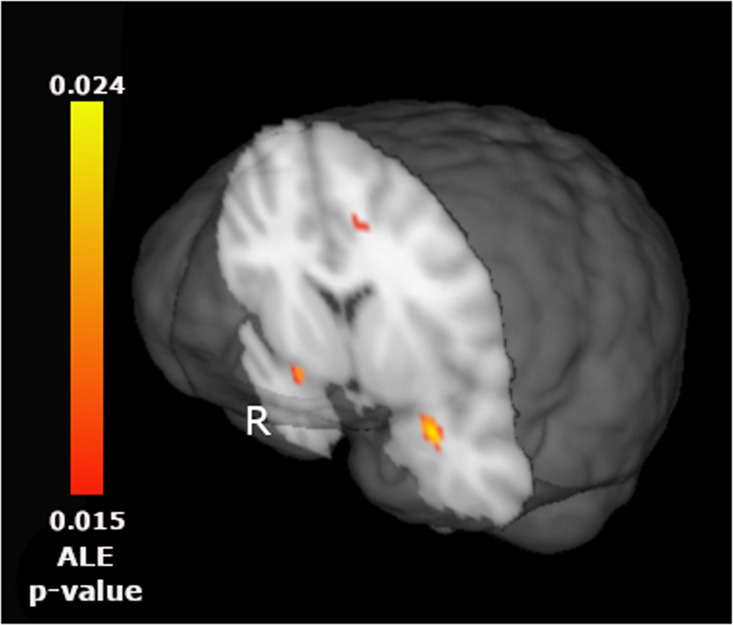
Results are cluster-wise corrected (uncorrected p value < 0.001, cluster-wise corrected p value < 0.05). R right side, ALE-p value activation likelihood estimation probability.
We were interested in the distinct pattern of activation related to emotional regulation, however only six studies specifically addressed emotional modulation in BPD28–33, too few for a meta-analysis. As impulsivity is a key feature of BPD17 with specific brain correlates only partially overlapping with the broader emotional circuits, we considered these studies separately. However, only four13,34–36 studies examined impulsivity.
Analyses restricted to studies of unmedicated individuals showed largely similar results of the emotional processing meta-analysis (Supplementary Material, Supplementary Fig. S2 and Supplementary Table S3). Another sensitivity analysis restricted to studies using active tasks did not produce significant results. There were too few studies using passive tasks (12 for BPD > HC and 2 for HC > BPD) to run a meta-analysis. Finally, the pooled analysis using both the HC > BPD and the BPD > HC contrasts in the same meta-analysis did not show significant results (Supplementary Material).
The leave-one-out procedure conducted on the subgroup of emotion processing studies (the only ones with statistically significant findings), showed that the clusters in the right amygdala remained significant 29 over 34 times, the cluster in the left amygdala, 27 times and the ACC/MFG cluster 24 times. Notably, excluding one particular study37 led to no significant cluster of convergence, excluding three studies8,38,39 led to only one significant cluster, and excluding other 13 studies preserved two out of three significant clusters (Table 3). The Fail-Safe N analysis showed that the addition of only two studies rendered the ACC/MFG cluster no longer significant. Similarly, three hypothetical studies would make the left amygdala finding null. Adding 33 studies would result in no remaining significant cluster of convergence (Supplementary Fig. S3).
Table 3.
Leave-one-out (LOO) analysis.
| Contribution to clusters | LOO | No. of remaining clusters | |||||
|---|---|---|---|---|---|---|---|
| R amy | L amy | ACC | R amy | L amy | ACC | ||
| Frick, et al. 2012 | x | 0 | |||||
| Minzenberg, et al. 2007 | x | x | x | 1 | |||
| Hazlett, et al. 2012 | x | x | 1 | ||||
| Schnell, et al. 2007 (b) | x | x | 1 | ||||
| Koenigsberg, et al. 2009 (a) | x | x | x | 2 | |||
| Cullen, et al. 2016 | x | x | x | 2 | |||
| Niedtfeld, et al. 2010 | x | x | x | 2 | |||
| Herpertz, et al. 2017 | x | x | x | 2 | |||
| Holtmann, et al. 2013 | x | x | x | 2 | |||
| Gottlich, et al. 2020 | x | x | x | 2 | |||
| Herpertz, et al. 2001 | x | x | 2 | ||||
| Mier, et al. 2013 | x | x | 2 | ||||
| Dudas, et al. 2017 | x | x | 2 | ||||
| Schulze, et al. 2011 | x | x | 2 | ||||
| Scherpiet, et al. 2014 | x | x | 2 | ||||
| Schnell, et al. 2007 (a) | x | x | 2 | ||||
| Wrege, et al. 2019 | x | x | 2 | ||||
| Domsalla, et al. 2014 | x | x | x | 3 | |||
| Peters, et al. 2018 | x | x | x | 3 | |||
| Guitart-Masip, 2009 | x | x | x | 3 | |||
| Koenigsberg, et al. 2009 (b) | x | x | x | 3 | |||
| Kraus, et al. 2010 | x | x | x | 3 | |||
| Winter, et al. 2015 | x | x | x | 3 | |||
| Krause-Utz, et al. 2018 | x | x | x | 3 | |||
| Koenigsberg, et al. 2014 | x | x | x | 3 | |||
| Buchheim, et al., 2008 | x | x | x | 3 | |||
| Beblo, et al. 2006 | x | x | x | 3 | |||
| Brown, et al. 2017 | x | x | x | 3 | |||
| Sosic-Vasic, et al. 2019 | x | x | x | 3 | |||
| Malejko, et al. 2018(b) | x | x | x | 3 | |||
| Lamers, et al. 2019 | x | x | x | 3 | |||
| Bertsch, et al. 2019 | x | x | x | 3 | |||
| Doell, et al. 2020 | x | x | x | 3 | |||
| van Zutphen, et al. 2017 | x | x | x | 3 | |||
| Total | 28 | 27 | 23 | ||||
The columns 2–4 indicates whether each article contributes directly to the cluster according to the gingerALE program; columns 5–7 indicates the effect of each article removal on the results in the three significant clusters; column 8 indicates the number of clusters remaining after leave each article out.
Secondary analysis: difference in convergences
Twenty-three experiments reported coordinates for single group analyses (23 for HC and 22 for BPD) (Supplementary Methods and Supplementary Table S1). Results for the meta-analysis within each group are reported in the Supplement (Supplementary Table S4 and Supplementary Fig. S4). No significant clusters were identified for the BPD > HC contrast. For the HC > BPD a significant cluster of difference in convergences between the two groups was highlighted in the right insula/ inferior frontal gyrus (IFG) (Supplementary Table S5 and Supplementary Fig. S5).
The number of eligible studies for all sensitivity analyses less than 17. Nevertheless, we computed exploratory sensitivity analyses (considering only emotion processing and respectively only active task experiments), which showed no significant results (see Supplementary Results) (Supplementary Tables S6 and S7 and Supplementary Fig. S6).
Discussion
We report, to our knowledge, the first meta-analysis comprising all fMRI neuroimaging studies for borderline personality disorder. The main findings underscore the substantial heterogeneity of this literature, in reference to the processes studied (e,g., emotion40,41, impulsivity36,42, attention and working memory43), as well as study populations (e.g., concomitant medication, comorbidities). Importantly, though impulsivity and emotional dysregulation are cardinal symptoms of BPD, they were assessed in few studies (four and six respectively). However, methodological differences can represent other important sources of heterogeneity. A variety of behavioral tasks were used, and it is likely that some were more reliable and robust in measuring the target processes than others, as demonstrated in a large-scale analysis of self-regulation measures44. Moreover, analytic pipelines including pre-processing, choices in data analysis like the type of multiple comparison corrections employed most likely diverged between studies, with direct consequences on the threshold for identifying statistical findings45–48. The relationship between analytic choices and reporting statistically significant findings is particularly relevant for ALE meta-analyses, which exclusively rely on these results and cannot consider non-significant one49.
Convergence of differences
Analyses restricted to more homogeneous subgroups highlighted significant clusters of convergence in the primary analysis. Specifically, we found dysfunctional pattern in the two amygdalae and ACC/MFG across emotion processing tasks. Of note, analyses limited to studies with non-medicated patients also resulted in a significant cluster of convergence in the right hippocamus/amygdala. Nonetheless, all the studies on unmedicated participants also investigated emotional processing, which might account for the overlap in results.
The role of the amygdala complex in emotional processing is well-established. Emotional responses were associated with activations of the amygdalae50,51, while effective emotional regulation strategies reduce amygdala reactivity52,53. Furthermore, anxiety disorders and mood disorders23,54–58 are characterized by amygdala hyperreactivity, normalized by effective pharmacological and psychological treatments59,60. Thus, our results support the role of amygdala dysfunction as a transdiagnostic mechanism, present in BPD, similarly to other disorders. It was hypothesized that behavioral alterations in emotional regulation and impulsivity in BPD rely on abnormal amygdala activity or a dysfunctional interaction between amygdala and prefrontal cortex, in line with findings on studies for emotional processing61,62. As a consistent finding in BPD and as marker of emotional dysfunction on BPD, amygdala altered activity was proposed as a potential predictor of treatment response as well as a target for neurofeedback interventions63,64. Finally, the development of new drug treatments has been hypothesized and tested considering their known action over amygdala activity65. A similar transdiagnostic role could be attributed to ACC, though this region was less frequently reported in prior studies. Activation in this region has been reported while retrieving emotionally negative life events66, during social exclusion67 and while processing negative emotions61,68 more generally. All these processes are affected in BPD, as well as in other emotional disorders.
Our findings partially confirm those of an earlier meta-analysis by Schulze and colleagues16. Divergences may stem from the fact the current meta-analysis included almost twice as many studies, (34 vs. 19 for the emotional processing sub-group) reflecting the large number of studies published in the last 4 years. Other sources of discrepancy include different inclusion criteria, meta-analytic approaches (as Schulze and colleagues used Anisotropic Effect Size Signed Differential Mapping69, which allows the combination of coordinates and unthresholded maps) and contrasts selected in the analysis.
Despite the considerably larger pool of studies, the robustness of the emotional processing findings is limited. Fail-Safe N analysis showed that as few as three additional null studies would render the clusters in the ACC and in the left amygdala no longer significant. Conversely, for the right amygdala, 33 studies with null findings would render the result not statistically significant. The Fail-Safe N is a proxy for potential publication bias (i.e., studies that were conducted, but remained unpublished, “in the file-drawer”, because of negative or null results). According to the adaptation of this method for ALE meta-analysis25, for findings to be robust to publication bias, at least twice the number of included studies should be necessary to make them non-significant. In the present report, additional evidence for publication bias comes from the high number of studies that could not be included due to not reporting significant findings or conducting only ROI analyses. Thus, the hypothetical null studies suggested by the Fail-Safe N might not even have to be searched in the file-drawer, but already published. Solutions for clarifying similar issues in the neuroimaging literature include access to unthresholded maps, for example by posting them in public repositories, so as to allow studies with null or negative findings to be included in neuroimaging meta-analyses. Likewise, pre-registration of ROI analyses49, by guaranteeing these analyses were not contingent to non-significant whole-brain results, could support their inclusion in meta-analyses. The results of the leave-one-out analysis for the emotional processing subgroup similarly point to limited robustness. The three significant clusters were maintained in only 13 iterations, but with the exclusion of a single study37, no results remain significant. This suggests that findings are heavily influenced by a few individual studies.
Finally, we failed to find convergence for the HC > BPD. While this contrast was reported in only 24 experiments, it is interesting to note that only four studies specifically discussed the dysfunction of emotional processing. As dysfunctions in emotional modulation are believed to be caused by a lack of activation in the prefrontal cortex in BPD, we speculate that a higher number of studies on emotional regulation would result in significant HC > BPD convergence.
Difference in convergence
The contrast HC > BPD resulted in a significant cluster of difference of convergence in the right insula/IFG complex. The activation of the inferior frontal gyrus has been often described for tasks related to emotional regulation and modulation18, thus is not surprising how control subjects showed a higher convergence in this region as compared to BPD. This might be due to a general lack of IFG activity in BPD or to a higher heterogeneity of brain activations in this group.
Convergence of difference or difference in convergence?
According to Muller and colleagues the difference between the convergence of difference and difference in convergence approaches are mirrored by the research question one wants to answer26. The former aims at identifying common difference between groups across the experiments, whereas the latter focuses to the convergence within the groups across the experiments and then to their possible differences. We confirm that these two approaches produce divergent findings, as previously shown70. Comparison of the two methods restricted to experiments reporting the data required to run both resulted in a significant cluster in the anterior cingulate for convergence of difference, and another in the left insula for difference of convergence, for the HC > BPD contrast, suggesting that divergences between the two methods are not imputable to different numbers of studies.
The discrepancy between the two approaches is probably rooted into the ALE meta-analysis method of taking only coordinates of significant clusters. We proposed that the convergence of differences method is more feasible for contrasting groups, because it generally relies on a larger number of studies and is usually more concordant with findings from individual studies70. In our work, differences might also be related to the relatively poor robustness of the primary analysis findings. For example, Hazellet and colleagues8, a study that if excluded resulted into two of the three significant clusters becoming non-significant, did not provide single group results, so could not be used for the difference in convergence analysis.
However, the two methods could also be viewed as complementary. In a recent ALE meta-analysis on depression the heterogeneity of the depressed patients was proposed as a possible reason for the lack of significant convergence71: the depressed groups of each study might be too neurobiologically different among each other thus the convergence of difference did not produce consistent results. Specifically, while we found no significant clusters with the difference of convergence method, we did find clusters of convergence for both HC and BPD with the convergence of difference method. This suggests that despite task-related heterogeneity, a common pattern of activation within BPD patients can be identified. We suggest that this result is a hint to the fact that the heterogeneity of BPD population is similar to the one of healthy controls although a more formalized and quantitative approach to heterogeneity should be applied (e.g.,72) to confirm this speculation.
Limitations
By design, ALE meta-analysis can only quantify convergence probabilities and not magnitude of activations. The method provides a probability of convergence, that is, concordance of statistically significant foci across experiments in terms of probability distributions centered at the each set of focus coordinates24. Moreover, coordinate based meta-analyses rely exclusively on coordinates for contrasts that reached statistical significance, as defined in each individual study. Consequently, these methodologies involve a significant loss information as compared to aggregation of fMRI unthresholded maps73 and cannot take into account publication bias25. Unfortunately, open sharing of unthresholded maps remains limited74. Another limitation is related to the considerable number of studies that were excluded due to reporting only on ROI analyses (N = 23). Though the ROI approach is widely employed in neuroimaging studies, particularly in small sample studies, it is associated with increased risk of false positives or overestimation of effects49. Moreover, its use is discouraged in coordinate based meta-analysis23,26. Unfortunately, requests for whole-brain results are often not honored54.
Conclusion
A meta-analysis of neuroimaging studies identified a consistent, albeit not robust, pattern of activation for emotional processing. No other common pattern of convergence of activation emerged, probably owing to the high between-study heterogeneity, ranging from tasks, populations and analytic approaches. Our findings mirror those of another ALE meta-analysis on unipolar depression71. Though this meta-analysis included a large number of neuroimaging experiments (99), overall analyses across cognitive and emotional processing experiments, as well as subgroup analyses, revealed no consistent pattern of convergence. As possible causes for the lack of significant convergence of activation, the authors list differences among individual studies (such as the use of uncorrected inference procedures, differences in experimental design and contrasts, or heterogeneous clinical populations) and meta-analytic approaches (such as different inclusion and exclusion criteria or too liberal statistical inference methods). Though we did report a pattern of amygdala and ACC dysfunction in studies of emotion processing, it was not robust to possible publication bias and single study effects. Amygdala dysfunction might represent a promising pathophysiological mechanism, as a well target for novel therapeutic strategies63–65, though, conversely, it might also indicate a transdiagnostic marker of difficulties in emotional processing and regulation. Further studies may disentangle this aspect particularly evaluating emotional regulation difficulties both in BPD and across psychopathological domains with a single paradigm. Network analyses have shown how difficulties in emotional regulation are the most relevant core feature of BPD17, applying the same approach to neuroimaging data across psychiatric disorders may help to clarify the specificity of the present findings. Perhaps more importantly than conducting new studies, public sharing of unthresholded maps75 from already conducted ones would represent a great advance in the understanding of the neurobiology of BPD.
Supplementary information
Acknowledgements
Although not directly financially supported the present research is in line with within the scope of the project “use-inspired basic research”, for which the Department of General Psychology of the University of Padova has been recognized as “Dipartimento di eccellenza” by the Ministry of University and Research. The Authors do not report founds for the present work.
Author contributions
G.D.G.: methodology, formal analysis, investigation, writing original draft. I.C.: conceptualization, methodology, writing - review & editing. E.D.R.: formal analysis, investigation, visualization. C.C.: methodology, formal analysis, investigation. C.G.: conceptualization, methodology, writing – original draft and review & editing, visualization.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Giorgia Degasperi, Ioana Alina Cristea
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01446-z.
References
- 1.Association, A. P. Diagnostic and statistical manual of mental disorders (DSM-5®). (American Psychiatric Pub, 2013). [DOI] [PubMed]
- 2.Grant BF, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2008;69:533. doi: 10.4088/JCP.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J. Personal. Disord. 2014;28:734–750. doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J. Personal. Disord. 2010;24:412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeGris J, Toplak M, Links PS. Affective decision making in women with borderline personality disorder. J. Personal. Disord. 2014;28:698–719. doi: 10.1521/pedi_2014_28_140. [DOI] [PubMed] [Google Scholar]
- 6.Brendel GR, Stern E, Silbersweig DA. Defining the neurocircuitry of borderline personality disorder: functional neuroimaging approaches. Dev. Psychopathol. 2005;17:1197–1206. doi: 10.1017/S095457940505056X. [DOI] [PubMed] [Google Scholar]
- 7.Herpertz SC, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol. Psychiatry. 2001;50:292–298. doi: 10.1016/S0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 8.Hazlett EA, et al. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol. Psychiatry. 2012;72:448–456. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RC, et al. Differential neural processing of social exclusion and inclusion in adolescents with non-suicidal self-injury and young adults with borderline personality disorder. Front Psychiatry. 2017;8:267. doi: 10.3389/fpsyt.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter D, et al. Dissociation in borderline personality disorder: disturbed cognitive and emotional inhibition and its neural correlates. Psychiatry Res. 2015;233:339–351. doi: 10.1016/j.pscychresns.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Cullen KR, et al. Brain activation in response to overt and covert fear and happy faces in women with borderline personality disorder. Brain Imaging Behav. 2016;10:319–331. doi: 10.1007/s11682-015-9406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherpiet S, et al. Altered emotion processing circuits during the anticipation of emotional stimuli in women with borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264:45–60. doi: 10.1007/s00406-013-0444-x. [DOI] [PubMed] [Google Scholar]
- 13.Herbort MC, et al. A negative relationship between ventral striatal loss anticipation response and impulsivity in borderline personality disorder. Neuroimage Clin. 2016;12:724–736. doi: 10.1016/j.nicl.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Eijk J, et al. Women with borderline personality disorder do not show altered BOLD responses during response inhibition. Psychiatry Res. 2015;234:378–389. doi: 10.1016/j.pscychresns.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Turkeltaub PE, et al. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze L, Schmahl C, Niedtfeld I. Neural correlates of disturbed emotion processing in borderline personality disorder: a multimodal meta-analysis. Biol. Psychiatry. 2016;79:97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Southward MW, Cheavens JS. Identifying core deficits in a dimensional model of borderline personality disorder features: a network analysis. Clin. Psychol. Sci. 2018;6:685–703. doi: 10.1177/2167702618769560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TW, Xue SW. Does emotion regulation engage the same neural circuit as working memory? A meta-analytical comparison between cognitive reappraisal of negative emotion and 2-back working memory task. PLoS ONE. 2018;13:e0203753. doi: 10.1371/journal.pone.0203753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharp C, et al. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:563–573.e561. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Krause-Utz A, et al. Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol. Med. 2012;42:2181–2192. doi: 10.1017/S0033291712000153. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells, G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2001).
- 23.Gentili C, Messerotti Benvenuti S, Lettieri G, Costa C, Cecchetti L. ROI and phobias: the effect of ROI approach on an ALE meta-analysis of specific phobias. Hum. Brain Mapp. 2019;40:1814–1828. doi: 10.1002/hbm.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eickhoff SB, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acar F, Seurinck R, Eickhoff SB, Moerkerke B. Assessing robustness against potential publication bias in Activation Likelihood Estimation (ALE) meta-analyses for fMRI. PLoS ONE. 2018;13:e0208177. doi: 10.1371/journal.pone.0208177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller VI, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eickhoff SB, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertsch K, et al. Out of control? Acting out anger is associated with deficient prefrontal emotional action control in male patients with borderline personality disorder. Neuropharmacology. 2019;156:107463. doi: 10.1016/j.neuropharm.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Koenigsberg HW, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol. Psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamers A, et al. Nonacceptance of negative emotions in women with borderline personality disorder: association with neuroactivity of the dorsal striatum. J. Psychiatry Neurosci. 2019;44:303–312. doi: 10.1503/jpn.180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niedtfeld I, et al. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol. Psychiatry. 2010;68:383–391. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Schulze L, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol. Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 33.van Zutphen L, et al. Always on guard: emotion regulation in women with borderline personality disorder compared to nonpatient controls and patients with cluster-C personality disorder. J. Psychiatry Neurosci. 2018;43:37–47. doi: 10.1503/jpn.170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King-Casas B, et al. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortensen JA, Evensmoen HR, Klensmeden G, Haberg AK. Outcome uncertainty and brain activity aberrance in the insula and anterior cingulate cortex are associated with dysfunctional impulsivity in borderline personality disorder. Front Hum. Neurosci. 2016;10:207. doi: 10.3389/fnhum.2016.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Zutphen, L. et al. Impulse control under emotion processing: an fMRI investigation in borderline personality disorder compared to non-patients and cluster-C personality disorder patients. Brain Imaging Behav. 10.1007/s11682-019-00161-0 (2019). [DOI] [PMC free article] [PubMed]
- 37.Frick C, et al. Hypersensitivity in borderline personality disorder during mindreading. PLoS ONE. 2012;7:e41650. doi: 10.1371/journal.pone.0041650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnell K, Dietrich T, Schnitker R, Daumann J, Herpertz SC. Processing of autobiographical memory retrieval cues in borderline personality disorder. J. Affect Disord. 2007;97:253–259. doi: 10.1016/j.jad.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Wrege, J. S. et al. Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cogn. Affect Behav. Neurosci.10.3758/s13415-019-00716-0 (2019). [DOI] [PMC free article] [PubMed]
- 41.Herpertz SC, et al. Brain mechanisms underlying reactive aggression in borderline personality disorder-sex matters. Biol. Psychiatry. 2017;82:257–266. doi: 10.1016/j.biopsych.2017.02.1175. [DOI] [PubMed] [Google Scholar]
- 42.van Zutphen L, et al. Always on guard: emotion regulation in women with borderline personality disorder compared to nonpatient controls and patients with cluster-C personality disorder. J. Psychiatry Neurosci. 2017;43:170008. doi: 10.1503/jpn.170008. [DOI] [PubMed] [Google Scholar]
- 43.Krause-Utz A, et al. Reduced amygdala reactivity and impaired working memory during dissociation in borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:401–415. doi: 10.1007/s00406-017-0806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enkavi AZ, et al. Large-scale analysis of test–retest reliabilities of self-regulation measures. Proc. Natl Acad. Sci. USA. 2019;116:5472–5477. doi: 10.1073/pnas.1818430116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carp J. On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front. Neurosci. 2012;6:149. doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carp J. Optimizing the order of operations for movement scrubbing: comment on Power et al. NeuroImage. 2013;76:436–438. doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 47.Skudlarski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: individual subjects. NeuroImage. 1999;9:311–329. doi: 10.1006/nimg.1999.0402. [DOI] [PubMed] [Google Scholar]
- 48.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl Acad. Sci. USA. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentili, C., Cecchetti, L., Handjaras, G., Lettieri, G. & Cristea, I. A. The case for preregistering all region of interest (ROI) analyses in neuroimaging research. Eur. J. Neurosci.10.1111/ejn.14954 (2020). [DOI] [PubMed]
- 50.Kirby LAJ, Robinson JL. Affective mapping: an activation likelihood estimation (ALE) meta-analysis. Brain Cogn. 2017;118:137–148. doi: 10.1016/j.bandc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Pico-Perez M, et al. Common and distinct neural correlates of fear extinction and cognitive reappraisal: a meta-analysis of fMRI studies. Neurosci. Biobehav Rev. 2019;104:102–115. doi: 10.1016/j.neubiorev.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 52.Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62:1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kral TRA, et al. Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. NeuroImage. 2018;181:301–313. doi: 10.1016/j.neuroimage.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gentili C, et al. Beyond emotions: a meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med. 2016;241:225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumeister P, et al. Specific amygdala response to masked fearful faces in post-traumatic stress relative to other anxiety disorders. Psychol. Med. 2018;48:1209–1217. doi: 10.1017/S0033291717002513. [DOI] [PubMed] [Google Scholar]
- 56.Wittmann A, et al. Effects of cognitive behavioral therapy on neural processing of agoraphobia-specific stimuli in panic disorder and agoraphobia. Psychother. Psychosom. 2018;87:350–365. doi: 10.1159/000493146. [DOI] [PubMed] [Google Scholar]
- 57.Wegbreit E, et al. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926–935. doi: 10.1001/jamapsychiatry.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janiri, D. et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry77, 172–179 (2020). [DOI] [PMC free article] [PubMed]
- 59.Furmark T, et al. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol. Psychiatry. 2005;58:132–142. doi: 10.1016/j.biopsych.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 60.Lipka J, Hoffmann M, Miltner WH, Straube T. Effects of cognitive-behavioral therapy on brain responses to subliminal and supraliminal threat and their functional significance in specific phobia. Biol. Psychiatry. 2014;76:869–877. doi: 10.1016/j.biopsych.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Göttlich, M. et al. Neural basis of shame and guilt experience in women with borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci.10.1007/s00406-020-01132-z (2020). [DOI] [PMC free article] [PubMed]
- 62.Westlund Schreiner M, et al. Neurocircuitry associated with symptom dimensions at baseline and with change in borderline personality disorder. Psychiatry Res. Neuroimaging. 2019;290:58–65. doi: 10.1016/j.pscychresns.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Schmitgen MM, et al. Individualized treatment response prediction of dialectical behavior therapy for borderline personality disorder using multimodal magnetic resonance imaging. Brain Behav. 2019;9:e01384. doi: 10.1002/brb3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaehringer J, et al. Improved emotion regulation after neurofeedback: a single-arm trial in patients with borderline personality disorder. Neuroimage Clin. 2019;24:102032. doi: 10.1016/j.nicl.2019.102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferber, S. G., Hazani, R., Shoval, G. & Weller, A. Targeting the endocannabinoid system in borderline personality disorder. Curr. Neuropharmacol.10.2174/1570159x18666200429234430 (2020). [DOI] [PMC free article] [PubMed]
- 66.Bozzatello P, et al. Autobiographical memories, identity disturbance and brain functioning in patients with borderline personality disorder: An fMRI study. Heliyon. 2019;5:e01323. doi: 10.1016/j.heliyon.2019.e01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malejko K, et al. Neural correlates of social inclusion in borderline personality disorder. Front Psychiatry. 2018;9:653. doi: 10.3389/fpsyt.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holtmann J, et al. Trait anxiety modulates fronto-limbic processing of emotional interference in borderline personality disorder. Front Hum. Neurosci. 2013;7:54. doi: 10.3389/fnhum.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radua J, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Costa, C., Cristea, I. A., Bò, E. D., Melloni, C. & Gentili, C. Brain activity during facial processing in autism spectrum disorder: an activation likelihood estimation (ALE) meta-analysis of neuroimaging studies. bioRxiv. 10.1101/2020.05.03.074351 (2020). [DOI] [PubMed]
- 71.Müller VI, et al. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry. 2017;74:47–55. doi: 10.1001/jamapsychiatry.2016.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ngo GH, et al. Beyond consensus: embracing heterogeneity in curated neuroimaging meta-analysis. Neuroimage. 2019;200:142–158. doi: 10.1016/j.neuroimage.2019.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 74.Nichols TE, et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 2017;20:299–303. doi: 10.1038/nn.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White, T., Blok, E. & Calhoun, V. D. Data sharing and privacy issues in neuroimaging research: opportunities, obstacles, challenges, and monsters under the bed. Human Brain Mapping, 10.1002/hbm.25120 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


