Abstract
Background
West African studies have suggested that national immunisation campaigns with oral polio vaccine (C-OPV) may non-specifically reduce all-cause child mortality rate by 15–25%. We investigated whether C-OPVs had similar non-specific effects in rural Bangladesh from 2004 to 2019.
Methods
Chakaria, is a health and demographic surveillance system (HDSS) in Southern Bangladesh. From 2004–2011 the HDSS covered a random sample of households; from 2012 to 2019 it covered a random sample of villages. Using Cox proportional hazards models, we calculated hazard ratios (HR) comparing mortality for children under 3 years of age after C-OPV versus before C-OPV to assess the effect of receiving a C-OPV. We allowed for different baseline hazard function in the two periods (2004–2011, 2012–2019), with separate models for each period.
Findings
There were 768 deaths (2.1%) amongst 36,176 children. The HR after C-OPV was 0.69 (95% confidence interval: 0.52–0.90). National campaigns providing vitamin A or measles vaccine did not have similar effects. Each additional dose of C-OPV was associated with a reduction in the mortality rate by 6% (−2 to 13%). The number needed to treat with C-OPV to save one life between 0 and 35 months of age was 88 (81–96).
Interpretation
This is the fourth study to show that C-OPV has beneficial non-specific effects on child survival. All studies have shown a beneficial effect of C-OPV on child health. Stopping OPV as planned after polio eradication without any mitigation plan could have detrimental effects for overall child health in low-income countries.
Funding
The Chakaria HDSS was funded by international sponsors. No sponsor had any influence on the preparation of the article.
Keywords: OPV, Bangladesh, Non-specific effects of vaccines, Eradication, Child mortality, Campaigns, Oral polio vaccine
Research in Context.
Evidence before this study
During the last 2–3 decades numerous campaigns with the live oral polio vaccine (C-OPV) have been conducted in low- and middle-income-countries with the goal to ultimately eradicate poliomyelitis. Search for studies revealed no investigation of the impact on child survival when these campaigns started. However, in recent years several studies from West Africa (Guinea-Bissau, Ghana, Burkina Faso) have suggested that C-OPVs are associated with 15–25% reduction in the mortality rates for children under five years of age.
Added value of this study
We tested the hypothesis that C-OPV had similar effects in child mortality outside West Africa. We analysed 2004–2019 data from Chakaria, a health and demographic surveillance system in Bangladesh. During this period 28 C-OPVs were conducted. Campaigns with vitamin A supplementation and measles vaccine were also conducted during this period. The number of polio cases during the study period was very low and Bangladesh was declared free from polio virus in 2014.
Campaigns with OPV-only were associated with a 31% (10–48%) reduction in mortality rate and each additional campaign reduced the mortality rate with 6% (−2 to 13%).The number needed to treat with C-OPV to save the life of one child was only 88 (91–96).
Implication of all the available evidence
This is the first study that analyses the effects of C-OPV outside West Africa. The results emphasise that C-OPVs may have played a significant role in the decline in child in low- and middle-income-countries. Stopping OPV after the eradication of poliomyelitis could have detrimental consequences for child survival.
Alt-text: Unlabelled box
1. Introduction
Epidemiological studies have shown that live attenuated vaccines have beneficial non-specific effects (NSEs) that cannot be explained by prevention of the vaccine-targeted infection. Several recent studies have suggested that vaccines could train the immune system in ways that may alter susceptibility to unrelated infections [1], [2], [3]. In 2014, WHO reviewed the potential NSEs of Bacille Calmette-Guérin (BCG), Diphtheria-Tetanus-Pertussis (DTP) and Measles Vaccine (MV) [4,5]. The two live vaccines, BCG and MV, were associated with large reductions in child mortality. WHO's Strategic Advisory Group of Experts on immunization (SAGE) recommended further research into the NSEs of vaccines [5]. The live Oral Polio Vaccine (OPV) was not included in the WHO- review.
In the last few decades, child mortality has declined remarkably in low-income countries. During this period numerous campaigns with OPV (C-OPV) have been conducted to eradicate polio infection. Studies from West Africa have indicated that C-OPVs may be associated with a decrease in the overall child mortality rate of 15–25% [6], [7], [8], [9], [10]. An intriguing implication of the NSEs of vaccines is that depriving children of beneficial immune-training by removing a live vaccine after eradication could cause harm [11]. This eradication paradox has become critical now because the world is about to eradicate polio infection and is gearing up to eliminate measles and rubella. Hence, it is urgent to assess the NSEs of the lives vaccines for these diseases before they are removed.
So far, C-OPVs have only been studied in West Africa (Ghana, Burkina Faso and Guinea-Bissau) [[6], [7], [8], [9], [10],12]. Since 2004, the icddr,b has maintained a health and demographic surveillance site (HDSS) in Chakaria, a rural area in Southern Bangladesh. During the study period Bangladesh had 18 cases of wild polio, all in 2006. Bangladesh was officially declared free of polio virus in 2014.
We aimed to study the impact of C-OPV on child survival and as in the West African studies, we hypothesised that C-OPV had strong beneficial effects whereas the effect of other campaigns was limited. If C-OPVs reduced the mortality rate it would further support the existence of important NSEs of OPV, also outside West Africa.
2. Methods
We followed the STROBE guideline for reporting of prospective observational studies [13].
2.1. Periods and data collection procedures
The Chakaria HDSS was initiated in 1999, but data collection was interrupted between 2001 and 2003. The present analysis therefore include data collected from 2004 to 2019. Data collection procedures changed in 2011.
First period: During 2004–2011, the HDSS covered a random sample of 7042 households amongst all 26,979 households in the study area. These households were followed with quarterly home visits. Second period: From 2012–2019, the HDSS covered a random sample of 49 villages amongst all 183 villages in the study area. All households from the chosen villages were followed with quarterly home visits. In the second period children were also registered during pregnancy.
The main analysis includes both periods, but we also present results from each period separately.
At the home visits information was collected on all individuals alive at the time of the visit. For the main analysis, we used this prospective part of the data. At the home visit information was also collected retrospectively for children, who were born since the last home visit, and who may have died or moved before being visited. This retrospective data would be less certain due to potential omissions and survival bias. We have therefore only used the retrospective data in a sensitivity analysis.
2.2. Information on OPV campaigns
We obtained information on national campaigns conducted during the study period from the Chakaria HDSS and from the local WHO office in Bangladesh. We used this information unless otherwise stated. We also had data on OPV campaigns from the WHO international office that provided a database of all OPV campaigns conducted globally. Compared to the data from Bangladesh, the global WHO data was less accurate and in some cases wrong, in the sense that there was certainty locally that some campaigns registered in the international data had not been carried out. As local campaign information may not be available for future analyses of OPV campaigns in other countries, we used information on OPV campaigns from the global data as a sensitivity analysis.
2.3. Information on other campaigns
Information on other campaigns providing vitamin A supplementation (VAS) and MV was obtained from the Chakaria HDSS.
2.4. Campaign administration
Campaign participation is usually high in Bangladesh. The participation rate was documented in the three OPV campaigns in 2012 and 2013; it was 85–89% (Supplementary Table 1). As in previous analyses of OPV campaigns we assumed that all children eligible for a particular campaign did receive the intervention.
OPV was mostly given alone, as C-OPV-only, but in two campaigns it was given with either VAS or MV. As done in the previous studies, we focussed on C-OPV-only and for simplicity denote this C-OPV. But we also conducted a sensitivity analysis including any received campaign OPV, i.e. C-OPV-only, C-OPV+VAS, and C-OPV+MV. Since Albendazole (ALZ) was always co-administered with VAS we did not include ALZ as a separate variable in any of the analyses.
2.5. Statistical analyses
National immunization campaigns are essentially a natural experiment since they allocate children to participate in campaigns based on their birthday and thereby eliminates many common causes of bias. For these reasons, most potential confounders, such as socio-economic status (SES) or education, would not confound the estimated association between campaign participation and risk of mortality, since the SES can only be linked with the mortality risk but not with the campaign participation. In this framework children are both controls and exposed to various campaigns as some children will contribute risk time to both the before and after campaign group. The analysis may be sensitive to time-varying covariates that either have a skewed distribution comparing before-campaign and after-campaign groups or are directly associated with the intervention, such as calendar year, seasonal trends and age.
Using Cox-proportional hazards (Cox) models with age as underlying time scale, we calculated the HR comparing mortality after C-OPV versus before C-OPV and present this as well as the mortality rates per 100 person-years, number of deaths and number of person-years. Thus, all analyses were inherently adjusted for age. The same child could contribute analysis time both in the before-campaign group and the after-campaign group. Children were followed until 36 months of age, movement, death or end of study period (31st of December 2011 or 31st of December 2019, for the two periods respectively), whichever came first. Interdependency of twins was adjusted for with robust standard errors.
All analyses were performed overall and by sex, as NSEs have often been found to be sex-differential [3]. In the main analysis for the full period 2004–2019, we allowed for different baseline hazard functions in the two periods (2004–2011, 2012–2019). As done in previous analyses [10], we investigated the effects in children below and above 1 year of age separately.
We present results from three Cox models for most of the analyses. The first and simplest model adjusted only for age. The second model also included an interaction term (c.year#i.agegroup) to the Cox model for the continuous covariate of years since 2004 (year) and age groups: 0–5, 6–11, 12–23, and 24–35 months of age (agegroup) (using finer timer intervals did not change the results, data not shown). The third model, representing the main analysis, further included adjustment for other campaigns in the multivariable analysis.
Several studies have indicated that sequence of vaccinations may play an important role for child health [14], [15], [16]. We therefore did an analysis of the most recent campaign received compared with no-campaign-received-yet as reference group, i.e. most recent campaign was updated at the time that a new campaign with a new type of intervention was carried out.
As done in previous studies, we also did an analysis to estimate the effects of each additional dose of C-OPV. We present the HR of 1, 2, and 3 or more doses compared to not yet having received any dose of C-OPV. The continuous trend per each additional dose of C-OPV was calculated using a numerical variable containing the number of C-OPV received (values from 0 to 12), yielding the effect of each additional dose of C-OPV received..
The Number Needed to Treat (NNT) was calculated as the inverse of the absolute risk reduction in the Kaplan-Meier survival estimates [17]. The proportional hazards assumption was assessed graphically and tested using Schoenfeld residuals; no test rejected the hypothesis of proportional hazards (Supplementary Fig. 1).
To allow mortality rates to be comparable for the two periods, we only included children from the first day seen after birth. Since children were registered during pregnancy in the second period, the children were on average younger at the time of inclusion in the second period compared to the first period.
We used a 5% level of significance and 95% confidence intervals for all analyses. All statistical analyses were performed using Stata version 16 (Stata Corp, College Station, Texas).
No ethical approval or consent was needed for the present study. All data was anonymised in the analysis process. The general data collection of the Chakaria HDSS is approved by the Ethical Review Committee of icddr,b.
3. Role of funding sources
The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
4. Results
In the main analysis, using prospectively collected data, 36,176 children below 3 years of age were included between 2004 and 2019. Of the children seen during a home visit, 768 died (2.1%) during 66,160 person-years of follow-up. The overall mortality rate for children aged 1 day to 3 years declined from 12 deaths per 1000 person-years in 2004 to 5 deaths in 2019, a decline of around 59% (Supplementary Table 2).
Between 2001 and 2019 a total of 42 national immunisation campaigns were conducted in Chakaria and included in the main analysis; 28 included OPV (26 were C-OPV-only), 13 VAS, 3 MV and 3 ALZ (Supplementary Table 1).
4.1. C-OPV
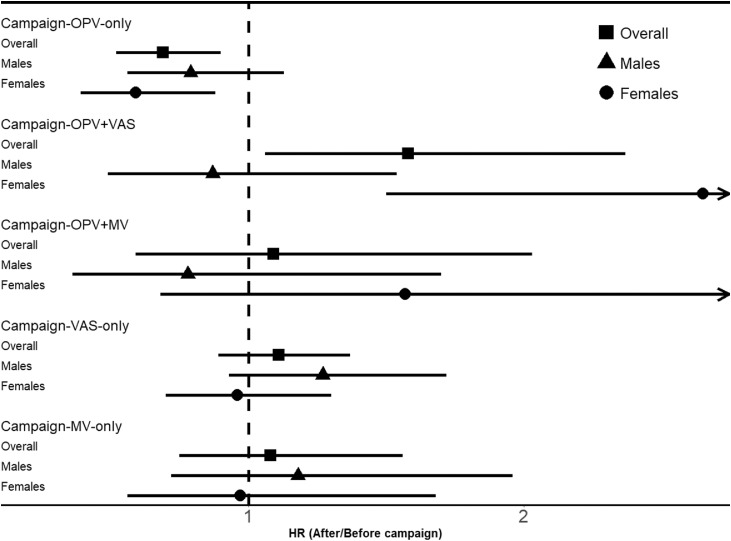
Comparing after-campaign mortality versus before-campaign mortality in the full multivariable model, the HR for C-OPV-only was 0.69 (95% confidence interval: 0.52–0.90) (Table 1, Fig. 1). The HR was separately significant for females, 0.59 (0.39–0.88), but not for males, 0.79 (0.56–1.13) (Fig. 1, Supplementary Table 3). The results were similar in the first, HR = 0.66 (0.46–0.96), and the second period, HR = 0.68 (0.44–1.04) (Supplementary Table 4). The beneficial effect was strongest for the 12–35 months old children, HR = 0.55 (0.35–0.88), whereas it was 0.76 (0.55–1.05) for infants (Table 1). Each additional dose of C-OPV was associated with a HR of 0.94 (0.87–1.02). There was a significant beneficial effect already after the first dose of C-OPV, HR = 0.59 (0.43–0.83), and each additional dose of C-OPV was associated with a HR of 0.94 (0.87–1.02) (Table 2). When we evaluated the most recently received campaign with OPV compared to not having received any campaign yet, the HR for C-OPV was 0.71 (0.54–0.92) (Table 3). In the analysis of any C-OPV received, i.e. OPV-only, OPV+VAS, and OPV+MV campaigns, the HR for any C-OPV was 0.78 (0.62–0.99).
Table 1.
Mortality rates (per 100 person-years) and hazard ratios (HR) for after-campaign versus before-campaign by age group from 2004 to 2019.
| Overall | ||||||
|---|---|---|---|---|---|---|
| Campaign | Mortality rates per 100 person years (deaths/person years) |
HR (After/Before-campaign) (95% CI)1 | HR (After/Before-campaign) (95% CI)2 | HR (After/Before-campaign) (95% CI)3 | ||
| After-campaign | Before-campaign | |||||
| Campaign-OPV-only | 0.73 (243/33,350) | 1.60 (525/32,810) | 0.84 (0.66–1.07) | 0.78 (0.62–0.98) | 0.69 (0.52–0.90) | |
| Campaign-OPV+VAS | 0.77 (39/5047) | 1.19 (729/61,112) | 1.44 (1.02–2.01) | 1.25 (0.87–1.79) | 1.58 (1.06–2.37) | |
| Campaign-OPV+MV | 0.36 (17/4771) | 1.22 (751/61,389) | 0.93 (0.54–1.60) | 0.82 (0.47–1.46) | 1.09 (0.59–2.03) | |
| Campaign-VAS-only | 0.60 (188/31,244) | 1.66 (580/34,915) | 1.03 (0.83–1.26) | 1.06 (0.85–1.32) | 1.11 (0.89–1.37) | |
| Campaign-MV-only | 0.51 (38/7430) | 1.24 (730/58,730) | 1.05 (0.73–1.49) | 1.08 (0.76–1.54) | 1.08 (0.75–1.56) | |
| 0–11 months | ||||||
| Campaign | Mortality rates per 100 person years (deaths/person years) |
HR (After/Before-campaign) (95% CI)1 | HR (After/Before-campaign) (95% CI)2 | HR (After/Before-campaign) (95% CI)3 | ||
| After-campaign | Before-campaign | |||||
| Campaign-OPV-only | 1.11 (70/6292) | 2.69 (430/16,008) | 0.84 (0.61–1.16) | 0.82 (0.60–1.11) | 0.76 (0.55–1.05) | |
| Campaign-OPV+VAS | 1.09 (11/1006) | 2.30 (489/21,295) | 1.13 (0.62–2.07) | 0.93 (0.50–1.73) | 1.18 (0.61–2.26) | |
| Campaign-OPV+MV | 5.31 (3/56) | 2.23 (497/22,244) | 10.6 (3.05–36.6) | 9.51 (2.73–33.2) | 11.8 (3.21–43.1) | |
| Campaign-VAS-only | 1.07 (41/3849) | 2.49 (459/18,452) | 1.11 (0.80–1.55) | 1.10 (0.78–1.54) | 1.10 (0.79–1.54) | |
| Campaign-MV-only | 1.10 (1/91) | 2.25 (499/22,209) | 1.33 (0.18–9.74) | 1.31 (0.18–9.68) | 1.45 (0.20–10.7) | |
| 12–35 months | ||||||
| Campaign | Mortality rates per 100 person years (deaths/person years) |
HR (After/Before-campaign) (95% CI)1 | HR (After/Before-campaign) (95% CI)2 | HR (After/Before-campaign) (95% CI)3 | ||
| After-campaign | Before-campaign | |||||
| Campaign-OPV-only | 0.64 (173/27,057) | 0.57 (95/16,802) | 0.84 (0.59–1.20) | 0.74 (0.52–1.05) | 0.55 (0.35–0.88) | |
| Campaign-OPV+VAS | 0.69 (28/4041) | 0.60 (240/39,818) | 1.63 (1.07–2.48) | 1.50 (0.94–2.37) | 2.17 (1.19–3.96) | |
| Campaign-OPV+MV | 0.30 (14/4714) | 0.65 (254/39,145) | 0.75 (0.42–1.34) | 0.66 (0.36–1.20) | 1.03 (0.51–2.06) | |
| Campaign-VAS-only | 0.54 (147/27,395) | 0.73 (121/16,464) | 0.98 (0.76–1.26) | 1.04 (0.79–1.37) | 1.13 (0.85–1.51) | |
| Campaign-MV-only | 0.50 (37/7339) | 0.63 (231/36,521) | 1.04 (0.73–1.49) | 1.08 (0.75–1.54) | 1.06 (0.73–1.55) | |
1Adjusting for age (underlying time).
2Adjusting for age (underlying time) and year*agegroup.
3Full multivariable model: adjusting for age (underlying time), OPV, OPV+VAS, OPV+MV, VAS, MV and year*agegroup.
Fig.1.
Hazard ratios (95% CI) for after-campaign versus before-campaign overall and by sex from 2004 to 2019 in the full multivariable model adjusting for age (underlying time), OPV, OPV+VAS, OPV+MV, VAS, MV and year*agegroup.
Table 2.
Hazard ratios (HR) for number of doses of C—OPV-only from 2004 to 2019.
| Overall | |||
|---|---|---|---|
| Campaign | HR (After/Before-campaign) (95% CI)1 | HR (After/Before-campaign) (95% CI)2 | HR (After/Before-campaign) (95% CI)3 |
| 1st campaign-OPV-only | 0.79 (0.61–1.04) | 0.69 (0.52–0.92) | 0.59 (0.43–0.83) |
| 2nd campaign-OPV-only | 0.97 (0.66–1.43) | 0.98 (0.68–1.41) | 0.86 (0.59–1.26) |
| 3rd campaign-OPV-only and more | 0.84 (0.51–1.38) | 0.84 (0.53–1.34) | 0.75 (0.47–1.20) |
| Campaign-OPV-only as a continuous trend | 0.96 (0.89–1.04) | 0.96 (0.89–1.04) | 0.94 (0.87–1.02) |
| Campaign-OPV+VAS | 1.32 (0.92–1.90) | ||
| Campaign-OPV+MV | 0.92 (0.50–1.67) | ||
| Campaign-VAS-only | 1.07 (0.86–1.33) | ||
| Campaign-MV-only | 1.20 (0.82–1.76) | ||
1Adjusting for age (underlying time).
2Adjusting for age (underlying time) and year*agegroup.
3Full multivariable model: adjusting for age (underlying time), OPV, OPV+VAS, OPV+MV, VAS, MV and year*agegroup.
Table 3.
Mortality rates (per 100 person-years) and hazard ratios (HR) comparing most recent campaign to no campaign yet from 2004 to 2019.
| Overall | |||
|---|---|---|---|
| Campaign | Mortality rates per 100 person years (deaths/person years) | HR (After/Before-campaign) (95% CI)1 | HR (After/Before-campaign) (95% CI)2 |
| No campaign yet | 2.28 (447/19,574) | 1.00 (ref.) | 1.00 (ref.) |
| Campaign-OPV-only | 0.83 (162/19,474) | 0.78 (0.60–1.01) | 0.71 (0.54–0.92) |
| Campaign-OPV+VAS | 2.60 (5/192) | 1.73 (0.71–4.22) | 1.51 (0.61–3.72) |
| Campaign-OPV+MV | 0.51 (4/792) | 0.99 (0.36–2.76) | 0.85 (0.30–2.41) |
| Campaign-VAS-only | 0.61 (129/21,029) | 0.87 (0.67–1.13) | 0.84 (0.65–1.09) |
| Campaign-MV-only | 0.41 (21/5099) | 0.91 (0.54–1.53) | 0.96 (0.57–1.61) |
1Adjusting for age (underlying time).
2Adjusting for age (underlying time) and year*agegroup.
In the overall analysis, the NNT to save one life between 0 and 35 months of age with C-OPV was 88 (81–96).
4.2. Sensitivity analyses
Using the global information on campaigns from the WHO international office rather than the local information resulted in an overall C-OPV-only HR of 0.90 (0.69–1.18) (data not shown).
In a sensitivity analysis, including also the retrospective data with 1051 deaths (2.8%) during 67,860 person-years, the mortality rate declined from 21 deaths per 1000 person-years in 2004 to 10 in 2019. The result was a C-OPV-only HR of 0.77 (0.61–0.97) (data not shown).
4.3. Other interventions
Other campaign interventions did not have a similar effect. The overall results were: OPV+VAS campaigns, HR 1.58 (1.06–2.37); OPV+MV campaigns, HR 1.09 (0.59–2.03); VAS-only campaigns, HR 1.11 (0.89–1.37); and MV-only campaigns, HR 1.08 (0.75–1.56) (Table 1, Fig. 1). The effect of C-OPV-VAS differed significantly, being worse for females (Supplementary Table 3) (test of interaction, p < 0.01). C-OPV+MV had a significant negative effect in infancy (Table 1), but only for females (data not shown); the estimate was based on few deaths and person-years between 9 and 11 months of age. The effect of C-OPV-only differed from the effects of OPV+VAS (test of interaction, p < 0.01) and of VAS-only campaigns (test of interaction, p < 0.01). (Fig. 1, Table 1).
5. Discussion
Mortality for children under 3 years of age declined almost 60% between 2004 and 2019. OPV-only campaigns were associated with a 31% (10 to 48%) reduction in mortality rate. Since there were essentially no polio infections in Bangladesh in this period, this is likely to be an entirely non-specific effect of C-OPV. The effect of C-OPVs was strongest for females. No other health campaign in this period had similar beneficial effects.
We could confirm our hypothesis: C-OPV had strong beneficial effects whereas the effect of other campaigns was limited. Although the Bangladeshi cohort had about 34% less follow-up time, it only included about one fourth of the number of deaths than in the study from Guinea-Bissau [10], but the overall effect was still statistically significant in Bangladesh.
Campaigns were not evenly distributed throughout the study period from 2004 to 2019. Due to this we had to adjust for the trend in mortality over the years.
Usually it is assumed that any observational study is potentially flawed due to uncontrolled confounding. However, in the framework presented here, where it is assumed that all eligible children participate in the campaigns, we have eliminated a good deal of this confounding by allocating children to the control and intervention group by their day of birth and by allowing children to be both controls and exposed. However, it is possible for time-varying covariates to alter the effect of C-OPV. We have therefore included such relevant covariates in the fully adjusted Cox models for the main analyses. We also used other campaigns as control exposures.
We analysed the community data in two different ways, first, using only the data collected prospectively in the main analysis (i.e. a landmark approach) [18] and second, including also the data collected retrospectively. In the latter analysis, there might have been survival bias in the period before the first home visit as already dead children may have been less likely to be registered, and there might have been uncertainty about whether children reported to be dead had died before or after a specific campaign. Using the landmark approach, where the children were only followed from the first time they were registered to be alive, reduced the number of deaths in the analysis, but most additional deaths in the retrospective analysis contributed only to the before-campaign period as it would be the youngest children who died before being seen alive. Results differed slightly, but non-significantly, between the landmark and the retrospective approach.
Chakaria did not have individual level information for the whole period about who received and who did not receive the various campaigns. Since coverage was high, we therefore simplified the analysis by assuming that all eligible children did receive the campaign vaccine. This will most likely lead to conservative estimates so the effect of C-OPV may in fact have been somewhat stronger. We did not include the routine vaccinations in the analysis since information about these vaccinations were only available for the interval between home visits and there would always be some children missing; hence, the data could not have been analysed in the same model. It is an important but unknown question whether the routine doses of OPV modify the effect of the C-OPV.
The negative results for females of campaigns with OPV+MV and OPV+VAS was based on small numbers and should be interpreted cautiously as there were only one campaign of each. DTP administered after MV has been associated with strong negative effects for females in both Bangladesh and elsewhere (16,19,20). Since information about all routine vaccinations was not available, this could not be tested in the present data set.
The overall results agree very well with the results from Guinea-Bissau that reported a HR of 0.75 (0.67–0.85) comparing time after C-OPV-only with time before C-OPV-only [10] and other West African studies [7,12]. In the studies from Guinea-Bissau repeated campaigns had a significant beneficial effect. In Bangladesh, repeated campaigns had a smaller effect (6% (−2 to 13%) reduction per C-OPV), possibly due to many more campaigns than in Guinea-Bissau (Supplementary Table 1).
Other live vaccines, like BCG and smallpox vaccine, have been found to induce innate immune training which reduces susceptibility to unrelated infections [1,2,21,22]. BCG reprograms monocytes through epigenetic changes to a more pro-inflammatory response; in animal models this response reduces mortality from challenge to unrelated infections [2] and in human experiments, randomisation to BCG reduced the viral load after a subsequent challenge with yellow-fever vaccine [22]. Similar studies have yet to be conducted for OPV. Other mechanisms might also be relevant including changes in microbiome due to the intestinal colonization with OPV. Interestingly, children, who received OPV0 and BCG within 48 h of birth, had significantly higher excretion of the antimicrobial peptide human cathelicidin LL37 (P < 0.05) in stool at age 6 weeks than children vaccinated later [23].
There has been little research in why NSEs often differ by sex and the immunological mechanism is unknown. Several studies have found a stronger beneficial effect of OPV for males than for females [9,24] but in the present analysis the effect of OPV was slightly stronger for females. Other live vaccines, such as MV [4,25,26] may also have a stronger beneficial effect for females.
Many studies now support that OPV is associated with beneficial NSEs [10,24,[27], [28], [29], [30]]. Historically, studies from Latin America showed that diarrhoea morality declined when OPV was first introduced. Large studies in the Soviet Union found that OPV and vaccine with non-pathogenic enterovirus reduced respiratory infections amongst adults [31], [32], [33]. More recently, we have found strong beneficial effects of C-OPV in several studies from urban Bissau [6,8,9,10] and in Burkina Faso [12] and Ghana [7]. This is the first study comparing the mortality rates before and after OPV campaigns outside West Africa. It is remarkable that the estimated effect of C-OPVs was very similar in spite of the major differences between Bangladesh and West Africa and presumably different underlying morbidity patterns and confounding structures [34].
When polio is eradicated, the plan is that OPV will be phased out globally and replaced by inactivated polio vaccine (IPV). The removal of a live vaccine with potential beneficial effects on child health may not be the best public health strategy [11,35]. A historic example of this is smallpox. When smallpox vaccine was stopped in the late 1970s, no study examined the effect on overall survival of withdrawing this vaccine. All studies of smallpox vaccine conducted after smallpox eradication have suggested that the smallpox vaccination had beneficial NSEs [36], [37], [38]. A novel polio vaccine type 2 (nOPV2), a modified version of the existing type 2 monovalent OPV, is underway. Clinical trials have shown it provides comparable protection against poliovirus while being more genetically stable and less likely to revert into a form which can cause paralysis in low immunity settings. It is urgent to assess whether nOPV may carry the same beneficial NSEs as OPV. If so, a switch to this vaccine could at the same time minimise the rare but serious risk that OPV may cause vaccine-derived polio, and maintain the non-specific health benefits of OPV.
Funding
This research study is funded by core donors who provide unrestricted support to icddr,b for its operations and research. Current donors providing unrestricted support include the Governments of Bangladesh, Canada, Sweden and the UK.
The work on non-specific effects of vaccines has been supported by the Danish Council for Development Research, Ministry of Foreign Affairs, Denmark [grant number 104.Dan.8.f.], Novo Nordisk Foundation and European Union FP7 support for OPTIMUNISE (grant: Health-F3-2011-261375).
Data sharing statement
The datasets used for these analyses are not public available, but access can be requested to the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge our core donors for their support and commitment to icddr,b's research efforts. The authors are grateful to the villages for their cooperation in providing invaluable information. The untiring efforts of the team members of the Chakaria HDSS in maintaining the surveillance system are gratefully acknowledged.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100886.
Appendix. Supplementary materials
References
- 1.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab – a big effect: non-specific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benn C.S., Fisker A.B., Rieckmann A., Sørup S., Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis. 2020;20(10):e274–e283. doi: 10.1016/S1473-3099(19)30742-X. Oct. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JPT, Soares-Weiser K, Reingold A. Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. http://www.who.int/immunization/sage/meetings/ 2014/april - accessed September 30,2020
- 5.Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 - conclusions and recommendations Week Epidemiol Rec. 2014;89:233–235. [PubMed] [Google Scholar]
- 6.Andersen A., Fisker A.B., Rodrigues A., Martins C., Ravn H., Lund N., Biering-Sørensen S., Benn C.S., Aaby P. National immunization campaigns with oral polio vaccine (OPV) reduce the general all-cause mortality rate: an analysis of the effect of campaign-OPV on child mortality within seven randomised trials. Front Public Health. 2018;6:13. doi: 10.3389/fpubh.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welaga P., Oduro A., Debpuur C., Aaby P., Ravn H., Andersen A. Fewer out-of-sequence vaccinations and reduction of child mortality in Northern Ghana. Vaccine. 2017;35:2496–2503. doi: 10.1016/j.vaccine.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Aaby P., Andersen A., Martins C.L., Fisker A.B., Rodrigues A., Whittle H.C. Does oral polio vaccine have non-specific effects on all-cause mortality? Natural experiments within a randomised controlled trial of early measles vaccine. BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benn C.S., Jacobsen L.H., Fisker A.B., Rodrigues A., Sartono E., Lund N. Campaigns with oral polio vaccine may lower mortality and create unexpected results. Vaccine. 2017;35:1113–1116. doi: 10.1016/j.vaccine.2016.11.006. 10.1016/j.vaccine.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen A., Fisker A.B., Nielsen S., Rodrigues A., Benn C.S., Aaby P. National immunisation campaigns with oral polio vaccine may reduce all-cause mortality: an analysis of 13 years of demographic surveillance data from an urban African area. Clin Infect Dis. 2020;19:ciaa1351. doi: 10.1093/cid/ciaa1351. Sep. [DOI] [PubMed] [Google Scholar]
- 11.Aaby P., Benn C.S. Stopping live vaccines after disease eradication may increase mortality. Vaccine. 2020;38(1):10–14. doi: 10.1016/j.vaccine.2019.10.034. Jan 3. [DOI] [PubMed] [Google Scholar]
- 12.Schoeps A., Nebié E., Fisker A.B., Sié A., Zakane A., Müller O., Aaby P., Becher H. No effect of an additional early dose of measles vaccine on hospitalization or mortality in children: a randomized controlled trial. Vaccine. 2018;36:1965–1971. doi: 10.1016/j.vaccine.2018.02.104. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. Oct 20PMID: 18064739. [DOI] [PubMed] [Google Scholar]
- 14.Thysen S.M., Rodrigues A., Aaby P., Fisker A.B. Out-of-sequence DTP and measles vaccinations and child mortality in Guinea-Bissau: a reanalysis. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2018-024893. Published 2019 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornshøj L., Benn C.S., Fernandes M. Vaccination coverage and out-of-sequence vaccinations in rural Guinea-Bissau: an observational cohort study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clipet-Jensen C., Andersen A., Jensen A.K.G., Aaby P., Zaman K. Out-of-sequence vaccinations with measles vaccine and diphtheria-tetanus-pertussis vaccine. A re-analysis of demographic surveillance data from rural Bangladesh. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa291. Mar 18:ciaa291. [DOI] [PubMed] [Google Scholar]
- 17.Saver J.L., Lewis R.J. Number needed to treat. Conveying the likelihood of a therapeutic effect. JAMA. 2019;321:798–799. doi: 10.1001/jama.2018.21971. [DOI] [PubMed] [Google Scholar]
- 18.Jensen H., Benn C.S., Lisse I.M., Rodrigues A., Andersen P.K., Aaby P. Survival bias in observational studies of the impact of routine immunizations on childhood survival. Trop Med Int Health. 2007;12(Jan (1)):5–14. doi: 10.1111/j.1365-3156.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanifi S.M.A., Biering-Sørensen S., Jensen A., Aaby P. Penta is associated with an increased female-male mortality ratio: cohort study from Bangladesh. Hum Vaccin Immunother. 2020;23(Jun):1–8. doi: 10.1080/21645515.2020.1763084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanifi S.M.A., Fisker A.B., Welaga P., Rieckmann A., Jensen A.G., Benn C.S., Aaby P. Diphtheria-tetanus-pertussis (DTP) vaccine is associated with increased female-male mortality rate ratios. A meta-analysis of studies of DTP administered before and after measles vaccine. J Infect Dis. 2020;30(Oct):jiaa684. doi: 10.1093/infdis/jiaa684. [DOI] [PubMed] [Google Scholar]
- 21.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A., Jacobs C., van Loenhout J., Xavier R.J., Aaby P., van der Meer J.W., van Crevel R., Netea M.G. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C.B.E.M., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van Crevel R., Netea M.G. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Alam M.J., Rashid M.M., Kabir Y., Raqib R., Ahmad S.M. On birth single dose of live attenuated OPV and BCG vaccination induces gut cathelicidin LL37 responses at 6 weeks of age: a natural experiment. Vaccine. 2015;33:18–21. doi: 10.1016/j.vaccine.2014.10.075. [DOI] [PubMed] [Google Scholar]
- 24.Lund N., Andersen A., Hansen A.S., Jepsen F.S., Barbosa A.G., Biering-Sørensen S. The effect of oral polio vaccine at birth on mortality. A randomized trial. Clin Infect Dis. 2015;61:1504–1511. doi: 10.1093/CID/civ61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaby P., Martins C.L., Garly M.L., Bale C., Andersen A., Rodrigues A. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisker A.B., Rodrigues A., Martins C., Byberg S., Thysen S., Pedersen M. Reduced mortality after general measles vaccination campaign in rural Guinea-Bissau. Pediatr Infect Dis J. 2015;34:1369–1376. doi: 10.1097/INF.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 27.Mogensen S.W., Andersen A., Rodrigues A., Benn C.S., Aaby P. The introduction of diphtheria-tetanus-pertussis and oral polio vaccine among young infants in an urban African community: a natural experiment. EBioMedicine. 2017;17:192–198. doi: 10.1016/j.ebiom.2017.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaby P., Hedegaard K., Sodemann M., Nhante E., Veirum J.E., Jakobsen M. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine. 2005;23:1746–1751. doi: 10.1016/j.vaccine.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Seppälä E., Viskari H., Hoppu S., Honkanen H., Huhtala H., Simell O. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine. 2011;29:8615–8618. doi: 10.1016/j.vaccine.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørup S., Stensballe L.G., Krause T.G., Aaby P., Benn C.B., Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis. 2016;3:ofv204. doi: 10.1093/ofid/ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ. 1974;8:123–132. [PubMed] [Google Scholar]
- 32.Chumakov M.P., Voroshilova M.K., Antsupova A.S., Boĭko V.M., Blinova M.I., Priĭmiagi L.S., Rodin V.I., Seĭbil' V.B., Siniak K.M., Smorodintsev A.A. Live enteroviral vaccines for the emergency nonspecific prevention of mass respiratory diseases during fall-winter epidemics of influenza and acute respiratory diseases. Zh Mikrobiol Epidemiol Immunobiol. 1992;(11–12):37–40. Russian. [PubMed] [Google Scholar]
- 33.Voroshilova M.K. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol. 1989;36:191–202. [PubMed] [Google Scholar]
- 34.Lawlor D.A., Tilling K., Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–1886. doi: 10.1093/ije/dyw314. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaby P., Benn C.S. Beneficial non-specific effects of oral polio vaccine (OPV): implications for the cessation of OPV? CID. 2017;65:420–421. doi: 10.1093/cid/cix340. [DOI] [PubMed] [Google Scholar]
- 36.Aaby P., Gustafson P., Roth A., Rodrigues A., Fernandes M., Sodemann M., Holmgren B., Stabell Benn C., Garly M.L., Lisse I.M., Jensen H. Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine. 2006;24:5718–5725. doi: 10.1016/j.vaccine.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 37.Sørup S., Villumsen M., Ravn H., Benn C.S., Sørensen T.I.A., Aaby P., Jess T., Roth A. Smallpox Vaccination and all-cause Infectious Disease Hospitalization: a Danish register-based cohort study. Int J Epidemiol. 2011;40:955–963. doi: 10.1093/ije/dyr063. [DOI] [PubMed] [Google Scholar]
- 38.Rieckmann A., Villumsen M., Sørup S., Haugaard L.K., Ravn H., Roth A., Baker J.L., Benn C.S., Aaby P. Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971- 2010. Int J Epidemiol. 2017;46:695–705. doi: 10.1093/ije/dyw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.