Abstract
Study design
Systematic Review & Meta-analysis.
Objectives
We aim to comparatively analyse the efficacy and safety of using leucocyte-poor platelet rich plasma (LP-PRP) against leucocyte-rich platelet rich plasma (LR-PRP) in the management of lateral epicondylitis.
Materials and methods
We conducted independent and duplicate electronic database searches including PubMed, Embase, Web of Science and Cochrane Library till September 2020 for randomised controlled trials analyzing the efficacy and safety of LP-PRP and LR-PRP in the management of lateral epicondylitis. Visual Analog Score(VAS) for pain, Disabilities of the Arm, Shoulder and Hand (DASH) Score, Patient Reported Tennis-Elbow Evaluation (PRETEE) Score, Mayo Elbow Performance Score(MEPS) and adverse events were the outcomes analyzed. Analysis was performed in R-platform using OpenMeta[Analyst] software.
Results
We performed a single arm meta-analysis of 26 studies involving 2034 patients. On analysis it was noted that significant improvement was noted in the VAS for pain (p < 0.001), DASH score (p < 0.001), PRETEE score (p < 0.001) and MEPS (p < 0.027) compared to their pre-operative state. No significant increase in adverse events were noted compared to the control group (p = 0.170). While stratifying the results based on the type of PRP used, no significant difference was noted between the use of LP-PRP or LR-PRP in any of the above-mentioned outcome measures.
Conclusion
PRP is a safe and effective treatment option for lateral epicondylitis with clinical improvements in pain and functional scores and both types of PRP (LR-PRP & LP-PRP) offer similar results.
Keywords: Platelet rich plasma, Lateral epicondylitis, Systematic review, Meta analysis
Introduction
Regenerative medicine has paved the way for less invasive techniques, minimal morbidity, and percutaneous administration of biological substances with osteoinductive and osteoconductive properties.1 Platelet-rich plasma (PRP) injection has evolved as a big boon in the treatment of musculoskeletal disorders. The usage of autologous PRP for the management of lateral epicondylitis was increasing among orthopedic surgeons and regenerative orthobiologists.2 With the promising evidence on the effects of PRP for lateral epicondylitis, extensive research is being conducted to identify the biomolecules present in PRP responsible for alleviating the disease process.3 There was no consensus on the technique of PRP preparation, the type of PRP to be used, the dose and frequency of PR injection and the amount of growth factors to be delivered at the pathological site for various diseases.4,5,6
A controversy on the usage of either leucocyte rich or leucocyte poor PRP for lateral epicondylitis prevails among all the orthobiologists. Leucocyte content in PRP solution induces an inflammatory regeneration through secretion of inflammatory cytokines and MMPs which exaggerate an inflammatory response at the site of action.7 Lana et al. devised MARSPILL classification to identify the variables responsible for therapeutic action of PRP.8 LR-PRP elicit catabolic, inflammatory, and detrimental environment while LP-PRP elicits an anabolic effect on the tissues being injected.9 In acute pathologies, LR-PRP helps in inflammatory regeneration and early angiogenesis at the injury site whereas LR-PRP in chronic pathologies leads to the formation of inordinate scar tissue at the injury site.10 The histological evaluation of chronic achilles tendinopathy of the rabbit model demonstrated that large collagen fibril formation with LR-PRP. The usage of LR-PRP in OA knee in vivo and in vitro demonstrated the detrimental effect of cartilage by increasing pro-inflammatory cytokines and anti-catabolic properties.9
There are no scientific evidence available for the usage of the right type of PRP (LR-PRP or LP-PRP) to treat lateral epicondylitis. Hence, with this meta-analysis, we aim to compare the efficacy and safety of LR-PRP vs LP-PRP in the management of lateral epicondylitis from the available literature.
Materials & Methods
This meta-analysis was conducted following the guidelines of the Back Review Group of Cochrane Collaboration11 and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12
Search strategy
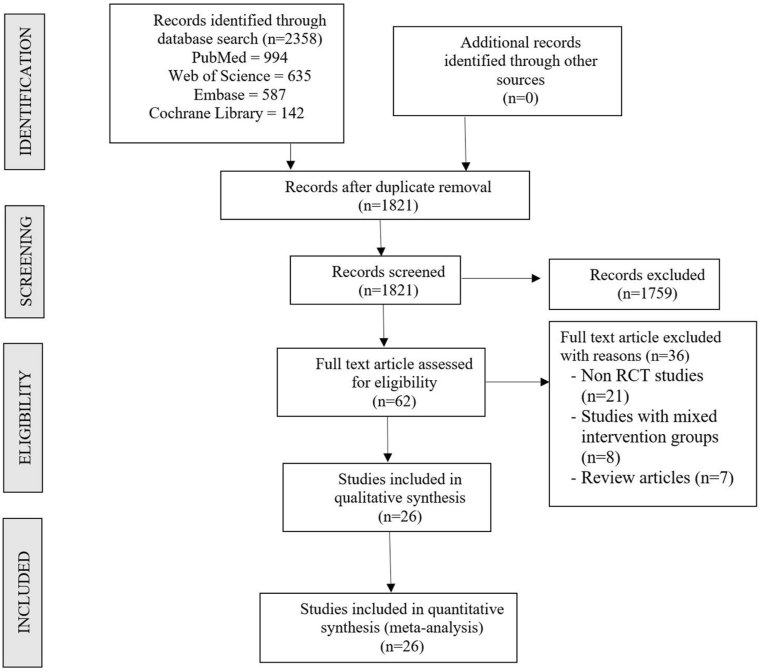
Two reviewers performed an independent electronic literature search for studies evaluating the efficacy and safety of using LP-PRP and LR-PRP in the management of lateral epicondylitis. We searched the following databases: PubMed, Embase, Web of Science and the Cochrane Library up to September 2020. No language or date restrictions were applied. Keywords used for the search were as follows: “Lateral Epicondylitis”, “Tennis Elbow”, “Platelet-Rich Plasma”, “PRP”, “Leucocyte rich”, “Leucocyte poor”, “Efficacy” and “Safety”. The reference list of the selected articles was also searched to identify studies not identified in the primary search. We also searched the grey literature sources like conference proceedings and meeting abstracts for potential studies to be included in the analysis. As per the inclusion and exclusion criteria, eligible studies were included for meta-analysis. The discrepancy between the authors was resolved through discussion until a consensus was obtained. A detailed study selection flow diagram is given in Fig. 1.
Fig. 1.
PRISMA flow diagram of the included studies.
Inclusion criteria
Studies were included for quantitative review if they met the following PICOS criteria:
Population: Patients with lateral epicondylitis.
Intervention: LP-PRP.
Comparator: LR-PRP.
Outcomes: Functional outcome measures like Visual Analog Score(VAS) for pain, Disabilities of the Arm, Shoulder, and Hand (DASH) Score, Patient Reported Tennis-Elbow Evaluation(PRETEE) Score, Mayo Elbow Performance Score(MEPS) and adverse events.
Study Design: Randomised Controlled Trials (RCTs).
Exclusion criteria
Trials were excluded if they had the following characteristics:
-
1.
Non randomized prospective and retrospective study designs
-
2.
Studies evaluating mixed interventions in treatment groups
Data extraction
Two reviewers retrieved independently relevant data from articles included for analysis. Following data were extracted:
-
1.
Study characteristics: year of publication, authors, country, number of patients enrolled.
-
2.
Baseline characteristics: mean age, gender proportions, method of preparation of PRP, number of injections used, injection frequency, volume of preparation injected, activator utilized, follow-up duration, and assessment parameters utilized.
-
3.
Efficacy Outcomes VAS for pain, DASH Score, PRETEE Score, MEPS
-
4.
Safety Outcomes: Adverse events in the included studies.
For missing data, we tried to contact the original author first. If the studies did not explicitly mention the type of PRP used as either LP-PRP or LR-PRP, we tried to identify them from their method of preparation of PRP and grouped them accordingly. If their method of preparation is not clear to categorize them, the corresponding author is contacted to ascertain the type of PRP they used in their study. Any disagreement in data collection was resolved until a consensus was attained by discussion.
Risk of bias and quality assessment
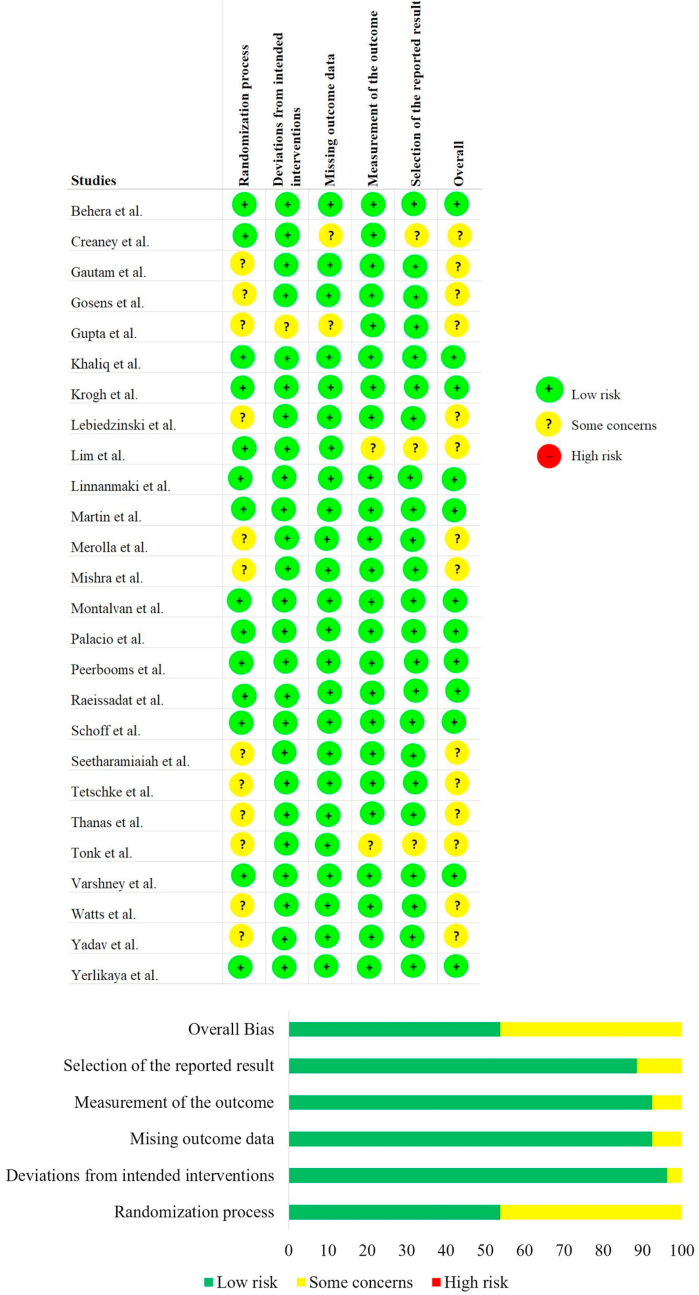
The methodological quality of the included studies was assessed independently by two reviewers using the Cochrane Collaboration's RoB-2 tool for RCTs which has five domains of bias assessment.13 We also analyzed the included studies for sponsorship bias to analyse the role of funding on the results of the study.14
Statistical analysis
Meta-analysis was conducted in the R platform with OpenMeta[Analyst].15 For dichotomous variable outcomes, risk ratio (RR) with 95% Confidence Interval (CI) was used and for continuous variable outcomes, weighted mean difference (WMD) with 95% CI was used. Heterogeneity was assessed using the I16 test.17 If I16 < 50% and p>0.1, we used a fixed-effects model to evaluate, otherwise, a random-effects was used. A p-value <0.05 was considered significant. Sensitivity analyses and subgroup analyses were performed to explore the source of heterogeneity when it existed. Publication bias was analyzed with a funnel plot for the outcomes in the included studies.
Results
Search results
Electronic database search resulted in 2358 articles, which after initial screening for duplicate removal gave a total of 1821 articles. Title and abstract screening were done in those 1821 articles and 1759 of them were excluded. 62 articles qualified for full-text review. On analyzing all the full-text qualified articles, it was noted that only one RCT by Yerlikaya et al.18 has directly compared the effectiveness of LP-PRP and LR-PRP in lateral epicondylitis. Hence a direct double-arm meta-analysis could not be performed to answer the research question. But we were able to identify 26 RCTs,19,20,21,22,23,24,25,26, 27, 28,29,3,30,31,32,33, 34, 35,36, 37, 38, 39, 40, 41, 42,18 after excluding 36 full-text eligible studies. These 26 RCTs with 2034 patients compared the effectiveness of LR-PRP and LP-PRP against various controls such as saline, steroid, and blood. To analyse the differential effectiveness of the LR-PRP and LP-PRP we performed a single-arm meta-analysis of the identified 26 RCTs studies to derive indirect evidence on the subject under study.
Of the 26 RCTs, 17 utilized LR-PRP while the rest of them used LP-PRP in their treatment arm. PRISMA flow diagram of study selection is given in Fig. 1. 7/26 studies under selection were from India19,22,35,38, 39, 40,42 while 10/26 studies were from European countries like Germany, Netherlands, Greece, Italy, Poland, Spain, and Turkey.
There was not much variability noted in the age of the included subjects within individual studies and among included studies. The mean age of the subjects in the included studies was 45.1 years with an overall range between 34 and 49 years. Included studies had a follow-up in the range between 3 weeks and 24 months. The general characteristics of the studies included were given in Table 1. The volume of the injectate ranged from 1.5 ml to 3.5 ml 22/26 studies used a single injection while the rest used two doses of injection. 19/26 used the single spin technique while the rest of them used the double spin method in the preparation of PRP. While 5 studies used calcium chloride as an activator,19,26,28,30,35 2 of them used sodium bicarbonate24,3 and the rest of them did not report the usage of an activator in their protocol. The preparation protocol of the PRP of the included studies was given in Table 2.
Table 1.
Characteristics of included studies.
| Sl.No | Author | Year | Country | Study design | Total patients | Treatment group | Type of PRP | Control group | Mean Age (years) | SD | Male | Female | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Behera et al. | 2015 | India | RCT | 25 | 15 | LP-PRP | 10 | 38 | 7 | 6 | 19 | 1 year |
| 2 | Creaney et al. | 2011 | UK | RCT | 150 | 80 | LR-PRP | 70 | 49 | 8 | 85 | 65 | 6 mon |
| 3 | Gautam et al. | 2015 | India | RCT | 30 | 15 | LP-PRP | 15 | ND | ND | ND | ND | 6 mo |
| 4 | Gosens et al. | 2011 | Netherlands | RCT | 100 | 51 | LR-PRP | 49 | 47 | 8.5 | 48 | 52 | 2 yrs |
| 5 | Gupta et al. | 2019 | India | RCT | 80 | 40 | LR-PRP | 40 | 40.8 | ND | 34 | 46 | 1 year |
| 6 | Khaliq et al. | 2015 | Pakistan | RCT | 102 | 51 | LR-PRP | 51 | 34 | 10 | 57 | 45 | 3 weeks |
| 7 | Krogh et al. | 2013 | Denmark | RCT | 40 | 20 | LR-PRP | 20 | 45 | 7.3 | 18 | 22 | 3 mo |
| 8 | Lebiedzinski et al. | 2015 | Poland | RCT | 99 | 53 | LP-PRP | 46 | 47 | 10 | 40 | 59 | 1 year |
| 9 | Lim et al. | 2018 | South Korea | RCT | 105 | 61 | LR-PRP | 59 | 50.1 | 9.41 | 61 | 65 | 24 weeks |
| 10 | Linnanmaki et al. | 2020 | Finland | RCT | 79 | 40 | LP-PRP | 39 | 46 | 6 | 35 | 44 | 52 weeks |
| 11 | Martin et al. | 2019 | Spain | RCT | 71 | 36 | LP-PRP | 35 | 48.26 | 7.64 | 33 | 38 | 1 year |
| 12 | Merolla et al. | 2017 | Italy | RCT | 101 | 50 | LR-PRP | 51 | 47 | 7.06 | 56 | 45 | 2 yrs |
| 13 | Mishra et al. | 2013 | USA | RCT | 230 | 116 | LR-PRP | 114 | 47.4 | 6 | ND | ND | 24 weeks |
| 14 | Montalvan et al. | 2016 | France | RCT | 50 | 25 | LP-PRP | 25 | 46 | 7.8 | 34 | 16 | 12 mo |
| 15 | Palacio et al. | 2015 | Brazil | RCT | 40 | 20 | LP-PRP | 20 | 47.9 | ND | ND | ND | 180 days |
| 16 | Peerbooms et al. | 2010 | Netherlands | RCT | 100 | 51 | LR-PRP | 49 | 47 | 7.6 | 48 | 52 | 1 yr |
| 17 | Raeissadat et al. | 2014 | Iran | RCT | 40 | 20 | LR-PRP | 20 | 46.25 | 7.5 | 8 | 32 | 14.5 mo |
| 18 | Schoff et al. | 2017 | Germany | RCT | 36 | 18 | LP-PRP | 18 | 52.6 | 11.4 | 18 | 18 | 6 mo |
| 19 | Seetharamiaiah et al. | 2017 | India | RCT | 60 | 30 | LR-PRP | 30 | ND | ND | ND | ND | 6 mo |
| 20 | Tetschke et al. | 2015 | Germany | RCT | 53 | 33 | LR-PRP | 19 | 52 | 12.6 | 22 | 31 | 1 yr |
| 21 | Thanas et al. | 2011 | Greece | RCT | 28 | 14 | LR-PRP | 14 | 36.6 | 8 | 20 | 6 mo | |
| 22 | Tonk et al. | 2014 | India | RCT | 81 | 39 | LR-PRP | 42 | 41.15 | 30 | 51 | 1 year | |
| 23 | Varshney et al. | 2016 | India | RCT | 83 | 33 | LR-PRP | 50 | 46 | ND | 45 | 38 | 6 mo |
| 24 | Watts et al. | 2018 | UK | RCT | 81 | 40 | LR-PRP | 41 | 47 | 4 | 47 | 34 | 52 weeks |
| 25 | Yadav et al. | 2015 | India | RCT | 60 | 30 | LR-PRP | 30 | 37 | ND | 17 | 43 | 3 mo |
| 26 | Yerlikaya et al. | 2018 | Turkey | RCT | 90 | 60 | LR-PRP | 30 | 45 | 8.9 | 26 | 64 | 2 mo |
LP-PRP – Leucocyte Poor Platelet Rich Plasma; LR-PRP – Leucocyte Rich Platelet Rich Plasma; NA – Not Available; RCT – Randomized Controlled Trial; SD – Standard Deviation; UK – United Kingdom; USA – United States of America.
Table 2.
PRP related parameters in the included studies.
| Sl.No | Author | Year | Type of PRP | Total patients | Volume injected (ml) | Number of injections | PRP preparation method | Activator | Comparator | Time between injections |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Behera et al. | 2015 | LP-PRP | 25 | 3 ml | 1 | Single spin | calcium chloride | Bupivacaine + Normal saline | |
| 2 | Creaney et al. | 2011 | LR-PRP | 150 | 1.5 ml | 1 | Single spin | NR | Blood | |
| 3 | Gautam et al. | 2015 | LP-PRP | 30 | 2 ml | 1 | single spin | NR | CS | |
| 4 | Gosens et al. | 2011 | LR-PRP | 100 | 2 ml | 1 | Single spin | NR | CS | |
| 5 | Gupta et al. | 2019 | LR-PRP | 80 | 3 ml | 1 | Double spin | citrate phosphate dextrose | CS | |
| 6 | Khaliq et al. | 2015 | LR-PRP | 102 | 3 ml | 1 | Single spin | NR | CS | |
| 7 | Krogh et al. | 2013 | LR-PRP | 40 | 3.5 ml | 1 | Single spin | sodium bicarbonate | Saline | |
| 8 | Lebiedzinski et al. | 2015 | LP-PRP | 99 | 2 ml | 1 | Double spin | NR | betamethasone | |
| 9 | Lim et al. | 2018 | LR-PRP | 105 | 2 ml | 1 | Single spin | calcium chloride | Saline | |
| 10 | Linnanmaki et al. | 2020 | LP-PRP | 79 | 2 ml | 1 | Single spin | NR | Saline | |
| 11 | Martin et al. | 2019 | LP-PRP | 71 | 5 ml | 2 | Single spin | calcium chloride | lidocaine | 6 months |
| 12 | Merolla et al. | 2017 | LR-PRP | 101 | 3 ml | 2 | Single spin | NR | Surgery | 2 weeks |
| 13 | Mishra et al. | 2013 | LR-PRP | 230 | 3 ml | 1 | Single spin | sodium bicarbonate | Bupivacaine | |
| 14 | Montalvan et al. | 2016 | LP-PRP | 50 | 2 ml | 2 | Single spin | calcium chloride | Saline | 4 weeks |
| 15 | Palacio et al. | 2015 | LP-PRP | 40 | 3 ml | 1 | Double spin | NR | CS | |
| 16 | Peerbooms et al. | 2010 | LR-PRP | 100 | 1 ml | 1 | Single spin | No activator | CS | |
| 17 | Raeissadat et al. | 2014 | LR-PRP | 40 | 2 ml | 1 | Double spin | NR | lidocaine | |
| 18 | Schoff et al. | 2017 | LP-PRP | 36 | 2 ml | 3 | Single spin | NR | Saline | 10 days |
| 19 | Seetharamiaiah et al. | 2017 | LR-PRP | 60 | 2 ml | 1 | Double spin | calcium chloride | Saline | |
| 20 | Tetschke et al. | 2015 | LR-PRP | 53 | 2 ml | 1 | Single spin | NR | Laser | |
| 21 | Thanas et al. | 2011 | LR-PRP | 28 | 3 ml | 1 | Single spin | No activator | Blood | |
| 22 | Tonk et al. | 2014 | LR-PRP | 81 | 3 ml | 1 | Double spin | No activator | Laser | |
| 23 | Varshney et al. | 2016 | LR-PRP | 83 | 2 ml | 1 | Single spin | NR | CS | |
| 24 | Watts et al. | 2018 | LR-PRP | 81 | 2 ml | 1 | Single spin | NR | Surgery | |
| 25 | Yadav et al. | 2015 | LR-PRP | 60 | 2 ml | 1 | Single spin | No activator | CS | |
| 26 | Yerlikaya et al. | 2018 | LR-PRP | 90 | 1.5 ml | 1 | Double spin | NR | Saline |
CS – Corticosteroid; LP-PRP – Leucocyte Poor Platelet Rich Plasma; LR-PRP – Leucocyte Rich Platelet Rich Plasma; NR – Not Reported.
Quality assessment
The methodological quality of the included studies was ascertained with the RoB2 tool of the Cochrane Collaboration as shown in Fig. 2. None of the included studies had an overall high risk of bias to be excluded from the analysis. On analysing the sponsorship bias, 10/26 of the included studies were externally funded. While 5/26 did not mentioned any data on the funding sources, rest 11/26 were non-funded studies. On further analysing the source of funding of those 10 studies, it was noted that 3 studies 27,28,31 received institutional research funding, and remaining 7 studies were funded directly or indirectly by commercial agents. Of the commercial sponsors we noted that Biomet Biologics Inc. (Warsaw, Indiana) funded 5 of the included studies involving LR-PRP 21,24,3,32,41 while 2 studies 30,34 involving LP-PRP was supported by Arthrex ®, Naples, FL, USA who were the manufactures of the extraction kits used in these trials. Although all these company sponsored trials reported favorable results on the part of the intervention under evaluation compared to the controls, similar results were noted by the non-funded and institution funded studies thereby making their results valid for inclusion into our study for analysis.
Fig. 2.
Methodological quality and risk of bias assessment of all the included studies.
Efficacy Outcomes
VAS for pain
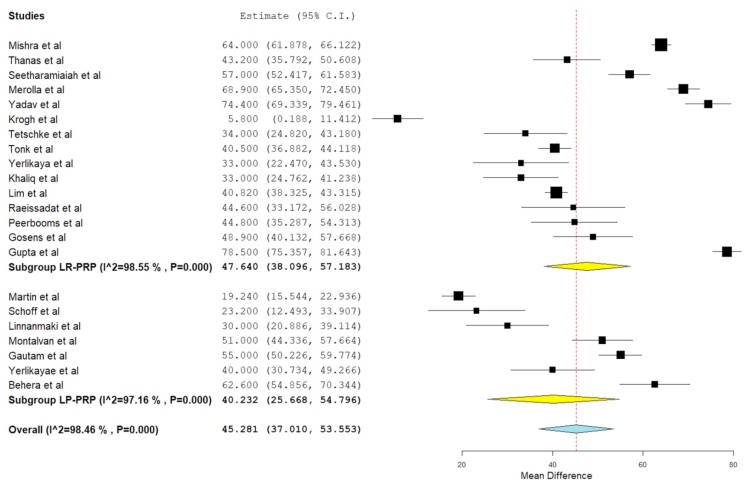
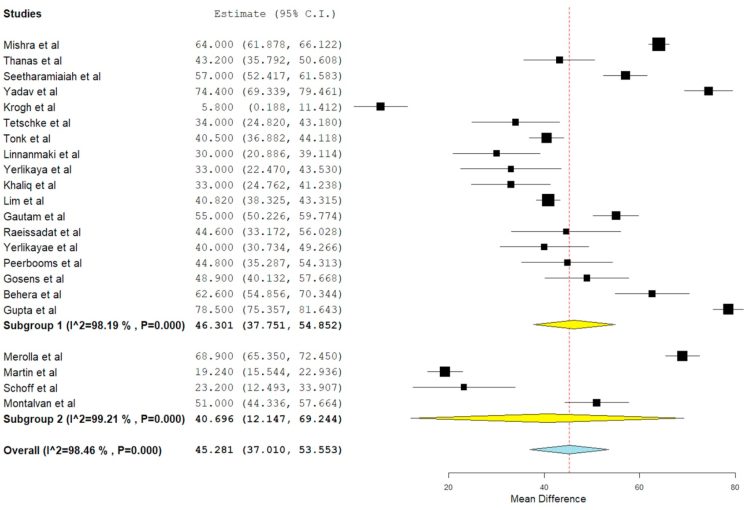
VAS score was reported in 22 of the included studies, 19,21,22,23,24,26, 27, 28,29,3,30,32,33, 34, 35,36, 37, 38,40,42,18 of which seven studies 24,28,29,3,35,37,42 utilized LP-PRP involving 351 patients while rest 15 studies utilized LR-PRP involving 1240 patients. There was significant heterogeneity observed between the included studies. (I16 = 98.46%, p = 0.001). Hence, a random-effects model was used for analysis. We found an overall significant improvement in VAS score compared to the pre-operative state irrespective of the nature of the PRP utilized (WMD = 45.281, 95% CI [37.010, 53.553], p < 0.001). We further stratified the analysis based on the nature of the PRP used by the studies and found significant improvement in the VAS Score in studies using LP-PRP (WMD = 40.232, 95% CI [25.668, 54.796], p < 0.001) and LR-PRP (WMD = 47.640, 95% CI [38.096, 57.183], p < 0.001) as shown in Fig. 3. However, no significant difference was found among the sub-groups analyzed.
Fig. 3.
Forest plot of the included studies stratified based on the nature of PRP used for VAS outcome at final follow-up.
DASH score
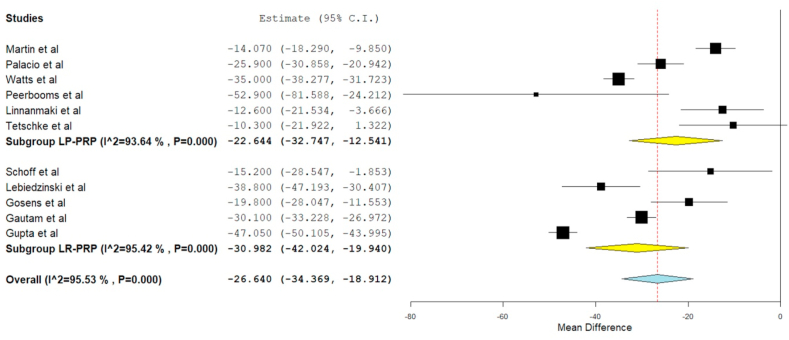
DASH score was reported in 11 of the included studies,21,22,25,27,28,31,32,34,36,40,41 of which six studies21,27,28,31,36,41 utilized LP-PRP involving 424 patients while rest 5 studies utilized LR-PRP involving 345 patients. There was a significant heterogeneity observed between the included studies. (I16 = 95.53%, p = 0.01). Hence, a random-effects model was used for analysis. We found an overall significant reduction in DASH score compared to the pre-operative state irrespective of the nature of the PRP utilized (WMD = −26.640, 95% CI [-34.369, −18.912], p < 0.001). We further stratified the analysis based on the nature of the PRP used by the studies and found significant improvement in the VAS Score in studies using LP-PRP (WMD = −22.644, 95% CI [-32.747, −12.541], p < 0.001) and LR-PRP (WMD = −30.982, 95% CI [-42.024, −19.940], p < 0.001) as shown in Fig. 4. However, no significant difference was found among the sub-groups analyzed.
Fig. 4.
Forest plot of the included studies stratified based on the nature of PRP used for DASH score outcome at final follow-up.
PRETEE score
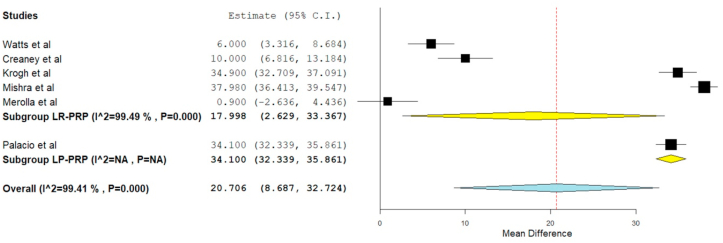
PRETEE score was reported in 6 of the included studies,20,24,29,3,31,41 of which five studies20,24,29,3,31 utilized LR-PRP involving 561 patients while only one other study41 utilized LP-PRP involving 81 patients. There was a significant heterogeneity observed between the included studies. (I16 = 99.41%, p = 0.01). Hence, a random-effects model was used for analysis. . We found an overall significant improvement in PRETEE score compared to the pre-operative state irrespective of the nature of the PRP utilized (WMD = 20.706, 95% CI [8.687, 32.724], p < 0.001). We further stratified the analysis based on the nature of the PRP used by the studies and found significant improvement in the PRETEE Score in studies using LP-PRP (WMD = 34.100, 95% CI [32.339, 35.861]) and LR-PRP (WMD = 17.998, 95% CI [2.629,33.367], p = 0.022) as shown in Fig. 5. However, no significant difference was found among the sub-groups analyzed.
Fig. 5.
Forest plot of the included studies stratified based on the nature of PRP used for PRETEE score outcome at final follow-up.
Mayo Elbow performance score
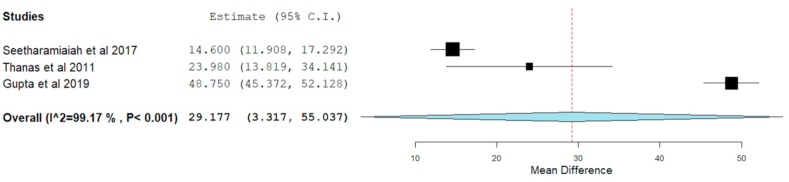
Three studies22,35,37 involving 168 patients utilizing LR-PRP reported functional outcomes based on Mayo Score without significant heterogeneity among them. (I16 = 99.169%, p = 0.001). Hence, a random-effects model was used for analysis. On analysis, significant improvement in Mayo score was noted in the PRP group compared to the preoperative state. (WMD = 29.177, 95% CI [3.317, 55.037], p = 0.027) as shown in Fig. 6.
Fig. 6.
Forest plot of the included studies describing MEPS outcome at final follow-up.
Safety
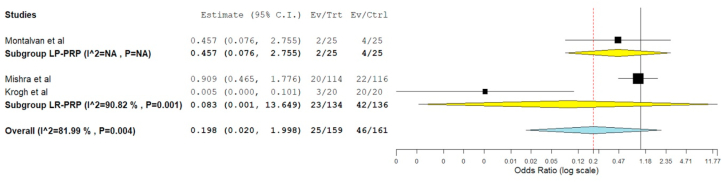
Three studies24,3,30 involving 320 patients reported adverse effects with significant heterogeneity among them (I16 = 81.99%, p = 0.004). Hence, a random-effects model was used for analysis. On analysis of the adverse events among the study participants, no significant difference was noted among the patient who received PRP compared to the controls. (RR = 0.198, 95% CI [0.020, 1.998], p = 0.170) as shown in Fig. 7. Of the three studies, two of them utilized LR-PRP24,3 while one used LP-PRP.30 Utilisation of LR-PRP (RR = 0.083, 95% CI [0.001, 13.649], p = 0.339) or LP-PRP (RR = 0.457, 95% CI [0.076, 2.755]) did not have a significant change in the adverse events noted among the studies.
Fig. 7.
Forest plot of the included studies comparing the safety of PRP therapy to the controls in lateral epicondylitis by adverse events reported in the included studies.
Subgroup analysis
We made a subgroup analysis to compare the efficacy of multiple injections used in 4/26 studies28,29,30,34 included in the analysis compared to a single injection of PRP in the rest of the studies for lateral epicondylitis and we did not find any significant benefit from usage of multiple doses of PRP (WMD = 40.696, 95% CI [12.147, 69.244], p = 0.005) for the condition compared to a single dose (WMD = 46.301, 95% CI [37.751, 54.852], p < 0.001) as given by the VAS scores on follow-up from the studies analyzed as shown in Fig. 8. We also categorised studies based on the single and double spin technique utilized to prepare the PRP. On subgroup analysis, we did not find any significant difference between the single (WMD = 43.805, 95% CI [33.930, 53.678], p < 0.001) and double spin technique (WMD = 49.208, 95% CI [32.534, 65.883], p < 0.001) on the outcome measures observed among the studies.
Fig. 8.
Forest plot of the subgroup analysis of the included studies comparing the role of single and multiple doses of PRP therapy in lateral epicondylitis.
Sensitivity analysis
A sensitivity analysis was performed in each outcome with significant heterogeneity among the reported results. All the results (VAS Score, DASH Score, PRETEE Score, and adverse events) were not significantly altered by sequentially omitting each study in the meta-analysis.
Publications bias
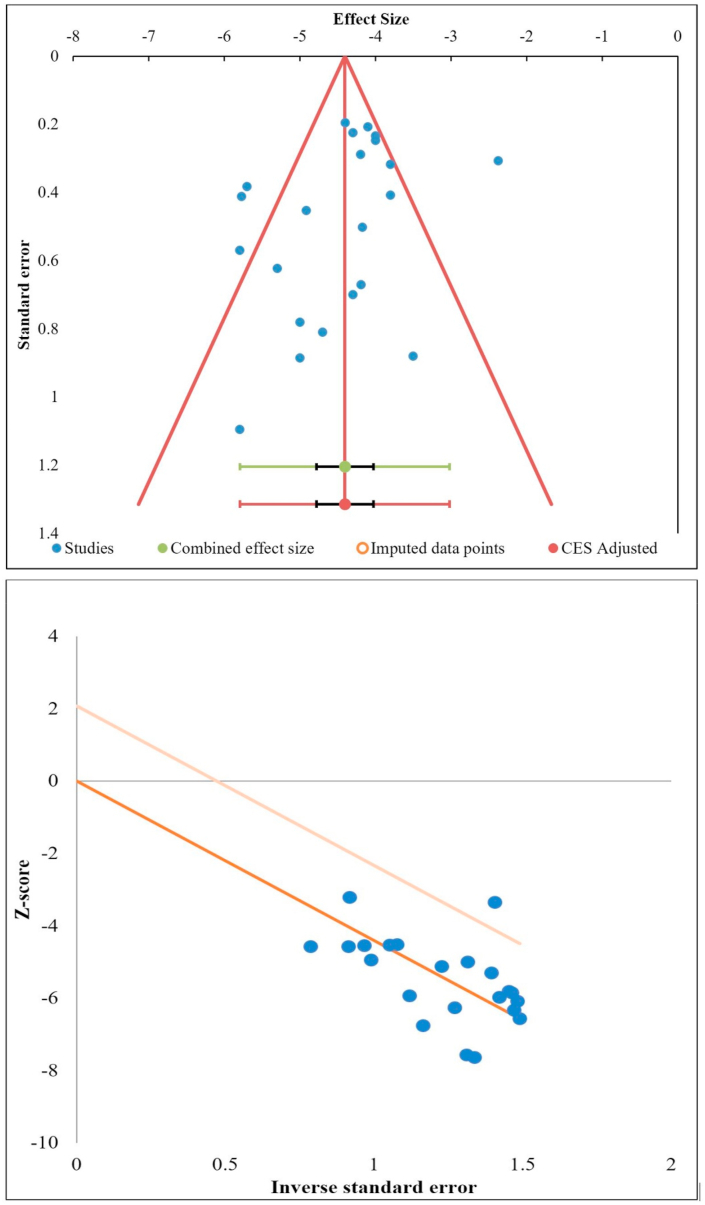
Publication bias was analyzed initially using the funnel for the VAS outcomes reported among the studies included in the analysis. Since the funnel plot was not symmetrical, we employed additional tests such as the Egger Regression test and Galbraith plot to verify the presence of publication bias since the funnel plot by itself might not be sufficient to estimate the publication bias effectively.43 There was no significant publication bias by the Egger Regression test (p = 0.129) and Galbraith plot as shown in Fig. 9.
Fig. 9.
Publication bias assessment with Funnel plot, Galbraith plot for VAS outcome of the included studies.
Discussion
The use of PRP for lateral epicondylitis started around 2006 and has been steadily increasing. The early results were promising and led to more frequent use and more studies, and after more than 10 years, we have a good number of RCTs to evaluate the role of PRP in lateral epicondylitis. In our meta-analysis, we found 26 RCTs eligible and had a total of 2034 patients. The various meta-analysis44,45 and RCTs have also established the positive role of PRP in lateral epicondylitis.24,3,32 The present need is to characterize the ideal PRP type, dose, and the number of injections required in lateral epicondylitis. We conducted this meta-analysis to specifically note the effect concerning the PRP type: LR-PRP versus LP-PRP.
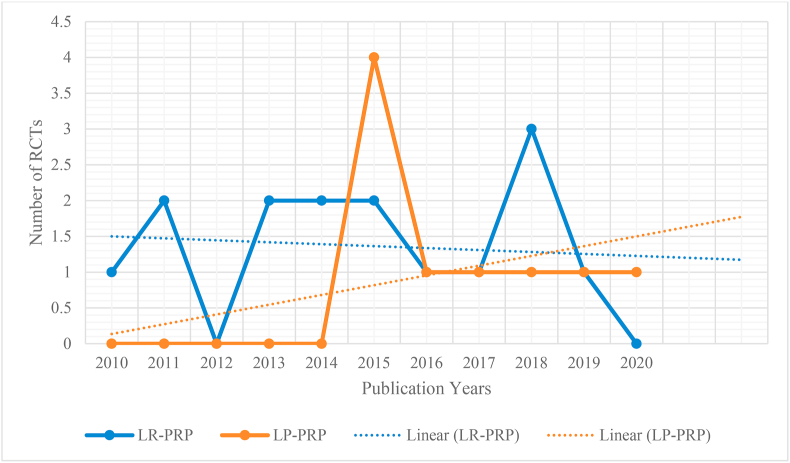
Mishra et al.46 who first published the results of PRP in lateral epicondylitis used LR-PRP and most of the studies which followed have used LR-PRP. Even in our meta-analysis 17 of the 26 studies have used LR-PRP. It is a well-discussed topic among PRP users and the consensus is to use LP-PRP for OA Knee and LR-PRP for tendinopathies. As far as lateral epicondylitis is concerned we felt that the preferred use of LR-PRP has been due to the fact that initial studies used LR-PRP and the other studies just followed them. This trend is clear from our meta-analysis (Fig. 10) and we can see a steady increase in the use of LP-PRP in recent years.
Fig. 10.
Chronology of use of type of PRP noted in the included studies.
In knee osteoarthritis, LP-PRP and LR PRP both have been used and both have shown good clinical outcomes. The proponents of LP PRP believe that the matrix metalloproteinases (MMPs) and other pro-inflammatory mediators in leucocytes could be deleterious to cartilage and some in vivo studies have demonstrated the same.16,47 Dragoo et al.48 and Filardo et al.49 have noted more undesirable side effects owing to greater inflammatory reactions in the LR-PRP group. The results of the meta-analysis and overall consensus is that both LR-PRP and LP-PRP are equally effective in osteoarthritis of knee as far as the outcomes are concerned, however, the short term pain and swelling reaction are more in LR-PRP compared to LP-PRP.
There is only one direct comparative study between LR-PRP and LP-PRP for lateral epicondylitis18 and the authors did not note any difference between both and concluded both to be effective. We comprehensively and systematically reviewed all the available literature to ascertain the effect of leucocyte concentration in the outcome of using PRP in lateral epicondylitis and found that.
-
1.
Significant improvement was noted in the VAS for pain (p < 0.001), DASH score (p < 0.001), PRETEE score (p < 0.001), and MEPS (p < 0.027) compared to their pre-injection state.
-
2.
No significant increase in adverse events was noted compared to the control group (p = 0.170).
-
3.
While stratifying the results based on the type of PRP used, no significant difference was noted between the use of LP-PRP or LR-PRP in any of the above-mentioned outcome measures.
There are some in vitro studies that have raised concerns regarding the leucocytes in PRP and the deleterious effects they can have on tendon healing. McCarrel et al.50 have noted that leucocytes in LR-PRP tend to have a negative effect on the anabolic effects of PRP by increasing Collagen 3 more than Collagen 1 and this indicates scar tissues, fibrosis, and poor mechanical strength. On the contrary, LP-PRP tends to increase the Collagen 1 more compared to Collagen 3 and thereby a favorable Collagen 1/Collagen 3 ratio for tendon healing. Similar results were also noted by Zhou et al.51 LR-PRP also tends to have catabolic effects on tendon cells as demonstrated by Zhou et al.51 wherein LR-PRP increased MMP-1 and MMP-13 levels in comparison to LP-PRP and control. Increased MMP-1 and MMP-3 expression were also noted by Mos et al.52 and this could be because of the large number of leucocytes in their platelet-rich clot releasate (PRCR). The third negative effect of leucocytes in PRP is the inflammatory effects it can have as seen by raised gene and protein expression of IL-6, IL-1β, and TNF-α in tendon cells.50,51
Despite the negative effects noted with the presence of leukocytes in PRP in various in vivo and in vitro studies, the most commonly used PRP for tendinopathies is LR-PRP and the reason for the same has no scientific explanation and could be because of personal preferences and following the initial studies.21,46,32 A meta-analysis by Fitzpatrick et al.44 which included studies till 2015 had 11 studies that used LR-PRP and only one study which used LP-PRP and they concluded by favoring the use of a single injection of LR-PRP under USG guidance. Our study specifically focussed on evaluating the role of both PRP types in achieving clinically relevant benefit and we observed that both the PRP types achieved good clinical outcomes.
Our analysis has some limitations. Heterogeneity was noted in the outcome parameters evaluated in the included studies which were mainly due to the variability in their period of the follow-up period. The other major limitation is that there was only one direct comparative study comparing LR-PRP and LP-PRP, and hence our single-arm meta-analysis design derived indirect evidence on the subject under study and may not truly estimate the superiority of one over the other and merely establishes that both are clinically effective treatment options.
Conclusion
PRP is a safe and effective treatment option for Lateral epicondylitis with clinical improvements in pain and functional scores and both types of PRP (LR-PRP & LP-PRP) offer similar results.
Funding
We din't receive any funding agency to support this study.
Ethics statement
Ethical approval is not required for systematic review and meta analysis.
Author contributions
(I) Conception and design: Sathsih Muthu, Madhan Jeyaraman, Sandeep Patel.
(II) Administrative support: Madhan Jeyaraman, Sathish Muthu.
(III) Provision of study materials or patients: Madhan Jeyaraman, Sathish Muthu.
(IV) Collection and assembly of data: Madhan Jeyaraman, Sathish Muthu, Sandeep Patel.
(V) Data analysis and interpretation: Preethi Selvaraj.
(VI) Manuscript writing: All authors.
(VII) Final approval of manuscript: All authors.
No human has participated in this study. This manuscript is framed based on the existing literature.
Declaration of competing interest
On behalf of all authors, corresponding author declares no conflict of interest.
Contributor Information
Sathish Muthu, Email: drsathishmuthu@gmail.com.
Sandeep Patel, Email: sandeepdrpatelortho@gmail.com.
Preethi Selvaraj, Email: preenessy@gmail.com.
Madhan Jeyaraman, Email: madhanjeyaraman@gmail.com.
References
- 1.Chen F.-M., Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra A.K., Skrepnik N.V., Edwards S.G. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463–471. doi: 10.1177/0363546513494359. [DOI] [PubMed] [Google Scholar]
- 4.Dhurat R., Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthetic Surg. 2014;7(4):189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics – a review of the literature. SICOT J. 3. doi:10.1051/sicotj/2017036. [DOI] [PMC free article] [PubMed]
- 6.Middleton K.K., Barro V., Muller B., Terada S., Fu F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- 7.Yin W., Qi X., Zhang Y. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med. 2016;14 doi: 10.1186/s12967-016-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lana J.F.S.D., Purita J., Paulus C. Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regen Med. 2017;12(5):565–574. doi: 10.2217/rme-2017-0042. [DOI] [PubMed] [Google Scholar]
- 9.Yan R., Gu Y., Ran J. Intratendon delivery of leukocyte-poor platelet-rich plasma improves healing compared with leukocyte-rich platelet-rich plasma in a rabbit achilles tendinopathy model. Am J Sports Med. 2017;45(8):1909–1920. doi: 10.1177/0363546517694357. [DOI] [PubMed] [Google Scholar]
- 10.Le A.D.K., Enweze L., DeBaun M.R., Dragoo J.L. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan A.D., Malmivaara A., Chou R. Updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine. 2015;40(21):1660–1673. doi: 10.1097/BRS.0000000000001061. 2015. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Savović J., Page M.J. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Lexchin J. Sponsorship bias in clinical research. Int J Risk Saf Med. 2012;24(4):233–242. doi: 10.3233/JRS-2012-0574. [DOI] [PubMed] [Google Scholar]
- 15.Closing the gap between methodologists and end-users: R as a computational back-end | wallace | journal of statistical software. 2020. https://www.jstatsoft.org/article/view/v049i05
- 16.Braun H.J., Kim H.J., Chu C.R., Dragoo J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. 2014;42(5):1204–1210. doi: 10.1177/0363546514525593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yerlikaya M., Talay Çaliş H., Tomruk Sütbeyaz S. Comparison of effects of leukocyte-rich and leukocyte-poor platelet-rich plasma on pain and functionality in patients with lateral epicondylitis. Arch Rheumatol. 2018;33(1):73–79. doi: 10.5606/ArchRheumatol.2018.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behera P., Dhillon M., Aggarwal S., Marwaha N., Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg. 2015;23(1):6–10. doi: 10.1177/230949901502300102. [DOI] [PubMed] [Google Scholar]
- 20.Creaney L., Wallace A., Curtis M., Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45(12):966–971. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 21.Gosens T., Peerbooms J.C., van Laar W., den Oudsten B.L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 22.Gupta P.K., Acharya A., Khanna V., Roy S., Khillan K., Sambandam S.N. PRP versus steroids in a deadlock for efficacy: long-term stability versus short-term intensity-results from a randomised trial. Musculoskelet Surg. 2019 doi: 10.1007/s12306-019-00619-w. Published online August 26. [DOI] [PubMed] [Google Scholar]
- 23.Khaliq A., Khan I., Inam M., Saeed M., Khan H., Iqbal M.J. Effectiveness of platelets rich plasma versus corticosteroids in lateral epicondylitis. J Pakistan Med Assoc. 2015;65(11 Suppl 3):S100–S104. [PubMed] [Google Scholar]
- 24.Krogh T.P., Fredberg U., Stengaard-Pedersen K., Christensen R., Jensen P., Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 25.Lebiedziński R., Synder M., Buchcic P., Polguj M., Grzegorzewski A., Sibiński M. A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. Int Orthop. 2015;39(11):2199–2203. doi: 10.1007/s00264-015-2861-0. [DOI] [PubMed] [Google Scholar]
- 26.Lim W., Park S.H., Kim B., Kang S.W., Lee J.W., Moon Y.L. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. J Orthop Res. 2018;36(3):913–920. doi: 10.1002/jor.23714. [DOI] [PubMed] [Google Scholar]
- 27.Linnanmäki L., Kanto K., Karjalainen T., Leppänen O.V., Lehtinen J. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clin Orthop Relat Res. 2020;478(8):1892–1900. doi: 10.1097/CORR.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin J.I., Atilano L., Merino J. Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: a randomized controlled trial. J Orthop Surg Res. 2019;14 doi: 10.1186/s13018-019-1153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merolla G., Dellabiancia F., Ricci A. Arthroscopic debridement versus platelet-rich plasma injection: a prospective, randomized, comparative study of chronic lateral epicondylitis with a nearly 2-year follow-up. Arthroscopy. 2017;33(7):1320–1329. doi: 10.1016/j.arthro.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Montalvan B., Le Goux P., Klouche S., Borgel D., Hardy P., Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology. 2016;55(2):279–285. doi: 10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
- 31.Palacio E.P., Schiavetti R.R., Kanematsu M., Ikeda T.M., Mizobuchi R.R., Galbiatti J.A. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51(1):90–95. doi: 10.1016/j.rboe.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peerbooms J.C., Sluimer J., Bruijn D.J., Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–262. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 33.Raeissadat S.A., Rayegani S.M., Hassanabadi H., Rahimi R., Sedighipour L., Rostami K. Is Platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schöffl V., Willauschus W., Sauer F. Autologous conditioned plasma versus placebo injection therapy in lateral epicondylitis of the elbow: a double blind, randomized study. Sportverletz Sportschaden. 2017;31(1):31–36. doi: 10.1055/s-0043-101042. [DOI] [PubMed] [Google Scholar]
- 35.Seetharamaiah V.B., Gantaguru A., Basavarajanna S. A comparative study to evaluate the efficacy of platelet-rich plasma and triamcinolone to treat tennis elbow. Indian J Orthop. 2017;51(3):304–311. doi: 10.4103/ortho.IJOrtho_181_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tetschke E., Rudolf M., Lohmann C.H., Stärke C. Autologous proliferative therapies in recalcitrant lateral epicondylitis. Am J Phys Med Rehabil. 2015;94(9):696–706. doi: 10.1097/PHM.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 37.Thanasas C., Papadimitriou G., Charalambidis C., Paraskevopoulos I., Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130–2134. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 38.Tonk G., Kumar A., Gupta A. Platelet rich plasma versus laser therapy in lateral epicondylitis of elbow. Indian J Orthop. 2014;48(4):390–393. doi: 10.4103/0019-5413.136260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varshney A., Maheshwari R., Juyal A., Agrawal A., Hayer P. Autologous platelet-rich plasma versus corticosteroid in the management of elbow epicondylitis: a randomized study. Int J Appl Basic Med Res. 2017;7(2):125–128. doi: 10.4103/2229-516X.205808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vk G. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg. 2015;23(1):1–5. doi: 10.1177/230949901502300101. [DOI] [PubMed] [Google Scholar]
- 41.Watts A.C., Morgan B.W., Birch A., Nuttall D., Trail I.A. Comparing leukocyte-rich platelet-rich plasma injection with surgical intervention for the management of refractory tennis elbow. A prospective randomised trial. Shoulder Elbow. 2020;12(1):46–53. doi: 10.1177/1758573218809467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav R., Kothari S.Y., Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05–RC07. doi: 10.7860/JCDR/2015/14087.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4(1):24. doi: 10.1186/s13643-015-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzpatrick J., Bulsara M., Zheng M.H. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45(1):226–233. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
- 45.Miller L.E., Parrish W.R., Roides B., Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3(1) doi: 10.1136/bmjsem-2017-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 47.Pifer M.A., Maerz T., Baker K.C., Anderson K. Matrix metalloproteinase content and activity in low-platelet, low-leukocyte and high-platelet, high-leukocyte platelet rich plasma (PRP) and the biologic response to PRP by human ligament fibroblasts. Am J Sports Med. 2014;42(5):1211–1218. doi: 10.1177/0363546514524710. [DOI] [PubMed] [Google Scholar]
- 48.Dragoo J.L., Braun H.J., Durham J.L. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40(6):1274–1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 49.Filardo G., Kon E., Pereira Ruiz M.T. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. doi: 10.1007/s00167-011-1837-x. [DOI] [PubMed] [Google Scholar]
- 50.McCarrel T.M., Mall N.A., Lee A.S., Cole B.J., Butty D.C., Fortier L.A. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med. 2014;44(8):1025–1036. doi: 10.1007/s40279-014-0195-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y., Zhang J., Wu H., Hogan M.V., Wang J.H.-C. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173. doi: 10.1186/s13287-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Mos M., van der Windt A.E., Jahr H. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]