Abstract
Background
Preoperative chemotherapy containing anthracyclines and taxanes is well established in early-stage breast cancer. Previous studies have suggested that the chemotherapy sequence may matter but definitive evidence is missing. ABCSG trial 34 evaluated the activity of the MUC1 vaccine tecemotide when added to neoadjuvant treatment; the study provided the opportunity for the second randomisation to compare two different anthracycline/taxane sequences.
Methods
HER2-negative early-stage breast cancer patients were recruited to this randomised multicentre Phase 2 study. Patients in the chemotherapy cohort (n = 311) were additionally randomised to a conventional or reversed sequence of epirubicin/cyclophosphamide and docetaxel. Residual cancer burden (RCB) with/without tecemotide was defined as primary study endpoint; RCB in the two chemotherapy groups was a key secondary endpoint.
Results
No significant differences in terms of RCB 0/I (40.1% vs. 37.2%; P = 0.61) or pathologic complete response (pCR) rates (24.3% vs. 25%, P = 0.89) were observed between conventional or reverse chemotherapy sequence. No new safety signals were reported, and upfront docetaxel did not result in decreased rates of treatment delay or discontinuation.
Conclusion
Upfront docetaxel did not improve chemotherapy activity or tolerability; these results suggest that upfront neoadjuvant treatment with anthracyclines remains a valid option.
Subject terms: Breast cancer, Chemotherapy
Background
Preoperative chemotherapy was developed in patients with locally advanced, inoperable breast cancer.1 Today, the neoadjuvant administration of systemic treatment has turned into a standard option whenever chemotherapy is indicated in principle since preoperative therapy improves breast conservation rates and provides information on response and disease biology.2
National Surgical Breast and Bowel Project (NSABP) trial B-27 investigated the addition of four cycles of pre- or postoperative docetaxel to four cycles of AC (doxorubicin/cyclophosphamide).3 Neoadjuvant treatment with AC-docetaxel yielded a significant increase in pathologic complete remission (pCR) rate from 12.9% (AC) to 26.1% (AC-docetaxel) and pCR correlated with improved overall survival (OS). This led to further evaluations of the prognostic role of pCR as a surrogate endpoint and two metanalyses have since confirmed the relationship of pCR and long-term outcome on an individual patient level in high-risk breast cancer subtypes while achieving pCR is apparently of less relevance in luminal disease.4,5 pCR, however, dichotomises responses which may not fully reflect the true prognosis of patients as non-pCR includes outcomes ranging from minimal residual disease (MRD) to progression. Therefore, the residual cancer burden (RCB) score was developed, wherein RCB 0 reflects pCR or in situ disease only and RCB I reflects a minimal amount of residual invasive cancer with comparable long-term outcome.6
As shown in NSABP B-27, the conventional sequence of chemotherapeutic drugs is the upfront administration of anthracyclines and cyclophosphamide followed by a taxane.3,7 This was challenged by observations that upfront treatment with taxanes may improve outcome8 and tolerability,9 thereby renewing interest in the question of chemotherapy sequencing. Of note, preclinical studies support an upfront taxane approach as well.10–13
ABCSG-34 is a randomised Phase 2 study evaluating the addition of tecemotide (liposomal BLP25; L-BLP25; Stimuvax®), a MUC1 vaccine, to standard preoperative treatment consisting either of endocrine therapy or chemotherapy.14 In the chemotherapy cohort, participants were subjected to second randomisation comparing four cycles of docetaxel after (conventional sequence) or before four cycles of epirubicin/cyclophosphamide (EC) (reverse sequence). Here, we present results of the secondary chemotherapy-sequencing randomisation.
Methods
Study design
ABCSG-34 is an academic, prospective, randomised, open-label, multicentre, Phase 2 trial evaluating the efficacy and safety of tecemotide as a component of neoadjuvant therapy in HER2-negative early-stage breast cancer. While postmenopausal patients with luminal A-like tumours received preoperative endocrine treatment with six months of letrozole, premenopausal patients and patients with luminal B-like or triple-negative breast cancer (TNBC) were scheduled for preoperative chemotherapy. For this study, luminal A like was defined as follows: high or intermediate expression of the oestrogen receptor (ER), grade 1 or 2, and a proliferation rate <14%. A cut-off of <10% was chosen to define negative ER expression.
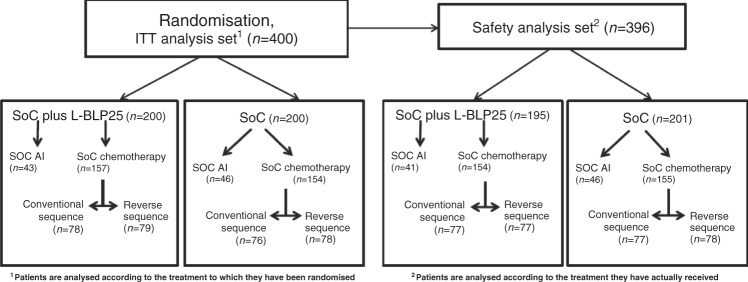
Patients accrued to the chemotherapy cohort were randomly assigned to a conventional chemotherapy sequence (four cycles of EC followed by four cycles of docetaxel) or to a reversed sequence thereof (upfront docetaxel followed by EC) (Consort diagram, Fig. 1).
Fig. 1. Consort diagram: ABCSG-34 trial overview.
ITT intent-to-treat, SoC Standard-of-Care, AI aromatase inhibitor.
The rate of patients with RCB 0/I score with or without tecemotide was defined as primary study endpoint; secondary endpoints included the rate of patients with RCB 0/I score in the two chemotherapy-sequencing arms, pCR rates, safety and quality-of-life (QoL) in patients with or without tecemotide.
Patients
Women aged 18 and older with histologically proven, invasive HER2-negative breast cancer without evidence of distant metastases scheduled to receive preoperative therapy were eligible.
Treatment
Patients accrued to the chemotherapy cohort received four consecutive cycles of EC (epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 q3w), followed by four cycles of docetaxel (100 mg/m2, 3qw) (conventional sequence), or the reversed sequence thereof. In addition, patients randomised to tecemotide received a single i.v. infusion of cyclophosphamide (300 mg/m2) 3 days prior to the first vaccination.
Response assessments
Pathological assessment of response to neoadjuvant treatment for the primary outcome analysis was performed by the RCB score;6 in short, the RCB score is a continuous variable based on the primary tumour bed dimensions, cellularity of the invasive tumour component, and axillary lymph node burden at the surgery. An RCB score of <1.36 describes minimal (RCB I) or absent (RCB 0) residual invasive disease. pCR was defined as ypT0/is ypN0 in the surgical specimen. RCB and pCR were both assessed locally after appropriate training of local pathologists; the quality of RCB 0/1 readouts was centrally reviewed by the ABCSG central trial pathologist.
Adverse events (AEs) and serious adverse events (SAEs) were classified according to World Health Organisation (WHO) criteria.
Statistical analysis
Patients were randomised 1:1 to standard neoadjuvant therapy with or without tecemotide using minimisation. All analyses, except for the safety analysis, were based on the intention to treat (ITT) principle with patients analysed according to the treatment to which they had been randomised to (Fig. 1). Only randomised patients who were evaluable at the time of final surgery were included in the efficacy analysis. The safety analysis set included all randomised patients with at least one administration of study treatment. The overall sample-size calculation of ABCSG-34 was based upon the primary endpoint; no sample-size calculation for the second randomisation (conventional vs. reverse chemotherapy sequence) was performed.
Patient and treatment characteristics, as well as safety data (AEs and SAEs) were described descriptively per chemotherapy treatment arm. Comparisons of proportions of RCB and pCR between treatment arms were performed with the chi-square test. Multivariate logistic regression models were used to adjust response for demographic or prognostic factors. Odds ratios (OR) with 95% confidence interval (CI) are provided.
Results
Patient characteristics
A total number of 311 patients were accrued to the chemotherapy cohort. The median age was 49 years (25–78), 184 patients (59.2%) were premenopausal, 126 (40.5%) had node-positive tumours at diagnosis, 51.8% had oestrogen-receptor positive breast cancer and 37.3% TNBC, respectively. Table 1 lists the patient’s characteristics for the entire chemotherapy cohort as well as for the sequencing arms. Apart from nodal status, no major inhomogeneities were observed between the groups.
Table 1.
Patient characteristics.
| Chemo conventional, N = 154 | Chemo reverse, N = 157 | Total, N = 311 | P value | |
|---|---|---|---|---|
| Age (years) | ||||
| N | 154 | 157 | 311 | |
| Mean | 49.1 | 49.5 | 49.3 | |
| SD | 10.7 | 11.3 | 11.0 | |
| Median | 49.0 | 48.0 | 49.0 | |
| Min | 26.0 | 25.0 | 25.0 | |
| Max | 78.0 | 75.0 | 78.0 | |
| Wilcoxon | 0.8836 | |||
| BMI | ||||
| N | 153 | 156 | 309 | |
| Mean | 25.5 | 25.6 | 25.6 | |
| SD | 5.0 | 5.0 | 5.0 | |
| Median | 24.5 | 24.5 | 24.5 | |
| Min | 16.5 | 15.0 | 15.0 | |
| Max | 42.8 | 40.5 | 42.8 | |
| Wilcoxon | 0.8605 | |||
| Menopausal status, n (%)a | ||||
| Perimenopausal | 56 (36.4%) | 56 (35.7%) | 112 (36.0%) | |
| Postmenopausal | 8 (5.2%) | 3 (1.9%) | 11 (3.5%) | |
| Premenopausal | 89 (57.8%) | 95 (60.5%) | 184 (59.2%) | |
| Missing | 1 (0.6%) | 3 (1.9%) | 4 (1.3%) | |
| Chi-square | 0.2915 | |||
| T-stage, n (%) | ||||
| T1 | 39 (25.3%) | 47 (29.9%) | 86 (27.7%) | |
| T2 | 99 (64.3%) | 90 (57.3%) | 189 (60.8%) | |
| T3 | 13 (8.4%) | 18 (11.5%) | 31 (10.0%) | |
| T4 | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) | |
| Fisher | 0.548 | |||
| Triple negative, n (%)b | ||||
| No | 91 (59.1%) | 85 (54.1%) | 176 (56.6%) | |
| Yes | 54 (35.1%) | 62 (39.5%) | 116 (37.3%) | |
| Missing | 9 (5.8%) | 10 (6.4%) | 19 (6.1%) | |
| Chi-square | 0.3888 | |||
| N-stage, n (%) | ||||
| Negative | 94 (61.0%) | 84 (53.5%) | 178 (57.2%) | |
| Positive | 57 (37.0%) | 69 (43.9%) | 126 (40.5%) | |
| Missing | 3 (1.9%) | 4 (2.5%) | 7 (2.3%) | |
| Chi-square | 0.1934 | |||
| Grading, n (%) | ||||
| G1 | 1 (0.6%) | 1 (0.6%) | 2 (0.6%) | |
| G2/Gx | 43 (27.9%) | 49 (31.2%) | 92 (29.6%) | |
| G3 | 107 (69.5%) | 105 (66.9%) | 212 (68.2%) | |
| Missing | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) | |
| Fisher | 0.8083 | |||
| HER2, n (%) | ||||
| Negative | 132 (85.7%) | 137 (87.3%) | 269 (86.5%) | |
| Positive | 1 (0.6%) | 1 (0.6%) | 2 (0.6%) | |
| Missing | 21 (13.6%) | 19 (12.1%) | 40 (12.9%) | |
| Fisher | 1 | |||
| ER, n (%) | ||||
| Negative | 68 (44.2%) | 76 (48.4%) | 144 (46.3%) | |
| Positive | 83 (53.9%) | 78 (49.7%) | 161 (51.8%) | |
| Missing | 3 (1.9%) | 3 (1.9%) | 6 (1.9%) | |
| Chi-square | 0.4502 | |||
| PgR, n (%) | ||||
| Negative | 76 (49.4%) | 81 (51.6%) | 157 (50.5%) | |
| Positive | 75 (48.7%) | 74 (47.1%) | 149 (47.9%) | |
| Missing | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) | |
| Chi-square | 0.736 | |||
| Ki67 | ||||
| N | 148 | 147 | 295 | |
| Mean | 50.4 | 49.6 | 50.0 | |
| SD | 24.3 | 23.9 | 24.1 | |
| Median | 50.0 | 50.0 | 50.0 | |
| Min | 2.0 | 3.0 | 2.0 | |
| Max | 90.0 | 95.0 | 95.0 | |
| Wilcoxon | 0.6744 | |||
N number of patients in the ITT analysis set, n number of patients, SD standard deviation, Min minimum, Max maximum.
For patients with bilateral breast cancer, information from the higher disease stage is used for descriptive summaries.
aWomen are considered postmenopausal if they have not had a menstrual period for >12 months due to natural causes or had a bilateral oophorectomy, and/or have serum levels of oestradiol, LH and FSH within the postmenopausal range.
bTNBC includes patients who have negative ER, PR and Her2 status—patients with missing information in any of these variables are not considered as TNBC.
In the overall chemotherapy cohort, 95.2% of patients had completed treatment as planned per protocol. Respective numbers are 95.5% in the conventional sequence arm and 94.9% in patients receiving upfront docetaxel. The mean number of chemotherapy cycles was 7.4 and again comparable (7.5 conventional sequence arm; 7.3 reverse sequence).
Residual cancer burden (RCB) and pathological complete remission (pCR) in patients treated with conventional or reversed chemotherapy sequence
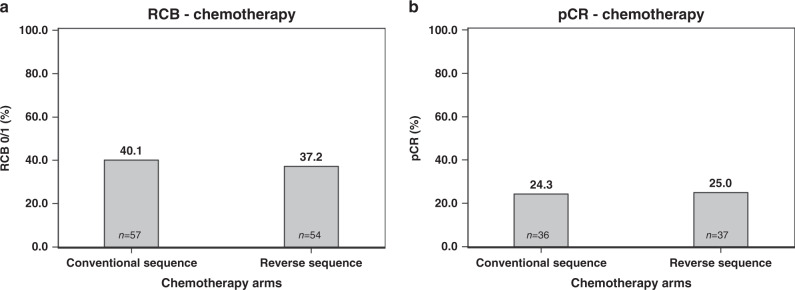
In patients with conventional chemotherapy sequence, an RCB 0/1 rate of 40.1% (n = 57) was recorded as compared with 37.2% (n = 54) in the upfront docetaxel group (P = 0.61, chi-square test, Fig. 2a). Respective numbers for pCR rates were 24.3% (n = 36, upfront anthracyclines) and 25.0% (n = 37, upfront docetaxel) (P = 0.89) (Fig. 2b).
Fig. 2. RCB 0/1 rate and pCR rate in patients with conventional and reverse chemotherapy sequence.
RCB residual cancer burden, pCR pathologic complete remission. Conventional vs. reverse in chemotherapy patients (a) RCB 0/I rate, (b) pCR rate.
Separate post hoc analyses of surgical outcome conducted in patients with TNBC and luminal breast cancer revealed minor outcome disparities between the two chemotherapy arms. In TNBC, a numerical benefit was observed in terms of RCB 0/1 and pCR rates favouring the conventional sequence group (RCB 0/1 rate conventional sequence 55.8% vs. reverse sequence 48.3%; pCR rate conventional sequence 45.3% vs. reverse sequence 31.2%), while numerically superior results with reverse chemotherapy sequence were observed in the in luminal breast cohort (RCB 0/1 rate conventional sequence 19.7% vs. reverse sequence 25.0%; pCR rate conventional sequence 1.6% vs. reverse sequence 14.5%), respectively.
Residual cancer burden (RCB) and pathological complete remission (pCR) in patients treated with conventional or reversed chemotherapy sequence with/without tecemotide
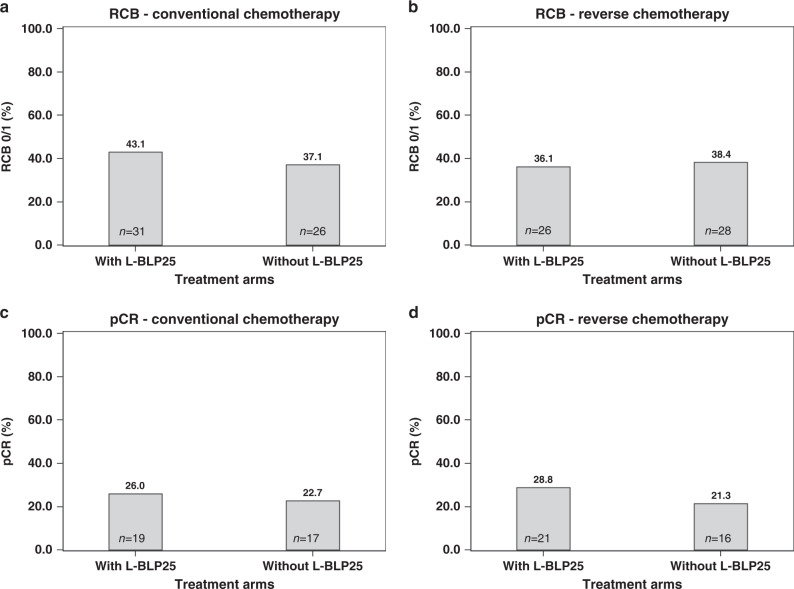
Regarding outcome in the overall chemotherapy cohort, no difference in terms of RCB 0/1 score was observed between patients with or without tecemotide (39.6% vs. 37.8%; P = 0.75, chi-square test). In the conventional sequence arm, an RCB score of 0/1 was recorded in 43.1% (n = 31) of patients with tecemotide as compared with 37.1% (n = 26) of patients without; in the upfront docetaxel group, corresponding numbers were 36.1% (n = 26) with and 38.4% (n = 28) without tecemotide, respectively (Fig. 3a, b).
Fig. 3. RCB 0/1 rate and pCR rate in patients with conventional and reverse chemotherapy sequence with or without L-BLP25.
RCB residual cancer burden, pCR pathologic complete remission. With vs. without L-BLP25 (a) conventional chemotherapy patients and RCB 0/I rate, (b) reverse chemotherapy patients and RCB 0/I rate, (c) conventional chemotherapy patients and pCR rate, (d) reverse chemotherapy patients and pCR rate.
A pCR was observed in 27.4% and 22.0% of patients with or without tecemotide in the chemotherapy cohort (P = 0.2815, chi-square test). When analysing the two sequencing arms separately, pCR rates were 26.0% (n = 19) and 22.7% (n = 17) in the conventional sequence arm in patients with or without tecemotide; corresponding numbers in the reverse sequence arm were 28.8% (n = 21) and 21.3% (n = 16), respectively (Fig. 3c, d).
Based on covariate analyses, within the subset of patients receiving chemotherapy, parameters significantly associated with RCB 0/I were Ki67 (OR 0.98; 95% CI 0.97–0.99) and progesterone receptor status (reference are negative patients; OR 0.24; 95% CI 0.09–0.64). No influence of tecemotide or chemotherapy sequence on outcome was detected in these models.
Safety
The safety population in the chemotherapy cohort consisted of 309 patients who had received at least one dose of study medication.
All patients had at least one AE in both treatment arms; regarding grade 3/4 AEs, corresponding numbers are 58.4% in the conventional and 52.3% in the reverse sequence arm, respectively. A numerically higher rate of neutropenia was observed with the conventional chemotherapy sequence (51/154 vs. 44/155), febrile neutropenia, however, was more common in the reverse sequence arm (13/155 vs. 3/154). Regarding non-haematological side effects, a slightly higher rate of eye disorders was observed with upfront docetaxel (56/155 vs. 43/154), while nausea was more common with upfront EC (108/154 vs. 91/155), as was cough (25/154 vs. 13/155). Skin disorders, again, were more commonly reported with upfront docetaxel (121/155 vs. 105/154). Grade 3/4 side effects occurring in ≥5 patients are summarised in Table 2; a complete list of AEs of patients in either sequencing arms is provided in Supplementary Table 1.
Table 2.
Grade 3/4 AEs occurring in ≥5 patients.
| by SOC and PT | Chemo conventional with L-BLP25, N = 154 | Chemo reverse with L-BLP25, N = 155 | Total, N = 309 |
|---|---|---|---|
| Number of patients with at least one grade 3/4 AE, n (%) | |||
| 90 (58.4%) | 81 (52.3%) | 171 (55.3%) | |
| Blood and lymphatic system disorders, n (%) | |||
| Febrile neutropenia | 3 (1.9%) | 13 (8.4%) | 16 (5.2%) |
| Leukopenia | 41 (26.6%) | 29 (18.7%) | 70 (22.7%) |
| Neutropenia | 50 (32.5%) | 37 (23.9%) | 87 (28.2%) |
| Gastrointestinal disorders, n (%) | |||
| Diarrhoea | 2 (1.3%) | 5 (3.2%) | 7 (2.3%) |
| General disorders and administration site conditions, n (%) | |||
| Asthenia | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) |
| Fatigue | 3 (1.9%) | 3 (1.9%) | 6 (1.9%) |
| Investigations, n (%) | |||
| Neutrophil count decreased | 2 (1.3%) | 3 (1.9%) | 5 (1.6%) |
| Musculoskeletal and connective tissue disorders, n (%) | |||
| Bone pain | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) |
| Myalgia | 3 (1.9%) | 2 (1.3%) | 5 (1.6%) |
| Respiratory, thoracic and mediastinal disorders, n (%) | |||
| Pulmonary embolism | 1 (0.6%) | 4 (2.6%) | 5 (1.6%) |
N number of patients in the safety analysis set, n number of patients, SOC system organ class, PT preferred term.
A numerically higher SAE rate was observed in the reverse sequence arm (54/155 (34.8%) vs. 41/154 (26.6%)). Overall, three cases of pulmonary embolism were observed (all in the reverse sequence group). SAEs occurring in ≥5 patients for the two sequencing arms are summarised in Table 3. Two deaths occurred on the trial (pulmonary embolism, n = 1; breast cancer progression, n = 1), both in the reverse sequence arm while none was observed in the upfront EC group.
Table 3.
SAEs occurring in ≥5 patients.
| by SOC and PT | Chemo conventional, N = 154 | Chemo reverse, N = 155 | Total, N = 309 |
|---|---|---|---|
| Number of patients with at least one SAEa, n (%) | |||
| 41 (26.6%) | 54 (34.8%) | 95 (30.7%) | |
| Blood and lymphatic system disorders, n (%) | |||
| Febrile neutropenia | 2 (1.3%) | 12 (7.7%) | 14 (4.5%) |
| Leukopenia | 4 (2.6%) | 4 (2.6%) | 8 (2.6%) |
| Neutropenia | 4 (2.6%) | 4 (2.6%) | 8 (2.6%) |
| Gastrointestinal disorders, n (%) | |||
| Diarrhoea | 5 (3.2%) | 4 (2.6%) | 9 (2.9%) |
| General disorders and administration site conditions, n (%) | |||
| Pyrexia | 4 (2.6%) | 1 (0.6%) | 5 (1.6%) |
| Musculoskeletal and connective tissue disorders, n (%) | |||
| Bone pain | 2 (1.3%) | 3 (1.9%) | 5 (1.6%) |
N number of patients in the safety analysis set, n number of patients, SOC system organ class, PT preferred term.
aSAE: any adverse event resulting in death, is immediately life-threatening, requires inpatient hospitalisation or prolongation of hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect in a child whose parent was exposed to a medicinal product prior to conception or during pregnancy or is considered otherwise medically significant such as important medical events that may not immediately be life-threatening or result in death or hospitalisation, but jeopardise the subject or require intervention to prevent one of the outcomes listed in the definition above.
Dose modifications, treatment delays and treatment discontinuations
With regards to chemotherapy discontinuation rates, 9/154 (5.8%) and 10/157 (6.4%) patients stopped docetaxel due to AEs in the conventional and reverse sequence arms, respectively; numerically more patients in the upfront docetaxel arm stopped taxane-based chemotherapy due to disease progression (4/157 vs. 1/154). In contrast, EC was discontinued due to disease progression in only two patients (both in the reverse sequence arm). EC was discontinued due to AEs in three patients, two in the conventional sequence arm and one with reverse chemotherapy sequence, respectively.
In the upfront EC group, more patients received epirubicin and cyclophosphamide without dose delays (60.4% vs. 38.9% epirubicin; 59.7% vs. 39.5% cyclophosphamide) while the rate of docetaxel dose delays was numerically lower in the reverse sequence arm (patients without dose delays conventional sequence 44.8% vs. reverse sequence 52.9%); regarding dose modifications, slightly more patients received EC without dose modifications in the conventional sequence arm (89.0% vs. 82.2%), while the rate of patients without dose modifications of docetaxel was numerically higher in the upfront taxane group (conventional sequence 66.9% vs. reverse sequence 79.6%).
Discussion
The prospective, randomised Phase 2 trial ABCSG-34 evaluated the addition of the MUC1 vaccine tecemotide to neoadjuvant treatment.14 In total, 311 patients were accrued to the chemotherapy cohort and randomised to either a conventional chemotherapy sequence of EC followed by docetaxel or a reverse sequence with upfront docetaxel, making this the largest randomised trial evaluating the role of EC/docetaxel sequencing in the neoadjuvant setting in a HER2-negative population to date (while Neo-tAnGo was the largest sequencing study in general). In our study, no significant efficacy differences were observed, and tolerability was comparable as well. These results need to be discussed in the light of experimental data as well as results of other studies evaluating chemotherapy sequencing in the neoadjuvant and adjuvant setting.
A cell culture model suggested that MCF-7 breast cancer cells resistant to doxorubicin were cross-resistant to paclitaxel and docetaxel due to an upregulation of P-glycoprotein; in a model of paclitaxel resistance, however, only limited cross-resistance to doxorubicin was observed.10 Anthracycline-induced senescence, cellular response to nonlethal stress resulting in persistent cytostasis, may constitute another reason for the assumed reduced activity of taxanes when administered after anthracyclines.11,12 In addition, in a study evaluating levels of circulating tumour cells (CTC) in patients receiving neoadjuvant chemotherapy, a decrease of CTC levels was observed during the initial anthracycline treatment phase. This was followed by a resurgence of CTC levels during the paclitaxel phase suggestive of resistance despite a further reduction in primary tumour size.13
In metastatic breast cancer, a prospective randomised Phase 3 trial compared the combination of doxorubicin plus paclitaxel to either doxorubicin or paclitaxel alone with a pre-specified crossover at the time of progression.15 No difference in terms of response rates, time to treatment failure and overall survival was observed between the two sequential arms. Furthermore, response rates after crossover were similar as well (20% in patients crossing-over from doxorubicin to paclitaxel and 22% with paclitaxel to doxorubicin, respectively). These results, however, may be impacted on by the well-established heterogeneity of the metastatic disease.16
In the neoadjuvant setting, the prospective randomised Phase 3 trial Neo-tAnGo randomised 831 patients to four cycles of EC followed by four cycles of dose-dense paclitaxel (with or without gemcitabine) or the reverse sequence thereof. Around one-quarter of patients were HER2-positive. In this study, upfront paclitaxel treatment resulted in significantly higher pCR rates (15% vs. 20%; P = 0.03), while no significant difference in terms of disease-free survival and OS was observed.17 Regarding tolerability, more dose reductions, and dose delays in the fourth cycle of paclitaxel were observed when anthracyclines were administered first (22% vs. 10% and 15% vs. 11%, respectively). These results are supported by data from a retrospective analysis of 1414 patients were also significantly higher pCR rates were reported when paclitaxel was administered before FEC/FAC (20.9% vs. 12.4%; P = 0.04).8 In contrast, no pCR increase was reported with upfront paclitaxel in a Phase 3 trial conducted in HER2-positive early-stage breast cancer; participants, however, received additional immunotherapy with trastuzumab, potentially reducing the relative importance of chemotherapy.18 Three small, randomised Phase 2 studies investigating doxorubicin followed by paclitaxel19,20 or weekly docetaxel21 or the reverse sequence thereof could not establish a significant improvement of response rates with the reversed sequence as well. Moreover, a French trial randomising 123 patients to four cycles of docetaxel followed by four cycles of anthracycline-containing chemotherapy or vice versa yielded results similar to our study.22 No difference in terms of response or breast conservation rate was observed but a higher neurotoxicity rate was reported in patients with upfront docetaxel (which was not seen in ABCSG-34).
Except for one small study,23 the majority of sequencing trials conducted in the adjuvant setting consistently suggested improved tolerability when taxanes were administered first, resulting in a higher relative chemotherapy dose-intensity and less treatment delays.9,24,25 Due to the size and design of these trials, however, no data regarding the influence of chemotherapy sequencing on long-term outcomes are available.
Results of ABCSG-34 are therefore in line with data from several smaller neoadjuvant studies suggesting that despite the preclinical rationale, upfront taxane therapy may not increase pCR rates in early-stage breast cancer. Of note, these studies often used suboptimal chemotherapy regimens (e.g., cyclophosphamide-free, weekly docetaxel). In contrast, the largest sequencing study hitherto, neo-tAnGo, reported contradicting results: here, a significantly higher pCR rate was observed when paclitaxel was administered first. Some relevant differences, however, need be kept in mind: Other than in ABCSG-34, a quarter of the study population participating in neo-tAnGo was HER2-positive; also, paclitaxel was chosen as taxane backbone and gemcitabine was added to half the subjects which may have altered tolerability. A Cochrane review investigating anthracycline/taxane sequencing yielded results similar to our trial: data from 1415 participants in five neoadjuvant trials, among them neo-tAnGo, were analysed and upfront administration of taxanes resulted in little to no difference in terms of OS, DFS, and pCR.26 neo-tAnGo and several other studies reported improved tolerability as indicted by fewer dose reductions and fewer dose delays with upfront taxane administration. This was not mirrored in ABCSG-34 where tolerability was overall comparable between the arms, and even a higher febrile neutropenia rate with upfront docetaxel was observed which may be explained by the numerically lower rate of docetaxel dose reductions in this group. Again, these differences might be due to the fact that docetaxel 100 mg/m² once every 3 weeks was chosen as chemotherapy backbone in ABCSG-34, and the sequence of anthracycline and taxanes may be more relevant when using paclitaxel and/or dose-dense regimens.
In ACSCG-34, none of the patients in the conventional sequence arm discontinued upfront EC for disease progression as opposed to 2.6% discontinuing upfront docetaxel in the reverse sequence arm. While these numbers are small, they are in line with results from the prospective randomised Phase 3 GeparSepto trial, where a discontinuation rate of 2% and 5% was reported with upfront neoadjuvant nab-paclitaxel and conventional paclitaxel, respectively.27 Further investigation of the primary progression rate in studies using an upfront taxane design may therefore be warranted. More recently, however, the upfront combination of paclitaxel and carboplatin became widely used in TNBC, thereby reducing the relevance of this issue.28
A major limitation of the trial is the fact that the secondary sequencing randomisation was not stratified for baseline characteristics such as subtype and nodal status, and no separate sample-size calculation was performed. This has led to an imbalance in the baseline characteristics between the two sequencing arms with a higher rate of N + patients in the reversed sequence group (44% and 37%, respectively); in addition, small patient numbers in the subgroups of TNBC and luminal breast cancer patients may have resulted in the numerically different effects of chemotherapy sequences in terms of RCB 0/1 and pCR rates. A further limitation is the lack of survival follow-up. Therefore, the results must be interpreted with due caution despite being in line with a recent Cochrane review.
Despite these limitations, the prospective randomised Phase 2 trial ABCSG-34 is to date the largest randomised trial evaluating the effect of EC/docetaxel sequencing in the neoadjuvant setting in a HER2-negative population. This study did not observe the hypothesised benefit of upfront docetaxel administration in terms of chemotherapy activity. In addition, tolerability was not improved as well. While no final conclusions can be drawn, upfront administration of anthracyclines followed by docetaxel, therefore, remains a potential treatment standard and results of this trial suggest that clinicians may choose upfront administration of EC or docetaxel as preferred.
Supplementary information
Acknowledgements
The authors would like to thank Dr. Martina Putz for her support in preparing the final version of this paper.
Author contributions
Conception and design: C.S., R.B., P.D., F.F., M.G. and M.H. Development of methodology: V.B.R., P.D., M.F., S.F., M.G., R.G., Z.B.H. and M.R. Acquisition of the data: all authors. Interpretation of the data: all authors. Paper writing: all authors. Paper approval: all authors.
Ethics approval and consent to participate
The study was conducted according to the principles of the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice. The protocol was conducted under EU directive 2001/20/EC and approved by the Ethics Committee of the Medical University of Vienna, Borschkegasse 8b/6, A-1090 Vienna, Austria, as the leading ethics committee. All subjects provided their written informed consent to participate in the study.
Consent to publish
Not applicable.
Data availability
As the regulatory sponsor of this trial, ABCSG has data sovereignty and individual participant data (including de-identified participant data, participant data with identifiers, data dictionary or other specified datasets) will not be shared.
Competing interests
R.B. reports advisory roles at AstraZeneca, Celgene, Daiichi, Eisai, Eli-Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche, Samsung, lecture honoraria: Accord, AstraZeneca, BMS, Celgene, Eli-Lilly, Novartis, Pfizer, Pierre-Fabre, Roche, Sandoz and received research support from Daiichi, Novartis and Roche, all outside of the submitted work. M.H. reports honoraria and/or travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, Pfizer, Novartis, Roche all outside the submitted work. A.L.P. reports honoraria and/or travel support from Abbvie, Amgen, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Eli-Lilly, MSD, Pfizer, Novartis, Roche, Sanofi and Takeda all outside the submitted work. D.E. reports honoraria/travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, MSD, Novartis, Pfizer, Roche and Myriad all outside of the submitted work. M.F. has received honoraria for advisory boards from AstraZeneca, Bayer, Biomedica, Boehringer Ingelheim, Eli-Lilly, Merck Sharp & Dohme, Myriad Genetics Inc., Pfizer and Roche. M.G. reports personal fees/travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, Invectys, Pfizer, Novartis, Puma, Nanostring, Roche, Medison, LifeBrain, all outside the submitted work; an immediate family member is employed by Sandoz. Z.B.-H. has advisory roles at Biomedica, Roche and Novartis, received lecture honoraria and travel support from Roche and research support from Boehringer Ingelheim. C.F.S., G.P., H.S., A.P., E.P., V.B.-R., R.G., M.R., M.K.T., V.W., P.S., P.D., F.F., R.E., R.J., M.B., C.T. and S.F. declare no competing interests.
Funding information
This study was supported by Merck KGaA, Darmstadt, Germany. The academic non-profit organisation ABCSG was the regulatory sponsor of this trial. Merck provided financial funding and tecemotide. The study was designed and conducted by ABCSG. Merck was not involved in the collection, management, analysis and interpretation of the data. ABCSG prepared and approved the paper and decided to submit the paper for the publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01284-2.
References
- 1.Bonadonna G. Karnofsky memorial lecture. Conceptual and practical advances in the management of breast cancer. J. Clin. Oncol. 1998;7:1380–1397. doi: 10.1200/JCO.1989.7.10.1380. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann. Oncol. 2007;18:1927–1934. doi: 10.1093/annonc/mdm201. [DOI] [PubMed] [Google Scholar]
- 3.Bear, H. D., Anderson, S., Smith, R. E., Geyer, C. E. Jr., Mamounas, E. P., Fisher, B. et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol.24, 2019–2027 (1999). [DOI] [PubMed]
- 4.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 5.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 6.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 7.Wildiers H, Forceville K, Paridaens R, Joensuu H. Taxanes and anthracyclines in early breast cancer: which first? Lancet Oncol. 2010;11:219–220. doi: 10.1016/S1470-2045(10)70025-5. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RH, Bianchini G, Hsu L, Cristofanilli M, Esteva FJ, Pusztai L, et al. Clinical outcome of two sequences of administering paclitaxel (P) and anthracyclines (A) as primary systemic therapy (PST) and adjuvant chemotherapy (ACT) in breast cancer (BC) patients: a retrospective analysis from the M. D. Anderson Cancer Center (MDACC) Cancer Res. 2010;70(24 Suppl):384s. [Google Scholar]
- 9.Puhalla S, Mrozek E, Young D, Ottman S, McVey A, Kendra K, et al. Randomized phase II adjuvant trial of dose-dense docetaxel before or after doxorubicin plus cyclophosphamide in axillary node-positive breast cancer. J. Clin. Oncol. 2008;26:1691–1697. doi: 10.1200/JCO.2007.14.3941. [DOI] [PubMed] [Google Scholar]
- 10.Guo B, Villeneuve DJ, Hembruff SL, Kirwan AF, Blais DE, Bonin M, et al. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel may be strongly compromised by prior doxorubicin exposure. Breast Cancer Res. Treat. 2004;85:31–51. doi: 10.1023/B:BREA.0000021046.29834.12. [DOI] [PubMed] [Google Scholar]
- 11.Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- 12.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J. Natl Cancer Inst. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camara O, Rengsberger M, Egbe A, Koch A, Gajda M, Hammer U, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann. Oncol. 2007;18:1484–1492. doi: 10.1093/annonc/mdm206. [DOI] [PubMed] [Google Scholar]
- 14.Singer CF, Pfeiler G, Hubalek M, Bartsch R, Stöger H, Pichler A, et al. Efficacy and safety of the therapeutic cancer vaccine tecemotide (L-BLP25) in early breast cancer: results from a prospective, randomized, neoadjuvant Phase II Study (ABCSG 34) Eur. J. Cancer. 2020;132:43–52. doi: 10.1016/j.ejca.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193) J. Clin. Oncol. 2003;21:588–592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Murtaza M, Dawson SJ, Pogrebniak K, Rueda OM, Provenzano E, Grant J, et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat. Commun. 2015;6:8760. doi: 10.1038/ncomms9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earl HM, Vallier AL, Hiller L, Fenwick N, Young J, Iddawela M, et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2×2 factorial randomised phase 3 trial. Lancet Oncol. 2014;15:201–212. doi: 10.1016/S1470-2045(13)70554-0. [DOI] [PubMed] [Google Scholar]
- 18.Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staems V, Singh B, Tsangaris T, Crawford JG, Novielli A, Ellis MJ, et al. A prospective randomized pilot study to evaluate predictors of response in serial core biopsies to single agent neoadjuvant doxorubicin or paclitaxel for patients with locally advanced breast cancer. Clin. Cancer Res. 2003;9:124–133. [PubMed] [Google Scholar]
- 20.Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J. Clin. Oncol. 2005;23:1951–1961. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 21.Miller KD, Soule SE, Calley C, Emerson RE, Hutchins GD, Kopecky K, et al. Randomized phase II trial of the anti-angiogenic potential of doxorubicin and docetaxel; primary chemotherapy as Biomarker Discovery Laboratory. Breast Cancer Res. Treat. 2005;89:187–197. doi: 10.1007/s10549-004-2044-y. [DOI] [PubMed] [Google Scholar]
- 22.Thiery-Vuillemin A, Llombart-Cussac A, Chaigneau L, Villanueva C, Bazan F, Montcuquet P, et al. Sequential taxane and anthracycline-containing neoadjuvant regimens: the sequential order impact. Breast. 2011;20:46–49. doi: 10.1016/j.breast.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso F, Ferreira Filho AF, Crown J, Dolci S, Paesmans M, Riva A, et al. Doxorubicin followed by docetaxel versus docetaxel followed by doxorubicin in the adjuvant treatment of node positive breast cancer: results of a feasibility study. Anticancer Res. 2001;21:789–795. [PubMed] [Google Scholar]
- 24.Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, et al. Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann. Oncol. 2007;18:52–57. doi: 10.1093/annonc/mdl355. [DOI] [PubMed] [Google Scholar]
- 25.Wildiers H, Dirix L, Neven P, Prové A, Clement P, Squifflet P, et al. Delivery of adjuvant sequential dose-dense FEC-Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Res. Treat. 2009;114:103–112. doi: 10.1007/s10549-008-9970-z. [DOI] [PubMed] [Google Scholar]
- 26.Zaheed, M., Wilcken, N., Willson, M. L., O’Connell, D. L. & Goodwin, A. Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer. Cochrane Database Syst. Rev. CD012873 (2019). [DOI] [PMC free article] [PubMed]
- 27.Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 28.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As the regulatory sponsor of this trial, ABCSG has data sovereignty and individual participant data (including de-identified participant data, participant data with identifiers, data dictionary or other specified datasets) will not be shared.