Abstract
Background
We investigated whether associations between prevalent diabetes and cancer risk are pertinent to older adults and whether associations differ across subgroups of age, body weight status or levels of physical activity.
Methods
We harmonised data from seven prospective cohort studies of older individuals in Europe and the United States participating in the CHANCES consortium. Cox proportional hazard regression was used to estimate the associations of prevalent diabetes with cancer risk (all cancers combined, and for colorectum, prostate and breast). We calculated summary risk estimates across cohorts using pooled analysis and random-effects meta-analysis.
Results
A total of 667,916 individuals were included with an overall median (P25–P75) age at recruitment of 62.3 (57–67) years. During a median follow-up time of 10.5 years, 114,404 total cancer cases were ascertained. Diabetes was not associated with the risk of all cancers combined (hazard ratio (HR) = 0.94; 95% confidence interval (CI): 0.86–1.04; I2 = 63.3%). Diabetes was positively associated with colorectal cancer risk in men (HR = 1.17; 95% CI: 1.08–1.26; I2 = 0%) and a similar HR in women (1.13; 95% CI: 0.82–1.56; I2 = 46%), but with a confidence interval including the null. Diabetes was inversely associated with prostate cancer risk (HR = 0.81; 95% CI: 0.77–0.85; I2 = 0%), but not with postmenopausal breast cancer (HR = 0.96; 95% CI: 0.89–1.03; I2 = 0%). In exploratory subgroup analyses, diabetes was inversely associated with prostate cancer risk only in men with overweight or obesity.
Conclusions
Prevalent diabetes was positively associated with colorectal cancer risk and inversely associated with prostate cancer risk in older Europeans and Americans.
Subject terms: Risk factors, Cancer epidemiology
Background
In 2015, 415 million people were estimated to live with diabetes, of whom more than 90% had type 2 diabetes, and this prevalence seems set to increase and affect all regions and countries of the world.1 The risk factors of type 2 diabetes include genetics, age, family history of diabetes, unhealthy diet, obesity and physical inactivity.2
A 2018 meta-analysis of 121 cohort studies reported that diabetes was positively associated with the incidence of all cancers combined, in both men and women. The pooled adjusted RRs were 1.19 (95% CI: 1.13–1.25) for men and 1.27 (95% CI: 1.21–1.32) for women.3 Several prospective studies and meta-analyses have suggested that type 2 diabetes significantly increases the risk of breast4 and colorectal cancers,5 but decreases the risk of prostate cancer.6 These are among the most frequent cancers and account together for 31% of total cancer cases globally.7 As noted in an umbrella review of meta-analyses,5 heterogeneity between studies investigating associations between diabetes and cancer risk was often large,5 which hampers the possibility of drawing robust conclusions across study settings. Different study designs with various definitions of prevalent diabetes and different levels of adjustment for confounders may at least partly explain the large heterogeneity in previous meta-analyses. The relationship between diabetes and cancer risk may also be different in older people, who likely lived with diabetes for longer durations. Yet, previous studies have not specifically considered associations in ageing populations, such as in our study, where study participants had a median age of 60 or older at recruitment in four of the seven included cohorts.
The current evidence suggests that the association between type 2 diabetes and cancer development may be partially explained by shared predisposing risk factors, such as physical inactivity and overweight (BMI ≥ 25 kg/m2) or obesity (BMI ≥ 30 kg/m2).8 Higher levels of physical activity were consistently associated with a lower risk of several cancers, in particular those of the breast and colorectum9,10 as well as type 2 diabetes.8 Similarly, obesity is an established risk factor for many cancers11,12 and type 2 diabetes.13 More insight is needed as to whether associations between diabetes and cancer are direct, i.e., due to specific metabolic consequences of diabetes, or modified by common underlying risk factors.
We utilised data from the Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) to evaluate the association between prevalent diabetes at enrolment into the cohorts with later cancer development while accounting for a common set of confounders. We assessed all cancers combined in seven cohorts, those of the colorectum and breast in four cohorts, and prostate in three cohorts. We focused on the most common cancers in Europe and the United States, for which respective data on incident cancer were available in at least three of the seven participating cohorts. We also assessed effect modification by age, body weight status and levels of physical activity.
Methods
Study population
The CHANCES project harmonised data from prospective cohort studies in Europe and the United States to investigate the determinants of healthy ageing. Details on CHANCES have been provided previously.14 Seven CHANCES cohorts provided data for the current analysis, summarised in Table 1:
the National Institutes of Health-AARP (NIH-AARP) study, which is a prospective cohort of men and women in the United States, aged 50–71 years at recruitment
the study centres in Greece, Spain, Sweden and the Netherlands of the EPIC-Elderly study, which is a subset of the European Prospective Investigation into Cancer and Nutrition (EPIC) project that consists of men and women aged 60 years or older at recruitment
the Epidemiological Study for Prevention, Early Detection, and Optimised Therapy of Chronic Diseases at Old Age (ESTHER), a population-based cohort covering the entire federal state of Saarland in Germany, aged 50–75 years at recruitment
the FINRISK study, from which we included men and women aged 24–74 years in Finland
the PRIME Belfast study, a cohort of male residents aged 50–60 years of Belfast and the surrounding area in the United Kingdom
the Northern Sweden MONICA study, which is a prospective cohort of men and women, aged 25–74 years in Sweden
the TROMSØ study, which recruited men and women in Norway between 1994 and 1995 (4th wave) aged 50–84 years.
Table 1.
Key characteristics of the cohorts in the CHANCES consortium.
| Characteristics | NIH-AARP | EPIC-Elderly | ESTHER | FINRISK | PRIME Belfast | Northern Sweden | TROMSØ |
|---|---|---|---|---|---|---|---|
| Overall subjects (n) | 566,279 | 25,308 | 9949 | 38,333 | 2745 | 10,839 | 10,463 |
| Men, n (%) | 339,656 (60.0) | 8061 (31.8) | 4482 (45.1) | 18,559 (48.4) | 2745 (100) | 5354 (49.4) | 4953 (47.3) |
| Women, n (%) | 226,623 (40.0) | 17,247 (68.2) | 4467 (54.9) | 19,774 (51.6) | 0 | 5485 (50.6) | 5510 (52.7) |
| Period of enrolment | 1995–1996 | 1992–2000 | 2000–2002 | 1982–2002 | 1991–1994 | 1985–2009 | 1994–1995 |
| Median follow-up (years) | 10.5 | 12.5 | 10.4 | 12.2 | 18.0 | 6.9 | 15.8 |
| Age at enrolment (years), p50 (P25–p75) | 63 (58–67) | 64 (61–67) | 63 (57–67) | 46 (36–56) | 54 (52–57) | 49 (37–60) | 62 (55–70) |
| BMI at baseline (kg/m2), mean ± SD | 27.1 ± 5.0 | 28.0 ± 4.6 | 27.7 ± 4.4 | – | 26.2 ± 3.4 | – | 26.1 ± 4.0 |
| Missing, n (%) | 13,932 (2.5) | 89 (0.4) | 16 (0.2) | – | 0 (0) | – | 236 (2.3) |
| Alcohol intake (g per day), mean ± SD | 13.2 ± 38.3 | 8.1 ± 16.2 | 6.7 ± 9.5 | 7.5 ± 13.7 | 21.8 ± 34.8 | 3.4 ± 5.4 | 3.5 ± 5.4 |
| Missing, n (%) | 0 (0) | 67 (0.3) | 966 (9.7) | 929 (2.4) | 0 (0) | 508 (4.7) | 2515 (24.0) |
| Smoking status | |||||||
| Never, n (%) | 196,444 (34.7) | 15,380 (60.8) | 4838 (48.6) | 17,556 (46.2) | 1028 (37.4) | 5495 (50.7) | 3476 (33.2) |
| Ever, n (%) | 348,052 (61.5) | 9504 (37.6) | 4837 (48.6) | 20,404 (53.4) | 1683 (61.3) | 5043 (46.5) | 6987 (66.8) |
| Missing, n (%) | 21,783 (3.8) | 424 (1.7) | 274 (2.8) | 726 (1.3) | 34 (1.2) | 301 (2.8) | 0 (0) |
| Vigorous physical activity | |||||||
| No, n (%) | 301,914 (53.3) | 15,320 (60.5) | 5751 (57.8) | – | 2414 (87.9) | – | 6996 (66.9) |
| Yes, n (%) | 257,838 (45.5) | 6162 (24.3) | 4168 (41.2) | – | 330 (12.0) | – | 3344 (31.9) |
| Missing, n (%) | 6500 (1.1) | 3826 (15.1) | 30 (0.3) | – | 1 (0.04) | – | 123 (1.2) |
| School level | |||||||
| Primary or less, n (%) | 4683 (0.8) | 17,518 (69.2) | 7242 (72.8) | 17,606 (46.0) | 0 | 3476 (32.1) | 5802 (55.5) |
| >Primary- <college/university, n (%) | 142,865 (25.2) | 5811 (23.0) | 1982 (19.9) | 16,857 (44.0) | 2411 (87.8) | 4982 (46.0) | 2907 (27.8) |
| College or university, n (%) | 401,756 (70.9) | 1777 (7.0) | 473 (4.8) | 3512 (9.0) | 308 (11.2) | 2226 (20.5) | 1681 (16.1) |
| Missing, n (%) | 16,975 (3.0) | 202 (0.8) | 252 (2.5) | 358 (1.0) | 26 (0.9) | 155 (1.4) | 73 (0.7) |
| History of diabetes, n (%) | |||||||
| No | 513,642 (90.7) | 22,755 (89.9) | 7461 (87.7) | 34,229 (89.3) | 2476 (90.2) | 14,486 (96.7) | 9705 (92.8) |
| Yes | 52,637 (9.3) | 2422 (9.6) | 1044 (12.3) | 4104 (10.7) | 269 (9.8) | 353 (3.3) | 759 (7.2) |
| Cancer cases | |||||||
| Total cancer cases | 102,799 | 3024 | 1135 | 3934 | 495 | 1064 | 2140 |
| Colorectal cancer cases | 8889 | 425 | 160 | – | 340 | – | – |
| Prostate cancer cases | 25,788 | 356 | 205 | – | – | – | – |
| Breast cancer cases | 10,888 | 476 | 165 | – | – | – | 165 |
| Cancer cases among diabetes | |||||||
| Total cancer cases | 9898 | 254 | 110 | 599 | 45 | 51 | 157 |
| Colorectal cancer cases | 1049 | 36 | 13 | – | – | – | 24 |
| Prostate cancer cases | 1857 | 19 | 18 | – | – | – | – |
| Breast cancer cases | 753 | 31 | 21 | – | – | – | 10 |
SD standard deviation, NIH-AARP National Institutes of Health-American Association of Retired Persons Diet and Health study, EPIC European Prospective Investigation into Cancer and Nutrition study.
Total cancers included all cancer (first primary) sites combined.
Data collection and assessment of the covariates
Data on age, sex, alcohol consumption, smoking status, physical activity, level of education and reproductive history (in women) were collected at baseline. Height and weight were measured at baseline in EPIC-Elderly, PRIME Belfast and TROMSØ. BMI was calculated as weight (kg) divided by height (metre) squared. ESTHER and NIH-AARP collected self-reported weight and height at baseline, while no anthropometry data were available from FINRISK and Northern Sweden MONICA. Information regarding physical activity was self-reported in all cohort studies and was assessed either in terms of intensity, meaning exercise intensive enough to cause sweating, or in terms of time engaged in sport activities, depending on data availability. Vigorous physical activity (dichotomous: yes, no) was defined as at least 1 h per week of physical activity intense enough to cause perspiration, being out of breath or a faster heartbeat. Only the latter was available from most cohorts, except FINRISK and Northern Sweden cohorts, and was the best available harmonised indicator of physical activity across cohorts.
At baseline, all cohorts collected data on self-reported and/or documented diabetes. In the NIH-AARP and the Northern Sweden MONICA cohort, we lacked information about whether diabetes was type 1 or type 2, but type 2 diabetes accounts for >90% of diabetes in this age group.15 Most cohorts (PRIME Belfast, FINRISK, Northern Sweden MONICA and TROMSØ) had information on documented diabetes. In the ESTHER cohort, 55 cases of diabetes (5% of all cases of diabetes) were self-reported, while NIH-AARP and EPIC studies collected self-reported diabetes. Documented diabetes was defined as a diagnosis of diabetes, based on the fulfilment of any of the following criteria:1 2-h plasma glucose ≥11.1 mmol/l (200 mg/ml) during an OGTT performed in accordance with the World Health Organization’s recommendations,16 using a glucose load containing the equivalent of 75 g of anhydrous glucose dissolved in water,2 HbA1c ≥48 mmol/mol (6.5%),3 fasting plasma glucose ≥7.0 mmol/l (126 mg/dl), fasting was defined as no caloric intake for at least 8 h,4 documented diagnosis of diabetes by a medical doctor,5 clinical or death certificate diagnosis indicating diabetes (Code 250 of ICD-8, Code 250 of ICD-9 and Code E11 of ICD-10) and6 documented current treatment of diabetes. For the remainder of the paper, diabetes refers to type 2 diabetes acknowledging that a low proportion of patients were also of type 1.
Outcome ascertainment
All cohorts participating in this study provided follow-up cancer incidence information for all anatomical sites combined. Four cohorts (EPIC-Elderly, ESTHER, TROMSØ and NIH-AARP) also provided information on site-specific incidence for breast and colorectal cancers, while three (EPIC-Elderly, ESTHER and NIH-AARP) also provided information on prostate cancer incidence. Study participants with history of cancer or prevalent cancer at baseline were excluded.
Cancer incidence was ascertained by active follow-up, record linkage with national/regional cancer registries, national hospital discharge register and causes of death register.17,18 The main endpoints were total cancer incidence as defined by codes C00-C97 according to the 10th edition of the ICD-10. Additional endpoints were incidence of colon and rectum (C18–20), (C25), breast (C50) and prostate cancer (C61). Vital status was obtained from regional or state registries for all cohorts.
Statistical analyses
Means and SDs, or percentages as appropriate, of selected baseline characteristics of the study population were computed in relation to diabetes status.
Cox proportional hazard models with age as the time metric were used to estimate cause-specific HR and 95% CI for associations of prevalent diabetes with total, colorectal, prostate and postmenopausal breast cancers. Age at entry was defined as the participant’s age at recruitment, and exit time was the age at diagnosis of cancer, death, loss to follow-up or censoring at the end of the follow-up period. The proportionality of hazards was verified based on the slope of the Schoenfeld residuals over time. Analyses were conducted for each individual cohort separately. Models were stratified by sex and adjusted for known or suspected cancer risk factors, including categories of smoking status, educational level, vigorous physical activity (yes/no), country (EPIC-Elderly only) and continuous variables of age, alcohol consumption and BMI (Table 1). The linearity assumption for the continuous covariates was verified using fractional polynomials. A dummy category for missing data was created for categorical covariates, and continuous missing data were replaced by the median value of the population data. The results across cohorts were then combined using DerSimonian and Laird random-effects meta-analysis.19 The heterogeneity of associations across studies was assessed using the I2 score.20
To investigate effect modification, all cohorts were pooled into one dataset. Stratified analyses were conducted according to age groups (<60, 60–<65, 65–<70 and ≥70 years), predefined WHO cut-off points of weight status (normal BMI, <25 kg/m2, overweight, 25–<30 kg/m2 and obese, ≥30 kg/m2) to facilitate clinical interpretation, and vigorous physical activity defined as at least 1 h per week of intense physical activity (yes/no). Multiplicative interaction was assessed by adding an interaction term between diabetes status and in turn age groups, weight status and levels of physical activity to the multivariable Cox proportional hazard models and the significance of these was assessed by likelihood ratio tests. In contrast to our main analysis, which was based on a strong prior hypothesis, we penalised the more exploratory subgroup analyses for multiple testing using Bonferroni correction and estimated the corresponding 98% CI (100–5/k, k = 24 tests).
For sensitivity analysis, we repeated our analyses using fixed-effect meta-analysis and pooled (individual-patient level) analyses to assess the robustness of the findings and to rule out sampling variability contributing to I2 in our random-effects meta-analysis. We also repeated our main analysis after excluding cancer cases ascertained within less than two years of follow-up to assess reverse causation. To assess whether defining diabetes (self-reported versus documented) affected associations with total cancer incidence, we performed the random-effects meta-analysis by cohorts with self-reported (or a mixture of documented and self-reported) diabetes status (i.e., NIH-AARP, EPIC-Elderly and ESTHER (since a certain proportion was self-reported)) versus cohorts with documented diabetes (FINRISK, PRIME Belfast, Northern Sweden and TROMSØ). This was not feasible for site-specific cancers, because not all cohorts could provide data on site-specific cancers and thus NIH-AARP and EPIC-Elderly dominated these analyses. Lastly, to assess whether imputation of missing values affected observed associations, we repeated the pooled analysis after excluding participants with missing values in any covariate (complete-case analysis).
All statistical tests were two-sided and P values < 0.05 were considered statistically significant (P < 0.002 in subgroup analyses). All analyses were performed using STATA version 14 (College Station, TX, USA).
Results
Population characteristics
Table 1 presents the key characteristics of the cohorts and study participants. A total of 663,916 participants (85.3% from NIH-AARP) were recruited between 1982 and 2009, with an overall median (P25–P75) age at recruitment of 62 (57–67) years (median age ranging from 46 years in FINRISK to 64 years in EPIC-Elderly). The proportion of individuals with diabetes was 9.3% (n = 61,587) ranging from 3.3% in the Northern Sweden study to 12.3% in ESTHER. During a median follow-up of 10.5 years, 114,404 total cancer cases were ascertained (89.7% in NIH-AARP). Selected characteristics of the participants by diabetes status are shown in Supplementary Table 1. Total cancer and colorectal cancer were more common in individuals with diabetes than those without, while breast and prostate cancer were less frequent. Individuals with diabetes were older, had a higher BMI, were less physically active, had a lower alcohol consumption and had attained a lower educational level.
Meta-analysis of diabetes and cancer risk
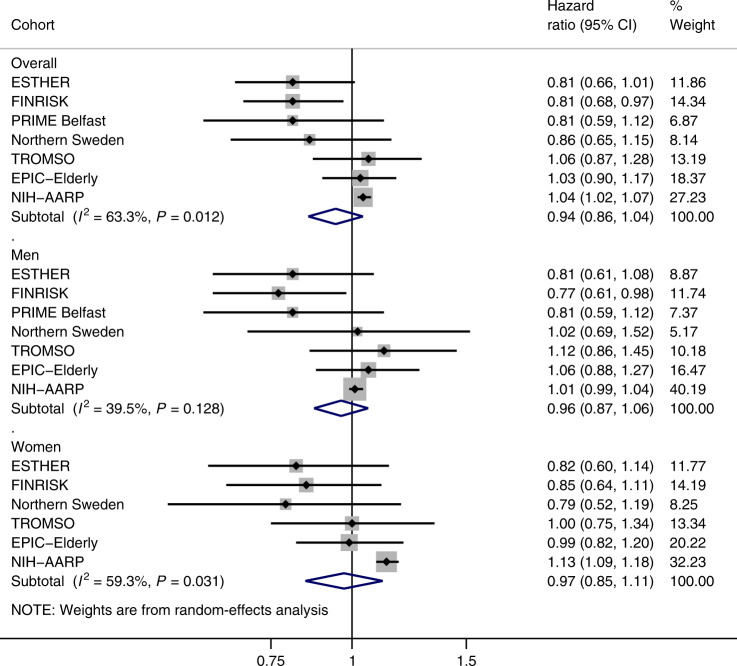
We found no evidence for an association between prevalent diabetes and total cancer incidence. After adjusting for age, sex, BMI, alcohol consumption, physical activity, smoking and education, the pooled HR from the random-effects meta-analysis was 0.94 (95% CI: 0.86–1.04) in men and women combined (Fig. 1). Similar risk estimates were obtained after stratification by sex (Fig. 1). Substantial heterogeneity was observed across studies for both sexes combined (I2 = 63.3%, P heterogeneity = 0.012). This was mostly due to the heterogeneity in women (I2 = 59.3%, P heterogeneity = 0.031), where the association was significantly positive in the NIH-AARP study and null in all other studies (Fig. 1).
Fig. 1. Random-effects meta-analysis of the association between diabetes and total cancer risk.
Models were adjusted for country, age (years), smoking status (never/ever), educational level (primary or less/primary or less than college or university/college or university), alcohol consumption (g/day), BMI, (kg/m2) and vigorous physical activity (yes/no). The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CI). Diamonds represent the combined HRs and 95% CI.
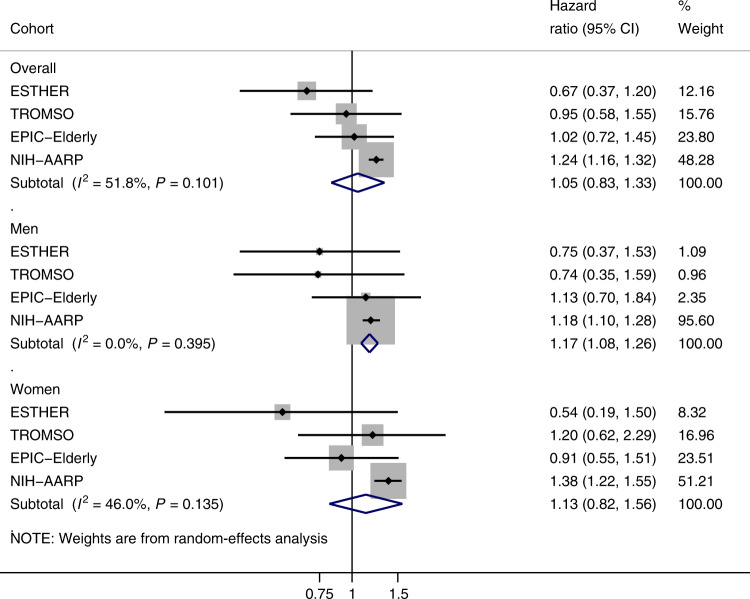
Diabetes was positively associated with colorectal cancer incidence in sex-stratified analyses with a pooled HR of 1.17 (95% CI: 1.08–1.26; I2 = 0%) in men, and a pooled HR in women equal to 1.13 (95% CI: 0.82–1.56; I2 = 46%) (Fig. 2).
Fig. 2. Random-effects meta-analysis of the association between diabetes and colorectal cancer risk.
Models were adjusted for country, age (years), smoking status (never/ever), educational level (primary or less/primary or less than college or university/college or university), alcohol consumption (g/day), BMI, (kg/m2) and vigorous physical activity (yes/no). The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CI). Diamonds represent the combined HRs and 95% CI.
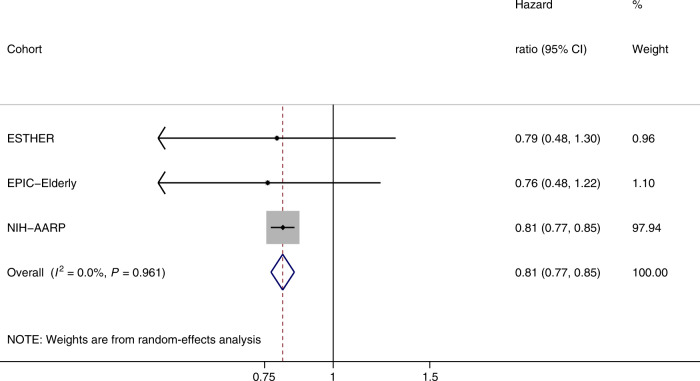
Diabetes was inversely associated with prostate cancer incidence with a pooled HR equal to 0.81 (95% CI: 0.77–0.85), and no heterogeneity across cohorts (I2 = 0%, P heterogeneity = 0.96) (Fig. 3).
Fig. 3. Random-effects meta-analysis of the association between diabetes and prostate cancer risk.
Models were adjusted for country, age (years), smoking status (never/ever), educational level (primary or less/primary or less than college or university/college or university), alcohol consumption (g/day), BMI, (kg/m2) and vigorous physical activity (yes/no). The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CI). Diamonds represent the combined HRs and 95% CI.
Regarding breast cancer in women, where postmenopausal cancer represented 93% of the cases, diabetes was not associated with risk. The corresponding pooled HR was equal to 0.96 (95% CI: 0.89–1.03) (Supplementary Fig. 1). There was no heterogeneity across cohorts (I2 = 0%, P heterogeneity = 0.86), and no notable changes in results were observed when stratifying by menopausal hormone therapy (P interaction = 0.12).
Sensitivity analyses
In contrast to the random-effects meta-analysis, we found a positive association between diabetes and total cancer incidence in both fixed-effect meta-analysis and pooled analysis (Supplementary Table 2). However, the I2 from the fixed-effect meta-analysis was 63% (P heterogeneity = 0.012), which suggests that variability in observed associations was due to between-study heterogeneity rather than chance, and the results of the random-effects meta-analysis more appropriately summarise the overall association across studies. Similarly, unlike the results of the random-effects meta-analysis, we found a positive association between diabetes and risk of colorectal cancer in women in the fixed-effect meta-analysis, but with a large variability of 46% (P heterogeneity = 0.135). In contrast, fixed-effect results for colorectal and prostate cancer in men and for breast cancer in women were comparable with the random-effects meta-analysis with no heterogeneity across studies (Supplementary Table 2).
Results after excluding the first two years of follow-up were unchanged (data not shown). Results of a complete-case analysis were comparable with the analysis using imputation (Supplementary Table 2). Stratification of cohorts by diabetes ascertainment (self-reported versus documented) resulted in similar pooled HRs for risk of total cancer and overlapping 95% CIs in both groups of cohorts (Supplementary Fig. 2).
Effect modification by age groups, body weight status and physical activity
After pooling all cohorts into one dataset, we found no evidence for effect modification of associations between diabetes and cancer incidence by age groups (Supplementary Table 3).
For BMI, a significant effect modification was observed for prostate cancer (P interaction <0.001), where the inverse association with diabetes remained among overweight (BMI = 25–<30 kg/m2; HR = 0.77, 98% CI: 0.71–0.83) and obese individuals (BMI ≥ 30 kg/m2; HR = 0.81, 98% CI: 0.73–0.89), but was attenuated among normal-weight individuals (BMI <25; HR = 0.94, 98% CI: 0.83–1.07) (Supplementary Table 4). There was some evidence for effect modification for total cancer in men and women combined (P interaction < 0.044), with a positive association, observed among obese individuals (HR = 1.06, 98% CI: 1.01–1.10) and overweight individuals (HR = 1.04, 95% CI: 1.00–1.07), but not in normal-weight individuals (HR = 1.01, 98% CI: 0.95–1.07) (Supplementary Table 4).
For physical activity, we observed little evidence for effect modification (Supplementary Table 5).
Discussion
In this meta-analysis of individual-level participant data, bringing together seven prospective cohort studies of middle-aged and older populations in Europe and the United States with a harmonised assessment of confounders and prevalent diabetes collected prior to cancer diagnoses, we found little evidence for an association between prevalent diabetes and development of all cancers combined. In analyses of data from the participating cohorts that provided information by anatomical site of cancer, we observed a positive association with risk of colorectal cancer in men, an inverse association with prostate cancer and no association for risk of postmenopausal breast cancer. In subgroup analyses testing effect modification by body weight status, we observed stronger inverse associations for prostate cancer among overweight and obese individuals, as compared to normal-weight individuals, with diabetes.
Previous research has shown that some cancers develop more commonly in patients with diabetes, while prostate cancer occurs less often in men with diabetes.5 The positive association between diabetes and colorectal cancer is consistent with previous findings.3,21 Our findings on the persistence of these associations into older age groups highlight the relevance of public health advice on diabetes prevention for reducing colorectal cancer risk. Further research into the underlying mechanisms linking diabetes with colorectal cancer development at various age groups would also be important.
For prostate cancer, our results confirm earlier evidence showing an inverse association between diabetes and prostate cancer incidence.5,22,23 Specifically, a meta-analysis of summary data based on 9 studies reported an inverse association between diabetes and prostate cancer, with the RR being slightly stronger for low-grade (RR = 0.74; 95% CI: 0.64–0.86) and localised disease (RR = 0.72; 95% CI 0.67–0.76) compared with high-grade (RR = 0.78; 95% CI: 0.67–0.90) and advanced disease (RR = 0.85; 95% CI: 0.75–0.97).24 Similarly, a meta-analysis of summary data reported a pooled RR of prostate cancer for the highest versus lowest category of fasting blood glucose of 0.88 (95% CI: 0.78–0.98, n = 15 studies).25 A large meta-analysis with bias analysis reported a RR of 0.83 (95% CI: 0.79–0.88) for associations between diabetes and prostate cancer incidence, but bias analysis for unmeasured confounding suggested that this association was unlikely to be causal.23
A Mendelian randomisation (MR) approach using genetic variants as proxies for glycaemic traits did not find strong evidence for the role of fasting glucose, glycated haemoglobin and diabetes in prostate cancer.26 Assuming that all assumptions for MR analysis were met, this suggests that the observed associations in our study are potentially not causal. Men with diabetes have a lower level of prostate-specific antigens than men without it,27 which may lead to the less frequent diagnosis of prostate cancer, in particular for low-grade and localised disease.
In contrast to some previous observational studies,21,28 we found no evidence for an association between diabetes and the risk of postmenopausal breast cancer. Nevertheless, our findings are consistent with an MR analysis, which also showed lack of association between genetically predicted diabetes and other glycaemic traits, and breast cancer risk.26 In the meta-analysis of Ling et al., bias analysis also suggested that associations between diabetes and breast cancer risk were unlikely to be causal.23
BMI and physical activity are modifiable factors that have been consistently related to risk of both diabetes29,30 and cancers.12,31 The strength of the association between diabetes and cancer risk may vary according to BMI and levels of physical activity.32 A prospective study reported that the increased risk of cancers associated with diabetes was limited to people less physically active or overweight/obese.33 In that study, diabetes was associated with risk of overall cancer with an HR of 1.15 (95% CI: 1.01–1.31) among men and women with diabetes, who reported low levels of physical activity (<2.0 h per week) and 1.21 (95% CI: 1.07–1.37) among overweight or obese people (BMI ≥ 25 kg/m2).33 In our study, the associations for diabetes with colorectal and breast cancer risks were generally similar across BMI categories, whereas, for total and prostate cancer, associations were stronger with higher BMI, suggesting that the interaction between BMI and diabetes status may have different consequences according to tissue type or organ. Consistent with this finding, Fall et al.34 also observed a strong inverse association with prostate cancer among men with diabetes and a high BMI. We note that BMI was considered as a common cause of both diabetes and cancer (i.e., a confounder). In an alternative and possibly less likely scenario, BMI could be a mediator of the diabetes–cancer association (e.g., by diabetes therapy leading to weight gain); stratifying on BMI would then return an estimate of the direct effect of diabetes on cancer risk, which is less straightforward to interpret. We found some evidence that vigorous physical activity appears to offset some of the positive associations between diabetes and risk of total cancer (Supplementary Table 5). However, after multiple testing correction, the confidence interval of the interaction term included the null. A similar lack of power (Supplementary Text 1) impacts our exploratory subgroup analysis of diabetes associated with total cancer across BMI categories, and therefore follow-up studies are required.
We found little evidence that cancer risk associated with diabetes varied across age groups in our study (Supplementary Table 3). However, the focus of the CHANCES consortium was on older adults, which resulted in a relatively narrow age range of individuals at recruitment (inter-decile range: 53–69 years).
It has been hypothesised that associations between diabetes and cancer may be direct, or indirect due in part to shared predisposing risk factors, such as obesity and physical inactivity.8 Indeed, plausible biologic mechanisms implicated in cancer initiation and progression include hyperinsulinaemia (either endogenous due to insulin resistance or exogenous due to administered insulin), hyperglycaemia and chronic inflammation.35 Insulin activates a number of signalling pathways, which may lead to cellular proliferation.36 Hyperglycaemia can affect tumour cells by increasing proliferation, inducing mutations35,37 and inducing the production of circulating growth factors (insulin/IGF-1) and inflammatory cytokines,38 which is associated with increased cancer risk.39 Investigations using detailed information on glycaemic control could help provide insight into the mechanisms behind the diabetes–cancer link. The inverse association observed between diabetes and prostate cancer may be explained by reduced testosterone levels among men with diabetes,40 although residual confounding cannot be excluded.
This study has several strengths, including the large study size and a large number of cancer cases among diabetes patients (11,176), thus providing sufficient statistical power for most analyses, and the prospective design of the cohorts with the availability of individual-level data allowing for data harmonisation and homogenous adjustment for confounding.
The results of this study should be interpreted in light of the following limitations. Site-specific data on incident cancer were only available in three (prostate cancer) and four (breast and colorectal cancers) of the seven cohorts, respectively. Second, Cox PH regression assumes that all individuals under observation experience either the primary outcome (here cancer) or non-informative censoring,41 which may not be best suited to estimating predictive relationships. We were, however, not able to estimate absolute risks due to a lack of data on specific censoring events. Instead, we estimated cause-specific hazard ratios to answer the aetiological question of whether the incidence of total cancer, and of common site-specific cancers, is higher among people with diabetes as compared to people without diabetes. This approach returns the total effect of the association between diabetes and the risk of cancer that is not mediated through other conditions. This should be a valid cause-specific estimate for relative risk, irrespective of whether people with diabetes also developed another condition, such as cardiovascular disease, prior or after diabetes. We acknowledge the lack of information on the duration and treatment of diabetes. Some treatments, in particular, biguanide metformin, may reduce cancer incidence.13 However, Kowal et al. provided evidence that metformin has no protective effect on the incidence of cancer among individuals with diabetes,42 or specifically with prostate cancer risk.43 No information was available about dynamic changes in or cumulative effects of risk factors such as BMI and physical activity during follow-up or about the subsequent initiation of medications that might affect cancer incidence. While this may have affected the identification of interactions, we doubt that any such effects could offer plausible explanations for the disparate findings across cancer sites and would rather attenuate any observed risk estimates. The disproportionate weight of the large NIH-AARP study, which contributed to a much larger number of cancer cases than the other studies combined, may also be a potential limitation. To counter this, a random-effects model was used in the main analysis, which gives smaller studies a relatively larger weight in the meta-analysis. However, the reported results of the supplementary fixed-effect meta-analysis and of the effect modification in the pooled analysis should be interpreted with caution because these findings may be driven by the NIH-AARP study. In some of the included cohorts, diabetes was assessed by self-report. However, sensitivity analysis revealed similar associations with total cancer when subdividing cohorts by diabetes ascertainment (self-reported versus documented). We also cannot exclude the possibility of residual confounding from unmeasured confounding factors, including diet, blood lipid concentrations and blood pressure, and by incomplete adjustment for physical activity, because both moderate-to-vigorous and light-intensity physical activity have also been shown to reduce cancer mortality44 and the risk of breast45 and total cancer.46
Conclusions
Overall, our analysis among older populations in Europe and the United States did not demonstrate an association between diabetes and the risk of all cancers combined or postmenopausal breast cancer. We corroborate and extend previous evidence that diabetes appears to be a risk factor for colorectal cancer among older populations. Our observed inverse association with prostate cancer risk contrasts with findings using an instrumental variable approach. Further research is needed to confirm our findings and to consider the duration and treatment of diabetes.
Supplementary information
Acknowledgements
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this paper and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Author contributions
A.A., H.F., M.J. and I.R. conceived and designed the work. A.A. performed data analysis. I.R. and H.F. contributed to data analysis. K.K.T., A.T., P.B., B.V.G., O.M., T.W., F.K., B.S., J.M.O.M., S.M., S.S., R.C.H.V., J.R.Q., L.M.M., R.S., K.K. and H.B. acquired the data, and all authors played an important role in interpreting the results. A.A., H.F., M.J. and I.R. drafted the paper. K.K.T., A.T., P.B., B.V.G., O.M., T.W., F.K., B.S., J.M.O.M., S.M., S.S., R.C.H.V., J.R.Q., L.M.M., R.S., K.K. and H.B. revised the paper. All authors approved the final version of the paper and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H.F. had full access to the data in the study and final responsibility for the decision to submit for publication.
Ethics approval and consent to participate
All studies participating in CHANCES were conducted in accordance with the Declaration of Helsinki. For each study, investigators satisfied the local requirements for ethical research, including obtaining informed consent from participants.
Consent to publish
Not applicable.
Data availability
The CHANCES participating cohorts’ data are available only to the collaborating scientists from the respective CHANCES participating centres. The data may be available upon request for some of the participating centres but not for all due to relevant data protection laws.
Competing interests
The authors declare no competing interests.
Funding information
Data used throughout the present study are derived from the CHANCES project. The project is coordinated by the Hellenic Health Foundation, Greece. The project received funding by the FP7 framework programme of DG-RESEARCH in the European Commission (grant agreement no. HEALTH-F3-2010-242244). EPIC Greece: funded by the Hellenic Health Foundation. EPIC Netherlands: funded by European Commission (DG SANCO), Dutch Ministry of Public Health, Welfare and Sports (VWS), The National Institute for Public Health and the Environment, the Dutch Cancer Society, the Netherlands Organisation for Health Research and Development (ZONMW) and World Cancer Research Fund (WCRF). EPIC Spain: supported by Health Research Fund (FIS) of the Spanish Ministry of Health RTICC ‘Red Temática de Investigación Cooperativa en Cáncer (grant numbers: Rd06/0020/0091 and Rd12/0036/0018), Regional Governments of Andalucía, Asturias, Basque Country, Murcia (project 6236) and Navarra, Instituto de Salud Carlos III, Redes de Investigacion Cooperativa (RD06/0020). EPIC Umea: funded by Region Västerbotten and the Swedish Research Council. ESTHER: funded by the Baden-Württemberg State Ministry of Science, Research and Arts (Stuttgart, Germany), the Federal Ministry of Education and Research (Berlin, Germany) and the Federal Ministry of Family Affairs, Senior Citizens, Women and Youth (Berlin, Germany). Data from FINRISK, Belfast and Northern Sweden were harmonised for this analysis in the MOnica Risk, Genetics, Archiving and Monograph (MORGAM) Project. The activities of the MORGAM Data Centre have been sustained also by recent funding from European Union FP7 project BiomarCaRE (HEALTH-F2-2011-278913). PRIME Belfast: supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), the Merck, Sharp & Dohme-Chibret Laboratory and the Northern Ireland Health & Social Care Research and Development Office. Northern Sweden MONICA Study: funded by Umeå University and the county councils of Norr and Västertbotten. Tromsø: funded by UiT The Arctic University of Norway, the National Screening Service and the Research Council of Norway. This study was in part funded by the World Cancer Research Fund (WCRF UK) as part of the World Cancer Research Fund International grant programme (IIG_2019_1978, Principal Investigator, Heinz Freisling).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01347-4.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61:2140–2154. doi: 10.1007/s00125-018-4664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouveri E, Papanas N, Maltezos E. The female breast and diabetes. Breast. 2011;20:205–211. doi: 10.1016/j.breast.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 6.Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16:151–158. doi: 10.1038/pcan.2012.40. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J. Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 9.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 10.Steindorf K, Ritte R, Eomois P-P, Lukanova A, Tjonneland A, Johnsen NF, et al. Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition. Int. J. Cancer. 2013;132:1667–1678. doi: 10.1002/ijc.27778. [DOI] [PubMed] [Google Scholar]
- 11.Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J. Oncol. 2013;2013:906495. doi: 10.1155/2013/906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Boffetta P, Bobak M, Borsch-Supan A, Brenner H, Eriksson S, Grodstein F, et al. The Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) project–design, population and data harmonization of a large-scale, international study. Eur. J. Epidemiol. 2014;29:929–936. doi: 10.1007/s10654-014-9977-1. [DOI] [PubMed] [Google Scholar]
- 15.Lai GY, Park Y, Hartge P, Hollenbeck AR, Freedman ND. The association between self-reported diabetes and cancer incidence in the. J. Clin. Endocrinol. Metab. 2013;98:E497–E502. doi: 10.1210/jc.2012-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy [Internet]. (WHO Guidelines Approved by the Guidelines Review Committee). http://www.ncbi.nlm.nih.gov/books/NBK169024/ (WHO, 2013). [PubMed]
- 17.FIN-EAS and FIN-WES: FINRISK Study [Internet]. https://www.thl.fi/publications/morgam/cohorts/full/finland/fin-fina.htm#dprocedure (2020).
- 18.Tsilidis, K. K., Papadimitriou, N., Capothanassi, D., Bamia, C., Benetou, V., Jenab, M. et al. Burden of Cancer In A Large Consortium Of Prospective Cohorts in Europe. J. Natl Cancer Inst.108, https://academic.oup.com/jnci/article/108/10/djw127/2412485 (2016). [DOI] [PubMed]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Dankner R, Boffetta P, Balicer RD, Boker LK, Sadeh M, Berlin A, et al. Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2.3 million adults. Am. J. Epidemiol. 2016;183:1098–1106. doi: 10.1093/aje/kwv290. [DOI] [PubMed] [Google Scholar]
- 22.Peila R, Rohan TE. Diabetes, glycated hemoglobin, and risk of cancer in the UK biobank study. Cancer Epidemiol. Biomark. Prev. 2020;29:1107–1119. doi: 10.1158/1055-9965.EPI-19-1623. [DOI] [PubMed] [Google Scholar]
- 23.Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, et al. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43:2313–2322. doi: 10.2337/dc20-0204. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Jiang H, Ding G, Zhang H, Zhang L, Mao S, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2013;99:241–249. doi: 10.1016/j.diabres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Jayedi A, Djafarian K, Rezagholizadeh F, Mirzababaei A, Hajimohammadi M, Shab-Bidar S. Fasting blood glucose and risk of prostate cancer: a systematic review and meta-analysis of dose-response. Diabetes Metab. 2018;44:320–327. doi: 10.1016/j.diabet.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Au Yeung SL, Schooling CM. Impact of glycemic traits, type 2 diabetes and metformin use on breast and prostate cancer risk: a Mendelian randomization study. BMJ Open Diabetes Res. Care. 2019;7:e000872. doi: 10.1136/bmjdrc-2019-000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dankner R, Boffetta P, Keinan-Boker L, Balicer RD, Berlin A, Olmer L, et al. Diabetes, prostate cancer screening and risk of low- and high-grade prostate cancer: an 11 year historical population follow-up study of more than 1 million men. Diabetologia. 2016;59:1683–1691. doi: 10.1007/s00125-016-3972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 29.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Meyerhardt JA, Giovannucci E, Jeon JY. Association between body mass index and prognosis of colorectal cancer: a meta-analysis of prospective cohort studies. PLoS ONE. 2015;10:e0120706. doi: 10.1371/journal.pone.0120706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freisling H, Arnold M, Soerjomataram I, O’Doherty MG, Ordonez-Mena JM, Bamia C, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br. J. Cancer. 2017;116:1486–1497. doi: 10.1038/bjc.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PJHL Peeters, Bazelier MT, Leufkens HGM, de Vries F, De Bruin ML. The risk of colorectal cancer in patients with type 2 diabetes: associations with treatment stage and obesity. Diabetes Care. 2015;38:495–502. doi: 10.2337/dc14-1175. [DOI] [PubMed] [Google Scholar]
- 33.Moe B, Nilsen TIL. Cancer risk in people with diabetes: Does physical activity and adiposity modify the association? Prospective data from the HUNT Study, Norway. J. Diabetes Complicat. 2015;29:176–179. doi: 10.1016/j.jdiacomp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Fall K, Garmo H, Gudbjornsdottir S, Stattin P, Zethelius B. Diabetes mellitus and prostate cancer risk; a nationwide case-control study within PCBaSe Sweden. Cancer Epidemiol. Biomark. Prev. 2013;22:1102–1109. doi: 10.1158/1055-9965.EPI-12-1046. [DOI] [PubMed] [Google Scholar]
- 35.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr. Relat. Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 36.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014;38:330–336. doi: 10.4093/dmj.2014.38.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C-X, Zhu H-H, Zhu Y-M. Diabetes and cancer: associations, mechanisms, and implications for medical practice. World J. Diabetes. 2014;5:372–380. doi: 10.4239/wjd.v5.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rastmanesh R, Hejazi J, Marotta F, Hara N. Type 2 diabetes: a protective factor for prostate cancer? An overview of proposed mechanisms. Clin. Genitourin. Cancer. 2014;12:143–148. doi: 10.1016/j.clgc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol. Drug Saf. 2015;24:865–874. doi: 10.1002/pds.3823. [DOI] [PubMed] [Google Scholar]
- 43.Feng Z, Zhou X, Liu N, Wang J, Chen X, Xu X. Metformin use and prostate cancer risk: a meta-analysis of cohort studies. Medicine. 2019;98:e14955. doi: 10.1097/MD.0000000000014955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu S, Cai X, Wu T, Sun Z, Guo H, Kirsten J, et al. Objectively-measured light-intensity physical activity and risk of cancer mortality: a meta-analysis of prospective cohort studies. Cancer Epidemiol. Biomark. Prev. 2020;29:1067–1073. doi: 10.1158/1055-9965.EPI-19-1446. [DOI] [PubMed] [Google Scholar]
- 45.Dallal CM, Brinton LA, Matthews CE, Lissowska J, Peplonska B, Hartman TJ, et al. Accelerometer-based measures of active and sedentary behavior in relation to breast cancer risk. Breast Cancer Res. Treat. 2012;134:1279–1290. doi: 10.1007/s10549-012-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dohrn I-M, Welmer A-K, Hagströmer M. Accelerometry-assessed physical activity and sedentary time and associations with chronic disease and hospital visits—a prospective cohort study with 15 years follow-up. Int. J. Behav. Nutr. Phys. Act. 2019;16:125. doi: 10.1186/s12966-019-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CHANCES participating cohorts’ data are available only to the collaborating scientists from the respective CHANCES participating centres. The data may be available upon request for some of the participating centres but not for all due to relevant data protection laws.