Highlights
-
•
In patients with ALK-rearranged NSCLC who received lorlatinib within the compassionate use program, the objective tumor response (OR) and disease control (DC) were observed in 43% and 94% cases, respectively.
-
•
Lorlatinib showed particularly high efficacy against brain metastases, with OR and DC for intracranial disease reaching 81% and 100%, respectively.
-
•
Patients with V.1 and V.3 ALK translocations had similar response to the therapy.
-
•
Complete lack of adverse events tended to correlate with poor outcome of lorlatinib treatment.
Keywords: Non-small cell lung cancer, ALK rearrangements, Lorlatinib, Brain metastases, Review
Abstract
Background
Lorlatinib is a novel potent ALK inhibitor, with only a few studies reporting the results of its clinical use.
Methods
This study describes the outcomes of lorlatinib treatment for 35 non-small cell lung cancer patients with ALK rearrangements, who had 2 (n = 5), 1 (n = 26) or none (n = 4) prior tyrosine kinase inhibitors and received lorlatinib mainly within the compassionate use program.
Results
Objective tumor response (OR) and disease control (DC) were registered in 15/35 (43%) and 33/35 (94%) patients, respectively; brain metastases were particularly responsive to the treatment (OR: 22/27 (81%); DC: 27/27 (100%)). Median progression free survival (PFS) was estimated to be 21.8 months, and median overall survival (OS) approached to 70.1 months. Only 4 out of 35 patients experienced no adverse effects; two of them were the only subjects who had no clinical benefit from lorlatinib. PFS and OS in the no-adverse-events lorlatinib users were strikingly lower as compared to the remaining patients (1.1 months vs. 23.7 months and 10.5 months vs. not reached, respectively; p < 0.0001 for both comparisons). ALK translocation variants were known for 28 patients; there was no statistical difference between patients with V.1 and V.3 rearrangements with regard to the OS or PFS.
Conclusion
Use of lorlatinib results in excellent disease outcomes, however caution must be taken for patients experiencing no adverse effects from this drug.
Introduction
ALK and ROS1 gene fusions account for 5–8% and 1–2% of non-small cell lung carcinomas (NSCLCs), respectively [1,2]. The invention of crizotinib led to a breakthrough in the treatment of these categories of patients, given that virtually all subjects with ALK/ROS1-rearranged NSCLC derive clinical benefit from this drug [3,4]. However, the efficacy of crizotinib, which was originally developed as a MET kinase inhibitor, is compromised by several factors [1,2,5]. Crizotinib is somewhat less potent as compared to newer ALK/ROS1-targeted drugs. Some of the tumors, which are exposed to crizotinib, escape from the therapy by developing secondary mutations in the ALK or ROS1 genes. Furthermore, crizotinib poorly penetrates through blood-brain barrier, therefore a significant portion of crizotinib-treated patients develop brain metastases. There is a number of novel tyrosine kinase inhibitors (TKIs), which were designed to address these disadvantages. In particular, studies on ALK-driven cancers demonstrated significant activity of ceritinib, alectinib, brigatinib and lorlatinib in crizotinib-treated and TKI-naïve NSCLCs [6], [7], [8], [9], [10], [11], [12]. ROS1-rearranged NSCLCs showed sensitivity to ceritinib, lorlatinib, entrectinib and some other TKIs in several clinical trials [1,12,[13], [14], [15]].
Lorlatinib (PF-06463922) is a potent ALK/ROS1-selective inhibitor, which retains activity against some ALK/ROS1 resistance mutations acquired during prior TKI therapy and is characterized by good penetration into the brain. Several studies demonstrated high efficacy of lorlatinib in both heavily pretreated and TKI-naïve NSCLCs [12,[16], [17], [18], [19], [20]]. Lin et al. [21] reported that lorlatinib renders significantly longer progression-free survival for patients, whose NSCLCs carry the variant 3 (V.3) of the ALK rearrangement; this phenomenon was explained by the property of ALK V.3 associated carcinomas to develop secondary ALK G1202R mutations, which are resistant to the majority of conventional TKIs, but are sensitive to lorlatinib [21]. Lorlatinib was accessible in Russia within years 2017–2019 mainly within the compassionate use program. Here we report a single-center experience оf the use of lorlatinib in ALK-rearranged NSCLC with the emphasis on ALK variant-specific disease outcomes.
Patients and methods
The patients were receiving lorlatinib therapy in the I.P. Pavlov Medical University (St.-Petersburg, Russia). The study included 35 subjects with ALK-rearranged NSCLC, with the first patients starting to receive this drug in March 2017 and the last person included in the lorlatinib treatment in December 2019. The mean age of the patients was 46.7 ± 2.3 years (range: 24–80 years). The median follow-up time, defined as the interval between the start of the therapy and the death or the date of the data cut-off (June 15, 2020), was equal to 17.5 months. Thirteen of these cases were submitted previously to the study of Peled et al. [22], with the data cut-off for these subjects being January 2019. In 12 out of 35 cases, lorlatinib dose reduction to 75 mg (n = 4) or 50 mg (n = 8) was required due to toxicity of the drug. Twenty eight patients provided to the study tumor samples; these tissues were subjected to ALK translocation variant genotyping by RT-PCR-based method as described by Iyevleva et al. [23]. Seven patients failed to preserve relevant biological material for ALK genetic testing, therefore they were included in the study based on the data from medical records (ALK fusion identified by FISH (n = 5) or IHC (n = 2)).
Treatment efficacy was evaluated using commonly accepted criteria for tumor response (RECIST), progression-free survival (PFS) and overall survival (OS). Adverse events were documented and graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Statistical analysis was done using MedCalc Statistical Software version 19.1.3. PFS (defined as the time from the start of lorlatinib therapy to disease progression or death) and OS (defined as the time from the diagnosis to death) were analyzed by Kaplan-Meier method and log-rank test. For PFS and OS analysis, patients without progression on lorlatinib or being alive at the end of the study were censored at the date of data cut-off. Duration of therapy, defined as the time interval between the start of lorlatinib treatment and the discontinuation of its use, was analyzed by Kaplan-Meier method.
Results
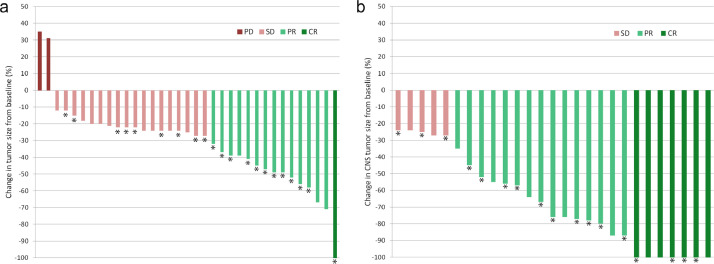
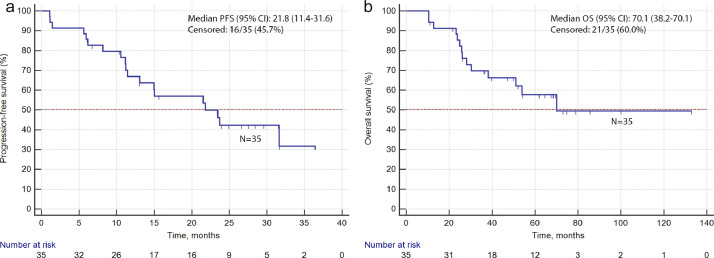
Characteristics of included patients are given in Table 1. Objective tumor response was observed in 15/35 patients (43%); the disease control was registered in 33/35 (94%) cases (Fig. 1). Twenty seven patients presented with intracranial metastases at the time of lorlatinib treatment; 22 (81%) showed objective response for brain metastatic lesions. Median PFS was estimated to be 21.8 months, and median OS was equal to 70.1 months (Fig. 2). Adverse events were observed in 31/35 (89%) patients; the most frequent adverse events were hypercholesterolemia (20 cases) and edema (13 cases).
Table 1.
Characteristics of NSCLC patients treated by lorlatinib and the treatment outcome.
| Characteristics of the patients | Treatment outcome |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients within a subgroup | CR | PR | SD | PD | Objective response (CR + PR) | Clinical benefit (CR + PR + SD) | Median PFS [95% CI] | Median OS [95% CI] | |
| Gender | N = 35 | ||||||||
| Male | 16 | 1 | 4 | 10 | 1 | 5 (31%) | 15 (94%) | 21.5 [6.2–23.7] | NR |
| Female | 19 | 0 | 10 | 8 | 1 | 10 (53%) | 18 (95%) | 23.5 [11.2–31.6] | 70.1 [26.0–70.1] |
| ALK fusion variant | N = 35 | ||||||||
| EML4ex13/ALKex20 (V.1) | 13 | 0 | 7 | 5 | 1 | 7 (54%) | 12 (92%) | 21.8 [8.2–23.7] | NR |
| EML4ex20/ALKex20 (V.2) | 4 | 1 | 1 | 2 | 0 | 2 (50%) | 4 (100%) | NR | NR |
| EML4ex6/ALKex20 (V.3) | 8 | 0 | 3 | 5 | 0 | 3 (38%) | 8 (100%) | NR | 54.0 [30.3–54.0] |
| Rare variants | 2 | 0 | 1 | 1 | 0 | 1 (50%) | 2 (100%) | 5.6 [5.6–23.5] | 51.2 [51.2–70.1] |
| Unknown | 8 | 0 | 2 | 5 | 1 | 2 (25%) | 7 (88%) | 13.1 [1.1–31.6] | NR |
| Treatment line | N = 35 | ||||||||
| 1st line | 2 | 0 | 1 | 1 | 0 | 1 (50%) | 2 (100%) | NR | NR |
| 2nd line | 16 | 1 | 6 | 8 | 1 | 7 (44%) | 15 (94%) | 15.0 [8.2–23.7] | 51.2 [25.8–51.2] |
| 3rd line | 12 | 0 | 5 | 7 | 0 | 5 (42%) | 12 (100%) | 31.6 [11.1–31.6] | NR |
| 4th line | 5 | 0 | 2 | 2 | 1 | 2 (40%) | 4 (80%) | 21.5 [1.1–21.8] | NR |
| Number of prior TKIs | N = 35 | ||||||||
| None | 4 | 1 | 1 | 2 | 0 | 2 (50%) | 4 (100%) | NR | NR |
| 1 TKI | 26 | 0 | 11 | 14 | 1 | 11 (42%) | 25 (96%) | 21.5 [11.1–23.7] | NR |
| 2 TKIs | 5 | 0 | 2 | 2 | 1 | 2 (40%) | 4 (80%) | 23.5 [1.1–31.6] | 70.1 [10.5–70.1] |
| Prior TKI treatment | N = 35 | ||||||||
| None | 4 | 1 | 1 | 2 | 0 | 2 (50%) | 4 (100%) | NR | NR |
| Crizotinib only | 12 | 0 | 7 | 4 | 1 | 7 (58%) | 11 (92%) | 23.7 [8.2–23.7] | NR |
| Ceritinib only | 14 | 0 | 4 | 10 | 0 | 4 (29%) | 14 (100%) | 15.0 [6.2–21.8] | NR |
| Crizotinib and other TKIsa | 5 | 0 | 2 | 2 | 1 | 2 (40%) | 4 (80%) | 23.5 [1.1–31.6] | 70.1 [10.5–70.1] |
| Prior chemotherapy | N = 35 | ||||||||
| No | 20 | 0 | 9 | 10 | 1 | 9 (45%) | 19 (95%) | 14.9 [10.6–31.6] | 70.1 [26.0–70.1] |
| Yes | 15 | 1 | 5 | 8 | 1 | 6 (40%) | 14 (93%) | NR | NR |
| Prior therapy | N = 35 | ||||||||
| None | 2 | 0 | 1 | 1 | 0 | 1 (50%) | 2 (100%) | NR | NR |
| Chemotherapy only | 2 | 1 | 0 | 1 | 0 | 1 (50%) | 2 (100%) | NR | NR |
| Crizotinib (with or without chemotherapy) | 12 | 0 | 7 | 4 | 1 | 7 (58%) | 11 (92%) | 23.7 [8.2–23.7] | NR |
| Ceritinib (with or without chemotherapy) | 14 | 0 | 4 | 10 | 0 | 4 (29%) | 14 (100%) | 15.0 [6.2–21.8] | NR |
| Crizotinib and ceritinib (with or without chemotherapy) | 3 | 0 | 2 | 1 | 0 | 2 (67%) | 3 (100%) | 23.5 [1.1–31.6] | 70.1 [12.8–70.1] |
| Crizotinib and alectinib (with or without chemotherapy) | 2 | 0 | 0 | 1 | 1 | 0 (0%) | 1 (50%) | 1.1 [1.1–1.1] | 10.5 [10.5–10.5] |
| CNS metastases | N = 35 | ||||||||
| Absent | 8 | 1 | 2 | 3 | 2 | 3 (33%) | 6 (75%) | 11.1 [1.1–11.1] | 23.2 [10.5–23.2] |
| Present | 27 | 0 | 12 | 15 | 0 | 12 (44%) | 27 (100%) | 23.5 [13.1–31.6] | NR |
| Adverse events | N = 35 | ||||||||
| Absent | 4b | 0 | 1 | 1 | 2b | 1 (25%) | 2 (50%) | 1.1 [1.1–8.2] | 10.5 [10.5–25.7] |
| Present | 31 | 1 | 13 | 17 | 0 | 14 (45%) | 31 (100%) | 23.7 [15.0–31.6] | NR |
| Types of adverse events | N = 35 | ||||||||
| Hypercholesterolemia | 20 | 0 | 7 | 13 | 0 | 7 (35%) | 20 (100%) | 31.6 [11.4–31.6] | NR |
| Edema | 13 | 1 | 6 | 6 | 0 | 7 (54%) | 13 (100%) | 23.5 [11.1–23.5] | 70.1 [23.8–70.1] |
| Weight gain | 4 | 0 | 4 | 0 | 0 | 4 (100%) | 4 (100%) | 31.6 [nd] | NR |
| Peripheral neuropathy | 2 | 0 | 0 | 2 | 0 | 0 (0%) | 2 (100%) | 11.1 [11.1–11.1] | 23.2 [23.2–23.2] |
| Psychosis | 2 | 0 | 2 | 0 | 0 | 2 (100%) | 2 (100%) | 21.8 [21.8–23.7] | NR |
| Hypercreatinemia | 1 | 0 | 1 | 0 | 0 | 1 (100%) | 1 (100%) | – | – |
| Pleuritis | 1 | 0 | 1 | 0 | 0 | 1 (100%) | 1 (100%) | – | – |
| Tumor response by RECIST (total) | N = 35 | 1 (3%) | 14 (40%) | 18 (51%) | 2 (6%) | 15 (43%) | 33 (94%) | 21.8 [11.4–31.6] | 70.1 [38.2–70.1] |
| CNS response | N = 27 | 7 (26%) | 15 (56%) | 5 (19%) | 0 (0%) | 22 (81%) | 27 (100%) | 23.5 [13.1–31.6] | NR |
Abbreviations: CR – complete response; NR - not reached; OS – overall survival; PD – progressive disease; PFS – progression-free survival; PR – partial response; SD – stable disease.
Crizotinib and ceritinib: 3; crizotinib and alectinib: 2.
Among 4 patients with the absence of adverse events, 3 subjects had ALK V.1 translocation variant, and one patient had ALK rearrangement determined only by IHC; the latter NSCLC and one NSCLC with ALK V.1 fusion showed the disease progression upon lorlatinib treatment.
Fig. 1.
Best percentage change in tumor size (a) and CNS lesions (b) from baseline. Patients continuing lorlatinib treatment are marked with asterisks.
Fig. 2.
PFS (a) and OS (b) in 35 patients receiving lorlatinib.
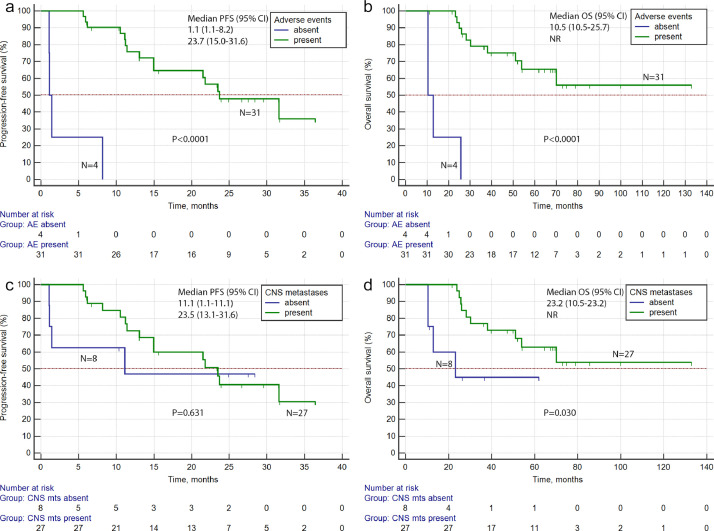
Only 4 patients experienced no adverse effects while being on lorlatinib therapy. It is of interest, that the only 2 patients, who showed progressive disease as the best response to lorlatinib, belonged to this group of subjects. PFS and OS in the no-adverse-events lorlatinib users were strikingly lower as compared to the remaining patients (1.1 months vs. 23.7 months and 10.5 months vs. not reached, respectively; p < 0.0001 for both comparisons) (Fig. 3a,b). PFS was higher in patients with brain metastases as compared to subjects without CNS involvement (23.5 months vs. 11.1 months; see also Fig. 3c). While comparison of PFS produced p values far below the statistical threshold, the presence of metastases in CNS correlated with statistically better overall survival (p = 0.030; Fig. 3d).
Fig. 3.
PFS and OS in patients with or without adverse events (a, b), and in patients with or without CNS metastases (c, d), upon lorlatinib treatment.
The information regarding the variant of the ALK translocation was available for the majority of patients included in the study (Table 1). There was no statistical difference between patients with V.1 and V.3 rearrangements with regard to the rate of objective response or PFS. Interestingly, PFS in patients with common ALK translocation variants (V1, V2 or V3; 23.7 months) was higher as compared to subjects with rare ALK variants (5.6 months) or unknown type of ALK rearrangement (13.1 months); however, this difference did not reach the level of statistical significance (p = 0.188 and p = 0.299, respectively).
The majority of included patients (26/35, 74%) received one TKI therapy prior lorlatinib treatment. Patients receiving prior crizotinib tended to have higher response rate (7/12, 58%) and PFS (23.7 months) as compared to subjects treated by ceritinib (4/14 (29%) and 15.0 months, respectively). However, the statistical tests did not confirm the significance of these observations (p = 0.233 and p = 0.586, respectively).
Seventeen patients, which were included in the study and benefited from lorlatinib treatment, progressed during the follow-up. The systemic progression, manifested by the enlargement of multiple metastatic lesions, was observed in 14 of these cases; 12 had treatment failure confined to visceral organs, while 2 patients experienced growth of both extracranial and intracranial tumor lesions. Three patients had oligometastatic progressive disease in the brain (n = 2) or lung (n = 1).
Discussion
NSCLC studies involving lorlatinib treatment are summarized in Table 2. Given the rarity of ALK rearrangements and the availability of several ALK inhibitors, clinical testing of novel ALK-targeted drugs presents a challenge. Published lorlatinib trials demonstrate significant variations with regard to the selection criteria for the patients, clinical characteristics of included subjects, prior treatment history etc., therefore their comparison is complicated.
Table 2.
Lorlatinib clinical studies involving patients with ALK-rearranged NSCLC.
| Study | Patients | Method of ALK testing | Prior TKIs (number of patients) | Main outcomes |
||
|---|---|---|---|---|---|---|
| Tumor responses by RECISTa | Duration of the effect | Overall survival | ||||
| Shaw et al., 2017, phase 1 study [17] | Dose-finding study involving 41 patients, who received prior TKI therapy (n = 40) or was TKI-naïve (n = 1) |
FISH or IHC | Crizotinib: 36 Ceritinib: >/= 20 Alectinib: >/= 9 Brigatinib: 2 |
OR: 19 (46%; 3 CR and 16 PR); SD: 8 (20%); PD: 11 (27%) OR in patients who received 1 TKI: 8/14 (57%); 2 or more TKIs: 11/26 (42%) Intracranial response: 8/19 (42%) |
PFS: 9.6 months 1 prior TKI: 13.5 months; 2 or more prior TKIs: 9.2 months |
|
| Solomon et al., 2018, phase 2 study [12] | 30 treatment-naïve patients | FISH or IHC | – | OR: 27 (90%; 1 CR and 26 PR); SD: 2 (7%); PD: 1 (3%) | PFS: not reached Duration of response: not reached |
|
| 59 patients who received previous crizotinib, with or without chemotherapy | FISH or IHC | Crizotinib: 59 | OR: 41 (69%; 1 CR and 40 PR); SD: 10 (17%); PD: 6 (10%) Intracranial response: 20/23 (87%) |
PFS: not reachedb Duration of response: not reached |
||
| 28 patients who received one previous non-crizotinib ALK tyrosine kinase inhibitor, with or without chemotherapy | FISH or IHC | Last TKI received: Alectinib: 13 Ceritinib: 13 Brigatinib: 1 Other: 1 |
OR: 9 (32%; 1 CR and 8 PR); SD: 10 (36%); PD: 7 (25%) | PFS: 5.5 months Duration of response: not reached |
||
| 111 patients with two or three previous ALK tyrosine kinase inhibitors, with or without chemotherapy | FISH or IHC | Last TKI received: Crizotinib: 18 Ceritinib: 34 Alectinib: 49 Brigatinib: 7 Other: 3 |
OR: 43 (39%; 2 CR and 41 PR); SD: 38 (34%); PD: 20 (18%) | PFS: 6.9 months Duration of response: not reached |
||
| Zhu et al., 2020, international real-world analysis (early access program) [24] | 76 patients, who failed all available ALK inhibitors (or had secondary ALK mutations rendering resistance to available inhibitors); some of these patients were also required to receive standard chemotherapy | Not indicated | Crizotinib: 66 Ceritinib: 46 Alectinib: 43 Brigatinib: 10 Other: 1 |
OR: 21 (78%; 2 CR and 19 PR); SD: 30 (39%); PD: 13 (17%) | PFS: 9.3 months 1 prior TKI: 9.3 months; 2 previous TKI: not reached; > 2 previous TKI: 11.2 months |
Not reached |
| Peled et al., 2020, international real-world analysis (early access program) [22] | 106 patients, who received prior TKI therapy | FISH (76%), IHC (31%), NGS (8%) or PCR (13%); 23 patients were tested by more than one method | Last therapy received: Crizotinib: 40 Ceritinib: 25 Alectinib: 15 Brigatinib: 13 |
Extracranial response: 52/87 (62%); intracranial response: 40/65 (62%) | Median duration of therapy: not reached; mean duration of therapy: 23.9 months |
89.1 months |
| Hochmair et al., 2020, multicenter real-world analysis (early access program, Austria) [20] | 37 patients, who received prior TKI therapy (1 line: 10; 2 lines: 13; 3 lines: 13; 4 lines: 1) | FISH (46%), IHC (35%), NGS (3%) or more than one method (16%) | Crizotinib: 25 Ceritinib: 21 Alectinib: 14 Brigatinib: 27 |
OR: 16 (43%; 1 CR and 15 PR); SD: 5 (14%); PD: 16 (43%) |
Median duration of therapy: 4.4 months | 41.8 months |
| Shaw et al., 2020, phase 3 study [19] | 149 treatment-naïve patients | IHC | – | OR: 113 (76%; 4 CR and 109 PR); SD: 19 (13%); PD: 10 (7%) Intracranial response: 14/17 (82%) |
Proportion of patients without disease progression at 12 months: 78% PFS: not reached |
|
| Present study, single-center real-world analysis (mainly patients included in the early access program, Russia) | 35 patients, who received prior TKI therapy (n = 31) or was TKI-naïve (n = 4) |
PCR (28 patients) FISH (5 patients) OR IHC (2 patients) | Crizotinib: 17 Ceritinib: 17 Alectinib: 2 |
OR: 15 (43%; 1 CR and 14 PR); SD: 18 (51%); PD: 2 (6%) Intracranial response: 22/27 (81%) |
PFS: 21.8 months; median duration of therapy: not reached; mean duration of therapy: 24.9 months | 70.1 months |
Abbreviations: CR – complete response; OR – objective response; OS – overall survival; PD – progressive disease; PFS – progression-free survival; PR – partial response; SD – stable disease.
The rate of tumor responses was calculated towards the total number of included patients, irrespective of the number of cases evaluable for response by the RECIST criteria.
11.1 months, as reported in the follow-up study [16].
While the present study did not differ from other repots with regard to response rates, it produced strikingly higher PFS (21.8 months) when compared to majority of similar investigations. The mere fact of observing high PFS for ALK-targeted drugs is not surprising: for example, the first-line alectinib trial resulted in PFS equal to 34.8 months [25]. However, most of previous lorlatinib trials produced somewhat lower PFS as compared to the current study. Several factors may play a role with regard to this difference. Interobserver variability may contribute to some extent to the estimation of PFS, especially given that the tumor progression upon continuing TKI treatment may be slow in some circumstances [26,27]. It is of notice, that our series of patients included apparently less pretreated subjects as compared to studies of Shaw et al. [12], Solomon et al. [17], Zhu et al. [24], and Hochmair et al. [20]; only a minority of included subjects experienced alectinib treatment and none received brigatinib prior to lorlatinib (Table 2). It appears that the TKI treatment history may dramatically influence PFS on lorlatinib. Indeed, recent first-line lorlatinib trial revealed that as many as 78% patients remained progression-free at 12 months; median PFS was not reached at the time of the data analysis, but it is very likely to significantly exceed historical estimates obtained on TKI-pretreated patients [19]. Absence of patients with experience of brigatinib therapy in our data set may also be of potential importance: recent real-world study suggested that prior treatment by brigatinib may compromise the efficacy of lorlatinib to a higher extent as compared to the use of other ALK-targeted TKIs [20]. The invitation of patients to the expanded access programs is often highly influenced by the preferences of primary physicians. Our patient series had remarkably high number of subjects with intracranial involvement. Lorlatinib is known to be particularly effective towards CNS metastases, therefore preferential recruitment of subjects with brain involvement could have led to some bias with regard to PFS. Indeed, patients with visceral-only tumor lesions fared surprisingly worse in this study as compared to NSCLC cases with brain metastases (Table 1); this could be explained by distinct biological properties and prior drug exposure of visceral cancer lesions. Furthermore, in contrast to earlier studies (Table 2), our report considered the pattern of the disease progression followed by the initial response to lorlatinib; as mentioned above, visceral systemic progression was more characteristic than the growth of brain lesions. Most of published lorlatinib trials involved NSCLCs, which were ALK-tested using FISH or IHC (Table 2). Our patient series is the only lorlatinib study, where the majority of ALK translocations were validated by genotyping. It is of interest, that patients with unknown variants of ALK rearrangements had numerically lower PFS as compared to subjects with common ALK translocations (Table 1). FISH and IHC generally produce concordant results with ALK genotyping procedures, however some occasional failures of these indirect methods may lead to false-positive detection of ALK rearrangements [28,29].
The study of Lin et al. [21] included 6 patients with V.1 and 15 subjects with V.3 ALK rearrangements, and demonstrated statistically longer PFS for NSCLCs with ALK V.3 fusions. Our study had comparable number of observations (13 patients with V.1 and 8 subjects with V.3 rearrangements, respectively), however failed to replicate this difference. Distinct pretreatment history may be a reason for the discrepancy between our observation and the report of Lin et al. [21]. Patients with the lack of adverse events had clearly worse outcomes of lorlatinib treatment as compared to subjects with the detectable toxicity of the drug (Table 1). Previous studies did not consider this type of associations (Table 2). The existence of correlations between the extent of adverse events and the degree of tumor response is not uncommon [30,31]. Our data may potentially call to consider an adjustment of lorlatinib dosage in patients with poor tumor response and complete lack of the toxicity of the drug. It is of interest that among 4 patients with no adverse event and poor response to lorlatinib 3 subjects had ALK V.1 gene fusion and 1 NSCLC carried ALK rearrangement detected only by IHC (Table 1). Although no conclusions can be drawn from this small number of observations, one may speculate that some subjects may have ultra-rapid drug turnover due to especial pharmacogenomic constitution or certain lifestyle factors [32], and these individual metabolic characteristics could critically affect the disease outcome only in patients with particular translocation variants.
Recent phase 3 randomized trial comparing lorlatinib and crizotinib resulted in the approval of the former drug for the first-line NSCLC treatment and is likely to be practice-changing. Lorlatinib clearly outperformed crizotinib for all treatment efficacy end-points, while showing more or less similar rate of adverse events. For example, the response rate in the lorlatinib arm was 76%, while only 58% of patients receiving crizotinib achieved objective reduction of tumor size as determined by RECIST criteria. The advantage of lorlatinib was particularly evident when considering patients with CNS involvement (intracranial response: 82% vs. 23%, respectively). Lorlatinib and crizotinib had distinct pattern of adverse events, with hypercholesterolemia, hypertriglyceridemia, increased weight and cognitive and mood disorders being characteristic for the former, and diarrhea, nausea, vomiting, low appetite and mild vision impairment being more frequently observed in the latter arm [19]. Given that lorlatinib demonstrated unprecedented duration of tumor response combined with generally manageable toxicity profile, it is very likely to be increasingly used in the first-line setting. However, the experience of treatment of ALK-rearranged NSCLC after the failure of lorlatinib is very limited for the time being. It remains to be seen, what effective options remain for the patients with acquired resistance to this drug. There are several ALK-targeted drugs, and the pattern of their antitumor activity may significantly depend on the type of prior treatment. Consequently, optimal sequencing of ALK-specific agents is critical for achieving maximal overall survival [33].
ALK-driven NSCLCs have high life expectancy, thanks to the availability of multiple treatment options [34]. Our study resulted in overall survival of 70.1 months, which may be regarded as an important advance in the NSCLC management. Further accumulation of the experience related to the clinical use of lorlatinib may help to define its position within the spectrum of ALK-targeted drugs.
Ethical approval
The study was approved by the local Ethics Committee. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients included in the study.
Funding
The study has been supported by the Russian Science Foundation (grant № 20–15–00244).
Authors’ contributions
Sergey V. Orlov: Conceptualization, Supervision, Data Curation, Writing – Review and Editing; Elena A. Filippova, Alexandra M. Lozhkina, Svetlana V. Odintsova: Investigation, Data Curation, Writing – Review and Editing; Aglaya G. Iyevleva, Tatiana N. Sokolova, Natalia V. Mitiushkina: Methodology, Formal Analysis, Writing – Original Draft; Vladislav I. Tiurin, Elena V. Preobrazhenskaya, Alexandr A. Romanko, Alexandr S. Martianov, Alexandr O. Ivantsov, Svetlana N. Aleksakhina: Methodology, Investigation, Data curation, Writing - Review and Editing; Alexandr V. Togo: Data Curation, Formal Analysis, Writing - Review and Editing; Evgeny N. Imyanitov: Conceptualization, Supervision, Writing - Review and Editing.
Declaration of Competing Interest
All other authors have no relevant financial or non-financial interests to disclose.
Acknowledgments
The study has been supported by the Russian Science Foundation (grant № 20-15-00244).
References
- 1.Morris T.A., Khoo C., Solomon B.J. Targeting ROS1 rearrangements in non-small cell lung cancer: crizotinib and newer generation tyrosine kinase inhibitors. Drugs. 2019;79:1277–1286. doi: 10.1007/s40265-019-01164-3. [DOI] [PubMed] [Google Scholar]
- 2.Rosas G., Ruiz R., Araujo J.M. ALK rearrangements: biology, detection and opportunities of therapy in non-small cell lung cancer. Crit. Rev. Oncol. Hematol. 2019;36:48–55. doi: 10.1016/j.critrevonc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Kwak E.L., Bang Y.J., Camidge D.R. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw A.T., Ou S.H., Bang Y.J. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remon J., Tabbò F., Jimenez B. Sequential blinded treatment decisions in ALK-positive non-small cell lung cancers in the era of precision medicine. Clin. Transl. Oncol. 2020;22:1425–1429. doi: 10.1007/s12094-020-02290-1. [DOI] [PubMed] [Google Scholar]
- 6.Shaw A.T., Gandhi L., Gadgeel S. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hida T., Nokihara H., Kondo M. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim D.W., Tiseo M., Ahn M.J. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J. Clin. Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 9.Peters S., Camidge D.R., Shaw A.T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 10.Shaw A.T., Kim T.M., Crinò L. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 11.Camidge D.R., Kim H.R., Ahn M.J. Brigatinib versus Crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 12.Solomon B.J., Besse B., Bauer T.M. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 13.Lim S.M., Kim H.R., Lee J.S. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J. Clin. Oncol. 2017;35:2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 14.Shaw A.T., Solomon B.J., Chiari R. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 15.Drilon A., Siena S., Dziadziuszko R. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw A.T., Solomon B.J., Besse B. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J. Clin. Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw A.T., Felip E., Bauer T.M. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou H.Y., Li Q., Engstrom L.D. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw A.T., Bauer T.M., de Marinis F. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 20.Hochmair M.J., Fabikan H., Illini O. Later-line treatment with lorlatinib in ALK- and ROS1-rearrangement-positive NSCLC: a retrospective, multicenter analysis. Pharmaceuticals (Basel) 2020;13:371. doi: 10.3390/ph13110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J.J., Zhu V.W., Yoda S. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J. Clin. Oncol. 2018;36:1199–1206. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peled N., Gillis R., Kilickap S. GLASS: global Lorlatinib for ALK(+) and ROS1(+) retrospective Study: real world data of 123 NSCLC patients. Lung Cancer. 2020;148:48–54. doi: 10.1016/j.lungcan.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Iyevleva A.G., Raskin G.A., Tiurin V.I. Novel ALK fusion partners in lung cancer. Cancer Lett. 2015;362:116–121. doi: 10.1016/j.canlet.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Zhu V.W., Lin Y.T., Kim D.W. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor-refractory ALK-positive or ROS1-positive NSCLC. J. Thorac. Oncol. 2020;15:1484–1496. doi: 10.1016/j.jtho.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Camidge D.R., Dziadziuszko R., Peters S. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 2019;14:1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Metro G., Tazza M., Matocci R. Optimal management of ALK-positive NSCLC progressing on crizotinib. Lung Cancer. 2017;106:58–66. doi: 10.1016/j.lungcan.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Yap T.A., Macklin-Doherty A., Popat S. Continuing EGFR inhibition beyond progression in advanced non-small cell lung cancer. Eur. J. Cancer. 2017;70:12–21. doi: 10.1016/j.ejca.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Thunnissen E., Lissenberg-Witte B.I., van den Heuvel M.M. ALK immunohistochemistry positive, FISH negative NSCLC is infrequent, but associated with impaired survival following treatment with crizotinib. Lung Cancer. 2019;138:13–18. doi: 10.1016/j.lungcan.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Vollbrecht C., Lenze D., Hummel M. RNA-based analysis of ALK fusions in non-small cell lung cancer cases showing IHC/FISH discordance. BMC Cancer. 2018;18:1158. doi: 10.1186/s12885-018-5070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S., Kurzrock R. Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014;40:883–891. doi: 10.1016/j.ctrv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka H., Hayashi T., Takigami K. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer. 2020;20:656. doi: 10.1186/s12885-020-07142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roden D.M., McLeod H.L., Relling M.V. Pharmacogenomics. Lancet. 2019;394:521–532. doi: 10.1016/S0140-6736(19)31276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauffmann-Guerrero D., Kahnert K., Huber R.M. Treatment sequencing for anaplastic lymphoma kinase-rearranged non-small-cell lung cancer. Drugs. 2021;81:87–100. doi: 10.1007/s40265-020-01445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duruisseaux M., Besse B., Cadranel J. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8:21903–21917. doi: 10.18632/oncotarget.15746. [DOI] [PMC free article] [PubMed] [Google Scholar]