Abstract
Electrochemistry has a lot of inherent advantages in organic synthesis and many redox reactions have been achieved under electrochemical condition. However, the electrochemical C−C bond cleavage and functionalization reactions are less studied. Here we develop electrochemical C−C bond cleavage and 1,3-difuntionalization of arylcyclopropanes under catalyst-free and external-oxidant-free conditions. 1,3-difluorination, 1,3-oxyfluorination and 1,3-dioxygenation of arylcyclopropanes are achieved with a high chemo- and regioselectivity by the strategic choice of nucleophiles. This protocol has good functional groups tolerance and can be scaled up. Mechanistic studies demonstrate that arylcyclopropane radical cation obtained from the anode oxidation and the subsequently generated benzyl carbonium are the key intermediates in this transformation. This development provides a scenario for constructing 1,3-difunctionalized molecules.
Subject terms: Chemical synthesis, Electrochemistry
Electrochemical oxidative C–C bond cleavage and functionalization are rarely developed due to the inertness and weak electronic bias of C–C bonds. In this study, the authors report the electrochemical C–C bond cleavage and 1,3-difluorination, 1,3-oxyfluorination and 1,3-dioxygenation of arylcyclopropanes under catalyst-free and external-oxidant-free conditions.
Introduction
C–C bonds are the basic skeleton of organic compounds and the direct functionalization via C–C bond deconstruction is quite meaningful for synthesis of complex molecules1,2. Cyclopropanes are important building blocks. The ring-opening of cyclopropanes driven by the release of ring strain has been widely applied in total synthesis3,4. Donor–acceptor cyclopropanes (DACs), which are activated by vicinal electron-donating and electron-withdrawing groups are predisposed to ring-opening under Lewis acid catalysis due to the inherent electronic bias5–7, whereas non-activated cyclopropanes, which are more regular in nature, are reluctant to ring-opening due to insufficient electronic bias. There are two methods for ring-opening of non-activatied cycolpropanes. One relies on oxidative addition by transition metals. However, these reaction are limited to ring-opening rearrangement or cycloaddition reactions and requires specific directing groups for regioselective ring-opening functionalization8. The other one relies on electrophilic activation with Lewis acidic species. However, most of the transformations are limited to electrophilic addition reactions9–17. Ring-opening functionalization of arylcyclopropanes initiated through the single electron oxidation followed by the yield of corresponding radical cations was discovered in the 1970s18,19. In recent years, this strategy was further applied in 1,3-aminofunctionalization, 1,3-oxoamination, and 1,3-oxochlorination of arylcycropropanes in the presence of oxidants or light. However, in most cases, its large scalability application was not studied and arylcycropropanes with electron-withdrawing groups could not be well compatible due to their high oxidative potential (Fig. 1a)20–24.
Fig. 1. 1,3-difunctionalization of cyclopropanes based on radical cation mechanism.
a Thermochemical and photochemical strategy. b Shono’s work. c This work: Electrochemical 1,3-difunctionalization of arylcyclopropanes (PC photoredox catalyst).
Organic electrochemistry is reviving due to their effortlessness of scalability, avoidance of stoichiometric oxidants or reducing agents, and flexible reaction tunability25. Various redox reactions have been achieved by the comsumption of traceless electrons under constant potential or current conditions26–38. As a main part of preparative electrosynthesis, anode processes such as C–H functionalization, oxidative coupling, decarboxylation, and olefin functionalization has been developed39–48. However, electrochemical oxidative C−C bond cleavage/functionalization are rarely developed due to the inertness and weak electronic bias of C−C bonds, which are always encumbered by other bonds8,48,49. Pioneering work was disclosed by Shono and coworkers who reported anodic oxidation of arylcyclopanes in methanol50. But only six examples were presented in this report (Fig. 1b). Our design for electrochemical C−C bond cleavage/functionalization based on the following mechanistic proposal (Fig. 1c): Firstly, arylcyclopropane is oxidized to a radical cation by anode, which results in the weakening of the Cα–Cβ bond, as the BDE of Cα–Cβ bond decreases more than 30 kcal/mol from the neutral cyclopropane to the corresponding radical cation51. Then the radical cation undergoes three-electron SN2 reaction to generate a benzyl radical52. Different from reported thermochemical and photochemical strategy, the benzyl radical can further lose one electron at anode and converted to a benzyl carbonium under electrochemical conditions43. The following nucleophilic attack to the benzyl carbonium can finally yield the 1,3-difunctionalization product. Fluorinated products could be prepared by employing Et3N·3HF as nucleophilic fluorine source53–58. In this work, we develop the electrochemical 1,3-difluorination, 1,3-oxyfluorination, and 1,3-dioxygenation of arylcyclopropanes with a high chemoselectivity and regioselectivity by the strategic choice of nucleophiles. Moreover, a wide variety of arylcyclopropanes with electron-donating and electron-withdrawing groups could be converted to the 1,3-difunctionalized molecules by following this protocol.
Results
Investigation of reaction conditions
We began our investigation by exploring the selective synthesis of 1,3-difluorination, 1,3-oxyfluorination, and 1,3-dioxygenation products from phenylcyclopropane. After extensive screening of various conditions (for more details, see Supplementary Table 1–5), with the use of Et3N·3HF as a fluorine source, 1,3-difluorination product 2 was obtained in 77% yield by conducting the electrolysis under constant current of 16 mA in an undivided cell equipped with platinum plate as both anode and cathode (Fig. 2, Entry 1). 1,3-oxyfluorination product 3 was obtained in 47% yield with the concomitant formation of other three 1,3-difuntionalization products under the existence of both Et3N·3HF and MeOH (Fig. 2, Entry 2). The yield of 1,3-oxyfluorination product 3 increased slightly when the reaction temperature decreased to 0 °C (Fig. 2, Entry 3). An obviously improved yield was observed by using carbon cloth as anode materials (Fig. 2, Entry 4). The influence of the concentration of phencyclopropane to the reaction could be neglected (Fig. 2, Entry 5). 1,3-oxyfluorination product 3 was finally observed in 76% yield by adjusting the ratio of Et3N·3HF/MeOH (Fig. 2, Entry 6). The good selectivity of the 1,3-oxyfluorination product 3 was speculated to be controlled by kinetics. At the first step, the reaction rate between MeOH and arylcyclopropane radical cation is larger than the reaction rate between Et3N·3HF and arylcyclopropane radical cation, which possibly due to the lower nucleophilicity of Et3N·3HF than MeOH57. Therefore, arylcropropane radical cation mainly reacts with MeOH instead of Et3N·3HF. According to the rate constant equation proposed by Mayr et al, the reaction rate of benzyl carbonium with MeOH or Et3N·3HF are both very fast and determined by diffusion rate59,60, so the fluorination is the major process in the second step because of the excess amount of fluorine source compared with methanol in the reaction system. Further condition screening demonstrated that 1,3-dioxygenation products could be obtained in 95% yield under the electrolysis in MeOH with Bu4NBF4 as supporting electrolyte (Fig. 2, Entry 7).
Fig. 2. Optimization of the reaction conditionsa.
aReaction conditions: electrode surface (1.5 × 1.5 cm2, J = 7.1 mA/cm2), 16 mA, undivided cell, 1 (0.5 mmol), PhCF3 (4.8 mL), 2 h, 2.4 F mol−1 (based on 1). bGC Yield using biphenyl as internal standard. c 1 (0.25 mmol), 1 h. dWithout PhCF3, Bu4NBF4 (0.25 mmol) was added as supporting electrolyte (CC Carbon cloth).
Substrate scope
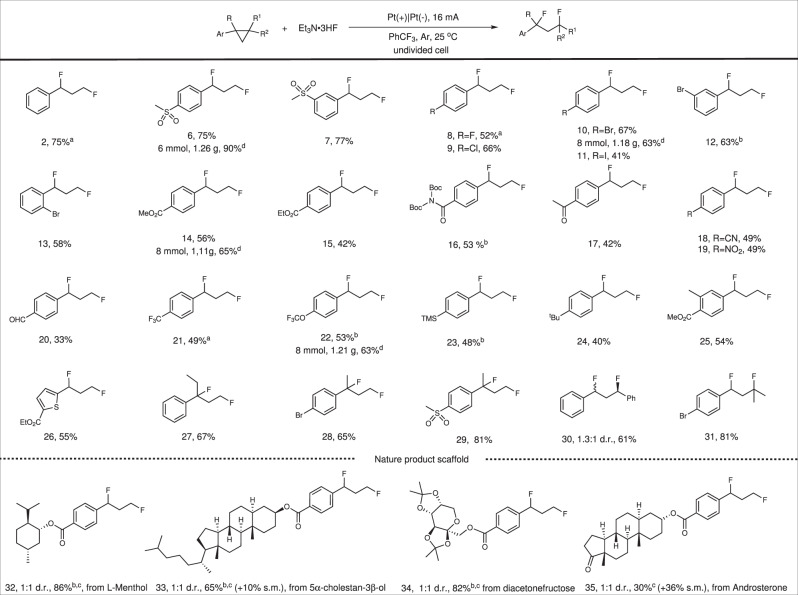
To investigate the substrate scope of the 1,3-difluorination reaction, we tested a wide range of arylcyclopropanes under the optimized condition (Fig. 3). Phenylcyclopropane gave 75% NMR yield (2). Methanesulfonyl group no matter at para or meta position was well amenable to this protocol in good yields (6 and 7). Halogen groups such as F, Cl, Br, and I were also compatible with this transformation (8–12), which provided an opportunity to further transformation through coupling reactions. Ortho substituted arylcyclopropane was also suitable reaction partner, furnishing the desired 1,3-difluorination product with 58% yield (13). Arylcyclopropanes with electron-withdrawing groups (such as ester, amide, acetyl, cyano, nitro, and aldehyde groups) were also amenable, furnishing the desired products in 42–56% yields by prolonging the reaction time (14–20), although these substrates have higher oxidative potential. However, Arylcyclopropanes with electron-donating groups (such as –OMe, SMe, OPh, and NHBoc) were not amenable in this transformation (for more details, see Supplementary Fig. 3). Trifluoromethyl and oxytrifluoromethyl substituted arylcyclopropanes gave the corresponding products in 49% and 53% yields, respectively (21–22). TMS group was retained in this transformation with 52% yield (23). Alkyl group such as tert-butyl group substituted arylcyclopropanes underwent the reaction in lower yield of 40% (24). 3-Methyl-4-ester substituted arylcyclopropane provided the desired product with 54% yield (25). Moreover, heterocyclic arylcyclopropane such as thienyl cyclopropane was also suitable for this reaction (26). Then, the feasibility of 1,1-disubstituted cyclopropanes and 1,2-disubstituted cyclopropanes were also tested. 1,1-disubstituted cyclopropanes were good reaction partners to this reaction, giving the 1,3-difluorination products with tertiary-carbon-fluorine bonds formation in good yields (27–29). Trans-1,2-diphenylcyclopropane provided 1,3-difluorination products with 61% yield and 1.3:1 diastereoselectivity (30) since 1,2-diphenylcyclopropane radical cation was once determined to be open geometries51. Tri-substituted cyclopropane was also tolerated in this transformation (31). Moreover, arylcyclopropanes containing complex nature product scaffolds were also compatible in this transformation. l-menthol scaffold substrate provided the desired product in 86% yield (32). 5α-cholestan-3β-ol scaffold substrate provided the coresponding product in 65% yield with 10% starting material recoverd (33). Protected sugar group was also compatible with 82% yield was obtained (34). Androsterone scaffold substrate gave the desired product in 33% yield with 36% starting material recoverd (35). The compatibility of these complex substrates further confirms the practicality of this transformation.
Fig. 3. Substrate scope of 1,3-difluorination reaction.
Reaction condition: Cyclopropanes (0.25 or 0.5 mmol), Et3N·3HF (1.2 mL), PhCF3 (4.8 mL), 16 mA, 40 min–20 h, isolated yields, electrode surface (1.5 × 1.5 cm2, J = 7.1 mA/cm2), undivided cell. aNMR yield using 1-fluoronaphthalene as internal standard. b0.5 mL DCE was added. c0.1 mmol scale, 2–6 h. s.m. starting material. dLarge-scale synthesis condition: carbon cloth anode, nickel foam cathode, cyclopropanes (6 or 8 mmol), Et3N·3HF (2.4 mL), PhCF3 (9.6 mL), 25–75 h, isolated yields. For more details, see Supplementary Experimental section.
The scalability of this method was demonstrated by using carbon cloth as anode and nickel foam as cathode instead of expensive platinum electrode with equivalent amount of Et3N·3HF (Fig. 3). The yields increased for most of the tested substrates when operated at gram scale. The yields increased from 75% to 90% for para-methanesulfonyl substituted phenylcyclopropane (6). The yields increased to 65% and 63% for ester (14) and oxytrifluoromethyl (22) substituted arylcyclopropanes. The yields would decrease slightly from 67% to 63% only when it came to 4-bromocyclopropylbenzene (10).
The scope of the 1,3-oxyfluorination reaction was also explored, as shown in Fig. 4. Para-bromine substituted phenylcyclopropane was compatible to give the target product in moderate yield (36). Both electro-donating group and electro-withdrawing groups could be tolerated in this transformation (37–40). Meta-trifluoromethyl substituted arylcyclopropane generated 1,3-oxyfluorination product 39 in 53% yield. This 1,3-oxyfluorination product could be potentially converted to fluorinated Cinacalcet following the reported methods61,62. Subsequently, the scope of the alcohols were examined as well. Alcohols such as EtOH, n-BuOH and long-chain primary alcohols (n-C6H13OH and n-C8H17OH) were transformed into desired products with good efficiency (41–44). 2-Chloro-1-ethanol afforded the desired product in 40% yield (45). Secondary alcohols (i-PrOH and cyclohexanol) were suitable substrates (46–47). Sterically hindered tertiary alcohol was also tolerant (48). In addition to alcohols, ethers could also participate in this reaction (Fig. 4). 1,3-Oxyfluorination could proceed smoothly when using ether as both oxygen nucleophiles and solvent (49–51). Cyclic ether such as tetrahydrofuran gave interesting product 49 in 43% yields accompanying with 14% 1,3-difluorination product. 1,2-Dimethoxyethane gave 1,3-oxyfluorination product 50 in 44% yield. The reaction system was separated into two phases and not conductive when using tetrahydropyran as both reactant and solvent. To solve the problem of solubility and conductivity, DCE was added to enhance the solubility and 1,3-oxyfluorination product 51 was obtained in 36% yield. Notably, C–O etheric bond was broken in these transformations. We proposed that oxonium is the key intermediate for cleavage of C–O etheric bond (for more details, see Supplementary Fig. 5)63,64. Finally, the scope of the 1,3-dioxygenation reaction was investigated (Fig. 4). Arylcyclopropanes with electron-donating groups (–OMe, –OPh) gave 74% and 93% yields, respectively (52–53). Para tert-butyl substituted arylcyclopropane underwent the reaction in 85% yield (54). Both TMS and halogen groups could be tolerated (55–56). The electronically deficient arylcyclopropane could also give 1,3-dioxygenation products with high yields (57–58).
Fig. 4. Substrate scope of 1,3-oxyfluorination and 1,3-dioxygenation reaction.
aCyclopropanes (0.25 mmol), Et3N·3HF (0.8 mL), ROH (0.2–0.75 mL), PhCF3 (4.8 mL), 16 mA, 1–2 h, isolated yields. bCyclopropanes (0.5 mmol), Et3N·3HF (1.2 mL), ether (4.8 mL), 4 h, isolated yields. c One milliliter of DCE was added. dCyclopropanes (0.25 mmol), Bu4NBF4 (0.25 mmol), MeOH (6 mL), 16 mA, 50 min−1h, isolated yields. For more details, see Supplementary Experimental section.
Discussion
To gain more insights into the aforementioned transformation, several mechanistic studies were conducted. Redox potentials of the cyclopropanes were tested by cyclic voltammetry experiments (Fig. 5a). The alkyl substituted cyclopropane 1c has a high oxidation potential and no oxidation peak can be observed under 3 V. In contrast, arylcyclopropanes even with strong electron withdrawing group (−NO2) have relatively lower oxidative potentials (Ep/2 = 1.96 V vs. Ag/AgCl/KClsat for 1 and Ep/2 = 2.32 V for 1a vs. Ag/AgCl/KClsat). These results illustrate that aryl group is crucial for the oxidation of the cyclopropane substrates. The charge distribution of phenylcyclopropane radical cation has been studied by DFT calculation (Fig. 5b). The results show that distal C atom in cyclopropane motif possess partial positive charge and are the potential nucleophilic attack sites. Considering the reaction between benzyl radical and dioxygen was very fast (rate constant 2.8 × 109 L mol−1 s−1)65, the reaction was conducted in the dioxygen atmosphere in order to trap the possible benzyl radical intermediate. The detection of the oxygenation products is highly inductive of the formation of the benzyl radical during the reaction (Fig. 5c). In addition, the existence of benzyl radical was also evidenced by the trapping experiment using BrCCl366,67. Furthermore, the electrolysis of phenylcyclopropane and Et3N·3HF in CH3CN resulted in the isolation of amidation product 59, which suggested the involvement of benzyl carbonium intermediate during the reaction (Fig. 5d)68,69.
Fig. 5. Mechanistic studies.
a Cyclic voltamemetry studies. b Electrostatic potential surface and charge of phenylcyclopropane radical cation (1·+). c Trap of benzyl radical intermediate. d Trap of benzyl carbonium intermediate.
In conclusion, we have developed a electrochemical C–C bond cleavage of arylcyclopropanes, enabling 1,3-difunctionalization of arylcyclopropanes to yield 1,3-difluorination, 1,3-oxyfluorination, and 1,3-dioxygenation products. Neither additional oxidant or catalyst were needed in this transformation. Productive gram-scale 1,3-difluorination reaction was conducted by using stoichiometric amount of commercial available Et3N·3HF as fluorine source. Mechanistic studies show that arylcyclopropane radical cation and benzyl carbonium play paramount role in this reaction. This study provides a simple strategy for constructing 1,3-difunctionalized molecules.
Methods
General procedure (2)
An oven-dried undivided three-necked bottle equipped with a stir bar. The bottle was equipped with platinum plate (15 mm × 15 mm × 0.3 mm) as both the anode and cathode and then charged with argon gas in glove box. Phenylcyclopropane (0.5 mmol), Et3N·3HF (1.2 mL) and PhCF3 (4.8 mL) were added. The reaction mixture was stirred and electrolyzed at a constant current of 16 mA at 25 °C for 2 h. The reaction was diluted with water. The organic layer was extracted with CH2Cl2, dried with anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The pure product was obtained by flash column chromatography on silica gel. Full experimental details can be found in the Supplementary Methods.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22031008, 21520102003) and the Hubei Province Natural Science Foundation of China (2017CFA010). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated. The numerical calculations in this paper have been done on the supercomputing system in the Supercomputing Center of Wuhan University.
Author contributions
A.L. and P.P. conceived the project and designed the experiments. P.P. developed the reactions, and contributed to the reaction scope and mechanistic studies. X.Y., K.Z., Z.L., and Y.C. contributed to the reaction scope and participated in the substrates synthesis. L.Z. and H.Z. discussed the results. H.Z. performed the theoretical calculation. P.P., H.Z., and A.L. wrote the paper, Supplementary methods, and related materials.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Wei Liu and the other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heng Zhang, Email: hengzhang@whu.edu.cn.
Aiwen Lei, Email: aiwenlei@whu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23401-8.
References
- 1.Roque JB, Kuroda Y, Göttemann LT, Sarpong R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science. 2018;361:171. doi: 10.1126/science.aat6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia Y, Lu G, Liu P, Dong G. Catalytic activation of carbon–carbon bonds in cyclopentanones. Nature. 2016;539:546–550. doi: 10.1038/nature19849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salaun J. Optically active cyclopropanes. Chem. Rev. 1989;89:1247–1270. [Google Scholar]
- 4.Wong HNC, et al. Use of cyclopropanes and their derivatives in organic synthesis. Chem. Rev. 1989;89:165–198. [Google Scholar]
- 5.Reissig H-U, Zimmer R. Donor−acceptor-substituted cyclopropane derivatives and their application in organic synthesis. Chem. Rev. 2003;103:1151–1196. doi: 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]
- 6.Yu M, Pagenkopf BL. Recent advances in donor–acceptor (DA) cyclopropanes. Tetrahedron. 2005;61:321–347. [Google Scholar]
- 7.Schneider TF, Kaschel J, Werz DB. A new golden age for donor–acceptor cyclopropanes. Angew. Chem. Int. Ed. 2014;53:5504–5523. doi: 10.1002/anie.201309886. [DOI] [PubMed] [Google Scholar]
- 8.Souillart L, Cramer N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- 9.Danishefsky S. Electrophilic cyclopropanes in organic synthesis. Acc. Chem. Res. 1979;12:66–72. [Google Scholar]
- 10.Lambert JB, Napoli JJ, Johnson KK, Taba KN. Scope, limitations, and mechanism of the homoconjugate electrophilic addition of hydrogen halides. J. Org. Chem. 1985;50:1291–1295. [Google Scholar]
- 11.Morton JGM, Dureen MA, Stephan DW. Ring-opening of cyclopropanes by “frustrated Lewis pairs”. Chem. Commun. 2010;46:8947–8949. doi: 10.1039/c0cc02862b. [DOI] [PubMed] [Google Scholar]
- 12.Rösner C, Hennecke U. Homohalocyclization: electrophilic bromine-induced cyclizations of cyclopropanes. Org. Lett. 2015;17:3226–3229. doi: 10.1021/acs.orglett.5b01315. [DOI] [PubMed] [Google Scholar]
- 13.Banik SM, Mennie KM, Jacobsen EN. Catalytic 1,3-difunctionalization via oxidative C–C bond activation. J. Am. Chem. Soc. 2017;139:9152–9155. doi: 10.1021/jacs.7b05160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilchenko NO, Hedberg M, Szabó KJ. Fluorinative ring-opening of cyclopropanes by hypervalent iodine reagents. An efficient method for 1,3-oxyfluorination and 1,3-difluorination. Chem. Sci. 2017;8:1056–1061. doi: 10.1039/c6sc03471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gieuw MH, Ke Z, Yeung Y-Y. Lewis base-promoted ring-opening 1,3-dioxygenation of unactivated cyclopropanes using a hypervalent iodine reagent. Angew. Chem. Int. Ed. 2018;57:3782–3786. doi: 10.1002/anie.201713422. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Xue X-S, Houk KN, Shi Z. Mild ring-opening 1,3-hydroborations of non-activated cyclopropanes. Angew. Chem. Int. Ed. 2018;57:16861–16865. doi: 10.1002/anie.201811036. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z-Y, et al. B(C6F5)3-catalyzed ring opening and isomerization of unactivated cyclopropanes. Angew. Chem. Int. Ed. 2017;56:4028–4032. doi: 10.1002/anie.201700864. [DOI] [PubMed] [Google Scholar]
- 18.Hixson SS, Garrett DW. Arylcyclopropane photochemistry. Photochemical addition of hydroxylic compounds to 1,2-diarylcyclopropanes. J. Am. Chem. Soc. 1974;96:4872–4879. [Google Scholar]
- 19.Rao VR, Hixson SS. Arylcyclopropane photochemistry. Electron-transfer-mediated photochemical addition of methanol to arylcyclopropanes. J. Am. Chem. Soc. 1979;101:6458–6459. [Google Scholar]
- 20.Pitts CR, Ling B, Snyder JA, Bragg AE, Lectka T. Aminofluorination of cyclopropanes: a multifold approach through a common, catalytically generated intermediate. J. Am. Chem. Soc. 2016;138:6598–6609. doi: 10.1021/jacs.6b02838. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, et al. Copper-catalyzed asymmetric aminocyanation of arylcyclopropanes for synthesis of γ-amino nitriles. ACS Catal. 2019;9:716–721. [Google Scholar]
- 22.Petzold D, Singh P, Almqvist F, König B. Visible-light-mediated synthesis of β-chloro ketones from aryl cyclopropanes. Angew. Chem. Int. Ed. 2019;58:8577–8580. doi: 10.1002/anie.201902473. [DOI] [PubMed] [Google Scholar]
- 23.Ge L, et al. Photoredox-catalyzed oxo-amination of aryl cyclopropanes. Nat. Commun. 2019;10:4367. doi: 10.1038/s41467-019-12403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, et al. Copper-catalyzed 1,3-aminoazidation of arylcyclopropanes: a facile access to 1,3-diamine derivatives. Org. Chem. Front. 2019;6:2934–2938. [Google Scholar]
- 25.Yan M, Kawamata Y, Baran PS. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong P, Xu H-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 2019;52:3339–3350. doi: 10.1021/acs.accounts.9b00472. [DOI] [PubMed] [Google Scholar]
- 27.Siu JC, Fu N, Lin S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 2020;53:547–560. doi: 10.1021/acs.accounts.9b00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Stahl SS. Electrochemical oxidation of organic molecules at lower overpotential: accessing broader functional group compatibility with electron–proton transfer mediators. Acc. Chem. Res. 2020;53:561–574. doi: 10.1021/acs.accounts.9b00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Y, Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 2019;52:3309–3324. doi: 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann L. Metalla-electrocatalyzed C–H activation by Earth-abundant 3d metals and beyond. Acc. Chem. Res. 2020;53:84–104. doi: 10.1021/acs.accounts.9b00510. [DOI] [PubMed] [Google Scholar]
- 31.Waldvogel SR, Lips S, Selt M, Riehl B, Kampf CJ. Electrochemical arylation reaction. Chem. Rev. 2018;118:6706–6765. doi: 10.1021/acs.chemrev.8b00233. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Xu K, Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 33.Moeller KD. Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 2018;118:4817–4833. doi: 10.1021/acs.chemrev.7b00656. [DOI] [PubMed] [Google Scholar]
- 34.Ma C, Fang P, Mei T-S. Recent advances in C–H functionalization using electrochemical transition metal catalysis. ACS Catal. 2018;8:7179–7189. [Google Scholar]
- 35.Möhle S, et al. Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. 2018;57:6018–6041. doi: 10.1002/anie.201712732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francke R, Little RD. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014;43:2492–2521. doi: 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida J-i, Kataoka K, Horcajada R, Nagaki A. Modern strategies in electroorganic synthesis. Chem. Rev. 2008;108:2265–2299. doi: 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- 38.Kärkäs MD. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 2018;47:5786–5865. doi: 10.1039/c7cs00619e. [DOI] [PubMed] [Google Scholar]
- 39.Xiong P, et al. Electrochemically enabled carbohydroxylation of alkenes with H2O and organotrifluoroborates. J. Am. Chem. Soc. 2018;140:16387–16391. doi: 10.1021/jacs.8b08592. [DOI] [PubMed] [Google Scholar]
- 40.Cai C-Y, Shu X-M, Xu H-C. Practical and stereoselective electrocatalytic 1,2-diamination of alkenes. Nat. Commun. 2019;10:4953. doi: 10.1038/s41467-019-13024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai C-Y, Xu H-C. Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles. Nat. Commun. 2018;9:3551. doi: 10.1038/s41467-018-06020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 43.Yuan Y, Chen Y, Tang S, Huang Z, Lei A. Electrochemical oxidative oxysulfenylation and aminosulfenylation of alkenes with hydrogen evolution. Sci. Adv. 2018;4:eaat5312. doi: 10.1126/sciadv.aat5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, et al. Electrochemical oxidation-induced etherification via C(sp3)-H/O-H cross-coupling. Sci. Adv. 2020;6:eaaz0590. doi: 10.1126/sciadv.aaz0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horn EJ, et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature. 2016;533:77–81. doi: 10.1038/nature17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang J, et al. Hindered dialkyl ether synthesis with electrogenerated carbocations. Nature. 2019;573:398–402. doi: 10.1038/s41586-019-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Tang S, Huang P, Lei A. Electrocatalytic oxidant-free dehydrogenative C−H/S−H cross-coupling. Angew. Chem. Int. Ed. 2017;56:3009–3013. doi: 10.1002/anie.201700012. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Wang P, Zeng L, Tang S, Lei A. Cobalt(II)-catalyzed electrooxidative C–H amination of arenes with alkylamines. J. Am. Chem. Soc. 2018;140:4195–4199. doi: 10.1021/jacs.7b13049. [DOI] [PubMed] [Google Scholar]
- 49.Adeli Y, et al. Electrochemically oxidative C–C bond cleavage of alkylarenes for anilines synthesis. ACS Catal. 2019;9:2063–2067. [Google Scholar]
- 50.Shono T, Matsumura Y. Organic synthesis by electrolysis. VI. Anodic oxidation of arylcyclopropanes. J. Org. Chem. 1970;35:4157–4160. [Google Scholar]
- 51.Dinnocenzo JP, Todd WP, Simpson TR, Gould IR. Nucleophilic cleavage of one-electron.sigma. bonds: stereochemistry and cleavage rates. J. Am. Chem. Soc. 1990;112:2462–2464. [Google Scholar]
- 52.Dinnocenzo JP, Zuilhof H, Lieberman DR, Simpson TR, McKechney MW. Three-electron SN2 reactions of arylcyclopropane cation radicals. 2. Steric and electronic effects of substitution1. J. Am. Chem. Soc. 1997;119:994–1004. [Google Scholar]
- 53.O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- 54.Fuchigami T, Inagi S. Selective electrochemical fluorination of organic molecules and macromolecules in ionic liquids. Chem. Commun. 2011;47:10211–10223. doi: 10.1039/c1cc12414e. [DOI] [PubMed] [Google Scholar]
- 55.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 56.Campbell MG, Ritter T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 2015;115:612–633. doi: 10.1021/cr500366b. [DOI] [PubMed] [Google Scholar]
- 57.Fuchigami T, Inagi S. Recent advances in electrochemical systems for selective fluorination of organic compounds. Acc. Chem. Res. 2020;53:322–334. doi: 10.1021/acs.accounts.9b00520. [DOI] [PubMed] [Google Scholar]
- 58.Doobary S, Sedikides AT, Caldora HP, Poole DL, Lennox AJJ. Electrochemical vicinal difluorination of alkenes: scalable and amenable to electron-rich substrates. Angew. Chem. Int. Ed. 2020;59:1155–1160. doi: 10.1002/anie.201912119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayr H, Ofial AR. Do general nucleophilicity scales exist? J. Phys. Org. Chem. 2008;21:584–595. [Google Scholar]
- 60.Nolte C, Ammer J, Mayr H. Nucleofugality and nucleophilicity of fluoride in protic solvents. J. Org. Chem. 2012;77:3325–3335. doi: 10.1021/jo300141z. [DOI] [PubMed] [Google Scholar]
- 61.Niwa H, Hida T, Yamada K. A new method for clevage of aliphatic methyl ethers. Tetrahedron Lett. 1981;22:4239–4240. [Google Scholar]
- 62.Cicco L, et al. Addition of highly polarized organometallic compounds to N-tert-butanesulfinyl imines in deep eutectic solvents under air: preparation of chiral amines of pharmaceutical interest. ChemSusChem. 2020;13:3583–3588. doi: 10.1002/cssc.202001142. [DOI] [PubMed] [Google Scholar]
- 63.Ye L, et al. Photoredox catalysis induced bisindolylation of ethers/alcohols via sequential C–H and C–O bond cleavage. Org. Lett. 2017;19:6164–6167. doi: 10.1021/acs.orglett.7b03073. [DOI] [PubMed] [Google Scholar]
- 64.An Z, et al. FeCl3-catalyzed synthesis of pyrrolo[1,2-a]quinoxaline derivatives from 1-(2-aminophenyl)pyrroles through annulation and cleavage of cyclic ethers. Chem. Commun. 2017;53:11572–11575. doi: 10.1039/c7cc07089f. [DOI] [PubMed] [Google Scholar]
- 65.Marchaj A, Kelley DG, Bakac A, Espenson JH. Kinetics of the reactios between alkyl radicals and molecular-oxygen in aqueous solution. J. Phys. Chem. 1991;95:4440–4441. [Google Scholar]
- 66.Lu Q, et al. Aerobic oxysulfonylation of alkenes leading to secondary and tertiary β-hydroxysulfones. Angew. Chem. Int. Ed. 2013;52:7156–7159. doi: 10.1002/anie.201301634. [DOI] [PubMed] [Google Scholar]
- 67.Cristol SJ, Seapy DG. New procedure for the transformation of alcohols to alkyl halides via xanthate esters and free-radical intermediates. J. Org. Chem. 1982;47:132–136. [Google Scholar]
- 68.Sakaguchi, S., Hirabayashi, T. & Ishii, Y. First Ritter-type reaction of alkylbenzenes using N-hydroxyphthalimide as a key catalyst. Chem. Commun. 5, 516–517 (2002). [DOI] [PubMed]
- 69.Michaudel Q, Thevenet D, Baran PS. Intermolecular Ritter-type C–H amination of unactivated sp3 carbons. J. Am. Chem. Soc. 2012;134:2547–2550. doi: 10.1021/ja212020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.