Highlights
-
•
Progression of colorectal cancer relies on the accumulation of gene mutations.
-
•
About 10% of metastatic patients present with an oligo-metastatic disease.
-
•
Next generation sequencing are clarifying the genetics of oligo-metastases.
-
•
“Regressive” genetic trajectories are found in oligo-metastatic progression.
Keywords: Oligo-metastases, Colorectal cancer, Genetics, NGS, Mutations
Abstract
Colorectal cancer (CRC) originates as consequence of multiple genetic alterations. Some of the involved genes have been extensively studied (APC, TP53, KRAS, SMAD4, PIK3CA, MMR genes) in highly heterogeneous and poly-metastatic cohorts. However, about 10% of metastatic CRC patients presents with an indolent oligo-metastatic disease differently from other patients with poly-metastatic and aggressive clinical course. Which are the genetic dynamics underlying the differences between oligo- and poly-metastatic CRC? The understanding of the genetic trajectories (primary→metastatic) of CRC, in patients selected to represent homogenous clinical models, is crucial to make genotype/phenotype correlations and to identify the molecular events pushing the disease towards an increasing malignant phenotype. This information is crucial to plan innovative therapeutic strategies aimed to reverse or inhibit these phenomena. In the present study, we review the genetic evolution of CRC with the intent to give a developmental perspective on the border line between oligo- and poly-metastatic diseases.
Graphical abstract
Introduction
Cancer is a genetic disease and the number and type of altered genes producing its phenotype, including proliferation, mobility, and resistance to drugs is high and heterogeneous, and mostly unknown. When studying metastatic patients, we focus on a specific setting and moment of the genetic evolution of cancer, excluding the sequential events that, in time and space, accumulated and progressed from the normal cell to the neoplastic one. In fact, it is quite impossible to follow in the same patient this genetic “cascade” because when clinically evident the tumor mass has already crossed a large part of its genetic history [1], [2], [3]. Thus, available studies compare normal cells, early, intermediate or advanced phase of cancers coming from different cohorts of patients. This introduces strong and uncontrollable biases linked to physiologic genetic polymorphisms (races, age, gender) and the presence of other comorbidities which often share common genetic mediators with cancer (diabetes, hypertension, obesity) [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. A strategy to partially encompass this difficulty has been recently applied with studies comparing primary and metastatic tumors surgically resected, if available, at different time points of clinical history in the same patient [15], [16], [17], [18], [19].
The understanding of the genetic evolution of ColoRectal Cancer (CRC) is crucial to identify the molecular events that push the disease towards an increasing malignant phenotype and, thus, to plan therapeutic strategies aimed to reverse or inhibit these phenomena. In the present study, we will review the genetic evolution of CRC with the intent to give a developmental perspective on the border line between oligo- and poly-metastatic CRC.
Genotype-phenotype correlations in CRC: comorbidities and cancer heterogeneity
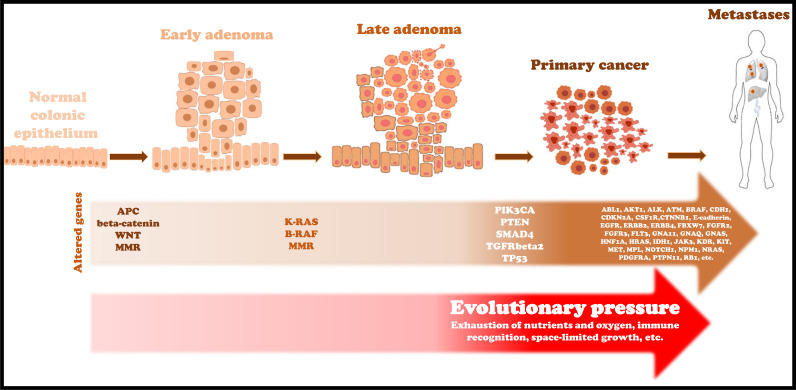
The genetic progression from normal mucosa to adenocarcinoma relies on the sequential accumulation of several gene mutations (“Volgestein model”) [20] initiating from “cancerized fields” induced by inherited mutations, natural DNA replication errors or mutagenic insults [21]. Insight into mutations and functions of all these genes and their relative products are beyond the scope of this review. Some of them are crucial to achieve the malignant phenotype (key driver genes) (Fig. 1) and are frequently found in cancerous cells. Table 1 summarizes the studies reporting the genetic comparison between primary and matched metastatic lesions in CRC.
Fig. 1.
Progressive accumulation of specific genetic alterations and environmental factors push the evolution from adenocarcinoma precursors to malignant lesions.
Table 1.
Results of studies reporting genetic evolution of matched primary and poly-metastatic CRC.
| Author | Year | No. of paired samples (PT/MT) | Patients’ characteristics at diagnosis | Site of metastases | NGS platform | Genetic sharing PT/MT (global concordance) | Four most frequent and shared mutations | Unshared altered genes in PT (found in primary only) | Unshared altered genes in MT (found in metastasis only) |
|---|---|---|---|---|---|---|---|---|---|
| Brannon AR et al. | 2014 | 69 | Four pts stage II, 3 stage III, 62 stage IV. Seventy-five percent of metastases were synchronous. Thirty pts were chemonaive. | Liver (only two ovary). | Illumina, HiSeq 2000. | 79% | APC, TP53, KRAS, PI3KCA were the most frequently mutated genes and most highly concordant between PT and MT (concordance of KRAS, NRAS, or BRAF was 100%). | ALK, APC, ASXL1, BAP1, CARD11, CBL, CEBPA, EPHA3, EPHA6, EPHA7, EPHB1, ERBB2, ERBB4, FLT1, FOXL2, GRIN2A, KDM6A, KDR, LGR6, MDM4, MITF, NFKB2, NOTCH3, PBRM1, PDGFRB, PIK3CA, PIK3CD, PIK3CG, SMAD4, STK11, TET1, TP53, TSHR. | APC, AR, ATM, ATRX, BCL6, BRCA2, EGFR, EPHA5, EPHA6, EPHB1, ERBB4, FAS, FH, FLT1, MAP2K1, MAP2K1, NF1, NFE2L2, NOTCH1, NTRK3, PIK3C2G, PIK3CA, PIK3CA, PIK3CD, PIK3CG, PIK3R1, PREX2, PTEN, PTPRS, REL, REL, SMAD4, SMAD4, SUFU, TBK1, TET1, TET2, TGFBR2, TP53, TSHR. |
| Lee SY et al. | 2014 | 15 | Stage IV. 6 pts had single liver metastasis. | Liver. | Illumina, HiSeq 2000. | *Mutational concordance showed only for each genes: APC: 100% TP53: 70% KRAS: 100% SMAD4: 75% | APC, TP53, KRAS, SMAD4. APC and KRAS mutations were ever concordant between PT and MT. | BRAF, CTNNB1, FBXW7, PIK3R1, TP53, SOX9. | ATR, BRAF, CDC42BPG, FBXW7, FLT4, KDR, PI3KCG, RB1, SMAD4, SOX9. |
| Kim R et al. | 2015 | 19 | Twelve pts were stage IV, 7 pts stage III. Data on treatments not reported. | Liver, lungs, lymphnodes, ovary. | Illumina, GAIIX. | 93.5% | APC and TP53 found concordant in 10/19 pairs.KRAS ever concordant (9/19 pts). PI3K ever condordant (3/19 pts). | ABCA3, ADAMTS20, APC, BRCA2, CX3CR1, DGKB, ERBB4, HSP90AB1, ITGA10, ITGAL, JAK1, LRP1B, MACF1, MAP3K, MAGI2, MARK1, NTRK2, PARP14, PIK3CG, RASA1, ROBO1, SMAD2, SMAD3, SMAD4, TEX14, TNKS, TP53, TTN, WNT2, ZNF217, ZNF831. | ADAMTS18, ADAMTS20, ADCY1, APC, BCL9, CASC5, CHD5, CIC, COL7A1, CSMD3, EPHA5, ETV4, FANCG, FBXW7, GPC5, HERC1, KIAA1409, KNTC1, MACF1, MAPK10, MAST4, MGA, MGMTk, MMP2, MPL, MUC16, NOS1, PCM1, PPM1H, PREX1, PRKCZ, PTPN13, PTPRC, PTPRD, RASA1, RB1CC1, ROBO1, RPS6KB2, SIRT6, SNX13, STIM1, TACR3, TCF12, TCF3, TOP2B, TOPBP1, TP53, TPO, TRAF4, TTN, VRTN, WNT2. |
| Vignot S et al. | 2015 | 13 | Stage IV. Six synchronous metastases, 7 methacronous. Patients received chemotherapy and/or radiotherapy (one pt) before surgery. | Liver and liver. Only local (1 pt), only peritoneum (1 pt). | Illumina, HiSeq 2000 | 78% | APC, TP53, KRAS, and SMAD4 were the most frequent mutated genes. Mutated APC had a concordance of 100%. | ALK, BRCA2, GNAS, NF1, RICTOR, STK11, TNKS. | BRCA2, CDH2, CDKN2A, EPHB1, GLUCY1A2, PI3KCG, RB1, RET, SMO. |
| Kovaleva V et al. | 2016 | 14 | Stage IV. Synchronous and/or metachronous liver and/or lung metastases. | Liver and lungs. | TruSeq Amplicon Cancer PanelTM, MiSeq (Illumina). | *From 0 to 100% (median 8.5%). | TP53, APC, KRAS, SMAD4. | ABL1, ATM, BRAF, EGFR, ERBB4, FBXW7, FGFR3, GNA11, GNAQ, HRAS, JAK3, KDR, KIT, MET, NOTCH1, NRAS, PDGFRA, PIK3CA, PTEN, RB1, RET, SMAD4, STK11, TP53, VHL. | ABL1, AKT1, ALK, ATM, BRAF, CDH1, CDKN2A, CSF1R, CTNNB1, EGFR, ERBB2, ERBB4, FBXW7, FGFR2, FGFR3, FLT3, GNA11, GNAQ, GNAS, HNF1A, HRAS, IDH1, JAK3, KDR, KIT, KRAS, MET, MLH1, MPL, NOTCH1, NPM1, NRAS, PDGFRA, PIK3CA, PTEN, PTPN11, RB1, RET, SMAD4, SMARCB1, SMO, SRC, STK11, TP53, VHL. |
NGS: Next Generation Sequencing; MT: Metastatic Tumor; PT: Primary Tumor; pts: patients; TMB: Tumor Mutation Burden.
when the data were not clearly reported they were derived from Venn Diagrams or descriptive tables.
Studies on correlations between genotype and phenotype in cancer are extremely difficult. In fact, except for very rare cases including some inherited forms of tumors (retinoblastoma, Wilms’ tumor, etc.), cancer arises as an acquired multi-genic disease. The first and most important limit on a methodologic point of view to make genotype/phenotype correlations is represented by the selection of “clean” human models of cancer. In fact, several genes involved in highly common diseases strongly contribute to cancer heterogeneity, i.e. hypertension [22, 23], diabetes [24], allergies [[8], [9], [10], [11], 25] and inflammatory chronic diseases [12], [13], [14]. These diseases interfere with cancer genetics; in fact, some genes involved in cancer-related phenomena (as proliferation and angiogenesis) are altered in hypertension and atherosclerotic plaque or they are induced as a consequence of hypoxia, oxidative stress and inflammation. HIF-1 (Hypoxia-Inducible Factor-1) and LOX-1 (Lectin-like OXidized low-density lipoprotein receptor-1) genes products are the most important mediator between chronic cardio-vascular diseases and cancer. HIF-1 induces the expression of multiple tumor angiogenic factors including VEGF (Vascular Endothelial Growth Factor) and other growth factors [26]. Furthermore, HIF-1 is also involved in promoting the oxidative stress through reactive oxygen species (ROS) and inflammatory pathways through nuclear factor-kappa B activation in atherosclerotic plaques [27]. Several pro-inflammatory genes and related products altered in diabetes, hypertension and chronic inflammatory diseases such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), can promote cancer progression directly or by stimulating tumor angiogenesis [28, 29]. LOX-1, a major receptor for ox-LDL in endothelial cells, cardiomyocytes, monocytes, platelets, and vascular smooth muscle is induced in some diseases including diabetes and atherosclerosis and has been involved in increased risk of colon, breast, and ovarian cancer [30]. On the other hand, LOX-1 is crucial for maintaining the transformed state in many different cancer cell lines [31], suggesting that LOX-1 is an important molecular factor in tumorigenesis. Recent studies highlight the role of LOX-1 in inducing angiogenesis, proliferation and metastatic spread in cancer [32], [33], [34], [35]. Most importantly, a significant overlap between cancer and atherosclerosis mutational gene signature has been found and, among common genes, oxidative stress and insulin gene networks emerge as central nodes [30]. Interestingly, PDGFs (Platelet-Derived Growth Factors), stored and released by platelets, act as potent mediators of metabolic and organic dysfunctions in hypertension, atherosclerosis, and diabetes where an increased serum level is registered and correlates with prognosis [36, 37]. PDGFs work as potent pro-angiogenic, mitogen and chemoattractant factors in many tumors. Germline and somatic mutations of PDGF pathways are found in dysmetabolic syndrome as well as in cancer. A complete dissertation of common mediators between cancer and inflammatory chronic diseases is beyond the scope of this review, however, very recently, RALB (RAS Like Proto-Oncogene B) gene has emerged as an important link between inflammation and cancer. In fact, it is involved in the inflammatory response and belongs to the RAS GTPase (RAt Sarcoma viral oncogene homolog Guanosine-5′-TriPhosphatase) superfamily crucial components of oncogenic RAS-induced transformation in many cancers [38]. The same has been documented for MYC (MYeloCytomatosis oncogene) oncogene which is not only involved in many aspects of the tumor phenotype but also in the release of a plethora of pro-inflammatory cytokines and VEGF-A [39]. Additionally, many inflammatory chronic diseases are characterized by activated RET (REarranged during Transfection) tyrosine kinase as frequently found in cancers [40]. The genetic links between altered body mass index and cancer has been extensively described elsewhere [7].
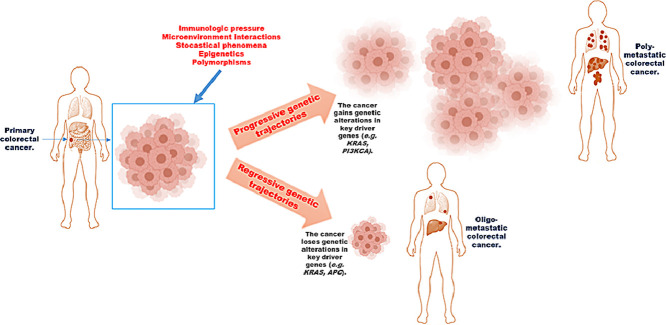
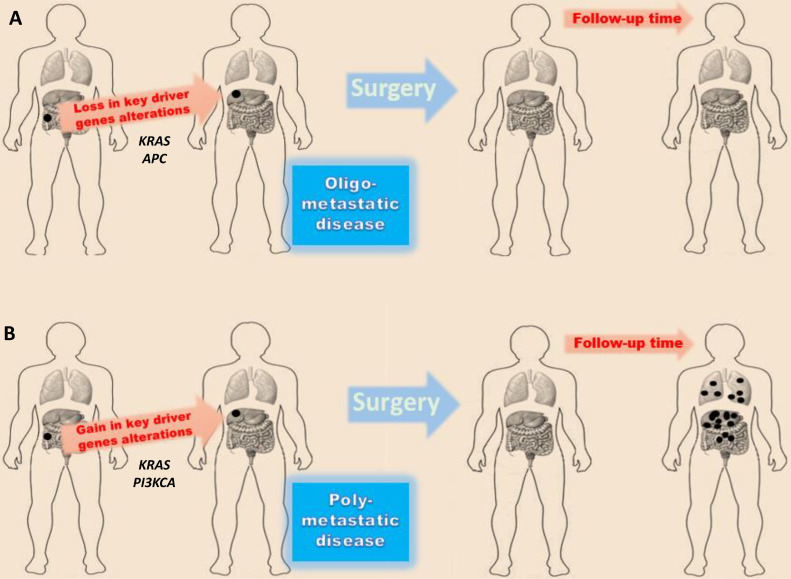
Altogether, these observations demonstrate that study of cancer genetics can be biased by the presence of comorbidities, which strongly contribute to increase heterogeneity. The mutational profile of matched primary and metastatic tumors in poly-metastatic CRC is a surrogate of tumor genetic evolution (Table 1). Two concepts emerge from studies included in Table 1: 1. Gain of mutations in key-driver genes in primary tumors is maintained in metastatic lesions, 2. Genetic results [in the same individuals (shared point mutations in primary vs metastatic tumors) and in different studies (type of mutated genes in primary and metastatic tumors)] are extremely heterogeneous. However, these studies suffered of uncontrolled clinical and pathological heterogeneity (different stages of disease, heterogeneous and sequential treatments, different comorbidities, different histotypes, etc.) certainly influencing the genotype/phenotype results’ interpretation. We previously performed two works where we made a rigorous effort to select patients having oligo-metastatic CRC as the only illness [41, 42]. Unlike what was observed in poly-metastatic CRC, we found “regressive” genetic trajectories in key driver genes (Fig. 2). As in a recent work [43], a “regressive” genetic trajectory was intended as the loss of the genetic alterations in a specific gene from the primary tumor to the metastatic one [i.e.: mutant KRAS (mutKRAS) in primary tumor→wild-type KRAS (wtKRAS) in metastases]; this issue was opposed to “progressive” genetic trajectory (i.e.: wtKRAS in primary tumor→mutKRAS in metastases) where the metastatic lesions gain genetic alterations..
Fig. 2.
Genetic trajectories underlying the different behavior among oligo- and poly-metastatic CRC. The figure represents two clinical models previously studied [42]. A) Patients without recurrence at 3-year follow-up, B) patients whose cancer recurred within 1 year after resection of the primary colorectal cancer and the single liver metastatic lesion.
Genetic border between oligo- and poly-metastatic CRC: dynamics and role of SMAD4, KRAS, APC, PI3KCA
About 10% of mCRC patients present with oligo-metastatic disease characterized by an indolent and long-term survival course. Oligometastases can be defined as tumor masses involving one to three lesions per organ with a maximum tumor diameter smaller than 7 cm [44, 45]. Based on available data, recently, consensus guidelines provided a more conservative definition (1–5 metastatic lesions, with a maximum tumor diameter smaller than 5 cm) [46]. Beside the tumor load, this definition has been enriched by the “rate of metastases development” which is low in the oligo-metastatic cancer [47, 48]. In clinical practice, the identification of an oligo-metastatic CRC is mostly retrospective since many patients [42] treated with a concomitant radical resection of primary tumor and oligometastases develop an aggressive and poly-metastatic disease within 1 year observation while others never experience disease poly-progression [“genuine” oligo-metastatic setting [49]]. Still to date, the genetic dynamics underlying the oligo- versus poly-metastatic status are unknown and very few data have been obtained on the genetic differences between these two clinical entities predominantly focused on the characterization and comparison of metastatic lesions [50]. In vitro assays and/or animal models are useful to explore and generate hypotheses but they are too far from the human mechanistic physiology. Furthermore, as above discussed, the presence of comorbidities in human models (hypertension, diabetes, allergies and inflammatory chronic diseases) induces strong interferences in cancer genetics and clinical outcomes complicating both epidemiologic and mechanistic studies. For this reason, in our previous studies [41, 42], we applied a very strict definition and selection of patients with oligo-metastatic CRC to investigate the mutational evolution of oligo-metastatic genetic landscape. Interestingly, we found in lung-limited oligo-metastatic CRC regressive mutational trajectories in APC (Adenomatous Polyposis Coli), KRAS and SMAD4 (caenorhabditis elegans Sma and drosophila Mothers Against Decapentaplegic 4) through an extended genomic characterization. Similarly, in liver-limited oligo-metastatic CRC, regressive trajectories were identified in KRAS and SMAD4 genes; in this work, the mutational evolution of oligo-metastatic patients was compared with patients developing poly-metastatic disease, in these patients, a PI3KCA (PhosphatIdyl 3-Kinase Catalytic subunit Alpha) mutation was always gained. Comparing these results with genomic referral patterns of poly-metastatic CRC (see Table 1) and our internal datasets, these altered key driver genes (APC, KRAS and SMAD4) are always shared between primary and metastatic lesions.
SMAD4
An interesting and surprising observation was the low incidence of SMAD4 mutations in primary CRC evolving towards an oligo-metastatic clinical behavior [41, 42]. SMAD4 gene is located on chromosome 18q21 and it is a downstream effector of the transforming growth factor (TGF)-β. TGF-β has contradictory effects during tumorigenesis: it inhibits cell growth and migration in normal cells but stimulates progression in transformed cells (“TGF-β paradox”). There are three forms of TGF-β: TGF-β1, -β2 and –β3. The most important is TGF-β1 whose gene is located on chromosome 19q13 and encodes for a peptide that plays a crucial role in angiogenesis, epithelial-to-mesenchymal transition (EMT) and proliferation during CRC progression [51]. Three types of TGF-β receptors (TGFβR) have been identified: type 1, 2 and 3. After ligand binding, TGF-βR2 recruits and phosphorylates TGF-βR1. Interestingly, TGF-βR1 is controlled through ubiquitination and it can be phosphorylated at different sites. After phosphorylation, it in turn phosphorylates either downstream proteins SMAD2 and 3 (“canonical” pathway) on serine residues at the C-terminus, or many kinases such as MAPKs, ERK, P38, JNK, PI3K, ROCK, etc. (“noncanonical” pathways, largely unknown) [52, 53]. SMAD2 and 3 complex with SMAD4 and translocate into the nucleus where they activate transcription of numerous target genes (including SERPINE1, LTBP2, CDKN1A, ARID3B, ATXN1, PTPRK, RAB6A, SMAD7, EHBP1, etc.) acting predominantly as a tumor-suppressor gene of TGF-β-mediated signals [54]. TGF-βR3 (betaglycan), is the most abundant TGFβ receptor. It can bind to all TGFβ receptors but has a short cytoplasmic domain lacking kinase activity. For this reason, it has been thought to act only by “sequestering” TGFβR2. However, recent evidences suggested that it works through a “non canonical” pathway. Alterations of the SMAD4 and TGF-βR2 genes have been reported as late events in CRC and are able to promote cancer progression [55, 56]. Thus, SMAD4 crucially concurs to the malignant phenotype of poly-metastatic CRC and, in advanced CRC, it is an independent negative prognostic factor for disease-free and overall survival [57], [58], [59].
KRAS
Another gene whose regressive trajectory contribute to the oligo-metastatic CRC phenotype is KRAS. It is one of the four isoform of the RAS gene and it is localized on chromosome 12p12 [60]. RAS proteins are involved in signal transduction. They cycle between two states: active GTP (Guanosine TriPhosphate)-bound and inactive GDP (Guanosine DiPhosphate)-bound. This switch mechanism is controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) leading to the activate and inactivate RAS forms, respectively [61]. GEFs and GAPs interact with an enormous variety of other molecules including also lipids; they regulate RAS status through recruitment of RAS at membrane level in response to P-Tyr-interactions or other signals. RAS proteins change their conformation according to GTP or GDP binding at two switch regions level (dynamics of this binary changes are beyond the scopes of this review) [62]. Interestingly, GDP has a very slow off-rate and this is the reason why RAS holds in an inactive state until a specific biochemical signal produces GEFs activation and thus GDP/GTP exchange. The binding of GTP determines the dissociation of GEFs; when bound to GTP, RAS becomes active and can bind to an effector partner and trigger a signal cascade. Major known RAS effectors are RAF (Rapidly Accelerated Fibrosarcoma) kinases stimulating proliferation, angiogenesis and migration. However, other effector proteins are: PI3K, RalGDS [including novel RAS effector 1A (NORE1A)], Af6, Bry2, phospholipase C (PLC), RAS and Rab interactor 1 (RIN1), T cell lymphoma invasion and metastasis-inducing protein (TIAM), and growth factor receptor 14 (Grb14) [63, 64].
APC
In the developmental analysis of regressive trajectories observed in oligo-metastatic CRC the loss of APC mutations is particularly interesting. It is a very rare phenomenon in poly-metastatic diseases. The APC gene, located on chromosome 5q21-q22, was identified through positional cloning of the familial adenomatous polyposis (FAP) [65]. However, subsequent studies demonstrated that also sporadically occurring CRCs had mutations in both APC alleles. APC is a large protein (312 kDa) interacting with beta-catenin a component of the cadherin adhesion complex and of the Wingless/Wnt pathway. In absence of Wnt signal, beta-catenin is bound to and phosphorilated by the “destruction/degradation complex” [APC, axin, CK1α (casein kinase), and GSK3β (glycogen synthase kinase 3β)]. Phosphorylated beta-catenin determines its ubiquitination [by β-TrCP (Beta-transducin repeats-containing proteins) an ubiquitin-ligase] and, finally, degradation (by proteasome). When Wnt signal is active, the Frizzled receptor turn off the GSK3-beta that cannot phosphorylate β-catenin which stabilizes and shuttles to the nucleus where it activates the TCF/LEF (T-Cell Factor/Lymphoid Enhancer Factor) transcription factor complex involved in promoting the transcription of several target genes including cyclin D1, c-myc, CRD-BP, etc. [66]. In particular, in the bowel, the main β-catenin-dependent transcription factor is TCF4 and its activity is fundamental to maintain the intestinal stem cell compartment [67]. Loss of APC function determines a disruption of the destruction complex and a consequent accumulation of beta-catenin resulting in a pathologic hyper-activation of the above referenced genes involved in cell growth and de-differentiation. Furthermore, APC is able to interact into the nucleus with beta-catenin promoting its removal from specific genomic loci (negative regulation of beta-catenin signal) [68]. However, the regulation of beta-catenin levels and activity are not the only tumorigenic role of APC. The APC C-terminus is involved in many interactions with DNA including i) binding of A/T-rich DNA which blocks entry into or progression through S-phase and ii) chromosomal stability through the proper attachment of metaphase chromosomes to the mitotic spindle. In fact, APC directly associates with kinetochore (through EB1 binding, a microtubule interacting protein), microtubule and centrosome [69, 70]. These functions strongly control DNA integrity and APC-deficient mouse cells have both dramatic quantitative alterations and structural rearrangements of chromosomes. Furthermore, APC localizes also at cell borders by interacting with actin through the scaffolding protein IQGAP1 [71]; this protein mediates APC interaction with Rac1 and Cdc42 (Ras-family GTPases) which in turn regulates actin structure. Additionally, APC participates with the mDia1 (Diaphanous-related formin-1) formin to nucleate actin filament formation and it interacts with both beta-catenin and plakoglobin at cell-cell junctions level [72, 73]: these properties are crucial for cell shape/polarity and migration.
PI3KCA
The hypothesis that the mutational regressive evolution of SMAD4, KRAS and APC could concur to depict the backbone genetic trajectory translating into different clinical behaviors (oligo- versus poly-metastatic CRC) is fascinating and it deserves to be further studied. Much more intuitive is the role of PIK3CA mutations that were gained in all CRC evolving towards to poly-metastatic CRC both in our and previous studies. The PI3KCA gene is located on the long (q) arm of chromosome 3 at position 26.32 and it encodes the p110 alpha (p110α) protein, which is a subunit of the PI3K (phosphatidylinositol 3-kinase) [74]. In fact, PI3K is composed of a heterodimer between a p110 catalytic subunit and a p85 regulatory subunit. It is involved in the phosphorylation of the inositol ring of the membrane-bound phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2), to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3) which participates to the activation of the AKT-dependent signaling cascade [75]. Interestingly, single amino acids changing somatic mutations in the PI3KCA gene are found in many other types of cancer, including cancer of the ovary, breast, lung, brain, and stomach [76], [77], [78]. Two common mutations occur in the same region and change the amino acid glutamate at position 542 or at position 545 of the p110α protein to the amino acid lysine (written as Glu542Lys and Glu545Lys, respectively) [79]. Two other common mutations occur in another region, changing the amino acid histidine at position 1047 of p110α to the amino acid arginine or leucine (written as His1047Arg and His1047Leu, respectively) [80]. Thus, PIK3CA gene mutations in poly-metastatic tumor clones result in production of an altered p110α subunit that allows PI3K to hyper-signal without regulation contributing to trigger malignant cells activities, including proliferation and migration.
Genetic evolution of CRC: role of DNA repair proteins in the border line poly- versus oligo-metastatic disease
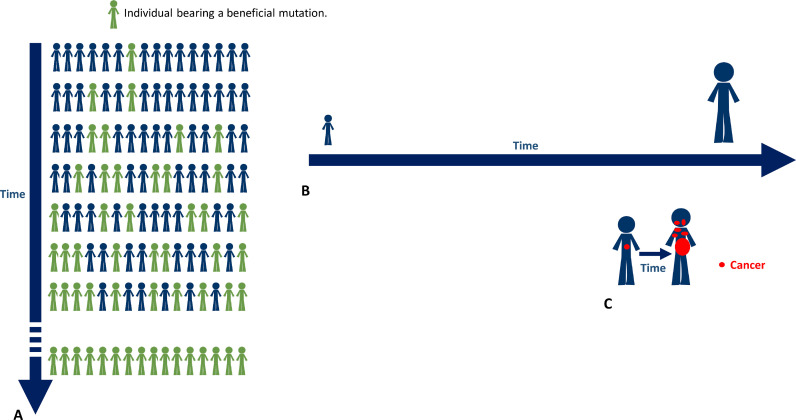
Cancer is a multi-genic disease resulting from the interrelation between genetic and environmental factors. It is well established that cancer development is prompted by DNA mutations which are also the basis of humans’ evolution [81, 82]. In other words, cancer may be intended as a consequence of a physiologically occurring effect (mutations) which is the source of genetic variability in humans. The time is a variable constantly present in mathematical modeling of evolution. The most simple and intuitive evolution model, at sites subject to selection, results in the following equation [83]: considering K as the number of chromosomes (K = 2Nc in a diploid population), Nc the census population size, µ the mutation rate, and P the probability that a mutation spreads and becomes fixed, R, the rate of neutral evolution is 2NcµP. The time enter into this model because μ in a population, assuming no back mutations, is the rate of new mutations in a gene during the time encompassing two generations or, more specifically, the number of non-synonymous mutations per base-pair/generation [84]. In humans, it has been estimated to be approximately 10−4 to 10−6. Thus, there are two types of time to consider in a biological system: “generational” and “individual”. The difference between them is obviously quantitative. In fact, the “generational” time is that encompassing between different generations (Fig. 3A); the “individual” time is the lifetime of a single organism (Fig. 3B). Beneficial mutations intended as stable gain of function-related mutations, displays its fitness effects through thousands of generations. In individuals, the normal mutation rate (intended as the number of non-synonymous somatic mutations accumulated per base-pair/gene during her/his lifetime) is unevaluable, but reasonably extremely low [85]. In fact, eukaryotic cells over hundred millions of years have learned to quickly and effectively repair damages induced by potent cosmic radiations and other mutagens [86, 87]. That is why many researchers use rapidly dividing organisms, as Escherichia Coli or yeast, to trace mutational events (both detrimental and beneficial) induced by environment, drugs, and radiations, to estimate the mutation rate in a short time [88]. In the “individual” biologic setting, the early phases of most malignant cancers are necessarily characterized by a pathologic alteration of genes related to the DNA repair (i.e. p53, MMR genes, BRCA, BAP1, etc.), this is a fundamental condition in order to increase the mutational propensity along with an individual allelic “susceptibility” to cancer. Alterations in genes preserving DNA integrity are found at high frequency when analyzing genetic profiles of primary malignant neoplasms evolving towards poly-metastatic spread; their aberrant function is necessary to activate the switch from an “individual” to “generational” time [89]. The most important is p53. In the late 70 s, TP53 (Tumor Protein 53 kDa) was discovered as a homologous of the simian virus 40 (SV-40) large T antigen able to transform normal cells [90]. Ten years later, it was indicated as the target gene of a frequent deletion involving the 17p chromosome in primary and metastatic CRC. Most of these CRCs had a missense mutation in the remaining allele [91, 92]. These data suggested it was a tumor-suppressor gene. In fact, forced expression of the wild-type protein was able to block oncogene-mediated transformation in many different experimental models. Interestingly, TP53 knockout (TP53−/−) mice or mice bearing loss-of-function mutations develop (with 100% incidence) spontaneous tumors whose histologic features depend on the genetic background and they have a strong susceptibility to carcinogen and γ-irradiation-induced tumors [93]. Notably, TP53 gene is the most commonly mutated gene in a broad variety of cancers and even if not directly altered, most tumors present with a deregulation or epigenetic alteration of TP53 pathway. For instance, TP53 function can be strongly influenced by alteration of its degradation through E3 ubiquitin ligase, MDM2 (Mouse Double Minute 2 homolog) the major negative regulator of TP53. Wild-type p53 is the guardian of the genome playing a critical role in maintaining DNA integrity. In response to DNA damage or other types of “stresses” including ribonucleotide depletion, hypoxia, oxidative stress, wrong hyper-proliferative stimuli, p53 stabilizes in response to MDM2, ATM, ATR and other stress sensors increasing its cellular concentration [94, 95]. After tetramerization, it can bind directly to DNA promoting the transcription of numerous genes involved in cell cycle arrest, apoptosis, and DNA repair. However, the role of p53 is pleiotropic being able to influence several different cellular phenomena including autophagy, metabolic processes, stem cell self-renewal and differentiation. P53 tetramer can undergo many different post-translational modifications (PTMs) (acetylation, methylation, phosphorylation, glycosylation, ubiquitylation, etc.) concurring to regulate its activity (DNA-binding affinity, p53 turnover, etc.) and protein-protein interactions (sites involved in c-ABL, BAX, BAK, etc.) [96]; some mutations may involve amino acid residues involved in PTMs [97]. Somatic mutations of p53 are frequent in CRC and they seem crucial in pushing the evolution towards the complete malignant phenotype and consist mainly on single-base substitutions determining reduction or loss of function [98]. Mismatch repair (MMR) proteins are fundamental to maintain DNA integrity and their transcription in genetically controlled by p53. The mechanism has been well characterized in E. Coli where MutS and MutL are responsible to correct mismatches of newly synthesized DNA [99]. Mammal cells have two homologous of MutS (MSH: MutS Homologue): the heterodimers MSH2-MSH6 and MSH2-MSH3 [100]. The first is able to recognize large deletions and insertions. The second repairs from two to eight nucleotides. Three homologues of MutL (MLH: MutL Homologue) have been identified: MLH1-PMS2 (MutLα), MLH1-PMS1 (MutLβ) and MLH1-MLH3 (MutLγ). The MLH1-PMS2 complex is the most active and it works after MSH2-MSH6 and MSH2-MSH3 intervention. MLH1, MSH2, MLH6, PMS1 and PMS2 (PostMeiotic Segregation 1 and 2) genes are located respectively on chromosome 3p21, 2p16, 2p15, 2q31-q33 and 7p22 [101]. Notably, germline mutations in at least one of the repair genes is found in about 90% of Lynch syndrome patients characterized by an increased risk of developing CRC, as well as cancers of the stomach, small intestine, liver, gallbladder ducts, brain, skin, and prostate, upper urinary tract, endometrial and ovarian cancer [102]. A complete dissertation of MMR genes mutations is beyond the scope of this review, however, PMS2, MLH1 and MLH2 can be affected by genomic rearrangements and point mutations (with missense, nonsense, frameshift and splice junction consequences) presenting in some cases with an autosomal-dominant inheritance [103, 104]. Recent studies have found also germline mutations in the promoter of these genes in patients with Lynch syndrome who were negative for exon mutations [105]. These mutations strongly decrease the activity of MSH2. Furthermore, hyper-methylation of MLH1 (epi‑mutation) promoter, in some cases originating during germination, produce gene silencing and protein function impairment [106].
Fig. 3.
A. The time to fix a single beneficial mutation in a condition of stable environment and no back mutations is about 1000 generations equivalent to about 25,000 years. B. The average life span of humans is about 75 years. C. The evolution of CRC from the precursor to the full and clinical evident malignant progeny is about 5–15 years.
Mutations of these genes confer to cancer a high adaptive evolutionistic power in the heterogeneous and changing surrounding environment. Many neoplastic phenomena (angiogenic switch, immune-system evasion, epithelial to mesenchymal transition, migration, invasion, etc.) take advantage from the gain in a “mutational plasticity” and they represent the manifestation of genetic trajectories evolving towards adaptation and high fitness levels of tumor cells. In fact, very recently, high-throughput genome sequencing techniques (Next Generation Sequencing - NGS) allowing the estimation of the mutation rate (through the evaluation of somatic mutation profiles and comparison with reference statistical classifiers) showed a tumor mutation burden (TMB) of malignant cells ever exceeding that of normal cells. Furthermore, in our previous studies and datasets [41, 42] the TMB of oligo-metastatic tumors was lower than that of poly-metastatic ones. In other words, the genetic dynamics of poly-metastatic disease evolution become similar to that of “generations’ evolution” allowing neoplastic cells to growth and adapt much faster than expected (Fig. 3C).
Immunological selection of tumor cells in an evolutionary perspective
A complete and exhaustive dissertation of relationships between cancer and immune system is beyond the scope of this review. However, recently, we had further demonstration of a fundamental concept from basic researches in oncology and immunology: the immune system response and cancer progression are strictly interrelated. Tumor immune microenvironment (TIM) shapes the clonal diversity of malignant cells in a dynamic process varying in space and time [107, 108]. In space, because of genetic and phenotypic diversity of cancer cells and TIMs among different tumor foci, in time, because of variations at different time points during progression (in the same or different metastatic sites). Interestingly, the more the presence of T-cells within the tumor, the more clonal selection of the tumor increases. Immuno-surveillance determines a continuous expansion and contractions of tumor subclones. High mutation-rate tumors rapidly acquire neoantigens, become immunogenic and undergo to negative immunologic selection. Cancer cells react to lymphocytes pressure i.) with a physiologic upregulation of immune checkpoint molecules and/or, if they growth at high rate, ii.) with a stochastic enrichment of immune-escaped clones [109]. In other words, the clash of immune system with cancer genome instability is crucial to drive cancer evolution towards a metastatic clinical setting. In fact, only immune-escaped clones are able to metastasize and give a progeny. This dynamic concept integrates the “cancer immunoediting” theory [110]. Thus, we can argue that some “immunoevasive” alterations (human leukocyte antigen-I down-regulation, overexpression of PD-1/PD-L1 pathway, transporter associated with antigen processing (TAP)-deficiency, etc.) [111] could directly associate with a metastatic progressive or regressive genotype (mutations in KRAS, SMAD4, PI3KCA, etc.) in the immunologically sculptured cells.
It was also recently reported that the mutational status of some oncogenes in the primary tumor (i.e.: KRAS) can influence the inflammatory status of the tumor and can influence the immune elimination of cancer cells driven by vaccination strategies [112]. Therefore, in addition to the developmental trajectories determined by the immune system of the host, a modification of the inflammatory background could be induced by the tumor itself based upon some initial mutational characteristics of the primary neoplasm. Furthermore, still to date, we cannot exclude that patient immunologic intervention might represent an evolutionistic “push” for CRC (along with intrinsic mutational heterogeneity and chemotherapy). Because of genomic instability, many cancer clones may regain over time the immunologic visibility; these clones are eliminated also in later stage of the disease while surviving residual clones leave the battlefield and reach secondary sites [106, 107]. Furthermore, another reflection can raise from the dynamic TIM interactions and the sculpturing action of lymphocytes. It is commonly observed in clinical practice that cancers with the same tissue origin display some common clinical features in terms of drug sensitivity, prognosis and prevalent organotropism (i.e., ovarian cancers generally metastasize to peritoneum, colon cancers to liver). In other words, tumors derived from the same tissue present a similar clinical course. The immune system response might also contribute to explain these characteristics. The high neoplastic heterogeneity might be counteracted by the lymphocytes whose biological behavior is based on evolutionarily solid and well-established processes. These concepts are important and innovative because they shift the focus of tumor evolution from “neutral developmental dynamics” (chaotic accumulation of genetic mutations) to the integration with a dynamic “immunologic model” triggered by the host.
Conclusions and perspectives
In our opinion, metastatic tumor clones might follow divergent genetic mutational trajectories conferring them different metastatic properties underlying the oligo- versus poly-metastatic clinical behaviours. These effects could be related to tumor genetic heterogeneity as well as to a complex and still unknown clash with immune system. Improvement in knowledge of these developmental dynamics can be pivotal in the comprehension of cancer mechanistic as well as in planning innovative therapeutic approaches. This can be realized through i.) the selection of clean human models to make informative genotype/phenotype correlations, ii.) whole tumoral tissue characterization, and iii.) availability of high-throughput analyses.
CRediT authorship contribution statement
Alessandro Ottaiano: Conceptualization, Data curation, Methodology, Validation, Writing - original draft. Mariachiara Santorsola: Methodology, Validation, Writing - original draft. Michele Caraglia: Conceptualization, Data curation, Writing - original draft. Luisa Circelli: Methodology. Valerio Gigantino: Validation. Gerardo Botti: Writing - original draft, Writing - review & editing. Guglielmo Nasti: Conceptualization, Data curation, Writing - original draft.
Declaration of Competing Interest
There is no conflict of interest to declare.
Acknowledgments
Funding
No funding to declare.
Acknowledgments
We acknowledge the LILT (Lega Italiana per la Lotta contro i Tumori – sezione di Napoli) for the precious collaboration.
References
- 1.Wood L.D., Parsons D.W., Jones S., Lin J., Sjöblom T., Leary R.J. The genomic landscapes of human breast and CRCs. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 2.Lin J., Gan C.M., Zhang X., Jones S., Sjöblom T., Wood L.D. A multidimensional analysis of genes mutated in breast and CRCs. Genome Res. 2007;17:1304–1318. doi: 10.1101/gr.6431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jass J.R. CRC a multipathway disease. Crit. Rev. Oncog. 2006;12:273–287. doi: 10.1615/critrevoncog.v12.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 4.Bastos P., Gomes T., Ribeiro L. Catechol-O-Methyltransferase (COMT): an update on its role in cancer, neurological and cardiovascular diseases. Rev. Physiol. Biochem. Pharmacol. 2017;173:1–39. doi: 10.1007/112_2017_2. [DOI] [PubMed] [Google Scholar]
- 5.Balzan S., Lubrano V. LOX-1 receptor: a potential link in atherosclerosis and cancer. Life Sci. 2018;198:79–86. doi: 10.1016/j.lfs.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang L., Zhang K., Chen J., Wang J., Huang H. Roles of platelet derived growth factor in vascular calcification. J. Cell. Physiol. 2018;233:2804–2814. doi: 10.1002/jcp.25985. [DOI] [PubMed] [Google Scholar]
- 7.Ottaiano A., De Divitiis C., Capozzi M., Avallone A., Pisano C., Pignata S. Obesity and cancer: biological links and treatment implications. Curr Cancer Drug Targets. 2018;18:231–238. doi: 10.2174/1568009617666170330125619. [DOI] [PubMed] [Google Scholar]
- 8.Nechama M., Kwon J., Wei S., Kyi A.T., Welner R.S., Ben-Dov I.Z. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity inIL-33-induced allergic airway inflammation. Nat. Commun. 2018;9:1603. doi: 10.1038/s41467-018-03886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marenholz I., Esparza-Gordillo J., Rüschendorf F., Bauerfeind A., Strachan D.P., Spycher B.D. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat. Commun. 2015;6:8804. doi: 10.1038/ncomms9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozłowska R., Bożek A., Jarząb J. Association between cancer and allergies. Allergy Asthma Clin. Immunol. 2016;12:39. doi: 10.1186/s13223-016-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou W.Y., Lai P.Y., Hu J.M., Hsu C.H., Chen Y.C., Tian Y.F. Association between atopic dermatitis and CRC risk: a nationwide cohort study. Medicine (Baltimore) 2020;99:e18530. doi: 10.1097/MD.0000000000018530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allavena P., Garlanda C., Borrello M.G., Sica A., Mantovani A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Borrello M.G., Degl'Innocenti D., Pierotti M.A. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2018;267:262–270. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 14.Marazzi I., Greenbaum B.D., Low D.H.P., Guccione E. Chromatin dependencies in cancer and inflammation. Nat. Rev. Mol. Cell Biol. 2018;19:245–261. doi: 10.1038/nrm.2017.113. [DOI] [PubMed] [Google Scholar]
- 15.Brannon A.R., Vakiani E., Sylvester B.E., Scott S.N., McDermott G., Shah R.H. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic CRC lesions. Genome Biol. 2014;15:454. doi: 10.1186/s13059-014-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.Y., Haq F., Kim D., Jun C., Jo H., Ahn S. Comparative genomic analysis of primary and synchronous metastatic CRCs. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R., Schell M.J., Teer J.K., Greenawalt D.M., Yang M., Yeatman T.J. Co-evolution of somatic variation in primary and metastatic CRC may expand biopsy indications in the molecular era. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0126670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignot S., Lefebvre C., Frampton G.M., Meurice G., Yelensky R., Palmer G. Comparative analysis of primary tumour and matched metastases in CRC patients: evaluation of concordance between genomic and transcriptional profiles. Eur. J. Cancer. 2015;51:791–799. doi: 10.1016/j.ejca.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Kovaleva V., Geissler A.L., Lutz L., Fritsch R., Makowiec F., Wiesemann S. Spatio-temporal mutation profiles of case-matched colorectal carcinomas and their metastases reveal unique de novo mutations in metachronous lung metastases by targeted next generation sequencing. Mol. Cancer. 2016;15:63. doi: 10.1186/s12943-016-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtius K., Wright N.A., Graham T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer. 2018;18:19–32. doi: 10.1038/nrc.2017.102. [DOI] [PubMed] [Google Scholar]
- 22.Sak K. The Val158Met polymorphism in COMT gene and cancer risk: role of endogenous and exogenous catechols. Drug Metab. Rev. 2017;49:56–83. doi: 10.1080/03602532.2016.1258075. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J., Yan M., Mehta J.L., Hu C. Angiogenesis is a link between atherosclerosis and tumorigenesis: role of LOX-1. Cardiovasc. Drugs Ther. 2011;25:461–468. doi: 10.1007/s10557-011-6343-3. [DOI] [PubMed] [Google Scholar]
- 24.Kashfi K., Rosen C.L., Aslan M. Obesity, type-2 diabetes and cancer: mechanistic insights. Crit. Rev. Oncog. 2019;24:285–305. doi: 10.1615/CritRevOncog.2019032959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner M.C. Epidemiology: allergy history, IgE, and cancer. Cancer Immunol. Immunother. 2012;61:1493–1510. doi: 10.1007/s00262-011-1180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T., Suo C., Zheng C., Zhang H. Hypoxia and metabolism in metastasis. Adv. Exp. Med. Biol. 2019;1136:87–95. doi: 10.1007/978-3-030-12734-3_6. [DOI] [PubMed] [Google Scholar]
- 27.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer. 2016;138:1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marazzi I., Greenbaum B.D., Low D.H.P., Guccione E. Chromatin dependencies in cancer and inflammation. Nat. Rev. Mol. Cell Biol. 2008;19:245–261. doi: 10.1038/nrm.2017.113. [DOI] [PubMed] [Google Scholar]
- 29.Lu J., Mitra S., Wang X., Khaidakov M., Mehta J.L. Oxidative stress and lectin-like Ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid. Redox. Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch H.A., Iliopoulos D., Joshi A., Zhang Y., Jaeger S.A., Bulyk M. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010;17:34861. doi: 10.1016/j.ccr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang M., Zhang P., Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Li D., Mehta J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 33.Hu C., Dandapat A., Mehta J.L. Angiotensin II induces capillary formation from endothelial cells via the LOX-1 dependent redox-sensitive pathway. Hypertension. 2007;50:952–957. doi: 10.1161/HYPERTENSIONAHA.107.096446. [DOI] [PubMed] [Google Scholar]
- 34.Khaidakov M., Szwedo J., Mitra S., Ayyadevara S., Dobretsov M., Lu J. Antiangiogenic and antimitotic effects of aspirin in hypoxia–reoxygenation modulation of the LOX-1-NADPH oxidase axis as a potential mechanism. J. Cardiovasc. Pharmacol. 2010;56:635–641. doi: 10.1097/FJC.0b013e3181f801e4. [DOI] [PubMed] [Google Scholar]
- 35.Kanata S., Akagi M., Nishimura S., Hayakawa S., Yoshida K., Sawamura T. Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochem. Biophys. Res. Commun. 2006;348:1003–1010. doi: 10.1016/j.bbrc.2006.07.133. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.J., Lee I.K., Jeon J.H. Vascular calcification-new insights into its mechanism. Int. J. Mol. Sci. 2020;21:2685. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma D., Brummel, -Ziedins K.E., Bouchard B.A., Holmes C.E. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J. Cell. Physiol. 2014;229:1005–1015. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 38.Neel N.F., Martin T.D., Stratford J.K., Zand T.P., Reiner D.J., Der C.J. The RalGEF-Ral effector signaling network: the road less traveled for anti-ras drug discovery. Genes. Cancer. 2011;2:275–287. doi: 10.1177/1947601911407329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kortlever R.M., Sodir N.M., Wilson C.H., Burkhart D.L., Pellegrinet L., Swigart L.B. Myc cooperates with Ras by programming inflammation and immune suppression. Cell. 2017;171:1301–1315. doi: 10.1016/j.cell.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulligan L.M. RET revisited: expanding the oncogenic portfolio. Nat. Rev. Cancer. 2014;14:173–186. doi: 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- 41.Ottaiano A., Circelli L., Lombardi A., Scala S., Martucci N., Galon J. Genetic trajectory and immune microenvironment of lung-specific oligometastatic CRC. Cell Death Dis. 2020;11:275. doi: 10.1038/s41419-020-2480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottaiano A., Caraglia M., Di Mauro A., Botti G., Lombardi A., Galon J. Evolution of mutational landscape and tumor immune-microenvironment in liver oligo-metastatic CRC. Cancers (Basel) 2020;12:3073. doi: 10.3390/cancers12103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ottaiano A., Nasti G., Santorsola M., Altieri V., Di Fruscio G., Circelli L. KRAS mutational regression is associated with oligo-metastatic status and good prognosis in metastatic colorectal cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.632962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellman S., Weichselbaum R.R. Oligometastases. J. Clin. Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 45.Niibe Y., Chang J.Y., Onishi H., Salama J., Hiraki T., Yamashita H. Oligometastases/oligo-recurrence of lung cancer. J. Clin. Oncol. 2014;13:8–10. [Google Scholar]
- 46.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother. Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Withers H.R., Lee S.P. Modeling growth kinetics and statistical distribution of oligometastases. Semin. Radiat. Oncol. 2016;16:111–119. doi: 10.1016/j.semradonc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Lussier Y.A., Khodarev N.N., Regan K., Corbin K., Li H., Ganai S. Oligo -and polymetastatic progression in lungmetastasis(es) patients is associated with specific MicroRNAs. PLoS ONE. 2012;7:e50141. doi: 10.1371/journal.pone.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 50.Pitroda S.P., Khodarev N.N., Huang L., Uppal A., Wightman S.C., Ganai S. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat. Commun. 2018;9:1793. doi: 10.1038/s41467-018-04278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caja L., Dituri F., Mancarella S., Caballero-Diaz D., Moustakas A., Giannelli G. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int. J. Mol. Sci. 2018;19:1294. doi: 10.3390/ijms19051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazono K., Katsuno Y., Koinuma D., Ehata S., Morikawa M. Intracellular and extracellular TGF-β signaling in cancer: some recent topics. Front. Med. 2018;12:387–411. doi: 10.1007/s11684-018-0646-8. [DOI] [PubMed] [Google Scholar]
- 53.Katsuno Y., Lamouille S., Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr. Opin. Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 54.Jung B., Staudacher J.J., Beauchamp D. Transforming growth factor β superfamily signaling in development of CRC. Gastroenterology. 2017;152:36–52. doi: 10.1053/j.gastro.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampropoulos P., Zizi-Sermpetzoglou A., Rizos S., Kostakis A., Nikiteas N., Papavassiliou A.G. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012;314:1–7. doi: 10.1016/j.canlet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 56.Bellam N., Pasche B. Tgf-beta signaling alterations and colon cancer. Cancer Treat. Res. 2010;155:85–103. doi: 10.1007/978-1-4419-6033-7_5. [DOI] [PubMed] [Google Scholar]
- 57.Kawaguchi Y., Kopetz S., Newhook T.E., De Bellis M., Chun Y.S., Tzeng C.D. Mutation status of RAS, TP53, and SMAD4 is superior to mutation status of RAS alone for predicting prognosis after resection of colorectal liver metastases. Clin. Cancer Res. 2019;25:5843–5851. doi: 10.1158/1078-0432.CCR-19-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oyanagi H., Shimada Y., Nagahashi M., Ichikawa H., Tajima Y., Abe K. SMAD4 alteration associates with invasive-front pathological markers and poor prognosis in CRC. Histopathology. 2019;74:873–882. doi: 10.1111/his.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarshekeh A.M., Advani S., Overman M., Manyam G., Kee B.K., Fogelman D.R. Association of SMAD4 mutation with patient demographics, tumor characteristics, and clinical outcomes in CRC. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0173345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uprety D., Adjei A.A.KRAS. From undruggable to a druggable Cancer Target. Cancer Treat. Rev. 2020;89 doi: 10.1016/j.ctrv.2020.102070. [DOI] [PubMed] [Google Scholar]
- 61.Hennig A., Markwart R., Esparza-Franco M.A., Ladds G., Rubio I. Ras activation revisited: role of GEF and GAP systems. Biol. Chem. 2015;396:831–848. doi: 10.1515/hsz-2014-0257. [DOI] [PubMed] [Google Scholar]
- 62.Gasper R., Wittinghofer F. The Ras switch in structural and historical perspective. Biol. Chem. 2019;401:143–163. doi: 10.1515/hsz-2019-0330. [DOI] [PubMed] [Google Scholar]
- 63.Pálfy G., Menyhárd D.K., Perczel A. Dynamically encoded reactivity of Ras enzymes: opening new frontiers for drug discovery. Cancer Metastasis Rev. 2020 doi: 10.1007/s10555-020-09917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casalou C., Ferreira A., Barral D.C. The role of ARF family proteins and their regulators and effectors in cancer progression: a therapeutic perspective. Front. Cell. Dev. Biol. 2020;8:217. doi: 10.3389/fcell.2020.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L., Shay J.W. Multiple roles of APC and its therapeutic implications in CRC. J. Natl. Cancer Inst. 2017;109:djw332. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanagan D.J., Vincan E., Phesse T.J. Wnt signaling in cancer: not a binary ON: OFF switch. Cancer Res. 2019;79:5901–5906. doi: 10.1158/0008-5472.CAN-19-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hrckulak D., Kolar M., Strnad H., Korinek V. TCF/LEF transcription factors: an update from the internet resources. Cancers (Basel) 2016;8:70. doi: 10.3390/cancers8070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verzi M.P., Hatzis P., Sulahian R., Philips J., Schuijers J., Shin H. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15157–15162. doi: 10.1073/pnas.1003822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S.K., Hwang J.H., Choi K. Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis. Adv. Biol. Regul. 2018;68:46–54. doi: 10.1016/j.jbior.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Sansregret L., Patterson J.O., Dewhurst S., López-García C., Koch A., McGranahane N. APC/C dysfunction limits excessive cancer chromosomal instability. Cancer Discov. 2017;7:218–233. doi: 10.1158/2159-8290.CD-16-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Islam F., Chaousis S., Wahab R., Gopalan V., Lam A.K. Protein interactions of FAM134B with EB1 and APC/beta-catenin in vitro in colon carcinoma. Mol. Carcinog. 2018;57:1480–1491. doi: 10.1002/mc.22871. [DOI] [PubMed] [Google Scholar]
- 72.Tirnauer J.S. A new cytoskeletal connection for APC: linked to actin through IQGAP. Dev. Cell. 2004;7:778–780. doi: 10.1016/j.devcel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Bartolini F., Moseley J.B., Schmoranzer J., Cassimeris L., Goode B.L., Gundersen G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papadatos-Pastos D., Rabbie R., Ross P., Sarker D. The role of the PI3K pathway in CRC. Crit. Rev. Oncol. Hematol. 2015;94:18–30. doi: 10.1016/j.critrevonc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barault L., Veyrie N., Jooste V., Lecorre D., Chapusot C., Ferraz J.M. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int. J. Cancer. 2008;122:2255–2259. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 77.Voutsina A., Tzardi M., Kalikaki A., Zafeiriou Z., Papadimitraki E., Papadakis M. Combined analysis of KRAS and PIK3CA mutations, MET and PTEN expression in primary tumors and corresponding metastases in CRC. Mod. Pathol. 2013;26:302–313. doi: 10.1038/modpathol.2012.150. [DOI] [PubMed] [Google Scholar]
- 78.Kim S.T., Ahn T.J., Lee E., Do I.G., Lee S.J., Park S.H. Exploratory biomarker analysis for treatment response in KRAS wild type metastatic CRC patients who received cetuximab plus irinotecan. BMC Cancer. 2015;15:747. doi: 10.1186/s12885-015-1759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guedes J.G., Veiga I., Rocha P., Pinto P., Pinto C., Pinheiro M. High resolution melting analysis of KRAS, BRAF and PIK3CA in KRAS exon 2 wild-type metastatic CRC. BMC Cancer. 2013;13:169. doi: 10.1186/1471-2407-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao L., Vogt P.K. Hot-spot mutations in p110alpha of phosphatidylinositol 3-kinase (pI3K): differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle. 2010;9:596–600. doi: 10.4161/cc.9.3.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reiter J.G., Baretti M., Gerold J.M., Makohon-Moore A.P., Daud A., Iacobuzio-Donahuee C.A. An analysis of genetic heterogeneity in untreated cancers. Nat. Rev. Cancer. 2019;19:639–650. doi: 10.1038/s41568-019-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lynch M., Ackerman M.S., Gout J.F., Long H., Sung W., Thomase W.K. Genetic drift, selection and the evolution of the mutation rate. Nat. Rev. Genet. 2016;17:704–714. doi: 10.1038/nrg.2016.104. [DOI] [PubMed] [Google Scholar]
- 84.Lanfear R., Kokko H., Eyre-Walker A. Population size and the rate of evolution. Trends Ecol. Evol. 2014;29:33–41. doi: 10.1016/j.tree.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Scally A. The mutation rate in human evolution and demographic inference. Curr. Opin. Genet. Dev. 2016;41:36–43. doi: 10.1016/j.gde.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Liu D., Keijzers G., Rasmussen L.J. DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. 2017;773:174–187. doi: 10.1016/j.mrrev.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Frigola J., Sabarinathan R., Mularoni L., Muiños F., Gonzalez-Perez A., López-Bigas N. Reduced mutation rate in exons due to differential mismatch repair. Nat. Genet. 2017;49:1684–1692. doi: 10.1038/ng.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thoma F. Light and dark in chromatin repair: repair of UV-induced DNA lesions by photolyase and nucleotide excision repair. EMBO J. 1999;18:6585–6598. doi: 10.1093/emboj/18.23.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lenski R.E. Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J. 2017;11:2181–2194. doi: 10.1038/ismej.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levine A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer. 2020;20:471–480. doi: 10.1038/s41568-020-0262-1. [DOI] [PubMed] [Google Scholar]
- 91.Nakayama M., Oshima M. Mutant p53 in colon cancer. J. Mol. Cell Biol. 2019;11:267–276. doi: 10.1093/jmcb/mjy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakayama M., Sakai E., Echizen K., Yamada Y., Oshima H., Han T.S. Intestinal cancer progression by mutant p53 through the acquisition of invasiveness associated with complex glandular formation. Oncogene. 2017;36:5885–5896. doi: 10.1038/onc.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taneja P., Zhu S., Maglic D., Fry E.A., Kendig R.D., Inoue K. Transgenic and knockout mice models to reveal the functions of tumor suppressor genes. Clin. Med. Insights Oncol. 2011;5:235–257. doi: 10.4137/CMO.S7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee J.T., Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010;17:86–92. doi: 10.1038/cdd.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saadatzadeh M.R., Elmi A.N., Pandya P.H., Bijangi-Vishehsaraei K., Ding J., Stamatkin C.W. The role of MDM2 in promoting genome stability versus instability. Int. J. Mol. Sci. 2017;18:2216. doi: 10.3390/ijms18102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollstein M., Hainaut P. Massively regulated genes: the example of TP53. J. Pathol. 2010;220:164–173. doi: 10.1002/path.2637. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen T.A., Menendez D., Resnick M.A., Anderson C.W. Mutant TP53 posttranslational modifications: challenges and opportunities. Hum. Mutat. 2014;35:738–755. doi: 10.1002/humu.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naccarati A., Polakova V., Pardini B., Vodickova L., Hemminki K., Kumar R. Mutations and polymorphisms in TP53 gene–an overview on the role in CRC. Mutagenesis. 2012;27:211–218. doi: 10.1093/mutage/ger067. [DOI] [PubMed] [Google Scholar]
- 99.Modrich P. Mechanisms in E. coli and human mismatch repair (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2016;55:8490–8501. doi: 10.1002/anie.201601412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sameer A.S., Nissar S., Fatima K. Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis. Eur. J. Cancer Prev. 2014;23:246–257. doi: 10.1097/CEJ.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 101.Pećina-Šlaus N., Kafka A., Salamon I., Bukovac A. Mismatch repair pathway, genome stability and cancer. Front. Mol. Biosci. 2020;7:122. doi: 10.3389/fmolb.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cerretelli G., Ager A., Arends M.J., Frayling I.M. Molecular pathology of Lynch syndrome. J. Pathol. 2020;250:518–531. doi: 10.1002/path.5422. [DOI] [PubMed] [Google Scholar]
- 103.Liu X., Yang H., Wu X., Huang K., Ma P., Jiang P. Molecular mutation characteristics of mismatch and homologous recombination repair genes in gastrointestinal cancer. Oncol. Lett. 2019;18:2789–2798. doi: 10.3892/ol.2019.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montazer Haghighi M., Radpour R., Aghajani K., Zali N., Molaei M., Zali M.R. Four novel germline mutations in the MLH1 and PMS2 mismatch repair genes in patients with hereditary nonpolyposis CRC. Int. J. Colorectal Dis. 2009;24:885–893. doi: 10.1007/s00384-009-0731-1. [DOI] [PubMed] [Google Scholar]
- 105.Loukola A., Vilkki S., Singh J., Launonen V., Aaltonen L.A. Germline and somatic mutation analysis of MLH3 in MSI-positive CRC. Am. J. Pathol. 2000;157:347–352. doi: 10.1016/S0002-9440(10)64546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ward R.L., Dobbins T., Lindor N.M., Rapkins R.W., Hitchins M.P. Identification of constitutional MLH1 epimutations and promoter variants in CRC patients from the colon cancer family registry. Genet. Med. 2013;15:25–35. doi: 10.1038/gim.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang A.W., McPherson A., Milne K., Kroeger D.R., Hamilton P.T., Miranda A. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173:1755–1769. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 108.Angelova M., Mlecnik B., Vasaturo A., Bindea G., Fredriksen T., Lafontaine L. et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765. doi: 10.1016/j.cell.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Lakatos E., Williams M.J., Schenck R.O. Evolutionary dynamics of neoantigens in growing tumors. Nat. Genet. 2020;52:1057–1066. doi: 10.1038/s41588-020-0687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 111.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:185–198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Correale P., Botta C., Staropoli N., Nardone V., Pastina P., Ulivieri C. Systemic inflammatory status predict the outcome of k-RAS WT metastatic CRC patients receiving the thymidylate synthase poly-epitope-peptide anticancer vaccine. Oncotarget. 2018;9:20539–20554. doi: 10.18632/oncotarget.24993. [DOI] [PMC free article] [PubMed] [Google Scholar]