Abstract
Nigeria, with a population of over 190 million people, is rated among the 10 countries with the highest burden of infectious and zoonotic diseases globally. In Nigeria, there exist a sub-optimal surveillance system to monitor and track priority zoonoses. We therefore conducted a prioritization of zoonotic diseases for the first time in Nigeria to guide prevention and control efforts. Towards this, a two-day in-country consultative meeting involving experts from the human, animal, and environmental health backgrounds prioritized zoonotic diseases using a modified semi-quantitative One Health Zoonotic Disease Prioritization tool in July 2017. Overall, 36 of 52 previously selected zoonoses were identified for prioritization. Five selection criteria were used to arrive at the relative importance of prioritized diseases based on their weighted score. Overall, this zoonotic disease prioritization process marks the first major step of bringing together experts from the human-animal-environment health spectrum in Nigeria. Importantly, the country ranked rabies, avian influenza, Ebola Virus Disease, swine influenza and anthrax as the first five priority zoonoses in Nigeria. Finally, this One Health approach to prioritizing important zoonoses is a step that will help to guide future tracking and monitoring of diseases of grave public health importance in Nigeria.
Keywords: Zoonotic diseases, Prioritization, Public Health, One Health, Nigeria
Highlights
-
•
Nigeria is among the top ten countries with the highest burden of infectious and zoonotic diseases globally.
-
•
One Health approach resulted in prioritization of important zoonoses and will guide their future tracking and monitoring.
-
•
Rabies, avian influenza, Ebola Virus Disease, swine influenza and anthrax as the first five priority zoonoses in Nigeria.
1. Introduction
Pathogenic agents transmissible in nature between vertebrate animals and humans pose significant public health and socio-economic threats globally [1]. These zoonotic agents are implicated in more than half of all pathogens infectious to humans, two-third of emergent and re-emergent infections and three-quarters of new diseases that have affected humans in the past 10 years [2,3] with the burden and impact varying in time and geographical settings [4]. The geographical variations require that each country identify and prioritize zoonotic diseases (ZDs) of major public health importance affecting its population.
In developing countries like Nigeria, the burden of zoonotic diseases is often underestimated due to weak surveillance, poor awareness and paucity of data [4]. Additionally, because of the diverse nature of these countries given their different geographic and environmental settings, they harbor a wide range of zoonotic disease pathogens with varying epidemiology and severity [5]. These challenges present limitations to effective prevention and control of zoonoses. In most instances, only zoonotic diseases that cause pandemics and attract global concerns gain the attention of national policy makers and international partners in control and prevention compared to endemic zoonotic diseases that impact rural communities sometimes more significantly [6,7]. For example, the first avian influenza outbreak in Nigeria in 2006 attracted huge investment from both the national government and international agencies due to its pandemic nature; while rabies, a preventable and endemic disease, with high mortality rates in different parts of the country attracts little or no attention [8]. Prioritizing zoonotic diseases is critical across countries for optimizing resources, improving surveillance, enhancing data quality, information dissemination and risk communication and promoting multi-sectoral collaboration. [5,[9], [10], [11]].
Nigeria is the most populous country in Africa with an estimated population of 188 million [12] and has one of the highest populations of livestock (19.5 million cattle, 72.5 million goats, 41.3 million goats) in the continent [13]. Agriculture is the economic mainstay of Nigeria, employing about 70% of the labor force with livestock production accounting for 6–8% of the national Gross Domestic Product (GDP) and 20–25% of agricultural GDP [13]. Despite these valuable contributions, the livestock sub-sector is neglected and therefore prone to myriad of problems, one of which is weak veterinary care and support system, thus, creating huge opportunities for several livestock diseases, including those of zoonotic importance, to thrive and spread. This becomes more pronounced because livestock farming is mainly subsistence [14]. Most households have close contact with domestic animals and are unaware of the public health implications of zoonotic diseases [15,16]. The situation is further exacerbated by the intensification of livestock production, due to the huge demand for animal protein, which favors the circulation of pathogens at the human-animal-ecosystem interface [17,18]. Given the aforementioned and other intrinsic as well as extrinsic factors (e.g. war, drought and climate change) beyond the control of government, Nigeria becomes highly vulnerable to the effect of several zoonotic diseases and ranks very high in the health burden of neglected zoonotic diseases [19].
In 2012, the government of Nigeria rolled out the Agriculture Transformation Agenda (ATA) in a bid to revamp the agriculture sector, ensure food security, diversify the economy and enhance foreign exchange earnings [20]. The Livestock Transformation Agenda (LITA), a subset of ATA, focused on intensification of livestock production without a concomitant intensification of zoonotic disease surveillance and other animal disease control measures. Weak surveillance systems, lack of coordination among human and animal health sectors and inadequate resources for public health systems have remained prominent barriers to effective response to public health threats posed by zoonotic diseases in Nigeria [15,21]. Ultimately, there is a lack of empirical data on zoonotic diseases in the country that can be used for evidence-based policy formulation and effective implementation of public health control measures and activities. In keeping with the Global Health Security Agenda, LITA necessitates the development of parallel strategies to prioritize and control zoonotic diseases of major public health and strengthening of existing surveillance systems for prioritized zoonoses [15,22].
To address the challenges, a zoonotic disease prioritization workshop using a one health approach was jointly organized. One Health is an approach that recognizes the interconnectivity between the health of people, animals and their shared environment. It adopts a collaborative, multisectoral, and transdisciplinary approach across local, regional, national, and global levels to optimize health systems and outcomes. The one health zoonotic prioritization workshop was initiated by the Nigeria Centre for Disease Control (NCDC) and the Federal Ministry of Agriculture and Rural Development's Department of Veterinary and Pest Control Services with support from the Africa Field Epidemiology Network (AFENET), Global Implementation Solution (GIS), US Centers for Disease Control and Prevention (CDC) and the Zoonotic Disease Unit (ZDU) of Kenya. The primary objectives of the prioritization process were to identify priority zoonotic diseases in Nigeria, strengthen the links between the human, animal and environment health sectors to jointly address these diseases and increase the coordination, collaboration and networking on zoonoses prevention and control activities among stakeholders. This paper therefore describes the outcome and product of a semi-quantitative, multi-sectoral process that led to the prioritization of zoonotic diseases in Nigeria, ranking them in order of relative importance towards guiding prevention and control strategies in the country.
2. Methods
The Nigerian zoonotic disease prioritization process was carried out via a scoping literature and document review and subsequent organization of a two-day, in-country facilitated consultative workshop of 61 one-health stakeholders and experts in July 2017 (Fig. 1). The experts were drawn from key stakeholders' agencies in public health, animal health and environmental health (Table 1) [4,15]. During the workshop, disease prioritization was conducted using the semi-quantitative One Health Zoonotic Disease Prioritization (OHZDP) tool developed by the USCDC [9,11], used by other countries [23,24,57] but modified in our context based on the Kenyan zoonotic disease prioritization process.[4,12]. In our prioritization process, we modified the OHZDP tool so that it could be administered to five groups consisting of 12–13 persons, rather than a maximum of 6–7 persons in the Kenyan prioritization. Though other disease prioritization approaches exist [25], we found the OHZDP process most suitable for Nigeria because of paucity of valid quantitative data for some zoonotic diseases and poor surveillance for most zoonotic diseases which made the application of other techniques impractical [4,9].
Fig. 1.
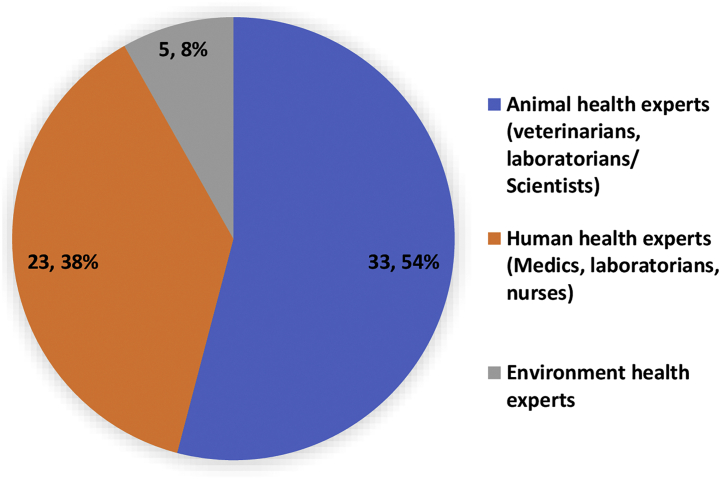
Participants of the Nigeria Zoonotic Disease Prioritization Process/Workshop by Professional Cadre, July 2017.
Table 1.
Participating Organizations of Nigeria's One Health Zoonotic Disease Prioritization Workshop — Abuja, Nigeria, 2017.
| Participating Organization | Abbreviations | Number of participants |
|---|---|---|
| Government Institutions/Ministries | ||
| Nigeria Centre for Disease Control | NCDC | 7 |
| Federal Ministry of Agriculture & Rural Development, Department of Veterinary and Pest Control Services | FMARD DV&PCS | 7 |
| National Veterinary Research Institute, Vom, Plateau State | NVRI | 4 |
| State Ministries of Health | SMOH | 5 |
| State Ministries of Agriculture | SMOA | 2 |
| Academia | ||
| Ahmadu Bello University | ABU | 7 |
| University of Ibadan | UI | 7 |
| University of Jos | UJ | 2 |
| University of Lagos | UNILAG | 1 |
| Usman Danfodio University | UDUS | 1 |
| Akwa Ibom State University | AKSU | 1 |
| Bayero University, Kano | BUK | 1 |
| University of Calabar | UNICAL | 1 |
| Nigeria Field Epidemiology and Laboratory Training Program | NFELTP | 2 |
| Partners | ||
| African Field Epidemiology Network | AFENET | 4 |
| United States Centers for Disease Control and Prevention | CDC | 4 |
| Global Implementation Solution | GIS | 2 |
| Measure Evaluation | ME | 1 |
| Zoonotic Disease Unit, Kenya | ZDU | 1 |
| World Health Organization | WHO | 1 |
Prior to the workshop, key institutions and ministries involved in zoonotic disease prevention, surveillance, research and diagnostics in both human, animal and environmental health, across the six geo-political zones of Nigeria were identified (Table 1). Subsequently, invitations were circulated to these institutions for selection of experts on zoonoses [4]. The selected experts indicated interest and willingness to participate in the prioritization process. Of the 61 participants that attended the workshop, 33 (54%) were animal health experts, 23 (38%) were human health experts, while 5 (8%) consisted of environmental health experts (Fig. 1). Participants were drawn from the academia 23(37.7%), Government ministries 20(32.8%), industry partners 12(19.7%) and researchers from research institutes 6(9.8%). During the workshop, participants were placed into five heterogenous groups of 12–13 participants per group ensuring even spread of all disciplines [4]. Participants from the same institution were put in different groups to minimize institutional and professional biases.
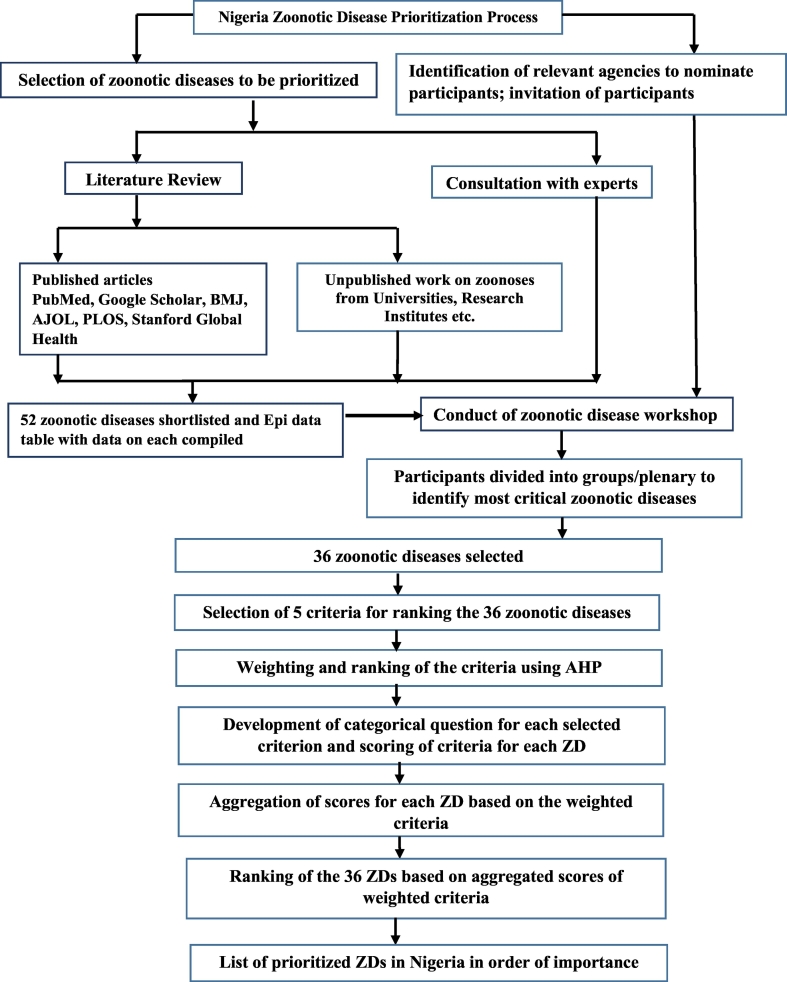
The prioritization process involved six steps (Fig. 2): i) selection of zoonotic diseases for prioritization; ii) selection of five measurable criteria for ranking these diseases; iii) ranking of the criteria using a semi-quantitative analytic hierarchy process; iv) development of categorical questions for each criterion and scoring each criterion for each disease; v) aggregation of scores in order to assign weights to each disease using decision tree analysis; and vi) ranking the zoonotic disease based on the weighted criteria.
Fig. 2.
Schematic representation of the Nigeria zoonotic disease prioritization process.
2.1. Literature review and selection of zoonotic diseases for prioritization
A literature review of existing published and unpublished works on zoonotic diseases in Nigeria and the West African region was carried out before the prioritization workshop to identify zoonoses of jurisdictional importance to the stakeholders and threat to the country. The literature review was conducted by three selected NFELTP [26] residents, with guidance from two One Health consultants from GIS and University of Ibadan, Nigeria. The review involved scoping literature searches in repositories of international human and animal health agencies like the World Organization for Animal Health (OIE) and World Health Organization, Google scholar, PubMed, BMJ, AJOL, PLoS, BioMed Central, CDC Publication and HINARI; and extensive review of available unpublished works on zoonoses from universities, research institutes and opinions from subject matter experts on zoonoses.
Publications from 2000 to 2017 were included in the review. The keywords' combination used for the search were: “zoonotic”, “diseases”, “zoonosis”, “endemic”, morbidity, “mortality” “Nigeria” and “west Africa”. First, online literature was reviewed to identify zoonotic diseases that have occurred in Nigeria. Next, literature searches were conducted on zoonotic diseases that can cause epidemics in Nigeria and those that are threat to Nigeria especially those reported within the west Africa subregion [[6], [16], [17], [18], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. After the listing of all identified zoonoses, searches were conducted on each of the listed zoonoses to obtain their epi-data. Upon assessment of publication abstract, articles on ZDs published in English and with relevant incidence/prevalence and/or socioeconomic burden data in humans and animals from Nigeria were included in listing the initial 52 zoonotic diseases [[37], [44], [45], [46], [47], [48], [49], [50], [51]] Table 2. The bibliographies of the articles that turned up in the initial search were also used to find other references relevant to zoonotic diseases in Nigeria. Articles without basic epi-data on animal and human cases and those reporting zoonotic events were excluded. Following the search, epi-data table was completed for each of the diseases covering incidences/prevalence and impacts [[52], [53], [54], [55]] in humans and animals or both in Nigeria and neighboring West African countries (Table 2).
Table 2.
Epidemiological Data for Zoonotic Diseases in Nigeria from Literature search.
| S/N | Disease/Condition | Prevalence in Nigeria |
Prevalence/Incidence in other African Countries |
Estimated impact or burden, Socio-economic and non-monetary losses |
|||
|---|---|---|---|---|---|---|---|
| Humans | Animals | Humans | Animals | CFR in humans | Production losses in animals |
||
| Intervention costs | |||||||
| 1 | Candidiasis | 7% (82%) is due to vaginitis and Tinea capitis | 6.30% | 56.3% in Nigeria | 51.2% in Cameroon | 30–50% | €10,530 and €51,033, depending on the certainty of infection and the duration of follow-up |
| 42.3% in pregnant women | 72.3% in Iran | 38–75% | |||||
| 2 | Staphylococcus infection | 21–30% | 18–34% | 21–30% in Nigeria, Cameroon | 81.8% in Cameroon | 20–40% | $35,300 per patient for community acquired infections |
| 28–40% | 28–40% | 10% in Malta, Tunisia and Algeria | 7.9% in Cote d'Ivoire | $28,800 per patient for nosocomial infections | |||
| 41% in Cape Verde | 18–41.2% in Egypt | ||||||
| 10% in Ethiopia | |||||||
| 3 | Vibrio infection | 0.7–1.1% | Nil | 0.0–11.7% | $39–64.2 m (2005) | ||
| Incidence rate = 4 per 1000 | Over 30% in vulnerable groups living in high risk areas | $91.9-156 m (2006) | |||||
| $60–72.7 m (2007) | |||||||
| 62% of total cholera cases and 56.7% deaths in Africa alone in 2007 | |||||||
| 4 | West Nile fever | 4.50% | 18% | 25% in Northeast Nigeria | 90.3% in Nigeria | 3–15% | $778 m (1999–2012) approx. $56 m per year |
| 97% in Chad | |||||||
| 92% in Senegal | |||||||
| 5 | Scabies | 8.0–18.5% | 0.09% in goats and cattle −12.77% in cheetah | 2.9% in Nigeria | 2–3.4% in dogs in Nigeria | >1.5million YLDS (Years Lived With Disability) | |
| 6 | Psittacosis | ||||||
| 7 | Rocky Mountain spotted fever | 15.80% | 1% in cattle to 43% in goats | 67.9% in Tanzania | 0.3–2.2% | $13.2 m from 2002 to 2011 | |
| 1.40% | |||||||
| 1.7 to 7 cases per million persons from 2000 to 2007 in the US | |||||||
| 8 | Glanders | 3.8% in Ethiopia | |||||
| 9 | Trichinosis | 2.2% in the US | 2.1% in Uganda | 2% | European Union spent $572 million in 1997 | ||
| 11–40% in Nigeria | 76 per billion persons per year(DALY) globally | ||||||
| 10 | Streptococcal infection | 51–60% in under fives | 12% | 30.7% in Nigeria | 29–45% | $224 to $539 million per year. | |
| 11 | Tuberculosis | 2.4–3% | 8.50% | 17.5% in Ethiopia 10.5% in Tanzania | 8% in Zaire (DRC) | 9.7–17% | $5b (2012) Globally, $1.8 (2008–2012) in Benue state, Nigeria. |
| 0.5–12.3% | 8.8–15.1% | 1.5 million deaths globally (2010) | |||||
| 10,000 human deaths in Africa(2015) | |||||||
| 12 | Brucellosis | 5.2–7.8% | 5.3–8.6% | 3.8% in Chad | 5–45% in Kenya | 0.8–2% | $ 575,605 (2016) in Nigeria |
| 24.10% | 41% in Togo | 4.9–9.6% Cameroon | $ 7.3(2015) in Sudan | ||||
| 298 Deaths in the US (1977) | |||||||
| 13 | Ebola | 2% | *Not documented in published literatures | 0.074% in Liberia | 31.8% in Gabon | 25–90% | $ 219 (2014–2017) in Sierra Leon |
| 0.032% in Sierra Leone | $ 1.4b in Nigeria (2014) | ||||||
| 5000 Gorilla deaths in Gabon (2003) | |||||||
| 9162 (2014–2015) human deaths in West Africa | |||||||
| 14 | Avian Influenza | 18.90% | 18.1–27.3% | 17.5–40% in Senegal | 16–88% in Egypt | 14–33% | $ 700 m(2006) in Nigeria |
| 35.3–93.4% | 29% in Cameroon | 222,796 bird deaths in Nigeria (2006) | |||||
| 161 (2003−2013)human deaths in Indonesia | |||||||
| 15 | Cryptosporidiosis | 21–30.5% | 16.6–28.1% | 3.5–22.3% in Chad | 18.9–50.6% in Ghana | 7.8–10.3% | $46 m hospitalization cost in USA |
| 11.7–28% | 14.1–25.4% | 1.5–14.4% in Cameroon | 1.58 m Euros in Netherlands | ||||
| 69 (1993)human deaths in Milwaukee | |||||||
| 12,400 (2010) human cases in Sweden | |||||||
| 16 | Leishmaniasis | 6.80% | 3.03–4.40% | 12.2–32.3% in Ghana | 2.7% in Mali | 8.8–17.7% | $1.2 m in Afghanistan |
| 1.1–2.6% | 7.5–38.3% in Cameroon | 5.9–11.7% in Algeria | 12,491,280 (2000−2011) human deaths in Brazil | ||||
| 582 (2006) human deaths in India | |||||||
| 17 | Echinococcosis | 5.10% | 12.45% | 5–10% in Argentina | 9.3–56% in Ethiopia | 0.50% | $2billion in Developing countries |
| 11.4–26.5% | 0.3–25% in Haiti | $459,659.6 (2008) in Iran | |||||
| 1200 (2000−2010) human deaths globally | |||||||
| 18 | Plague | 7.80% | 0.10% | 50–80% in London | 17.9 in Peru | 8.1–66.6% | 52 (1994) deaths in India |
| 0.5% in Tanzania | 1.2–5.0% | 60million deaths in China in the 14th century | |||||
| 19 | Tularemia | *Not documented in published literatures | *Not documented in published literatures | 2.22–7.46% in Turkey | 1.3–16% in the US | 3–35% | $12.6 (1993) billion following bioterrorist attack in the US |
| 441 (1995–2005) human cases in Sweden | |||||||
| 5(2004) animal deaths in Germany | |||||||
| 3.70% | 2.2–13.5% in Turkey | 30% if untreated | $3.9–5.5 billion per 100,000 people exposed | ||||
| 20 | Cat Scratch Fever | *Not documented in Published literatures | 10–45.5% | 0.77–28% in the US | 13–60% in Kenya | 3–40% in Italy | $3.5 m (2000) in the US |
| 26–28% | 6% in Turkey | 49.5% in the US | 500 hospitalized humans in the US | ||||
| 21 | Rabies | 505 cases (1912–1978) | 24 of 41 (1980–1982) | 1.3% in Malawi | 1.4 per 1000 in Chad | 100% | $46 per DALYs averted |
| 6 of 149 (4%) (2004–2013) | |||||||
| 44.90% | 12.6 cases per million in Ethiopia | 412.83 cases in Ethiopia | 55,000 human death per year in Africa and Asia | ||||
| 169 cases (1969–1978) | 44 cases (1987–2001) | 1.8 cases per million in Ghana | |||||
| 2 of 81 (3.3%) (2000–2010) | $583.5 million intervention costs / year in Africa & Asia | ||||||
| 1.58% | 2.3 cases per 100,000 in Ethiopia | ||||||
| 2.8%7 | 7.89%9; 7.98% | Average economic loss of $49–52 in Ethiopia | |||||
| 16.7%10 | |||||||
| 22 | Lassa fever | 623(3.4 cases/million) | 5.80% | 26% in Ivory Coast | 19.4% in Mali | 37.9% - 50.0% | Affects 100,000 to 500,000 persons/year in West Africa |
| 12% | 8–52% in Sierra Leone | > 50% CFR | |||||
| 0.44–42% | 0–80% in Sierra Leone | 31% | |||||
| 23 | Yellow fever | 20% | 7–64% | 0% in Africa in 2015 | 13% in Congo Basin | 81% | 200,000 cases per year in South America and Africa |
| 661–884 lab confirmed cases in Angola in 2016 | 52% CFR in Western Nigeria | ||||||
| 3–26% | 10% | 37 cases in DRC | 20–50% | ||||
| 37–67% | 2 cases in Kenya | 13–22% | |||||
| 6% in Kenya | |||||||
| 24 | Western equine encephalitis | No lit available | No lit available | No lit available | No lit available | ||
| 25 | Streptotrichosis | No Lit available. | 3.11% | 5.3% in India | 13.55% in Bangladesh | N/A | Economic loss of over N40,000 per cattle ($103 @ N390/1$) |
| 3.2–8% | |||||||
| 5.5% in India | |||||||
| 13.6% in Iran | |||||||
| 5.8–9.6% in Egypt | |||||||
| 8.3% in Southern Ethiopia | |||||||
| 26 | Clostridial disease | 14–43% among HIV | 26.60% | 6.7% in India | 22.5–36% in India | 4.7–13.8% | €1,222,376 attributable cost in outbreak setting in the Netherlands |
| 9.2%in South Africa | 4.0–4.5% in Egypt | 1.2–2.2% | $3.2 billion Annual management cost in US | ||||
| 15.6–58.4% in Egypt | |||||||
| 27 | Shigellosis | 20.70% | 10.60% | 2.3%, 13.3%, 6.9% in Ethiopia | 6% in Uganda | 9.80% | 163.2 million episode in developing countries with 1.1 million death annually |
| 4218 cases in Sierra Leone | |||||||
| 15.50% | 22.50% | 7.4% in Ethiopia | 0–2.73% | ||||
| 24%; 408/100,000 person years of observation in Kenya | 20.8 in Kenya | ||||||
| 34% | 2.1–6.1% | ||||||
| 28 | Toxoplasmosis | 24% | 13.9%,29.1% | 75.7%, 94.4% in Ethiopia | 55.18–58.18% in Ethiopia | Indeterminate | 26 cases of cerebral toxoplasmosis in Dakar Cameroon |
| 27.4% - 40.8% | 40.40% | 5.87% in Zambia | 79.0% in Kenya | 2.6% ocular toxoplasmosis in Ghana | |||
| 32.4–38.7% | 19.6–88.7% in Cameroon | 8.33% in Ethiopia | 29% Maternal-fetal transmission rate | ||||
| 14% | 10–20% in Egypt | 29% in Burkina Faso | |||||
| 4.6–6.7% | |||||||
| 29 | Rat bite fever | No lit available | No lit available | No lit available | No lit available | 10% | No lit avaiable |
| 30 | Newcastle disease | No available Lit. | 17% | No available Lit. | 28.3–34.5% in Cameroon | No available Lit. | 40 cases of conjunctivitis of 90 poultry workers |
| 25–35.7%96 | 43.8–54.4% in Senegal | ||||||
| 31 | Hantavirus | No available Lit. | No available Lit. | 1.2–4.4% in Guinea | 0.24% in Sierra Leone | 40% | 200,000 cases estimated per year |
| 1% in Southern Africa | 0.16% in Guinea | No treatment available | |||||
| 2.4 & 3.9% in DR Congo & Côte d'Ivoire104 | 57.50% | ||||||
| 32 | African trypanosomiasis | No data | Cattle (Jos) 46.8% | No data | 40.90% | 4.30% | costs Africa US$5 billion a year and Africa spends every year at least $30 million to control cattle trypanosomiasis Direct losses due to Trypanosomiasis are estimated to between US$ 1–1.2 billion each year. |
| 33 | Aspergillosis | 36.94% -51.25% | 47.87% in apparently healthy birds | No data | No data | 58% | No data |
| 34 | Anthrax | No data | No data | No data | 90% Serengeti lions, 87% hyena Tanzania | 50% | No data |
| 35 | Leptospirosis | 13.5% Enugu | 27.2% horses | Uganda 35% | No data | 1–5% | No data |
| 3.5% abattoir cattle Kaduna | |||||||
| 36 | Visceral larva migrans | No data | 33.8% dogs in Nigeria | 7.70% | No data | Most cases of visceral larva migrans are subclinical, Fatalities are rare but have occurred in cases with severe pneumonia, cardiac involvement or neurological disease12 | Puppies can die occasionally from the effects of larval migration (especially pneumonia) and rarely from intestinal complications. |
| In dogs, maternal transmission is very efficient | |||||||
| 37 | Cutaneous larvae migrans | No data | No data | No data | No data | No data | No data |
| 38 | Dermatophytosis | 5% primary school children Kwara state | 39.8% domesticated animals | 23.4% children in Ethiopia | No data | No data | No data |
| 17.6% Horses Kaduna state | 11.2% children in Nairobi | ||||||
| 39 | Pasteurellosis | No data | No data | No data | Sheep 37.1%, goats 21.9% | No data | No data |
| Bovine 97% ovine 86% in Ethiopia | |||||||
| 2.85% camels in Cairo | |||||||
| 40 | Diphyllobothriosis | No data | 32.1% Ekiti | No data | 7.1% dogs in Ghana | No data | No data |
| 41 | Clonorchis | No data | No data | No data | No data | No data | No data |
| 42 | Salmonellosis | 5.70% | 43.6% (95%CI [39.7–48.3%]) | 8.72%, 5.68%, and 1.08% in children, adults, and carriers respectively (Ethiopia) | 44·0% in Ghana | 1.03%/10 years | Salmonella-contaminated meats and poultry, was estimated to cost Americans around one billion dollars in 1987 |
| 43 | Escherichia coli O157 | 5.00% | Cattle- 49.4% | 7.5%- South Africa | 44–50% in pigs (S/Africa) | case-fatality rate ranging from 3 to 5% | The annual cost of illness due to O157 STEC was $405 million (in 2003 dollars) |
| Sheep-6.3% | 5.4–20% in cattle | ||||||
| Goats- 2.5% | |||||||
| 44 | Rift valley fever | 6.7–31.2% | 3.3–18.7% | 29.3% in Tanzania | 7.67% in Burkina Faso | 14%- 30% | $250 m (1998), $540 m (2007- combined for East Africa) livestock trade losses. |
| 16.8% in Saudi Arabia | 600 deaths in Egypt in 1977 | ||||||
| 608 human deaths in 1997& 2007 | |||||||
| 45 | Swine influenza | Humans are not susceptible | 9% of serum samples and 48% of tissue samples | Humans are not susceptible | 52.96%(ELISA); 11.5% (PCR) | Humans are not susceptible | potential to cause losses of up to |
| US$910836.70 in a single year | |||||||
| 46 | Dengue | 30.8% (among febrile children) | 48% of monkeys and 25% of galagos | 17.8% in Somalia | Largely unknown due to sparse data | 2–5% (treated) | $0.85 billion and $1.15 billion, of which control cost constitute 42%–59%. (Singapore) |
| 17.2% (among healthy children) | 50% (untreated) | ||||||
| 47 | Fascioliasis | Cattle (27.68%) | 7.3% in the Nile delta, Egypt | 37% in Sudan, 45% in Cameroon | Rarely kills in humans | The cost due to condemnation of goat-livers has been estimated to be US$ 115 per thousand livers | |
| 48 | Cysticercosis/ Taeniasis | 8.60% | porcine cysticercosis (20.5%) | 45.3% in Tanzania | 24.6 and 32.2% for Ag-ELISA and Ab-ELISA, respectively (Cameroon) | £4.0 million annually (England) | |
| 49 | Abattoir fever | 44% (Sokoto) | Total prevalence rate herd prevalence rates (14.5%) and (57.1%) | 16% in Egypt | 13% of cattle, 23% of goats, 33% of sheep, 0% of buffalo (Cairo, Egypt) | vary between 5 and 50% | The total intervention cost in agriculture amounted approximately 35,000 Euro per DALY occurred (controlling the Q fever epidemic in 2007–2011 in the Netherlands) |
| 50 | Listeriosis | (23.3%) of HIV/AIDS patients | 91.8% (poultry) | 20% | |||
| 51 | Campylobacteriosis | 8.30% | 20% | 15.4% in Ethiopia | 43.6% domestic fowls, goats (33.3%) and sheep (23%) | <0.01% to 8.8% | €10.9 million annually |
| 52 | Giardiasis | 33.20% | 27.68% | 5.8% in Ghana | |||
The database of 52 zoonotic diseases (Table 2) was presented to workshop participants in a plenary session for validation. The participants reviewed the list, and the number was pruned to 36 diseases known to have been reported in Nigeria and the West African region in the last 20 years and whose ecological and epidemiological conditions of occurrence exist in Nigeria.
2.2. Selection of measurable criteria for ranking the diseases
Each of the five (5) groups of participants was given a list of eight criteria (Table 3), adapted from published works on zoonotic disease prioritization [4,5,15], and tasked to select a set of five criteria as suggested by the OHZDP tool to justify prioritization. Giving participants a list of eight criteria to choose from was a modification of the OHZDP process aimed at fast-tracking the generation of suitable criteria from existing listings from other countries who have used the tool. Participants were also asked to suggest additional criteria where they deemed necessary. Each group selected a lead who moderated the discussions and a note taker who took minute of the discussions and conclusions by their group. The criteria selected by each group were subsequently discussed at a plenary session to produce a combined final list of five criteria including, epidemic potential, severity, economic impact, burden of zoonotic diseases and ability to prevent and control, that were used to evaluate the diseases (Table 4). To arrive at these criteria, three criteria chosen by all five groups were first selected; subsequently, two criteria, each cutting across three groups and two groups respectively were selected by majority vote after a debate for and against. Consequently, three criteria were unanimously discarded at plenary: i) transmission potential between humans and animals; ii) bioterrorism and iii) amenability to collaboration already established.
Table 3.
List of sample criteria given to each group for zoonotic disease prioritization.
| Criteria | Summary |
|---|---|
| (i) Transmission potential between humans and animals | Horizontal transmission of disease from humans to animals or vice versa. |
| (ii) Burden of disease in humans | Disease causes high prevalence or incidence rate/year in humans. |
| (iii) Epidemic/pandemic potential in humans | Capacity of the infectious agent to spread across state, national, or political borders. |
| (iv) Bioterrorism potential | Capacity of the infectious agent to be used as a weapon against human populations. |
| (v) Amenability to collaborate/collaboration already established | Capacity of public health organizations (including the Government) to work together to diminish burden of disease. |
| (vi) Socio-economic burden of disease | Social as well as financial costs that are associated with the disease. There are numerous indicators associated with socio-economic burden of disease. |
| (vii) Severity of illness in humans | Morbidity and/or mortality associated with the disease. |
| (viii) Ability to prevent and control the zoonotic disease in the country | Capacity of the country to prevent, contain and control the disease. |
Table 4.
Criteria selected by the zoonotic disease (ZD) prioritization multi-sectoral working group in order of importance; the categorical questions and response options for each criterion.
| S/N | Criterion | Categorical Questions | Response to categorical question |
|---|---|---|---|
| 1 | Epidemic Potential | 1. Has the ZD caused epidemics in the past (X) years? | a) <5 years: 3 b) 5–10 years: 2 c) >10 years: 1 d) No outbreak reported: 0 |
| 2. Has the ZD caused a pandemic in the past (X)? | a) <10 years: 2 b) ≥10 years: 1 c) No reported pandemic: 0 | ||
| 3. Has the ZD presented resistance to treatment in humans or pathogenic mutations in the past 0–10 years? | a) Yes: 1 b) No/no data: 0 | ||
| 4. Has the ZD been detected in a new location or population (human or animal) in the country or neighboring countries in the past 10 years? | a) Yes: 1 b) No: 0 | ||
| 5. Is the ZD pathogen capable of sustained human-to-human transmission? | a) Yes: b) No: 0 | ||
| 2 | Severity | 6. What is the ZD case fatality rate in humans? | a) <10%: 1 b) 10–25%: 2 c) 26–50%:3 d) >50%: 4 |
| 7. Can the ZD result in long-term disability? | a) Yes: 1 b) No: 0 | ||
| 8. Has the ZD pathogen mutated in the past 5 years leading to increased severity? | a) Yes: 1 b) No/no data: 0 | ||
| 3 | Socio-Economic Impact | 9. Is the ZD prevalent in key animal species; fish/poultry (>10%) and other livestock (>5%)? | a) Yes: 1 b) No: 0 |
| 10. Does the ZD cause mortality in livestock animals? | a) Yes: 1 b) No: 0 | ||
| 11. Does the ZD cause >20% decrease in animal productivity/production? | a) Yes: 1 b) No: 0 | ||
| 12. Is the ZD a transboundary animal disease (TAD) with trade limitations? | a) Yes: 1 b) No: 0 | ||
| 4 | Burden of Zoonotic Disease | 13. Is the overall ZD incidence/prevalence in humans >5% per year? | a) Yes: 1 b) No: 0 |
| 14. Is the overall ZD incidence/prevalence higher in age groups (0–20 years) as compared to (>21 years) | a) Yes: 1 b) No: 0 | ||
| 15. Is the ZD listed in country specific information about zoonoses surveillance in humans/animals? | a) Both: 2 b) Either: 1 c) None: 0 | ||
| 5 | Ability to Prevent and Control | 16. Is there a known wildlife reservoir for the disease? | a) Yes: 1 b) No: 0 |
| 17. Is there an effective treatment of the ZD pathogen in humans and animals? | a) Both: 2 b) Either: 1 c) None: 0 | ||
| 18. Is there an effective vaccine/control for the ZD pathogen in humans and animals? | a) Both: 2 b) Either: 1 c) None: 0 | ||
| 19. Is the animal host (domestic or wild) in close proximity to humans? | a) Yes: 1 b) No: 0 |
First, ‘transmission potential between humans and animals’ was deemed a general criterion that cuts across all zoonotic diseases and it was agreed that its variation across diseases may not be sufficient for ranking zoonotic diseases compared to other criteria. Secondly, most participants argued that ‘bioterrorism potential’ was not a major concern in Nigeria's setting. It was agreed that this criterion was adequately captured in the “epidemic/pandemic potential” and “socio-economic burden of the disease” as these criteria were some of the considerations for using a disease pathogen for bioterrorism [56]. Finally, it was agreed at plenary that “amenability to collaborate/collaboration already established” was contained in “ability to prevent and control the zoonotic disease in the country” and thus, should not stand alone as a separate criterion.
Subsequently, each group conducted criteria ranking using the Analytical Hierarchy process (AHP) in the OHZDP tool [20] to assign weights to the five criteria based on their relative importance as perceived by each group [57,58]. The most important criterion was assigned the highest weight, and the least important criterion the lowest weight. The group results were then combined at plenary to produce the overall rank and weights for each criterion (Table 4) [4]. A final consistency ratio of less than 0.1 was acceptable in the analytic hierarchy process.
2.3. Development of questions for each criterion
Participants in each of the five groups developed a set of categorical questions that adequately addressed each criterion to be applied to each of the pathogens/diseases (Table 4). The outcome was 19 categorical questions that addressed all the five criteria. In the five groups, each disease/pathogen received a score for each question. A slight modification of the standard OHZDP tool methods allowed us to develop multiple questions for some criteria (Table 4). The questions had binomial (yes/no) or ordered multinomial response levels with a maximum of 5 categories (e.g. scoring 0–4) for each question [15]. For criteria with multiple questions, scores were summed up to obtain the total score [4]. To score the pathogens using the criteria questions, participants scored the pathogens against each of the categorical questions.
2.4. Ranking the zoonotic disease based on aggregation of scores of the weighted criteria
The final step of the prioritization (Table 5) was to multiply categorical question scores for each pathogen by the weight given to the specific criteria. This was done using decision tree analysis in the OHZDP tool based on Microsoft® Excel. For each disease, the weighted scores for each criterion were summed up to obtain a total weighted score by group. Subsequently, an average weighted score (from all five groups) was obtained and normalized in relation to the maximum score, yielding a normalized final score within a range of 1 to 0 that was used to rank the diseases. Afterwards, the final ranked disease list was reviewed during a plenary session and adopted as the prioritized zoonotic diseases for the country.
Table 5.
Summary of steps involved in arriving at prioritized zoonotic diseases in Nigeria.
| S/N | Activities/calculations involved in arriving at each conclusion | Conclusions/step arrived at |
|---|---|---|
| 1 | Extensive literature review of existing published and unpublished works on zoonotic diseases in Nigeria and the West African region | Zoonotic diseases to be prioritized |
| 2 | Selection of ranking criteria by groups, discussions, consensus and adoption of five criteria at plenary for ranking. | Development of ranking criteria |
| 3 | Conduct of semi-quantitative analytic hierarchy process using a Microsoft Excel® program from the OHZDP tool to assign weights to the five criteria based on their relative importance as perceived by each the groups. | Weighting criteria by pair-wise comparison through analytical hierarchical process |
| 4 | Development of categorical questions that adequately addressed each criterion to be applied to each of the pathogens/diseases with slight modification of the standard OHZDP tool methods allowing for development of multiple questions for some criteria. Scoring of the pathogens using the criteria questions; participants scored the pathogens against each of the categorical questions | Scoring each zoonotic disease based on the criteria |
| 5 | Multiplication of the categorical question scores for each pathogen by the weight given to that specific criteria using OHZDP decision tree analysis based on Microsoft® Excel. Summation of weighted scores for each criterion for each disease to obtain a total weighted score by group | Aggregation of scores |
| 6 | Average weighted score (from all five groups) was obtained and normalized in relation to the maximum score, yielding a normalized final score within a range of 1 to 0 that was used to rank the diseases. | Ranking of the zoonotic diseases |
| 7 | Final ranked disease list was reviewed during a plenary session and adopted for the country | Adoption of ranked zoonotic diseases list |
2.5. Sensitivity analysis
To assess the strength of the prioritization outcome and sensitivity of the process, the variability in weighting of the selection criteria, consensus building in groups and scoring of disease data by expert opinion were evaluated [4,20] using the sensitivity analysis. First, the five criteria were given equal weights of 1 to obtain normalized scores for each of the preselected zoonotic disease [4]. Next, normalized scores for each zoonotic disease were obtained by systematically removing each of the five criteria from the process. Finally, each of the five groups was removed separately to assess the impact each group had on the final normalized scores. Spearman's correlation was used to assess the relationship between normalized scores obtained using the OHZDP tool that produced the ranked priority disease list reported here and the adjusted scores, assessing impact of criteria weight and contribution of each criterion and group respectively. Spearman's correlation coefficient was considered significant at p-values < 0.05 [4,9].
This study did not require ethical approval because the activity was not a human subject research but an expert elicitation process for assembling information on zoonotic disease prioritization using data that was publicly available. The process entailed experts' opinions and perceptions based on their knowledge of the field. No personal data was collected from the experts that participated in producing the final document. Consequently, informed consent was not sought and all data was analyzed anonymously. However, participants were verbally informed that the process and outcomes of the prioritization would be shared with the global community through scientific publications and conferences. No objections were raised. Participants were duly acknowledged.
3. Results
Thirty-six zoonotic diseases were shortlisted for prioritization (Table 6). Overall, bacterial zoonoses accounted for 15 (41.7%) of the zoonoses shortlisted for prioritization while viral, helminth and protozoan diseases constituted 10 (27.8%), seven (19.4%) and three (8.3%), respectively. Fungal zoonoses accounted for the least number of zoonoses shortlisted for prioritization, one (2.7%). Rabies, avian influenza, Ebola viral disease (EVD), swine influenza and anthrax emerged the first five priority diseases in the final prioritized list; while leptospirosis, toxoplasmosis and cryptosporidiosis ranked the least. Generally, zoonoses with scanty data for both animals and humans or those for which data were limited to animals alone or were unavailable such as dengue fever, campylobacteriosis, leishmaniasis and toxoplasmosis ranked lowest since determination of the weighting and ranking criteria were dependent on available data.
Table 6.
Prioritized zoonotic disease list for Nigeria ranked by criteria and final normalized scores, 2017.
| Disease | Severity | Epidemic Potential | Burden of Disease | Ability to Prevent and Control | Socio-economic Impact | Normalized Final Score |
|---|---|---|---|---|---|---|
| Rabies | 1 | 5 | 1 | 3 | 4 | 1.00 |
| Avian influenza | 2 | 2 | 3 | 7 | 1 | 0.88 |
| EVD | 1 | 1 | 6 | 9 | 9 | 0.71 |
| Swine influenza | 5 | 3 | 5 | 7 | 1 | 0.68 |
| Anthrax | 4 | 6 | 4 | 5 | 2 | 0.67 |
| Bovine tuberculosis | 5 | 7 | 3 | 3 | 3 | 0.67 |
| African trypanosomosis | 5 | 9 | 3 | 1 | 4 | 0.65 |
| Lassa fever | 2 | 3 | 5 | 6 | 9 | 0.65 |
| Colibacillosis | 6 | 4 | 3 | 6 | 5 | 0.61 |
| Brucellosis | 4 | 10 | 4 | 4 | 4 | 0.57 |
| Cysticercosis/ Taeniasis | 6 | 11 | 2 | 2 | 6 | 0.55 |
| Staphylococcal disease of animal origins | 7 | 6 | 6 | 1 | 4 | 0.53 |
| Rift valley fever | 7 | 6 | 3 | 6 | 5 | 0.51 |
| Clostridia disease | 3 | 9 | 6 | 6 | 5 | 0.50 |
| Salmonellosis | 7 | 8 | 4 | 4 | 4 | 0.50 |
| Visceral larva migrans | 5 | 11 | 5 | 1 | 7 | 0.48 |
| Schistosomiasis | 6 | 8 | 4 | 2 | 9 | 0.48 |
| Cutaneous larva migrans | 6 | 11 | 4 | 1 | 8 | 0.46 |
| Yellow fever | 3 | 8 | 5 | 8 | 8 | 0.45 |
| Listeriosis | 4 | 11 | 7 | 3 | 5 | 0.45 |
| Dermatophytosis | 7 | 9 | 5 | 3 | 6 | 0.43 |
| West Nile fever | 6 | 7 | 5 | 7 | 6 | 0.42 |
| Dengue | 6 | 3 | 5 | 10 | 9 | 0.42 |
| Campylobacteriosis | 6 | 9 | 6 | 3 | 7 | 0.41 |
| Pasteurellosis | 8 | 11 | 6 | 2 | 3 | 0.40 |
| Psittacosis/Ornithosis | 6 | 11 | 6 | 3 | 6 | 0.39 |
| Streptococcal diseases of animal origin | 7 | 9 | 6 | 3 | 7 | 0.37 |
| Echinococcosis | 5 | 10 | 5 | 6 | 9 | 0.36 |
| Rotavirus Infections | 8 | 10 | 3 | 7 | 6 | 0.36 |
| Yersiniosis | 7 | 11 | 6 | 3 | 8 | 0.31 |
| Q Fever | 7 | 11 | 7 | 3 | 7 | 0.30 |
| Trichinosis | 6 | 11 | 7 | 3 | 9 | 0.30 |
| Leishmaniasis | 5 | 10 | 7 | 8 | 7 | 0.29 |
| Leptospirosis | 7 | 10 | 7 | 5 | 7 | 0.28 |
| Cryptosporidiosis | 7 | 9 | 6 | 9 | 8 | 0.23 |
| Toxoplasmosis | 6 | 11 | 6 | 9 | 8 | 0.23 |
A panel of five criteria comprising “epidemic potential, severity, economic impact, burden of zoonotic diseases and ability to prevent and control” made the final list of criteria for the prioritization. Diseases with case fatality rates (CFR) > 50% such as rabies, EVD and Lassa fever ranked highest on severity when compared with those of CFR < 10–25% (Table 6). In addition, viral hemorrhagic zoonoses such as EVD and Lassa fever with tendency to cause long-term disability also scored high on severity. Furthermore, avian influenza ranked high on severity owing to the ability of the pathogen to mutate to a more severe form in animals and humans.
For epidemic potential, zoonotic diseases that have capacity for sustained human-to-human transmission such as EVD and Lassa fever, and those that have caused epidemic in Nigeria in <5 years prior to the prioritization process such as avian influenza, EVD and Lassa fever ranked highest compared to others on the list. In addition to these characteristics, EVD ranked first overall regarding epidemic potential because it was detected in Nigeria among other countries (new location and population) for the first time in 10 years.
Viral zoonoses such as EVD, avian influenza, yellow fever and dengue fever ranked lowest on the ability to prevent and control criterion. This is because they are known to have wildlife reservoirs which perpetuate them in nature and make their control and possible elimination difficult. In addition, these diseases have no known treatments currently. Conversely, most bacterial and protozoan diseases with effective treatment /control in humans and animals ranked highest.
Transboundary animal diseases such as avian influenza, swine influenza, anthrax and tuberculosis, whose outbreaks in livestock and poultry cause productivity and market losses resulting from high mortality and associated trade bans, ranked highest on the socio-economic impact scale. On the other hand, lower scores were assigned to EVD, Lassa fever, dengue, yellow fever, trichinosis and echinococcosis because they have low prevalence in livestock and poultry and do not cause significant productivity and market losses.
Of the eight suggested selection criteria (Table 3), five were adopted for the prioritization process (Table 4). The weighting and ranking of the selection criteria by the five groups were later summarized (Table 7). Overall, the criteria assessing epidemic potential of a disease with a final weight of 0.23, ranked highest while socioeconomic impact of a disease, with a final weight of 0.17, ranked the least (Table 7). Although severity of disease was ranked first across four groups, it however ranked second overall with a final weight of 0.22, due to it being ranked fourth by one of the groups.
Table 7.
Ranking of zoonotic disease selection criteria using analytical hierarchical process with weighted scores and (ranks) for each group, 2017.⁎
| Criteria | Group: weighted scores (ranks) |
Final weight | Overall ranking | ||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |||
| Epidemic Potential | 0.28 (2) | 0.31 (2) | 0.28 (2) | 0.46 (1) | 0.31 (2) | 0.23 | 1 |
| Severity | 0.42 (1) | 0.08 (4) | 0.37 (1) | 0.27 (2) | 0.42 (1) | 0.22 | 2 |
| Burden | 0.17 (3) | 0.41 (1) | 0.16 (3) | 0.05 (5) | 0.16 (3) | 0.2 | 3 |
| Ability to Prevent and Control | 0.09 (4) | 0.16 (3) | 0.11 (4) | 0.07 (4) | 0.06 (4) | 0.18 | 4 |
| Socio-economic impact | 0.04 (5) | 0.04 (5) | 0.08 (5) | 0.15 (3) | 0.04 (5) | 0.17 | 5 |
| Consistency ratio | 0.04 | 0.06 | 0.04 | 0.08 | 0.02 | ||
Consistency ratio < 0.1 is significant.
3.1. Sensitivity analysis
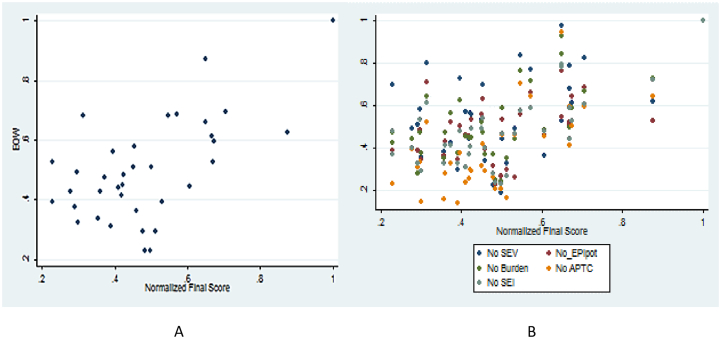
The overall consistency ratio between normalized scores and adjusted scores calculated by the OHZDP tool when comparing weighted and unweighted criteria was 0.06. There was moderate correlation between normalized scores and adjusted scores, calculated by the OHZDP tool, seen when comparing weighted and unweighted criteria (r = 0.59, p < 0.05), there was strong positive correlation when excluding each criterion from the model (r = 0.78–0.91, p < 0.05), and when excluding each group from the model (r = 0.53, p < 0.05).
There was minimal variability in the scores and ranking between the weighted and unweighted criteria and all top 10 diseases were ranked. There was more variability of scores between the groups with statistically different scores for the diseases (Fig. 3A). However, in terms of ranking, for the top 10 diseases, each of the group scored 8. Rabies and avian influenza were consistently ranked first and second across the groups and across the criteria while tuberculosis, African trypanosomosis and E. coli O157 were ranked 13, 11 and 3 by one group and 3,4 and 21 by another group respectively. Comparing other criteria, least variability was observed when epidemic potential was excluded from the model (Fig. 3B).
Fig. 3.
Comparison of normalized scores obtained from the weighted criteria (A) using equal weights; (B) excluding each of the five criteria.
4. Discussion
There have been various methods used for prioritizing zoonotic diseases in several developed (e.g. Canada, Japan, The Netherlands, Switzerland and USA) and few developing (Ethiopia, Kenya and Vietnam) countries of the world [11,[57], [59], [60], [61], [62], [63]]. To our knowledge, this is the first report that prioritized zoonotic diseases in the most populous African nation, Nigeria in the West African sub-region. Following a two-day meeting of a cross section of a multidisciplinary team of human, animals and environmental health experts selected from diverse backgrounds, a ranking process of prioritization of zoonotic diseases was done. The ranking process employed a structured technique by these team of experts to develop a systematic, interactive semi-quantitative consensus that had been previously used and validated [4,5,9,15,57]. The result of this prioritization process led to the ranking in order of importance: rabies, avian influenza, EVD, swine influenza and anthrax as the five most important zoonotic diseases in Nigeria, in accordance with the GHSA Action Package Prevent −2 [22]. The findings of this prioritization process strongly favored coordinated, collaborative and multi-sectoral interventions towards the control and prevention of zoonotic diseases through a One Health approach in Nigeria as recommended by the joint external evaluation of the IHR core capacities of the Federal republic of Nigeria [64].
In general, our ranking process prioritized zoonotic diseases due to viruses over bacterial or parasitic pathogens with the topmost four being viral infections while none of the lowest seven was of viral origin. Indeed, rabies has been consistently recognized among the top three zoonotic diseases in similar prioritization surveys reported from Ethiopia, Kenya and Vietnam [4,5,11,15]. There were important features common across the different processes. Diseases either “more prone to cause epidemics or more prevalent in certain regions” were ranked highly [65,66]. In our prioritization, EVD ranked high given the recent outbreaks in West Africa. Similarly, avian influenza that has caused major outbreaks in poultry and affected humans in Nigeria and East Asia was ranked highly in this and the Vietnamese reports [5]. Conversely Rift Valley fever (RVF) and Streptococcus suis infection ranked high in Kenya and Vietnam, possibly due to their regional distribution and significance, respectively [4,5]. In East Africa with large pastoral populations, brucellosis and echinococcosis were prioritized and ranked highly compared to their ranking in our case.
Compared to the WHO global burden of zoonotic diseases list for Nigeria using DALYs and death rates, none of the viral diseases in our prioritized list was found in the WHO list rather, only parasitic diseases such as trypanosomosis, Chagas disease, schistosomosis, leishmaniasis, lymphatic filariasis and onchocercosis were listed [67]. Additionally, only three of the WHO listed zoonotic diseases - trypanosomosis, taeniosis and leishmaniasis [1] featured in our prioritized zoonotic diseases list, ranking 3rd, 10th and 11th, respectively, in the burden of diseases ranking, and 7th, 11th and 33rd in the overall ranking. The variations between the global burden estimates and our prioritized list may have arisen from the different methods employed and the sources of data used in the two scenarios.
Of all the bacterial infections, only anthrax was ranked among the top five in our prioritization list; even though potential risk for bioterrorism was not a consideration and it is not a major human affliction in Nigeria. However, the socioeconomic importance of the disease, especially when large outbreaks occur in animals, and the associated severity and epidemic potential may have resulted in the high ranking. It is unclear the role played by higher proportion of veterinarians in the prioritization of anthrax in our work. Although Nigeria is among the top 30 countries with the highest burden of human tuberculosis (TB) globally [68], prevalence of zoonotic tuberculosis caused by M. bovis is not known despite bovine TB infection reported in about 5.7–11.7% of its cattle herds [[40], [46]] consequently, bovine TB was not listed among the top five priority diseases. This is probably due to its low epidemic potential as well as perceived severity by the groups. Similarly, common cosmopolitan, mostly foodborne bacterial diseases (Campylobacteriosis, Clostridia disease, colibacillosis, Salmonellosis, Staphylococcal diseases, Streptococcal disease, listeriosis and leptospirosis) were not among the five prioritized zoonotic diseases, suggesting ubiquitous global health problems were rarely prioritized at developing country or regional level.
This outcome conforms with the overarching goal of this work which aimed at identifying and prioritizing endemic zoonotic diseases which impact health and socio-economic well-being of Nigerians for collaborative intervention and management. Consequently, the prioritization process weighted diseases with prevention and control measures higher than those that lack any form of prevention, treatment or curative measures. Not surprisingly, higher weights were assigned to diseases with larger geographical occurrence nationally, as opposed to those that have occurred regionally or globally. The directionality of these choices stem from: the perceived ease and lower cost of addressing preventable zoonotic diseases compared to those lacking any form of prevention; perceived higher burden and risk of impact of endemic diseases compared to diseases that occur regionally and globally and increased risk of impact of diseases with wider geographic spread nationally compared with localized diseases of equal severity. The final list of prioritized zoonotic diseases provides the framework for the conceptualization, design and implementation of prevention and control programs for zoonoses. It also allows for allocation of resources to enhance the management, control and possible elimination of zoonotic diseases in Nigeria [40].
Although important concepts within or common to animal and human health such as antimicrobial resistance and non-communicable diseases such as snakebite were not included, we are aware that this list would not be static. It is anticipated that the list will be reviewed at regular intervals to accommodate other diseases and relevant concepts as new data emerge. For example, monkey pox was not considered in our prioritization process due to its absence in Nigeria since 1979 [69]. However, it resurfaced two months after the prioritization process; with three confirmed cases reported in one of the southern states of Nigeria in September 2017 [70]. As at January 25, 2018, the outbreak had spread with 216 suspected cases reported across 24 states of Nigeria and 80 cases and 5 deaths confirmed in 14 states [71]. Similarly, a nationwide outbreak of yellow fever occurred, shortly after the prioritization process, from September 2017 to March 2018 across the 36 states of the Federation and the Federal Capital Territory [33,34]. A total of 1640 suspected, and 99 confirmed cases was recorded in 334 (43%) local government areas of Nigeria with a case fatality rate of 24.4% among confirmed cases. Yellow fever ranked 19th on the prioritized list but may have ranked higher if the outbreak was recorded prior to the prioritization. These incidences highlight the dynamic trend in the emergence and re-emergence of zoonotic diseases across the globe which necessitates periodic review of the prioritized list as deemed necessary.
The modified OHZDP tool used in this process facilitated a multi-stakeholder collaborative decision-making process that allowed groups of experts to provide input from a wide range of experience for ranking of criteria and consequently, ranking of the zoonotic diseases. The consultative nature of the process, we believe, would enhance ownership of the final list of prioritized diseases by different sectors in Nigeria for resource allocation, and convergence of human and animal health zoonotic disease management programs. Although various stakeholders in different regions have used the OHZDP semi-quantitative tool for prioritization [4,5,15,57], some applied varying degrees of modifications based on country-specific peculiarities, which may make it difficult to compare outputs across countries. In our case, we used a slightly modified version of the OHZDP tool which relied more on group consensus over individual opinion. The modified tool was similar to that used in Kenya [4] but differed slightly in application from those used in Ethiopia [15], Vietnam [5] and Japan [57]. Consequently, our prioritization outputs, like those obtained in Kenya, highlighted endemic diseases with high local public health importance such as rabies and not just diseases with high global attention such as the epidemic-prone diseases or those that impact negatively on trade.
The process employed has certain limitations. For instance, the zoonotic disease surveillance system in Nigeria is primarily passive with high rate of underreporting and limited diagnosis. This, coupled with manual, paper-based reporting at peripheral level provides a major challenge in using health system data to gauge true burden of zoonotic disease, majority of which are neglected in nature. This is why our review focused mainly on online data sources though it is a known fact that many diseases occur in Africa that are not detected by rigorous surveillance, or when detected are not published (on time, delayed or never) [72]. Furthermore, inappropriate group composition may lead to subjectively biased results especially if the groups disproportionately comprise of many participants from same background or even with few but highly influential participants from a given discipline. To avert this challenge, we ensured the use of multiple groups, and that participants within groups were balanced by cadre, professional background and geographical location. Similarly, to curb the disproportionate influence of anchors on the conclusions made, active contributions and participation through voting by raising of hands and name calling of seemingly silent participants for inputs was required for consensus building. Secondly, the measures of disease occurrence and burden (prevalence, mortality rate, case fatality rate, DALYs and zDALY [73] etc) required for decision tree analysis were not available for some diseases and where available, were limited to studies that may not be representative of the entire country. In such cases, experts provided estimates based on data from institutional research work, the West African region or from diseases closest in epidemiology to those being examined which may have introduced some error. Thirdly, overestimation of true burden of certain diseases based on their epidemiology in Nigeria could have resulted in the country ranking such diseases high. For example, in Nigeria, the population of dogs is not well known, and reporting of dog rabies is suboptimal making it difficult to ascertain the true burden of canine rabies. However, rabies ranked highest in the list due to its endemicity, perceived nationwide spread and the associated severity with a case fatality rate of 100% [49]. Conversely, the use of disease metrics such as case fatality rates and DALYs determined with data from the developed world could underestimate the public health burden of some of the endemic zoonotic diseases. Finally, using the modified OHZDP tool and semi-quantitative techniques limited the comparability of our output with outputs from other countries especially those that used the standard OHZDP tool version.
5. Conclusion
Using a systematic, semi-quantitative approach, a team of animal, human and environment health practitioners prioritized zoonotic diseases in Nigeria, ranking rabies, avian influenza, EVD, swine influenza and anthrax as the most important. It is recommended that coordinated collaborative multi-sectoral interventions towards the control and prevention of zoonotic diseases should be implemented through a One Health approach in the country. The data gained from this process will be used to optimize human and financial resources for the prevention, detection and control of zoonotic diseases in Nigeria. Following the prioritization, a multisectoral and multi-agency one-health technical working group coordinated by the Nigeria Centre for Disease Control has been established at the National level and have been actively meeting to move the Nigerian one health agenda forward.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This prioritization work was made possible by the generous support of Kaitlin Sandhaus and Dr. Kariuki Njenga of the Global implementation solution (GIS), Washington, USA. We also acknowledge the contributing authors.
Contributor Information
Charles Akataobi Michael, Email: drcedon@yahoo.com.
The Nigeria Zoonotic Diseases Prioritization Group:
Charles Akataobi Michael, Kaitlin Sandhaus, Philip M. Ricks, Albert Ogunkoya, Sola Aruna, Aisha Abubakar, Yusuf Bidemi, Kariuki Njenga, Garba Ibrahim, Olukemi Adekanmbi, Ifeoma Nwadiuto, Idris S. Hadejia, Gatai Nganda, Jarlath U. Umoh, Kwaga Jacob, Olajide Owolodun, Okafor Christoper, T.Z. Gandi Benjamin Tule, Habib Abdulrazak, Dooshima Kwange, Sabitu Kabiru, Babasola Olugasa, Gidado M. Muhammed, Tony Joannis, Sunday Omilabu, Junaid Kabir, G.A.T. Ogundipe, Olubunmi Ojo, Obasanya Joshua, Aisha Abubakar Sadiq, Olayinka Adebola, Abdullahi A. Magaji, Aisha Nasir, Dan Duvall, S. Tekki, Sati Ngulukun, Dotun Soruuke, Abiodun Egumenu, Ibro Idiona, Oyiri Ferdinand, Olufemi Abayomi, Ilori Elsie, Visa I. Tyakaray, Angela Oyo-Ita, Godson Ana, Olaniran Alabi, Mabel Aworh, John Kvagai, Gana Chinyere, and Okara Gloria
References
- 1.WHO, Zoonoses and the Human-Animal-Ecosystems Interface. WHO. http://www.who.int/zoonoses/en/. Accessed 27 July 2017.
- 2.Gowtage-Sequeria M.W., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. J. December 2005;11(12) doi: 10.3201/eid1112.050997. CDC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. WHO | Zoonoses. Veterinary Public Health (VPH). WHO. http://www.who.int/zoonoses/vph/en/. Accessed 29 July 2017.
- 4.Munyua P., Bitek A., Osoro E., Pieracci E.G., Muema J., Mwatondo A. Prioritization of zoonotic diseases in Kenya, 2015. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trang Do Thuy, Siembieda Jennifer, Hung Pham, Ky Van Dang, Bandyopahyay Santanu, Olowokure Babatunde. Prioritization of zoonotic diseases of public health significance in Vietnam. J. Infect. Dev. Countries. 2015;9(12):1315–1322. doi: 10.3855/jidc.6582. [DOI] [PubMed] [Google Scholar]
- 6.Welburn S.C., Beange I., Ducrotoy M.J., Okello A.L. The neglected zoonoses--the case for integrated control and advocacy. Clin Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015;21(5):433–443. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Neglected and Endemic Zoonoses https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2865085/ Accessed 17 August 2017.
- 8.Richard O.G., Olaniyi A.J., Paul M.P., Odinya A.V., Adamu D.A., Atinuke D.M. A review on human deaths associated with rabies in Nigeria. J. Vacc. Vaccin. 2013 doi: 10.4172/2157-7560.1000262. [DOI] [Google Scholar]
- 9.Rist C.L., Arriola C.S., Rubin C. Prioritizing zoonoses: a proposed one health tool for collaborative decision-making. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salyer S.J., Silver R., Simone K., Barton Behravesh C. Prioritizing zoonoses for global health capacity building—themes from one health zoonotic disease workshops in 7 countries, 2014–2016. Emerg. Infect. Dis. 2017;23(13) doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC One health zoonotic disease prioritization process overview. One Health. 2020 https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/fact-sheet.html Accessed 22 April 2021. [Google Scholar]
- 12.The Most Populated Countries In Africa WorldAtlas. http://www.worldatlas.com/articles/the-most-populated-countries-in-africa.html Accessed 27 September 2017.
- 13.Onusi A. Retreat On Livestock And Dairy Development In Nigeria – Keynote Address Delivered By The Hon. Minister Of Agriculture And Rural Development, Chief Audu Ogbeh – Federal Ministry of Agriculture and Rural Development. http://fmard.gov.ng/retreat-on-livestock-and-dairy-development-in-nigeria-keynote-address-delivered-by-the-hon-minister-of-agriculture-and-rural-development-chief-audu-ogbeh/ Accessed 17 August 2017.
- 14.Apata T.G., Folayan A., Apata O.M., Akinlua J. 85th Annu Conf Agric Econ Soc Warwick Univ. 18–20 April 2011. The economic role of Nigeria’s subsistence agriculture in the transition process: implications for rural development.http://ageconsearch.umn.edu/bitstream/108942/2/64apata_folayan_apata_akinlua.pdf Accessed 27 September 2017. [Google Scholar]
- 15.Pieracci E.G., Hall A.J., Gharpure R., Haile A., Walelign E., Deressa A. Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health. 2016;2:131–135. doi: 10.1016/j.onehlt.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel C.J., Cardwell D.M., Moeller R.B., Gray G.C. Humans and cattle: a review of bovine zoonoses. Vector Borne Zoonotic Dis. 2014;14(1):1–19. doi: 10.1089/vbz.2012.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones B.A., Grace D., Kock R., Alonso S., Rushton J., Said M.Y. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. U. S. A. 2013;110(21):8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klous G., Huss A., Heederik D.J.J., Coutinho R.A. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health. 2016;2:65–76. doi: 10.1016/j.onehlt.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngutor K.S. Benefits of animal intervention strategies in the control of neglected zoonotic diseases in Nigeria. J. Public Health Epidemiol. 2016;8(7):121–126. [Google Scholar]
- 20.A review of agricultural transformation agenda in Nigeria: the case of public and private sector participation (PDF Download Available). ResearchGate. https://www.researchgate.net/publication/267507156_A_Review_of_Agricultural_Transformation_Agenda_in_Nigeria_The_Case_of_Public_and_Private_Sector_Participation. Accessed 17 August 2017.
- 21.Gebreyes W.A., Dupouy-Camet J., Newport M.J., Oliveira C.J.B., Schlesinger L.S., Saif Y.M. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 2014;8(11) doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC Global Health Global Health Security Agenda: GHSA Zoonotic Disease Action Package (GHSA Action Package Prevent-2) https://www.cdc.gov/globalhealth/security/actionpackages/zoonotic_disease.htm Accessed 28 September 2017.
- 23.Yasobant S., Saxena D., Bruchhausen W., Memon F.Z., Falkenberg T. Multi-sectoral prioritization of zoonotic diseases: one health perspective from Ahmedabad, India. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekamatte M., Krishnasamy V., Bulage L., Kihembo C., Nantima N., Monje F. Multisectoral prioritization of zoonotic diseases in Uganda, 2017: a One Health perspective. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookes V.J., Del Rio Vilas V.J., Ward M.P. Disease prioritization: what is the state of the art? Epidemiol. Infect. 2015;143(14):2911–2922. doi: 10.1017/S0950268815000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguku Patrick, Oyemakinde Akin, Sabitu Kabiru, Olayinka Adebola, Ajayi Ikeoluwapo, Fawole Olufunmilayo. Training and service in public health, Nigeria field epidemiology and laboratory training, 2008–2014. Pan Afr. Med. J. 2014;18(Suppl .1):2. doi: 10.11694/pamj.supp.2014.18.1.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith T.C., Harper A.L., Nair R., Wardyn S.E., Hanson B.M., Ferguson D.D. Emerging swine zoonoses. Vector Borne Zoonotic Dis. Larchmt. N. 2011;11(9):1225–1234. doi: 10.1089/vbz.2010.0182. [DOI] [PubMed] [Google Scholar]
- 28.Murphy F.A. Emerging zoonoses: the challenge for public health and biodefense. Prev. Vet. Med. 2008;86(3–4):216–223. doi: 10.1016/j.prevetmed.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafond K.E., Nair H., Rasooly M.H., Valente F., Booy R., Rahman M. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owolodun O.A., Gerber P.F., Giménez-Lirola L.G., Kwaga J.K.P., Opriessnig T. First report of hepatitis E virus circulation in domestic pigs in Nigeria. Am. J. Trop. Med. Hyg. 2014;91(4):699–704. doi: 10.4269/ajtmh.14-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karshima S.N. Helminths of zoonotic importance in slaughtered food animals in Nigeria: a systematic review and meta-analysis. J. Helminthol. 2019;93(3):295–305. doi: 10.1017/S0022149X18000196. [DOI] [PubMed] [Google Scholar]
- 32.Sule W.F., Oluwayelu D.O., Adedokun R.A.M., Rufai N., McCracken F., Mansfield K.L. High seroprevelance of West Nile virus antibodies observed in horses from southwestern Nigeria. Vector Borne Zoonotic Dis. Larchmt. N. 2015;15(3):218–220. doi: 10.1089/vbz.2014.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogbu O., Ajuluchukwu E., Uneke C.J. Lassa fever in West African sub-region: an overview. J. Vector Borne Dis. 2007;44(1):1–11. [PubMed] [Google Scholar]
- 34.Cadmus S.I.B., Yakubu M.K., Magaji A.A., Jenkins A.O., van Soolingen D. Mycobacterium bovis, but also M. africanum present in raw milk of pastoral cattle in north-central Nigeria. Trop. Anim. Health Prod. 2010;42(6):1047–1048. doi: 10.1007/s11250-010-9533-2. [DOI] [PubMed] [Google Scholar]
- 35.Hotez P.J., Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3(8) doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maudlin I., Eisler M.C., Welburn S.C. Neglected and endemic zoonoses. Philos. Trans. R Soc. B Biol. Sci. 2009;364(1530):2777–2787. doi: 10.1098/rstb.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ugbomoiko U.S., Ariza L., Heukelbach J. Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008;4:49. doi: 10.1186/1746-6148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olugasa B.O., Aiyedun J.O., Emikpe B.O. Prevalence of antibody against rabies among confined, free-roaming and stray dogs in a transit city of Nigeria. Vet. Ital. 2011;47(4):453–460. [PubMed] [Google Scholar]
- 39.Meslin F.X., Stöhr K., Heymann D. Public health implications of emerging zoonoses. Rev. Sci. Tech. Int. Off. Epizoot. 2000;19(1):310–317. doi: 10.20506/rst.19.1.1214. [DOI] [PubMed] [Google Scholar]
- 40.Cadmus S.I.B., Agada C.A., Onoja I.I., Salisu I. Risk factors associated with bovine tuberculosis in some selected herds in Nigeria. Trop. Anim. Health Prod. 2010;42(4):547–549. doi: 10.1007/s11250-009-9463-z. [DOI] [PubMed] [Google Scholar]
- 41.Odetokun I.A., Ballhausen B., Adetunji V.O., Ghali-Mohammed I., Adelowo M.T., Adetunji S.A. Staphylococcus aureus in two municipal abattoirs in Nigeria: risk perception, spread and public health implications. Vet. Microbiol. 2018;216:52–59. doi: 10.1016/j.vetmic.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Meseko C., Olaleye D., Capua I., Cattoli G. Swine influenza in sub-saharan Africa—current knowledge and emerging insights. Zoonoses Public Health. 2014;61(4):229–237. doi: 10.1111/zph.12068. [DOI] [PubMed] [Google Scholar]
- 43.Greger M. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit. Rev. Microbiol. 2007;33(4):243–299. doi: 10.1080/10408410701647594. [DOI] [PubMed] [Google Scholar]
- 44.Ayinmode A.B., Obebe O.O., Olayemi E. Prevalence of potentially zoonotic gastrointestinal parasites in canine faeces in Ibadan, Nigeria. Ghana Med. J. 2016;50(4):201–206. doi: 10.4314/gmj.v50i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducrotoy M.J., Bertu W.J., Ocholi R.A., Gusi A.M., Bryssinckx W., Welburn S. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl. Trop. Dis. 2014;8(7) doi: 10.1371/journal.pntd.0003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akinseye V.O., Adebayo M.D., Genesis O.O., Adelakun O.D., Cadmus S.I.B. Prevalence and risk factors of mycobacterial infections in farm and trade cattle in southwestern Nigeria. Trop. Anim. Health Prod. 2017 doi: 10.1007/s11250-017-1492-4. [DOI] [PubMed] [Google Scholar]
- 47.Tambo E., Adetunde O.T., Olalubi O.A. Re-emerging Lassa fever outbreaks in Nigeria: re-enforcing “One Health” community surveillance and emergency response practice. Infect. Dis. Poverty. 2018;7 doi: 10.1186/s40249-018-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorusso V., Wijnveld M., Majekodunmi A.O., Dongkum C., Fajinmi A., Dogo A.G. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasit. Vectors. 2016;9 doi: 10.1186/s13071-016-1504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eke C.B., Omotowo I.B., Ukoha O.M., Ibe B.C. Human rabies: still a neglected preventable disease in Nigeria. Niger. J. Clin. Pract. 2015;18(2):268. doi: 10.4103/1119-3077.151064. [DOI] [PubMed] [Google Scholar]
- 50.Aworh M.K., Okolocha E., Kwaga J., Fasina F., Lazarus D., Suleman I. Human brucellosis: seroprevalence and associated exposure factors among abattoir workers in Abuja, Nigeria - 2011. Pan Afr. Med. J. 2013;16 doi: 10.11604/pamj.2013.16.103.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adesokan H.K., Akinseye V.O., Sulaimon M.A. Knowledge and practices about zoonotic tuberculosis prevention and associated determinants amongst livestock workers in Nigeria; 2015. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hambolu S.E., Dzikwi A.A., Kwaga J.K.P., Kazeem H.M., Umoh J.U., Hambolu D.A. Rabies and dog bites cases in Lagos state Nigeria: a prevalence and retrospective studies (2006–2011) Global J. Health Sci. 2014;6(1):107–114. doi: 10.5539/gjhs.v6n1p107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maurice N.A., Luka P.D., Maurice M.N., Ngbede E.O., Zhakom P.N., Mshelbwala P.P. Rabies in a set of eight-week old puppies in Nigeria: the need for review of current dog antirabies vaccination schedule. Afr. J. Infect. Dis. 2018;12(2):72–77. doi: 10.21010/ajid.v12i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayoola M.C., Akinseye V.O., Cadmus E., Awosanya E., Popoola O.A., Akinyemi O.O. Prevalence of bovine brucellosis in slaughtered cattle and barriers to better protection of abattoir workers in Ibadan, South-Western Nigeria. Pan Afr. Med. J. 2017;28 doi: 10.11604/pamj.2017.28.68.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olugasa B.O., Oshinowo O.Y., Odigie E.A. Preventive and social cost implications of Ebola Virus Disease (EVD) outbreak on selected organizations in Lagos state, Nigeria. Pan Afr. Med. J. 2015;22(Suppl. 1) doi: 10.11694/pamj.supp.2015.22.1.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CDC Bioterrorism Agents/Diseases (by Category) | Emergency Preparedness & Response. 2017. https://emergency.cdc.gov/agent/agentlist-category.asp Accessed 12 September 2017.
- 57.Kadohira M., Hill G., Yoshizaki R., Ota S., Yoshikawa Y. Stakeholder prioritization of zoonoses in Japan with analytic hierarchy process method. Epidemiol. Infect. 2015;143(7):1477–1485. doi: 10.1017/S0950268814002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saaty T.L. Decision making with the analytic hierarchy process. Int. J. Serv. Sci. 2008;1(1):83–98. [Google Scholar]
- 59.Havelaar A.H., van Rosse F., Bucura C., Toetenel M.A., Haagsma J.A., Kurowicka D. Prioritizing emerging zoonoses in the Netherlands. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stebler N., Schuepbach-Regula G., Braam P., Falzon L.C. Use of a modified Delphi panel to identify and weight criteria for prioritization of zoonotic diseases in Switzerland. Prev. Vet. Med. 2015;121(1–2):165–169. doi: 10.1016/j.prevetmed.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Ng V., Sargeant J.M. A stakeholder-informed approach to the identification of criteria for the prioritization of zoonoses in Canada. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox R., Sanchez J., Revie C.W. Multi-criteria decision analysis tools for prioritising emerging or re-emerging infectious diseases associated with climate change in Canada. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trang D.T., Siembieda J., Huong N.T., Hung P., Ky V.D., Bandyopahyay S. Prioritization of zoonotic diseases of public health significance in Vietnam. J. Infect. Dev. Countries. 2015;9(12):1315–1322. doi: 10.3855/jidc.6582. [DOI] [PubMed] [Google Scholar]
- 64.WHO Joint External Evaluation of the IHR Core Capacities of the Federal Republic of Nigeria. 2017. http://apps.who.int/iris/bitstream/handle/10665/259382/WHO-WHE-CPI-REP-2017.46-eng.pdf?sequence=1 Accessed 17 April 2018.
- 65.Nguku P.M., Sharif S.K., Mutonga D., Amwayi S., Omolo J., Mohammed O. An investigation of a major outbreak of Rift Valley fever in Kenya: 2006–2007. Am. J. Trop. Med. Hyg. 2010;83(2 Suppl):5–13. doi: 10.4269/ajtmh.2010.09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anyangu A.S., Hannah Gould L., Sharif S.K., Nguku P.M., Omolo J.O., Mutonga D. Risk factors for severe rift valley fever infection in Kenya, 2007. Am. J. Trop. Med. Hyg. 2010;83(2 Suppl):14–21. doi: 10.4269/ajtmh.2010.09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO | Disease and Injury Country Estimates. WHO. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/. Accessed 20 September 2018.
- 68.World Health Organisation . WHO; Geneva, Switzerland: 2017. WHO | Global Tuberculosis Report 2017.http://www.who.int/tb/publications/global_report/en/ Accessed 10 January 2017. [Google Scholar]
- 69.Weinstein RA, Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin. Infect. Dis.. 1765–1771. [DOI] [PubMed]
- 70.Nigeria Centre for Disease Control Update on monkeypox - Government Confirms Three Cases. http://www.ncdc.gov.ng/news/projects/bids_and_tenders e.
- 71.Nigeria Center for Disease Control Monkeypox outbreak in Nigeria - a situation report. NCDC Dis. Situat. Rep. 2018;016(04):1–4. [Google Scholar]
- 72.Influenza in Africa. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000182 Accessed 15 September 2018.
- 73.Torgerson P.R., Rüegg S., Devleesschauwer B., Abela-Ridder B., Havelaar A.H., Shaw A.P.M. zDALY: an adjusted indicator to estimate the burden of zoonotic diseases. One Health. 2017;5:40–45. doi: 10.1016/j.onehlt.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]