Abstract
Objective
To summarize the evidence for dextrose prolotherapy in knee osteoarthritis.
Data sources
The authors searched PubMed and Embase from inception to September 2020. All publications in the English language were included without demographic limits.
Study selection
Randomized clinical trials comparing the effects of any active interventions or placebo versus dextrose prolotherapy in patients with knee osteoarthritis were included.
Data extraction
Potential articles were screened for eligibility, and data was extracted independently. The risk of bias was assessed using the Cochrane Risk of Bias tool. Meta-analysis was performed on clinical trials with similar parameters. The Strength of Recommendation Taxonomy (SORT) was used for evaluating the strength of recommendations.
Data synthesis
In total, eleven articles (n = 837 patients) met the search criteria and were included. The risk-of-bias analysis revealed two studies to be of low risk. The overall effectiveness was calculated using a meta-analysis method. Prolotherapy was no different from platelet-rich plasma on the pain subscale at the 6-month time point. Prolotherapy was inferior to platelet-rich plasma at 6 months (MD 0.45, 95% CI 0.06–0.85, p = 0.03) on the stiffness subscale. Prolotherapy was found to be safe with no major adverse effects.
Conclusion
Prolotherapy in knee osteoarthritis confers potential benefits for pain but the studies are at high risk of bias. Based on two well-designed studies, dextrose prolotherapy may be considered in knee osteoarthritis (strength of recommendation B). This treatment is safe and may be considered in patients with limited alternative options (strength of recommendation C).
Keywords: Dextrose prolotherapy, Osteoarthritis, Knee, Systematic review, Meta-analysis
Introduction
Knee osteoarthritis (OA) is a chronic, progressive, and disabling joint disease, often resulting in a poor quality of life and an enormous social and economic burden to both patients and their caregivers. It is common in the adult population with a lifetime risk of symptomatic knee OA of 45%.1 Contrary to previous belief that OA was simply a degenerative joint disease, ongoing research has shown that the pathogenesis of OA is much more complex than just a degenerative process.2 This has prompted the development of new treatment strategies as there is currently no cure for OA. At present, available treatments are focused mainly on symptom relief and improvement of joint disabilities rather than modifying the disease progression. There is ongoing research on various disease-modifying treatments that regulate cartilage catabolism and anabolism, inflammation control, and remodeling of subchondral bone.3 In recent years, different injection-based therapies for knee OA have been studied. These include dextrose prolotherapy, ozone, botulinum toxin, platelet-rich plasma (PRP), and hyaluronic acid.4, 5, 6, 7 Most of these interventions are costly, with questionable therapeutic efficacy for symptom control.
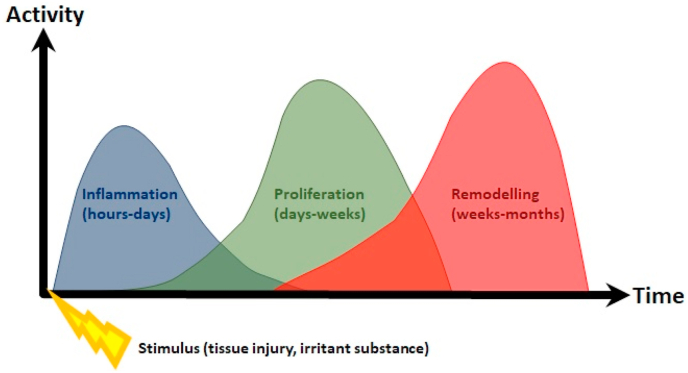
Dextrose is low-cost and widely-available in the clinical setting. Intuitively, dextrose prolotherapy appears to be a promising alternative injection-based therapeutic procedure for managing chronic painful musculoskeletal conditions. Modern applications of prolotherapy date back to the 1950s, with increased interest by physicians and patients in the 1990s.8 At present, there is incomplete understanding regarding the mechanism behind prolotherapy. However, initiation of a local inflammatory cascade, leading to tissue proliferation and remodeling, is thought to be involved in the healing process.9 During this procedure, a small amount of an irritant solution is injected at multiple sites corresponding to painful tendons and ligament insertions, or intra-articularly, where it is thought to incite the body's healing response (Fig. 1). Despite many reports of clinical success with dextrose prolotherapy in an array of musculoskeletal issues, dextrose prolotherapy has yet to be seen as a mainstream treatment for knee OA in recent guidelines. In the 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee, the use of prolotherapy was conditionally recommended against in patients with knee OA.10 Likewise, the Osteoarthritis Research Society International (OARSI) guidelines strongly recommended against dextrose prolotherapy due to extremely-low quality evidence.11
Fig. 1.
Relationship of prolotherapy to the tissue injury timeline.
Despite the various guidelines recommending against dextrose prolotherapy, randomized trials of prolotherapy continued to emerge in recent years with a growing body of literature review, especially in 2016 and 2017. These reviews suggested positive benefits of using prolotherapy for various functional domains of OA.12,13 This systematic review aimed to re-evaluate randomized studies examining efficacy of dextrose prolotherapy compared against controls or other active interventions in the management of knee OA, in light of findings from a spate of new trials that have been published in this field since the last systematic review.7
Methods
We conducted a systematic review and meta-analysis of relevant studies following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Search strategy and selection criteria
We identified potential studies by performing a thorough systematic search of the PubMed and Embase databases. The search period spanned from inception to January 31, 2021. The search terms included [(prolotherapy) OR (dextrose prolotherapy)] AND [(knee osteoarthritis) OR (knee) OR (knee arthrosis)].
One investigator (E-N) ran the search strategy and removed the duplicates. Two authors (TC-W and E-N) assessed all titles and abstracts to determine if the articles met the inclusion criteria. The full text of potentially eligible articles was then retrieved and independently screened by the same two investigators. Any disagreement was resolved through mutual discussion. If a consensus could not be achieved, the third author (YL-T) would have the casting vote. The reference lists of the full-text articles were further screened for relevant articles for inclusion.
Inclusion criteria
Our inclusion criteria included
-
1.
All randomized trials that compared the use of dextrose prolotherapy versus other injectates (active and placebo) or interventions in the treatment of knee OA;
-
2.
Participants at least 21 years of age;
-
3.
OA diagnosis and severity grading as defined by the various study authors;
-
4.
Follow-up duration of all timepoints
Exclusion criteria
We excluded articles published in languages other than the English language, reviews, case series, case reports, conference abstracts, and studies performed for knee pathologies other than OA.
Data extraction
E-N extracted the data independently, which was separately verified by YL-T. Disagreements on data extraction were resolved through consensus discussion between E-N and YL-T. If a consensus could not be achieved, then a third author (TC-W) would have the casting vote. Relevant information from each included article was extracted and recorded in an electronic spreadsheet. These information were: (1) first author and year of publication, (2) sample size, (3) average age of participants, (4) symptom duration, (5) OA severity on radiographs, (6) the total number of injections, (7) volume of injectate per dose, (8) type of injectate, (9) control, (10) injection technique, (11) interval of injection, (12) outcome measures, and (13) occurrence of adverse events.
Methodological quality and risk of bias assessment
For each included study, two investigators independently assessed the methodological quality and risk of bias based on the Cochrane Handbook for Systematic Reviews of Interventions recommendations. We used Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2).14 The RoB 2 tool provides a framework consisting of various domains for assessing the risk of bias. The domains included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. The Strength of Recommendation Taxonomy (SORT) was used to evaluate the strength of recommendations based on the available evidence and the quality of included studies.15 An A-level recommendation is based on consistent and good-quality patient-oriented evidence; a B-level recommendation is based on inconsistent or limited-quality patient-oriented evidence; and a C-level recommendation is based on consensus, usual practice, opinion, disease-oriented evidence, or case series for studies of diagnosis, treatment, prevention, or screening.
Statistical analysis
Data was then analyzed using the Review Manager software (RevMan version 5.4). Mean differences (MD) were used to evaluate effect sizes for continuous outcomes. We accepted an I2 value of >50% to denote significant heterogeneity. In cases where heterogeneity was attributable to differences in the subjects, interventions, or study design, we used random-effects analyses. Otherwise, we chose fixed-effects analyses. A probability value of <0.05 was considered to be statistically significant. Outcome measures recorded up till the 3-month timepoint were considered to be sub-acute, and those beyond that considered as chronic. When data was deemed incomplete or needed clarification, we contacted the corresponding authors through electronic mail.
Results
Study selection
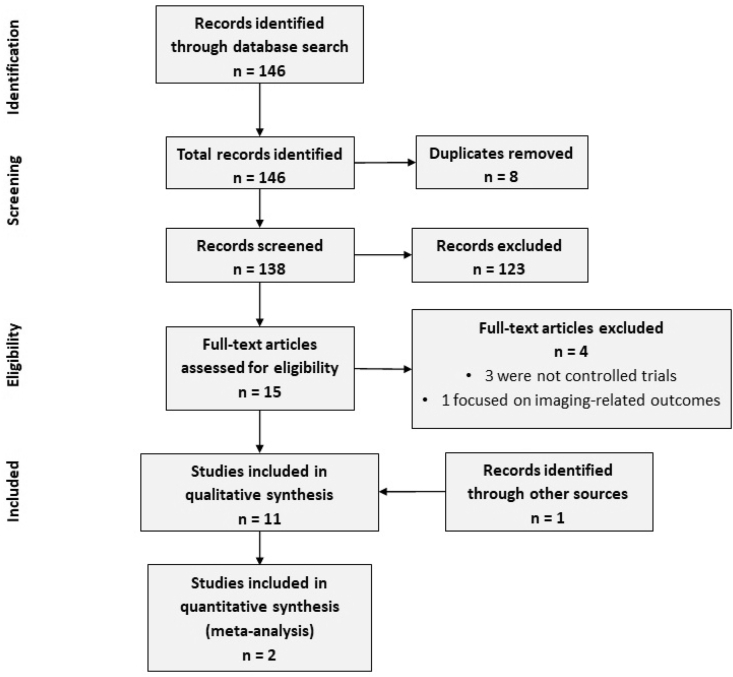
Our search yielded 146 articles. After removing duplicates, 138 studies remained and were screened using their title and abstract, leaving 15 studies for full-text review. Of these, ten were considered eligible. Manual screening of references of these included articles did not reveal any further relevant studies. However, one candidate was discovered in a prior systematic review that had been among the results generated through our database search. Eleven articles were included in the systematic review (Fig. 2). We sought clarifications from the authors of two studies but did not receive any response.16,17
Fig. 2.
PRISMA flow sheet.
Study characteristics
The 11 included studies were published between 2000 and 2020 and included 837 patients in total. Study size ranged from 31 to 120 patients. Patients with varying grades of severity of OA were included. The clinical details of the various studies are summarized and we highlight pertinent information below (Table 1).
Table 1.
Summary of trial design and protocol characteristics for included studies.
| # | Study | Design | Participant characteristics | Injection characteristics | Injectate characteristics |
|---|---|---|---|---|---|
| 1 | Eroğlu 2016 | Three-armed RCT; Dextrose prolotherapy vs PRP vs saline control; Follow-up at 3 and 6 months after first injection | Total 58 (Kellgren-Lawrence 1–3); Dextrose: 20, PRP: 18, Saline: 20 | Dextrose: Up to 15 EA injections using peppering technique based on major tender points followed by 1 IA injection from infero-medial approach; 3 procedures at 3-weekly intervals. PRP: Up to 15 EA injections using peppering tehcnique based on major tender points followed by 1 IA injection from infero-medial approach; 3 procedures at 3-weekly intervals. Saline: Up to 15 EA injections using peppering tehcnique based on major tender points followed by 1 IA injection from infero-medial approach; 3 procedures at 3-weekly intervals | Dextrose: 22.5 mLs of solution (concentration not disclosed) injected EA followed by 6 mLs of IA solution. PRP: 22.5 mLs of solution (2 centrifugations of 1500 rpm × 6 mins then 3500 rpm × 6 mins, activated with 10% calcium chloride) injected EA followed by 6 mLs of IA solution. Saline: 22.5 mLs of solution (reported concentration 0.09%) injected EA followed by 6 mLs of IA solution |
| 2 | Hashemi 2015 | RCT; Hypertonic dextrose prolotherapy vs ozone prolotherapy (prolozone); Follow-up at 3 months after last injection | Total 80 (Kellgren-Lawrence 1–2); Hypertonic dextrose: 40, Prolozone: 40 | Hypertonic dextrose: 1 IA injection from inferomedial approach; 3 procedures at intervals of 7–10 days. Prolozone: 1 IA injection from inferomedial approach; 3 procedures at intervals of 7–10 days | Hypertonic dextrose: 7 cm3 of 12.5% dextrose. Prolozone: 5–7 cm3 of 15 g/mL ozone-oxygen mixture |
| 3 | Hosseini 2019 | RCT; Hypertonic dextrose-saline vs hyaluronic acid prolotherapy; Follow-up at 3 months | Total 104 (Kellgren-Lawrence ≥2); Hypertonic dextrose-saline: 52, Hyaluronic acid: 52 | Hypertonic dextrose-saline: 4 peri-articular injections using fan-wise technique (2 injections at superolateral patella, 1 at medial knee joint line, 1 at anterior fibular head); 3 procedures at days 0, 7, and 14. Hyaluronic acid: 1 IA injection from inferomedial approach; 3 procedures at days 0, 7, and 14 | Hypertonic dextrose-saline: 10 mLs of 12.5% dextrose (2.5 cc at each point). Hyaluronic acid: 2.5 mLs of hyaluronic acid |
| 4 | Pishgahi 2020 | Three-arm RCT; Dextrose prolotherapy vs PRP vs autologous conditioned serum (ACS); Follow-up at 1 and 6 months | Total 92 (Radiologic grade 2–4); Dextrose: 30, PRP: 30, ACS: 32 | Dextrose: 1 IA injection from supra-lateral approach; 3 procedures at weekly intervals. PRP: 1 IA injection from supra-lateral approach; 2 procedures at weekly intervals. ACS: 1 IA injection from supra-lateral approach; 2 procedures at weekly intervals | Dextrose: 2 mLs of 50% dextrose mixed with 2 mLs water and 1 mL of 2% lidocaine. PRP: 4X concentration of platelets from 20 mLs of venous blood. ACS: 2 mLs of conditioned supernatant from 20 mLs of venous blood |
| 5 | Rabago 2013 | Three-arm partially-blinded RCT; Dextrose prolotherapy vs saline control vs home exercise; Follow-up at 5, 9, and 12 weeks in person, 26 and 52 weeks by telephone | Total 98 (Moderate-severe pain); Dextrose: 30, Saline: 29. Exercise: 31 | Dextrose: Up to 15 EA injections using peppering tehcnique at ligament-bone insertions followed by 1 IA injection from infero-medial approach; 3–5 procedures at 1,5, and 9 weeks (optional sessions at 13 and 17 weeks). Saline: Up to 15 EA injections using peppering tehcnique at ligament-bone insertions followed by 1 IA injection from infero-medial approach; 3–5 procedures at 1,5, and 9 weeks (optional sessions at 13 and 17 weeks) | Dextrose: 22.5 mLs containing 6.75 mLs of 50% dextrose, 4.5 mLs of 1% lidocaine, and 11.25 mLs of 0.9% saline (EA); 6 mLs from a 10 mL solution containing 5 mLs of 50% dextrose and 5 mLs of 1% lidocaine/saline (IA). Saline: 22.5 mLs containing 18 mLs of 0.9% sodium chloride and 4.5 mLs of 1% lidocaine (EA); 6 mLs from a 10 mL solution containing 5 mLs of 0.9% sodium chloride and 5 mLs of 1% lidocaine (IA) |
| 6 | Rahimzadeh 2014 | Three-arm double-blind RCT; Erythropoietin prolotherapy (EPO) vs dextrose prolotherapy vs pulsed radiofrequency; Follow-up at 2, 4, and 12 weeks | Total 70 (Kellgren-Lawrence 1–3 or clinical class I-III); EPO: 20, Dextrose: 24, Pulsed radiofrequency: 26 | EPO: 1 IA injection from anteroposterior approach at superolateral patella, fluoroscopic guidance; single procedure. Dextrose: 1 IA injection from anteroposterior approach at superolateral patella, fluoroscopic guidance; single procedure. Pulsed radiofrequency: 1 IA injection from anteroposterior approach at superolateral patella, fluoroscopic guidance; single procedure | EPO: 4000 international units. Dextrose: 5 cc 25% dextrose with 5 cc of 0.5% ropivacaine. Pulsed radiofrequency: 20 ms, 2 Hz, 45 V, 15 min s, 42 °C, 2 cycles |
| 7 | Rahimzadeh 2018 | Double-blind RCT; Platelet-rich plasma (PRP) vs dextrose prolotherapy; Follow-up at 1, 2, and 6 months | Total 42 (Kellgren-Lawrence 1–2); PRP: 21, Dextrose: 21 | PRP: 1 IA injection from upper outer quadrant approach; 2 procedures at monthly intervals. Dextrose: 1 IA injection from upper outer quadrant approach; 2 procedures at monthly intervals | PRP: 7 mLs of separated plasma from 20 mLs of venous blood centrifuged for 20 min s at 3200 rpm and then 5 min s at 1500 rpm. Dextrose: 7 mLs of 25% dextrose |
| 8 | Reeves 2000 | Double-blind RCT; Dextrose prolotherapy vs placebo injection; Follow-up at 12 months | Total 31 (Radiologic grade ≥2); Group numbers undisclosed | Dextrose: 1 IA injection from inferomedial approach; 3 procedures at 2-monthly intervals (optional open-label fontinuation at 6, 8, and 10 months). Placebo: 1 IA injection from inferomedial approach; 3 procedures at 2-monthly intervals | Dextrose: 9 cc of 10% dextrose and lidocaine solution (0.075%) in bacteriostatic water. Placebo: 9 cc of lidocaine solution (0.075%) in bacteriostatic water |
| 9 | Rezasoltani 2020 | Four-arm RCT; Physiotherapy modalities vs botulinum neurotoxin A vs hyaluronic acid vs dextrose prolotherapy; Follow-up at 1 and 4 weeks, and 3 months | Total 120 (Kellgren-Lawrence 3–4); Modalities: 30, Botulinum neurotoxin: 30, Hyaluronic acid: 30, Dextrose: 30 | Botulinum neurotoxin: 1 IA injection, ultrasound-guided; single procedure. Hyaluronic acid: 1 IA injection, ultrasound-guided; 3 procedures at weekly intervals. Dextrose: 1 IA injection, ultrasound-guided; 3 procedures at monthly intervals | Botulinum neurotoxin: 250 units of Dysport diluted with 5 mLs of normal saline. Hyaluronic acid: 2 mLs of hyaluronic acid. Dextrose: 8 mLs of 20% dextrose and 2 mLs of 2% lidocaine |
| 10 | Sit 2020 | Double-blind RCT; Hypertonic dextrose prolotherapy vs normal saline; Follow-up at 16, 26, and 52 weeks | Total 76 (Pain score ≥ 3/6); Dextrose: 38, Normal saline: 38 | Dextrose: 1 IA injection from suprapatellar approach, ultrasound-guided; single procedure. Normal saline: 1 IA injection from suprapatellar approach, ultrasound-guided; single procedure | Dextrose: 5 mLs of 25% dextrose/water. Normal saline: 5 mLs of normal saline |
| 11 | Sert 2020 | Three-arm RCT; Hypertonic dextrose prolotherapy vs normal saline control vs exercise control; Follow-up at 6 and 18 weeks | Total 66 (Kellgren-Lawrence 2–3); Dextrose: 21, Normal saline: 22, Exercise: 19 | Dextrose: 1 IA injection from superolateral approach followed by multiple EA peppering injections to multiple pre-marked sites; 3 procedures at 3-weekly intervals. Normal saline: 1 IA injection from superolateral approach followed by multiple EA peppering injections to multiple pre-marked sites; 3 procedures at 3-weekly intervals | Dextrose: 5 mLs of 25% dextrose/saline solution for IA injection, followed by total 10 mLs of 15% dextrose/saline solution for EA injection. Normal saline: 2.5 mLs of normal saline with 2.5 mLs of 1% lidocaine for IA injection, followed by total 10 mLs (5 mLs of normal saline with 5 mLs of 1% lidocaine) for EA injection |
Abbreviations: EA: Extra-articular, IA: Intra-articular, PRP: Platelet-rich plasma; RCT: randomised control trial.
Methodological quality and risk of bias
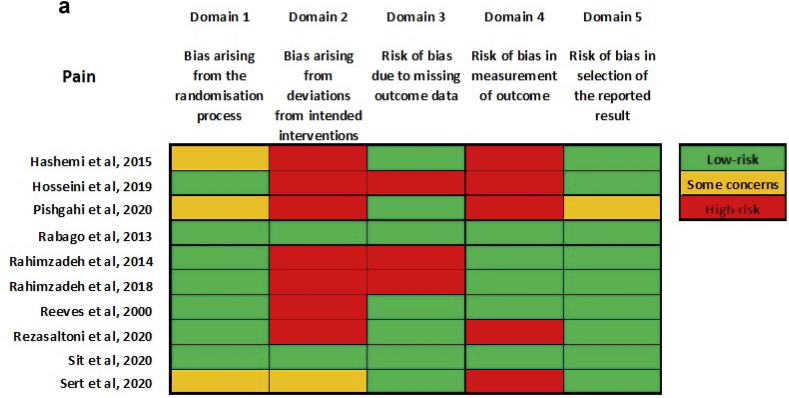
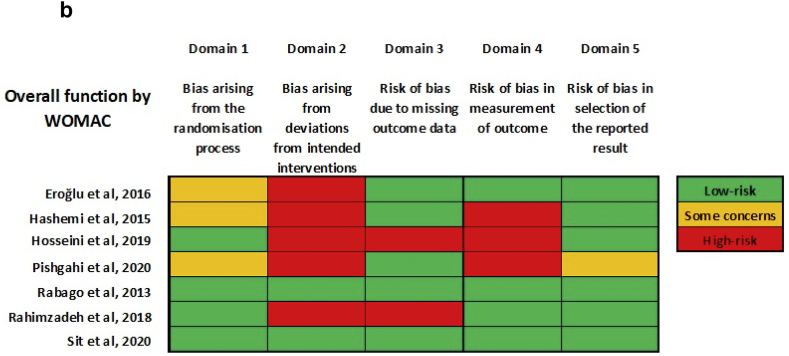
The overall risk of bias was high for both pain and overall function (by reported Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores). (Fig. 3a, Fig. 3ba & b). Most studies were at high risk for bias arising from deviations from intended interventions. Most studies were at low risk for bias in the selection of reported results. None of the studies provided a detailed publicly-accessible trial protocol, including planned a priori statistical analysis methods on trial registration websites such as ClinicalTrials.gov.
Fig. 3a.
Risk-of-bias summary for outcome: pain.
Fig. 3b.
Risk-of-bias summary for outcome: function (by WOMAC).
Concentration of dextrose
The concentration of dextrose ranged from 10% to 25% for intra-articular injections, while extra-articular injections consisted of dextrose with a range of concentration from 12.5% to 15%. One study did not report the concentration of dextrose injected.18
Injection regime
The number of injections and the intervals between injections were heterogenous across studies. The number of injections ranged from one to three, with just under half of the studies using the three-injection regime. The interval between injections ranged from one week to two months, with no particular intervals forming a significant majority.
Site(s) of injections
The choice of injection site was heterogenous across studies. Intra-articular dextrose prolotherapy was performed in seven studies.16,17,19, 20, 21, 22, 23 Both intra and extra-articular dextrose prolotherapy were performed in three studies,18,24,25 while extra-articular dextrose prolotherapy was performed in one study.26
Dextrose prolotherapy vs control
Five studies included a control in the study design.16,18,23, 24, 25 One study showed that improvement in total WOMAC and WOMAC subscales did not reach statistical significance and there was no inter-group difference post-treatment.18 (Table 2) Four studies with follow-up of up to one year, using outcome measures such as pain scores, WOMAC, SF-36, and range of motion (ROM) reported significant improvements after dextrose prolotherapy compared to controls.16,23, 24, 25 In one study, although a significant difference was reported in WOMAC and pain intensity, objective outcome measures such as timed-up-and-go, 30-s chair stand, and 40 m fast-paced walk were similar pre- and post-intervention.16
Table 2.
Summary of outcomes for included studies.
| # | Study | Outcomes charted | Summary of findings | Safety aspects |
|---|---|---|---|---|
| 1 | Eroğlu 2016 | Primary: WOMAC | Intra-group: No significant differences in all groups before and after intervention for both the composite score and the individual subscales. Inter-group: No significant difference for all outcomes at all time points | No severe adverse events reported |
| 2 | Hashemi 2015 | Primary: VAS, WOMAC | Intra-group: Significant differences in both groups before and after intervention for both outcomes. Inter-group: No significant difference for both outcomes | Undeclared |
| 3 | Hosseini 2019 | Primary: VAS, WOMAC | Intra-group: Significant differences in both groups before and after intervention for both outcomes. Inter-group: Significant improvement in hyaluronic acid group over dextrose group for both outcomes | No serious adverse events reported |
| 4 | Pishgahi 2020 | Primary: VAS, WOMAC (Persian version) | Intra-group: No significant improvement in pain or function at 1 and 6 months for dextrose group Significant improvement in pain for PRP group at 1 month but not sustained at 6; significant improvement in function at 1 and 6 months Significant changes in pain and function at 1 and 6 months for ACS group. Inter-group: PRP showed significant improvement over dextrose for function but not pain ACS showed significant improvement over dextrose for pain and function as well as over PRP for pain | Undeclared |
| 5 | Rabago 2013 | Primary: WOMAC. Secondary: Knee pain scale (KPS). Tertiary: Procedure-related pain severity, daily oipiod usage, treatment satisfaction | Intra-group: Significant improvements in WOMAC scores across all groups. Inter-group: Dextrose group had significantly-better WOMAC scores vs saline and exercise controls when adjusted for sex, age, and body mass Dextrose group had significantly-better KPS scores vs saline and exercise controls | Mild-moderate post-injection pain 3 patients had self-limited bruising No other side effects or adverse events |
| 6 | Rahimzadeh 2014 | Primary: VAS, range of movement, patient satisfaction | Intra-group: Pain, ROM and patient satisfaction improved significantly in both the EPO and pulsed RF groups by the 12-week endpoint No improvements in the dextrose group. Inter-group: Pain and ROM improved significantly more for the EPO group as compared to the other two groups | No serious adverse events reported |
| 7 | Rahimzadeh 2018 | Primary: WOMAC and pain/stiffiness/functional subscores | Intra-group: Improvement in all measures for both groups between baseline and 6-month follow-up. Inter-group: All scores were significantly better for the PRP group as compared to the prolotherapy group | No serious adverse events reported |
| 8 | Reeves 2000 | Primary: VAS, frequency of leg buckling, flexion by goniometry, radiological findings of joint narrowing, osteophytosis, and anterior displacement difference | Intra-group: Pain improved in both groups but was only statistically significant in the dextrose group Radiographic features at 1-year timepoint were found to demonstrate improvement in osteoarthritic severity in the dextrose group. Inter-group: Multivariate analysis showed statistically-superior effect in the dextrose group for all non-radiographic outcomes | Some patients reported pain from distension of the joint capsule One patient had a postinjection flare that required IA steroid and orthopedic referral (subsequently found to have received placebo) |
| 9 | Rezasoltani 2020 | Primary: VAS. Secondary: Knee Injury and Osteoarthritis Outcome Score (KOOS) (Persian) | Intra-group: Pain and KOOS scores fell in all groups but was most marked in botulinum neurotoxin and dextrose prolotherapy groups. Inter-group: Hyaluronic acid was the least effective in terms of both pain improvement and KOOS total + subscales | No serious adverse events reported |
| 10 | Sit 2020 | Primary: WOMAC pain subscale (Chinese). Secondary: VAS, WOMAC and stiffness/functional subscales (Chinese), physical function measures, EuroQol-5D | Inter-group: Statistically-significant improvements in WOMAC pain subscale, functional subscale, composite score, pain, and EuroQol-5D VAS favouring dextrose over normal saline | Eight serious adverse events reported (2 in dextrose group and 6 in saline group) - but none were related to the interventions |
| 11 | Sert 2020 | Primary: WOMAC pain subscale. Secondary: WOMAC stiffness/functional subscales, VAS for pain and stiffness, SF-36 | Inter-group: Statistically-significant improvements in pain for dextrose group compared to both saline and exercise controls Statistically-significant improvements in stiffness and physical functioning for dextrose group compared to the exercise control | Undeclared |
Abbreviations: EA: Extra-articular, IA: Intra-articular, PRP: Platelet-rich plasma; ROM: Range of motion; WOMAC: Western Ontario and McMaster Universities Arthritis Index.
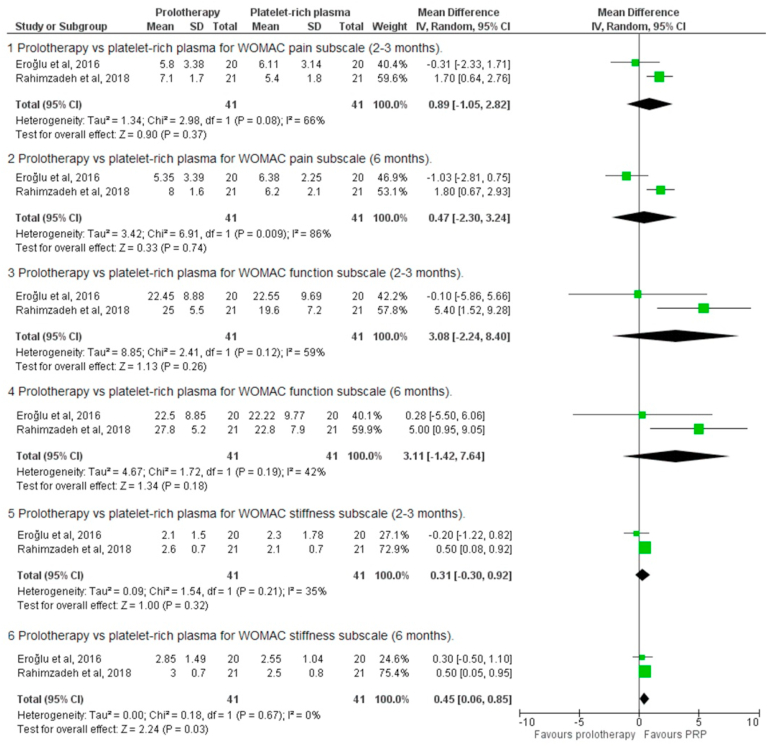
Dextrose prolotherapy vs platelet-rich plasma
There were two studies that compared dextrose prolotherapy against PRP.18,21 In one study with a six-month follow-up, combined peri-articular and intra-articular dextrose prolotherapy was not superior to PRP.18 In the prolotherapy arm, the pre and post-injection pain, stiffness, and function subscale scores of the WOMAC were similar across all time points. In a separate study involving intra-articular dextrose prolotherapy and PRP, dextrose prolotherapy did not significantly change pain scores or WOMAC at one month and six months post-intervention.20 Pain was significantly reduced at one month but not at six months post-intervention in the PRP arm. This observation was reversed for the WOMAC. The pooled results of two studies (n = 82) showed a significant difference in WOMAC function and stiffness subscales in favor of PRP.18,22
We proceeded with a meta-analysis of the prolotherapy versus PRP studies. At the 2-3-month time point, there were no significant differences between the two interventions for the WOMAC pain subscale (MD 0.89, 95% CI -1.05-2.82, p = 0.37), WOMAC function subscale (MD 3.08, 95% CI -2.24-8.40, p = 0.26), and WOMAC stiffness subscale (MD 0.0.31, 95% CI -0.3-0.92, p = 0.32. At the 6-month time point, there were no significant differences for the pain subscale (MD 0.47, 95% CI -2.30-3.24, p = 0.74) and the function subscale (MD 3.11, 95% CI 0.-1.42-7.64, p = 0.18), but PRP was superior on the stiffness subscale (MD 0.45, 95% CI 0.06–0.85, p = 0.03). (Fig. 4).
Fig. 4.
Meta-analysis of WOMAC sub-scale outcomes.
Dextrose prolotherapy vs hyaluronic acid
Two studies compared the use of dextrose prolotherapy and intra-articular hyaluronic acid. In the study looking at peri-articular dextrose prolotherapy versus intra-articular hyaluronic acid, the authors reported that pain and WOMAC scores significantly improved in both groups three months post-intervention.26 The hyaluronic acid group showed significantly more improvement.
Dextrose prolotherapy vs botulinum toxin
Both intra-articular botulinum toxin and intra-articular dextrose prolotherapy resulted in a significant decrease in pain three months post-intervention.17 Using the Knee Injury and Osteoarthritis Outcome Score (KOOS) as an outcome measure, dextrose prolotherapy resulted in a significant improvement in symptoms other than pain, physical function for daily living, and for sports activities at three months. Patients in the intra-articular botulinum toxin arm reported significant improvement in the preceding domains apart from stiffness. Small effect size was noted in stiffness in both groups. Apart from stiffness, the effect size was generally larger in the dextrose prolotherapy group than the botulinum toxin group.
Dextrose prolotherapy vs pulsed radiofrequency
Pain was reduced after both intra-articular dextrose prolotherapy and intra-articular pulsed radiofrequency. The former did not attain statistical significance. Knee ROM improved after both interventions, and again it was not significant in the dextrose prolotherapy arm.21
Dextrose prolotherapy vs erythropoietin
Pain was reduced after both intra-articular dextrose prolotherapy and intra-articular erythropoietin into the knee joint, but the difference in dextrose prolotherapy was not significant at three months. Knee ROM improved after both interventions, and again it was not significant in the dextrose prolotherapy arm.21
Dextrose prolotherapy vs intra-articular ozone
One study reported the use of intra-articular dextrose prolotherapy and intra-articular ozone.19 Follow-up was performed three months after the interventions. Pain intensity was reduced significantly in both treatment arms. WOMAC scores increased significantly in both treatment arms. There was no significant difference between the two groups.
Dextrose prolotherapy vs physical therapy
Physical therapy and intra-articular dextrose prolotherapy resulted in a significant decrease in pain and all the various KOOS subscales three months post-intervention.17 However, the effect size of physical therapy on stiffness was larger compared to dextrose prolotherapy, whereas the effect size of physical therapy on physical function for daily living was smaller.
Adverse outcomes
Three studies did not report on adverse outcomes.19,20,25 Overall, there were no serious adverse events reported apart from self-limiting post-injection pain and bruises.
Discussion
Current practice and understanding
This systematic review provides an update of current knowledge regarding the use of dextrose prolotherapy in knee OA. Overall, it appears that dextrose prolotherapy is effective at reducing pain and improving function in patients with knee OA; however, the results are at high risk for bias.
It was previously noted in a systematic review that studies (n = 4) were moderately heterogenous.13 The authors concluded that dextrose prolotherapy conferred a positive and significant beneficial effect in treating symptomatic knee OA. Separately, another systematic review evaluated ten studies, and the authors concluded that there is moderate evidence to suggest that dextrose prolotherapy is safe and can help achieve significant symptomatic control in patients with knee OA.27
A recent 2019 systematic review of dextrose prolotherapy in knee OA described statistically significant outcomes for prolotherapy with positive functional and pain outcomes.7 However, more studies have emerged since then, and we believe this warranted an update of the current body of literature. The five intercurrent studies have had relatively large participant numbers, with most findings favoring dextrose prolotherapy, but have also discussed other injectable and conventional treatments.16,17,20,25,26
Results from our systematic review indicated that most authors favored intra-articular injections with a three-injection regime. Half of the studies used dextrose solutions of less than 20%, while the rest used dextrose solutions of between 20% and 25%. Intervals between injections were disparate, ranging from a single injection, to weekly, monthly, or bimonthly, implying a lack of consistency in clinical trials or clinical practice.
The WOMAC and visual analog scales were the most commonly used outcome measures amongst the studies. The WOMAC is a self-administered questionnaire consisting of 24 items divided into three subscales: pain, stiffness, and physical functions. Beyond self-reported data, only one study was found to have reported outcome measures that were objective, such as the 30-s chair stand performance test, 40 m fast-paced walk test, and timed-up-and-go test.16 Although patient-reported outcomes are essential, we opine that objective outcome measures remain crucial in clinical trials.
Mechanism of action
Despite the number of studies performed to date, the precise mechanism of dextrose prolotherapy remains to be elucidated. There are three potential mechanisms proposed by researchers. Firstly, the basic concept of prolotherapy is the regeneration and repair of tissue by inducing inflammation using irritants. One study has shown that 10% dextrose appeared to result in the repair of articular cartilage defects in rabbits.28 Investigators have also demonstrated that 20% dextrose exerted a regenerative effect in the healing of injured Achilles tendons in rats through the proliferation of fibroblasts. In human in-vitro studies, a high glucose environment resulted in increased production of platelet-derived growth factors by the vascular endothelium and mesangial cells.29,30 Proving that these processes are directly responsible for in-vivo changes in human patients remains challenging, however.
Secondly, there is mixed evidence for the prochondrogenic effect of dextrose prolotherapy. Intra-articular dextrose prolotherapy did not reverse or slow cartilage loss for patients with knee OA compared to a control group as measured by MRI.31 On the other hand, a small study in patients with severe symptomatic knee OA found that intra-articular dextrose prolotherapy showed improvement in knee cartilage quality via direct arthroscopic visualisation and biopsy of cartilage, consistent with chondrogenesis.32
The third proposed mechanism is a direct pain-modulating effect of dextrose. A double blinded RCT showed that caudal epidural dextrose (5%) injection (without local anesthetic) resulted in pain reduction amongst patients with chronic low back pain and either gluteal or leg pain. The onset of analgesia occurred as early as 15 min after the injection, thus lending weight to the hypothesis that dextrose may have a direct sensorineural effect.33
Safety of intervention
Our findings regarding the safety of this intervention echoed those of previous systematic reviews in that dextrose prolotherapy was not known to cause any direct complications.7,13,27 We recognize that powering such studies for safety outcomes may be challenging. Until further data suggests otherwise, it is reasonable to assume that dextrose prolotherapy is safe in treating patients with knee OA.
Ideal injectable treatment for OA
Amongst various intra-articular injections that have been studied, only intra-articular corticosteroids received a strong recommendation from the ACR guidelines.10 Both intra-articular corticosteroids and hyaluronic acid are conditionally recommended in the OARSI guidelines.11 Our systematic review has included two studies that compared dextrose prolotherapy against intra-articular hyaluronic acid; however, we did not find any studies comparing dextrose prolotherapy against intra-articular corticosteroids.17,26
From this systematic review, many questions regarding the peri-procedural characteristics of dextrose prolotherapy remain unanswered. These include injection sites, the optimal concentration of dextrose, the number of injections, and the interval between injections. Given the technical complexity inherent in administering dextrose prolotherapy, it is likely that the impact of operator skill and experience may also play a role in intervention efficacy.
Risk of bias
The risk of bias in the included studies was generally high. These pertained to deviations from intended interventions, missing outcome data, and measurements of outcome. In particular, many reported trials did not discuss their management of missing data or trial deviations, and drop-outs were not clearly declared or discussed. The blinding of outcome assessors also was not well-documented. This had a significant impact on our ability to appraise the quality of the studies positively. Addressing missing data and the blinding of outcome assessors is imperative for future trials.
Limitations and strengths
This study's limitations are related to the studies' heterogeneity in terms of study design, injection sites, and techniques, varying concentrations of dextrose prolotherapy, and outcome measures used. Meta-analysis was limited to only two studies due to this heterogeneity. Although there are some clear practice patterns emerging, such as intra-articular injection and a three-injection regimen, these choices are not evidence-based and can at best be viewed as an expert opinion.
Despite that, this systematic review's strength included five studies published in the last two years, providing an updated overview of functional outcomes and the safety profile of dextrose prolotherapy. We were able to conclude that there were substantial findings in our review to support the safety of dextrose prolotherapy. In addition, we highlighted trends in risk-of-bias analysis for researchers to be aware of in future design of study methodology pertaining to the use of dextrose prolotherapy in knee OA.
Recommendations
While a significant proportion of studies are at risk for bias, there are two well-conducted studies which showed pain reduction and improved function. Despite good study design, we lowered the strength of our recommendation as study interventions differed between the two studies. It is possible that a single intra-articular injection of 25% dextrose may result in benefit in terms of pain and function for patients with knee OA. (Strength of recommendation B).
Due to the low-risk nature of the procedure in experienced hands and its affordability, we opined that it may be considered in patients who have failed conservative therapies, who are poor surgical candidates, or those who have declined definitive surgery. (Strength of recommendation C).
We recommend that future studies should be performed with controls and more widely accepted interventions such as intra-articular corticosteroids. Efforts should also be directed towards refining and optimizing injection protocols such as site and frequency. Studies with adequate follow-up for up to one year would be ideal for assessing the durability of treatment effects. We suggest that both subjective and objective outcome measures be used, such as VAS for pain, WOMAC with subscales for functional aspects, and the 6-min walk test for ambulatory distance. (Strength of recommendation not applicable – no recommendation could be made for both subjective and objective outcomes due to a lack of robust evidence).
Conclusions
Despite the addition of new studies, variation in study protocols and intervention choices, heterogenous documentation of outcomes, and a high degree of bias made it difficult to consolidate the therapeutic benefit of using dextrose prolotherapy. As a result, we can only make a recommendation that dextrose prolotherapy may be considered in knee osteoarthritis. More high-quality randomized controlled trials are warranted to establish the benefits of this intervention. To improve study quality, future studies should include blinding of outcome assessors, and better documentation of missing data and drop-outs. Dextrose prolotherapy is, however, deemed to be safe with no serious adverse events.
Declaration of competing interest
Nil grants/funding
The authors declare no financial benefits
The material in this manuscript has not been presented previously.
References
- 1.Murphy L., Schwartz T.A., Helmick C.G. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni P., Martson A., Vidya R. Pathophysiological landscape of osteoarthritis. Adv Clin Chem. 2021;100:37–90. doi: 10.1016/bs.acc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z., Ding C., Li T., Yu S.P. Current status and future prospects for disease modification in osteoarthritis. Rheumatology. 2018;57(suppl_4):iv108–iv123. doi: 10.1093/rheumatology/kex496. [DOI] [PubMed] [Google Scholar]
- 4.Sconza C., Respizzi S., Virelli L. Oxygen-ozone therapy for the treatment of knee osteoarthritis: a systematic review of randomized controlled trials. Arthroscopy. 2020;36(1):277–286. doi: 10.1016/j.arthro.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Zhai S., Huang B., Yu K. The efficacy and safety of Botulinum Toxin Type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48(4) doi: 10.1177/0300060519895868. 300060519895868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belk J.W., Kraeutler M.J., Houck D.A. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 7.Arias-Vázquez P.I., Tovilla-Zárate C.A., Legorreta-Ramírez B.G. Prolotherapy for knee osteoarthritis using hypertonic dextrose vs other interventional treatments: systematic review of clinical trials. Adv Rheumatol. 2019;59(1):39. doi: 10.1186/s42358-019-0083-7. [DOI] [PubMed] [Google Scholar]
- 8.Rabago D., Slattengren A., Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37(1):65–80. doi: 10.1016/j.pop.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser R.A., Lackner J.B., Steilen-Matias D. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139–159. doi: 10.4137/CMAMD.S39160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolasinski S.L., Neogi T., Hochberg M.C. American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheum. 2019;72(2):220–233. doi: 10.1002/art.41142. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannuru R.R., Osani M.C., Vaysbrot E.E. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Rabago D., Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19(6):34. doi: 10.1007/s11926-017-0659-3. [DOI] [PubMed] [Google Scholar]
- 13.Sit R.W., Chung V.C.H., Reeves K.D. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: a systematic review and meta-analysis. Sci Rep. 2016;6:25247. doi: 10.1038/srep25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Savović J., Page M.J. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Ebell M.H., Siwek J., Weiss B.D. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548–556. PMID: 14971837. [PubMed] [Google Scholar]
- 16.Sit R.W.S., Wu R.W.K., Rabago D. Efficacy of intra-articular hypertonic dextrose (prolotherapy) for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2020;18(3):235–242. doi: 10.1370/afm.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezasoltani Z., Azizi S., Najafi S. Physical therapy, intra-articular dextrose prolotherapy, botulinum neurotoxin, and hyaluronic acid for knee osteoarthritis: randomized clinical trial. Int J Rehabil Res. 2020;43(3):219–227. doi: 10.1097/MRR.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 18.Eroğlu A., Sari A., Durmuş B. Platelet-rich plasma vs prolotherapy in the management of knee osteoarthritis: randomized placebo-controlled trial. Spor Hekimliği Dergisi. 2016;51(2):34–43. doi: 10.5152/tjsm.2016.005. [DOI] [Google Scholar]
- 19.Hashemi M., Jalili P., Mennati S. The effects of prolotherapy with hypertonic dextrose versus prolozone (intraarticular ozone) in patients with knee osteoarthritis. Anesthesiol Pain Med. 2015;5(5) doi: 10.5812/aapm.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pishgahi A., Abolhasan R., Shakouri S.K. Effect of dextrose prolotherapy, platelet rich plasma and autologous conditioned serum on knee osteoarthritis: a randomized clinical trial. Iran J Allergy, Asthma Immunol. 2020;19(3):243–252. doi: 10.18502/ijaai.v19i3.3452. [DOI] [PubMed] [Google Scholar]
- 21.Rahimzadeh P., Imani F., Faiz S.H. Investigation the efficacy of intra-articular prolotherapy with erythropoietin and dextrose and intra-articular pulsed radiofrequency on pain level reduction and range of motion improvement in primary osteoarthritis of knee. J Res Med Sci. 2014;19(8):696–702. PMID: 25422652. [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimzadeh P., Imani F., Faiz S.H.R. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018;13:73–79. doi: 10.2147/CIA.S147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves K.D., Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Alternative Ther Health Med. 2000;6(2):68–74. 77-80. PMID: 10710805. [PubMed] [Google Scholar]
- 24.Rabago D., Patterson J.J., Mundt M. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013 May-Jun 2013;11(3):229–237. doi: 10.1370/afm.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sert A.T., Sen E.I., Esmaeilzadeh S., Ozcan E. The effects of dextrose prolotherapy in symptomatic knee osteoarthritis: a randomized controlled study. J Alternative Compl Med. 2020;26(5):409–417. doi: 10.1089/acm.2019.0335. [DOI] [PubMed] [Google Scholar]
- 26.Hosseini B., Taheri M., Pourroustaei Ardekani R. Periarticular hypertonic dextrose vs intraarticular hyaluronic acid injections: a comparison of two minimally invasive techniques in the treatment of symptomatic knee osteoarthritis. Open Access Rheumatol. 2019;11:269–274. doi: 10.2147/OARRR.S215576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan F., Trebinjac S., Murrell W.D., Maffulli N. The effectiveness of prolotherapy in treating knee osteoarthritis in adults: a systematic review. Br Med Bull. 2017;122(1):91–108. doi: 10.1093/bmb/ldx006. [DOI] [PubMed] [Google Scholar]
- 28.Kim S., Kim E., Kim S. The effects of hyperosmolar dextrose and autologous serum injection in the experimental articular defect of rabbit. J Korean Acad Rehabil Med. 2006;30(2):173–178. [Google Scholar]
- 29.Di Paolo S., Gesualdo L., Ranieri E. High glucose concentration induces the overexpression of transforming growth factor-beta through the activation of a platelet-derived growth factor loop in human mesangial cells. Am J Pathol. 1996;149(6):2095–2106. PMID: 8952542. [PMC free article] [PubMed] [Google Scholar]
- 30.Okuda Y., Adrogue H.J., Nakajima T. Increased production of PDGF by angiotensin and high glucose in human vascular endothelium. Life Sci. 1996;59(17):1455–1461. doi: 10.1016/0024-3205(96)00473-0. [DOI] [PubMed] [Google Scholar]
- 31.Rabago D., Kijowski R., Woods M. Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil. 2013;94(11):2075–2082. doi: 10.1016/j.apmr.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topol G.A., Podesta L.A., Reeves K.D. Chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. Pharm Manag PM R. 2016;8(11):1072–1082. doi: 10.1016/j.pmrj.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Maniquis-Smigel L., Reeves K.D., Rosen H.J. Analgesic effect and potential cumulative benefit from caudal epidural D5W in consecutive participants with chronic low-back and buttock/leg pain. J Alternative Compl Med. 2018;24(12):1189–1196. doi: 10.1089/acm.2018.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]