Abstract
Objective:
Prior studies of universal masking have not measured face-mask compliance. We performed a quality improvement study to monitor and improve face-mask compliance among healthcare personnel (HCP) during the coronavirus disease 2019 (COVID-19) pandemic.
Design:
Mixed-methods study.
Setting:
Tertiary-care center in West Haven, Connecticut.
Patients:
HCP including physicians, nurses, and ancillary staff.
Methods:
Face-mask compliance was measured through direct observations during a 4-week baseline period after universal masking was mandated. Frontline and management HCP completed semistructured interviews from which a multimodal intervention was developed. Direct observations were repeated during a 14-week period following implementation of the multimodal intervention. Differences between units were evaluated with χ2 testing using the Bonferroni correction. Face-mask compliance between baseline and intervention periods was compared using time-series regression.
Results:
Among 1,561 observations during the baseline period, median weekly face-mask compliance was 82.2% (range, 80.8%–84.4%). Semistructured interviews were performed with 16 HCP. Qualitative analysis informed the development of a multimodal intervention consisting of audit and passive feedback, active discussion, and increased communication from leadership. Among 2,651 observations during the intervention period, median weekly face-mask compliance was 92.6% (range, 84.6%–97.9%). There was no difference in weekly face-mask compliance between COVID-19 and non–COVID-19 units. The multimodal intervention was associated with an increase in face-mask compliance (β = 0.023; P = .002).
Conclusions:
Face-mask compliance remained suboptimal among HCP despite a facility-wide mandate for universal masking. A multimodal intervention consisting of audit and passive feedback, active discussion, and increased communication from leadership was effective in increasing face-mask compliance among HCP.
The use of face masks among healthcare personnel (HCP) protects against acquisition of severe acute respiratory coronavirus virus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19).1 The Centers for Disease Control and Prevention recommends that HCP wear a face mask at all times in healthcare settings.7 This recommendation is driven, in part, by evidence suggesting that most infections with SARS-CoV-2 are transmitted through close contact via respiratory droplets.6 The importance of universal masking among HCP is heightened by the frequency of asymptomatic and presymptomatic transmission of SARS-CoV-2.2–4
Universal masking among HCP may reduce healthcare-associated transmission of SARS-CoV-2. In one study, a decrease in the cumulative incidence rate of healthcare-acquired COVID-19 was observed following the implementation of universal masking.8 In another report, universal masking was associated with a significantly lower rate of SARS-CoV-2 positivity among HCP.9 Universal masking, when combined with a comprehensive infection prevention program, was also shown to reduce cases of healthcare-associated COVID-19.10
Nevertheless, there is a paucity of data regarding face-mask compliance among HCP. Many reports of universal masking represent expert opinions.11–13 Prior quantitative studies of universal masking have not measured face-mask compliance among HCP or variation in face-mask compliance over time.8–10 Furthermore, existing international data may have limited generalizability due to differences in culture and healthcare-related behaviors in the United States.14
Similar to monitoring hand hygiene compliance, monitoring face-mask compliance among HCP is critical to establish baseline estimates and to maximize the effectiveness of universal masking. Whereas hand hygiene is widely considered the most effective method of preventing healthcare-associated infections, hand hygiene compliance among HCP remains poor.15 Accordingly, many strategies have been proposed to enhance hand hygiene compliance.16 We hypothesize that analogous approaches are needed to optimize face-mask compliance, particularly given the potential for complacency among HCP and the duration of the COVID-19 pandemic.17,18 We therefore conducted a quality improvement study to improve face-mask compliance among HCP at a tertiary-care teaching center during the COVID-19 pandemic.
Methods
Study setting
We conducted a quality improvement study at the Department of Veterans Affairs Connecticut Healthcare System, a 191-bed facility in West Haven, Connecticut. Approximately 3,160 persons are employed by the study site, and an additional 685 students and 675 medical and dental residents from affiliated institutions receive onsite clinical training. Universal masking was mandated by the Veterans Health Administration on May 7, 2020. This study was deemed quality improvement by the Veterans Affairs Health Services Research and Development Service, which waived further institutional review board approval. This work has been reported using Standards for QUality Improvement Reporting Excellence (SQUIRE) guidelines.19
Baseline period
Face-mask compliance among HCP was observed directly by a hospital epidemiologist (R.D.) or infectious diseases fellow (K.G., J.T., or K.P.) using a standardized form over a 4-week period beginning July 20, 2020. The fellow observed face-mask compliance during their infection prevention rotation. HCP included physicians, nurses, phlebotomists, physical therapists, and staff from nutrition and environmental services. Observations occurred between 08:00 and 18:00 on weekdays in clinical care areas. Clinical care areas included COVID-19 units (eg, emergency department, medical intensive care unit, and medicine units) and non–COVID-19 units (eg, surgical intensive care unit, other medicine units, and specialty care clinics).
Face-mask compliance in clinical care areas was measured before entering a patient room, while in a patient room, after exiting a patient room, or while in a charting room. Face-mask compliance was categorized as compliant or noncompliant. Compliance was defined as wearing an undamaged surgical mask that covered the nose and mouth. Noncompliance was defined as wearing a damaged surgical mask, cloth mask, gaiter, or multiple masks (eg, a surgical mask over an N95 respirator); wearing a surgical mask around the chin or elsewhere such that the nose or mouth were uncovered; or not wearing a surgical mask. Observations were not conducted during aerosol-generating procedures. Each HCP could contribute 1 observation per day. If 1 HCP was observed repeatedly, the earliest observation was categorized.
Semistructured interviews
To inform strategies to improve face-mask compliance, HCP across 2 strata (frontline and management) were invited to participate in semistructured interviews starting August 13, 2020 by adapting a previously evaluated mixed-methods framework.20,21 The management group consisted of nurse managers, and frontline staff included HCP who provided direct patient care. A convenience sample from each stratum was selected and interviewed individually by phone. A moderator (K.G.) conducted each interview using a structured 18-question script for frontline staff and 16-question script for management with an assistant (R.D.) who took notes. Participants completed quantitative questions on a 10-point scale (1, strongly disagree; 10, strongly agree) that assessed barriers to and facilitators of face-mask compliance. Interviews lasted ˜30 minutes and were audio recorded and transcribed. Participants were informed that they were being audio recorded, but that their identities would remain confidential. Participation was voluntary and without monetary compensation.
Qualitative responses from semistructured interviews were analyzed using an immersion and crystallization technique.22 Individual responses were coded into themes for each question and aggregated by strata per question. An Excel (Microsoft, Redmond, WA) database was developed that listed thematic responses from the interviews as well as direct quotes that supported each theme. Responses from quantitative questions were also descriptively analyzed. After all themes were identified and quantitative data were summarized, major themes for each question were reviewed by the investigative team and used to develop a feasible and acceptable infection prevention intervention to increase face-mask compliance among HCP.
Intervention period
Following the implementation of the infection prevention intervention designed to increase face-mask compliance, direct observations of face-mask compliance among HCP were repeated over a 14-week period beginning September 21, 2020 by the same hospital epidemiologist (R.D.) and infectious diseases fellow (K.G.) using the protocol described in the baseline period. When a cover story was needed, observers mentioned they were looking for a colleague or involved in patient care.
Statistical analysis
We calculated the total number of observations during baseline and intervention periods across all clinical care areas. Face-mask compliance was calculated as a percentage of the number of observations categorized as compliant (numerator) divided by the total number of observations (denominator). Face-mask compliance among HCP during the baseline and intervention periods were reported by week. Differences in face-mask compliance between COVID-19 units and non–COVID-19 units were compared using the χ2 test. The Bonferroni correction was applied to account for multiple comparisons.23 Weekly face-mask compliance was graphed relative to the number of confirmed cases of COVID-19 in Connecticut using publicly available data from the Connecticut Department of Public Health.24
We used an interrupted time-series design, which is suited to addressing secular trends and evaluating multiple interventions, to compare face-mask compliance between baseline and intervention periods.25–27 We used segmented regression models to assess changes in face-mask compliance associated with the infection prevention intervention. Time-series analyses provided results as changes in level (acute changes in face-mask compliance immediately after the infection prevention intervention) of face-mask compliance while controlling for secular trend. We adjusted for serial autocorrelation using the Durbin-Watson statistic.28 All analyses were conducted using SAS Proc Autoreg, version 9.4 software (SAS Institute, Cary, NC).
Results
We performed 1,561 observations during the baseline period: 714 observations from COVID-19 units and 847 observations from non–COVID-19 units. Most observations occurred among nursing staff. During the baseline period, the median number of observations per week across all clinical care areas was 408 (range, 262–483), and median weekly face-mask compliance was 82.2% (range, 80.8%–84.4%). There was no difference in weekly face-mask compliance between COVID-19 and non–COVID-19 units during the baseline period (Supplementary Table 1).
Table 1.
Most Common Thematic Responses From Semistructured Interviews of Management and Frontline Staff from a Tertiary-Care Teaching Center (n=16)
| Domain and Stratum | Numerical Response, Median (Range) |
Supporting Quote |
|---|---|---|
| Concern for potential exposure to SARS-CoV-2 | ||
| Management | 5 (3–10) | “[T]here haven’t been any healthcare workers in the [emergency department] who contracted coronavirus, which is good evidence that PPE is working…” |
| Frontline | 5 (1–10) | “I’m going to keep doing what I’m doing and I should be ok. I know there’s still a risk there, so it’s important to be vigilant.” |
| Confidence in understanding of institutional policy on universal masking | ||
| Management | 10 (8–10) | “I get all the information given to me firsthand, so I have to be knowledgeable about the policy and make sure my staff is adhering to it.” |
| Frontline | 10 (8–10) | “On campus we’re always supposed to have a mask on.” |
| Perceived compliance in accordance with universal masking policy | ||
| Management | 9 (8–10) | “Sometimes [non-compliance is] inadvertent. Sitting in my office I always have it on my ear and sometimes I forget to put it back over.” |
| Frontline | 9 (7–10) | “Sometimes 12 hours in a mask, you want to pull it under your nose…to take a breath.” |
| Importance of universal masking among staff/colleagues | ||
| Management | 10 (10) | “[It is critical] to make sure they were wearing PPE properly.” |
| Frontline | 10 (5–10) | “[High] because one of our coworkers in our unit actually had COVID.” |
| Feasibility of universal masking | ||
| Management | 9 (9–10) | “I think we can achieve 90%. [But] you’re with your friends [or] in the break room talking, you might not be paying attention.” |
| Frontline | 10 (7–10) | “It’s feasible, but we have to acknowledge that people are human and we need breaks.” |
| Significance of the following barriers to mask adherence | ||
| Difficulty communicating | ||
| Management | 8 (6–10) | “Number 1 reason for [noncompliance].” |
| Frontline | 5 (2–10) | “Some people are hard of hearing. I have had to take it off to talk to patients at least 2 or 3 times to read my lips…” |
| Difficulty breathing | ||
| Management | 6 (1–8) | “Not that [common], more so N95…” |
| Frontline | 2 (1–6) | “Some [have trouble to] breathe.” |
| Other discomfort (eg, fogging glasses) | ||
| Management | 6 (3–9) | “Heat is definitely one, the glasses…” |
| Frontline | 7 (1–9) | “It bothers me that they’re hot and uncomfortable.” |
| Anxiety | ||
| Management | 3.5 (1–6) | “At this point… everyone’s so used to wearing it…” |
| Frontline | 1 (1–5) | “I haven’t heard complaints.” |
| Usefulness of proposed interventions to improve mask compliance | ||
| Increased breaks | ||
| Management | 8 (5–10) | “I definitely think that would be helpful if they were able to get a break alone or distance and pull mask down. If feasible…” |
| Frontline | 8 (1–10) | “Yes, but not feasible.” |
| Increased education | ||
| Management | 4 (1–10) | “My personal experience with staff, they’re well educated.” |
| Frontline | 3 (1–10) | “I feel they’re educated, but there’s nothing wrong with re-education.” |
| Disciplinary action | ||
| Management | 7 (5–8) | “I guess it would motivate them to adhere to the policy….” |
| Frontline | 6 (1–10) | “It might help some people, maybe.” |
| Positive reinforcement | ||
| Management | 9 (8–10) | “Reinforcing people, thank you for wearing mask… I think that’s the best strategy to share the information, give positive reinforcement.” |
| Frontline | 7 (1–10) | “They’ve been doing that.” |
| Increased communication from hospital leadership | ||
| Management | 10 (1–10) | “I think that’s something that would promote mask adherence. [It would also help] having Infection Prevention weigh in from their perspective…” |
| Frontline | 7 (3–10) | “Some people like to hear it from higher up, some people could care less.” |
| Audit and feedback | ||
| Management | 9 (7–10) | “It’s good to get the feedback and I do find it valuable… [it] gives us an objective number and helps us improve our adherence.” |
| Frontline | 7 (4–10) | “I’m on for hand hygiene, so that would be helpful. Auditing would be a nice reminder.” |
Of 21 HCP who were selected for semistructured interviews, we completed 16 (76%) interviews from a convenience sample of 8 nurse managers in the management group and 8 registered nurses in the frontline group. Frontline and management staff reported moderate concern for potential exposure to SARS-CoV-2 among HCP on their units and perceived high mask compliance among themselves and their colleagues (Table 1). Significant barriers to mask compliance included difficulty communicating and other discomforts such as fogging glasses. Increased education was not deemed useful to improve face-mask compliance among frontline and management staff.
Qualitative data from semistructured interviews informed the development of a multimodal intervention consisting of the 3 components: (1) audit and passive feedback, (2) active discussion, and (3) increased communication from leadership (Table 2). Passive feedback was provided at the unit level by adding face-mask compliance to infection prevention dashboards that shared information on compliance with hand hygiene, personal protective equipment, and medical device best practices as well as time since last healthcare-associated infection. Active discussion was provided by infection prevention personnel to nurse managers from each unit on a routine basis no less than once per week. Communication regarding the importance of face-mask compliance was provided by the facility director, chief of staff, associate director of patient care services or associate director of the study site on a weekly basis.
Table 2.
Timeline of Interventions Relevant to Face-Mask Compliance Among Healthcare Personnel
| Component | Description | Date of Implementation |
|---|---|---|
| Universal masking mandate | Use of face mask by all persons in healthcare facility | May 7, 2020 |
| Standard pandemic precautions | Face mask and face shield with all face-to-face patient encounters | August 5, 2020 |
| Multimodal intervention | ||
| Audit and passive feedback | Direct observations with weekly and monthly feedback on unit-level infection prevention dashboards | September 21, 2020 |
| Active discussion | Direct communication between infection prevention and nurse manager highlighting weekly or monthly face-mask compliance | September 21, 2020 |
| Increased communication | Weekly messaging regarding the importance of face-mask compliance from hospital leadership via e-mail, virtual team huddles, staff meetings, and walking roundsa | September 21, 2020 |
| Universal face shielding | Use of face shields plus face mask within ˜2 m (6 feet) of others in all healthcare facility locations | November 9, 2020 |
Hospital leadership included the facility director, chief of staff, associate director of patient care services, and associate director.
In total, 2,651 observations were performed during the intervention period, including 1,377 observations from COVID-19 units and 1,274 observations from non–COVID-19 units. The median number of observations per week across all clinical care areas was 151 (range, 92–339) during the intervention period, and median weekly face-mask compliance was 92.6% (range, 84.6%–97.9%). There was no difference in weekly face-mask compliance between COVID-19 and non–COVID-19 units during the intervention period (Supplementary Table 1).
Face-mask compliance increased prior to the autumn surge in cases of COVID-19 throughout Connecticut (Fig. 1). In time series regression analysis, we found no evidence of positive (P = .59) or negative (P = .41) autocorrelation using the Durbin-Watson statistic. The multimodal intervention was associated with an immediate increase in face-mask compliance among HCP (β = 0.02; P = .002) (Fig. 2). No significant secular trend was observed in face-mask compliance over the study period (β = 0.002; P = .08).
Fig. 1.
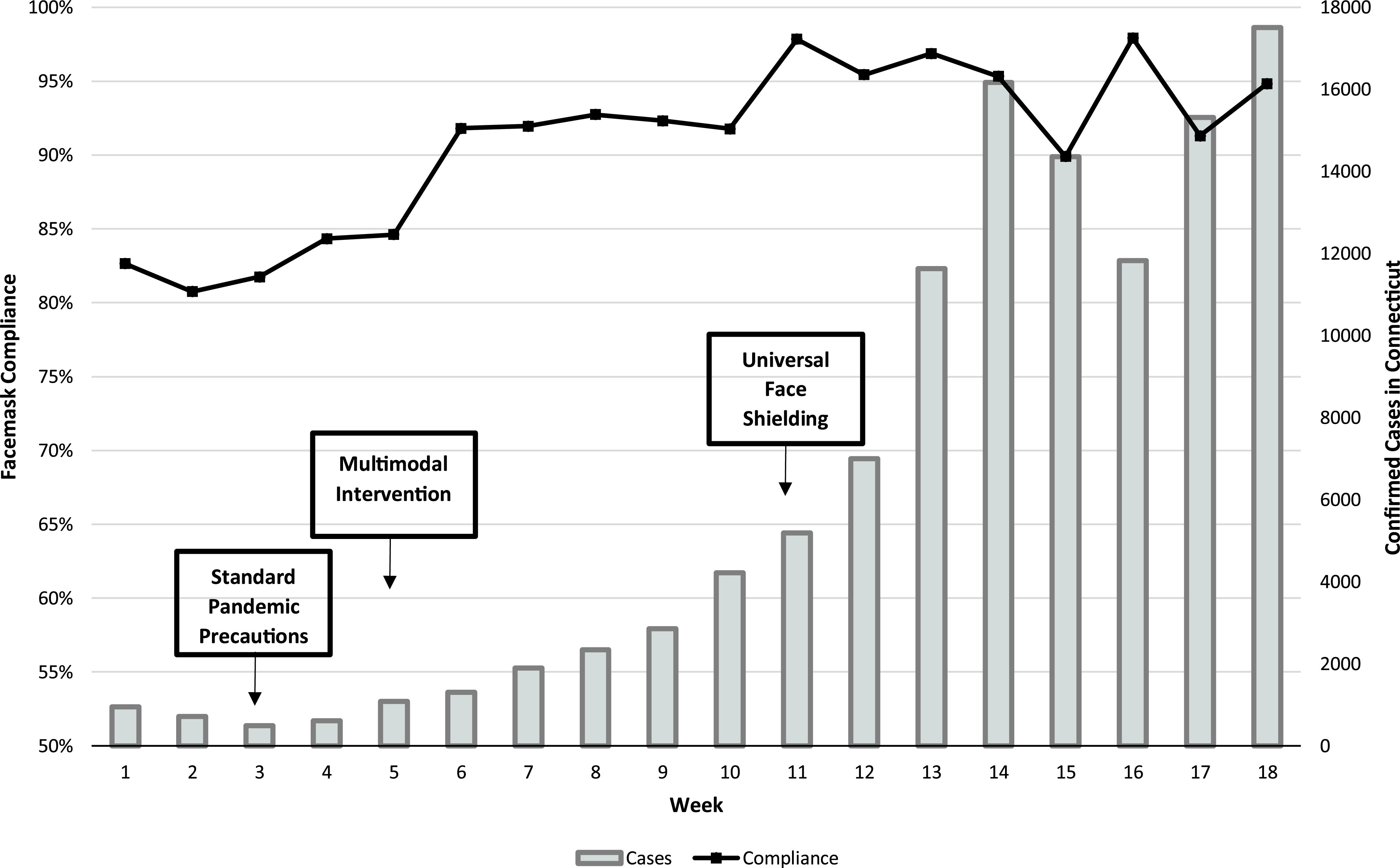
Weekly face-mask compliance among healthcare personnel from a tertiary-care teaching center in which universal masking was mandated ˜10 weeks prior to the observation period.
Fig. 2.
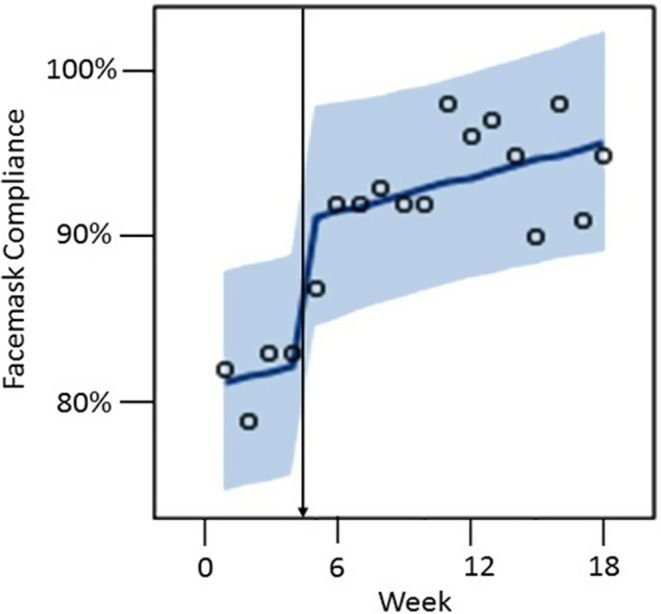
Observed outcomes from time series analysis. Observed values are designated by circles, predicted values by the solid line, and 95% confidence limits by the shaded area. The vertical arrow denotes the multimodal intervention.
Discussion
Universal masking is among the most effective methods of reducing transmission of COVID-19. Nevertheless, studies evaluating compliance with universal masking in healthcare settings are lacking. We show that nearly 1 in 5 HCP were noncompliant despite an institutional mandate for universal masking that was accompanied by an extensive promotional campaign. This campaign included education, staff meetings, widespread announcements with signage, town halls, and mass distribution of face masks. Following the development and implementation of a multimodal intervention informed by frontline and management staff, an immediate and sustained increase in face-mask compliance was observed over a 14-week period. Collectively, our data underscore the potential benefit of adapting principles of hand hygiene promotion, namely audit and passive feedback, active discussion, and increased communication from leadership, to improve face-mask compliance among HCP during the COVID-19 pandemic.
Consistent with prior work, this study demonstrates that the effectiveness of mandates depends on the timely enforcement of compliance.28 We show that institutional mandates for universal masking can be monitored and enforced with limited expansion of existing infection prevention practices, such as providing face-mask compliance metrics on unit-level infection prevention dashboards. This approach may minimize strain on infection prevention personnel and HCP who are already overburdened by COVID-19. The process of interviewing HCP about methods that may change behavior also engaged staff members. In contrast to prior reports, this process revealed that additional education about the rationale for face masks may not be necessary.29 Moreover, active discussion with unit managers during which trends in face-mask compliance were discussed was part of the multimodal intervention. This was done to promote collaboration and communication between infection prevention staff and HCP.17
Our data must be interpreted in the context of local transmission of SARS-CoV-2. Following a peak hospital census of COVID-19 inpatients in the spring, the numbers of COVID-19 inpatients steadily declined at our institution and statewide until the late fall.24 We hypothesize that mask compliance, like other protective behaviors, was influenced by risk perception among HCP.30 This notion is supported by qualitative data suggesting that risk perception among HCP was low due to the prevalence of COVID-19 during the study weeks in the summer, perceived effectiveness of personal protective equipment, and confidence in other HCP being compliant with transmission-based precautions for COVID-19. These data also align with direct observations demonstrating optimal compliance in patient care settings but frequent noncompliance in charting rooms, where HCP often congregated with one another.
Notably, universal face shielding was introduced at the study institution as a method to reduce staff-to-staff transmission of SARS-CoV-2 during a time of increasing community transmission of SARS-CoV-2. An unintended consequence of universal face shielding may have been to complement our multimodal intervention to improve face-mask compliance. Median weekly face-mask compliance was 95.4% after the introduction of universal face shielding during weeks 11–18. In contrast, median weekly compliance was 91.9% during weeks 5–10 of the intervention period. Although numerous factors likely contributed to the observed values across weeks, the requirement for eye protection with face masks may have inadvertently supported the use of face masks. This trend was also evident in the non–COVID-19 units when standard pandemic precautions were implemented in weeks 3-4.
We acknowledge that direct observations of face-mask compliance are fraught with challenges. The use of direct observations may be limited by insufficient staffing, observations during daytime hours and weekdays, time and cost, and lack of consensus on standardized definitions. Variation in policies regarding masking may also be a factor.31 Nevertheless, face-mask compliance is a key process measure that may provide actionable data for quality improvement interventions in healthcare settings. Moreover, direct observations are relatively simple to perform and have minimal technological requirements. Given the prolonged duration of the COVID-19 pandemic and challenges associated with the distribution of COVID-19 vaccines, monitoring and improving face-mask compliance may become a fundamental activity of infection prevention programs.32,33
This study has several limitations. The Hawthorne effect, unbalanced data collection, and observer bias may reduce internal validity. Although the Hawthorne effect was minimized by employing observer personnel who were unfamiliar to HCP, HCP may nevertheless have known they were being observed, particularly during the intervention period. Balanced data collection was facilitated by large numbers of observations that were obtained during weekly intervals, and observer bias was reduced by the application of standardized definitions. Our standardized definitions considered unmasked HCP who were alone in a private room as being compliant with the policy that did not require them to mask, but the number of observations from this group were limited. Although all interviews were confidential, participants may have been inclined to provide desirable responses. Additionally, if external factors influenced face-mask compliance during the intervention period, such as risk perception associated with the second wave of COVID-19, then our findings would overestimate the benefit of the multimodal intervention. However, time-series analyses limit confounding to factors changing near the intervention time and related to the outcome. These factors were unlikely, and the observed increase in face-mask compliance preceded the surge in cases of COVID-19. Finally, evaluating face-mask compliance by type of HCP was beyond the scope of this work, and our study was limited by the lack of a control group.
In summary, face-mask compliance among HCP remained suboptimal across COVID-19 and non–COVID-19 units despite an institutional mandate for universal masking accompanied by an extensive promotional campaign. A multimodal intervention informed by key stakeholders consisting of audit and passive feedback, active discussion, and increased communication from leadership appeared effective in improving face-mask compliance. Infection prevention programs should consider applying principles of hand hygiene promotion to maximize the benefit of universal masking policies.
Acknowledgments
We thank the Hospital Epidemiology and Infection Prevention Program at the Veterans Affairs Connecticut Healthcare System for their support of this study. The statements contained in this article reflect the views of the authors and do not represent the official positions of the US Department of Veterans Affairs or other author affiliate organizations.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.205.
click here to view supplementary material
References
- 1. Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020;395:1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis 2020;26:e201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–675. [DOI] [PubMed] [Google Scholar]
- 4. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. Morbid Mortal Wkly Rep 2020;69:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scientific brief: SARS-CoV-2 and potential airborne transmission. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html. Accessed November 18, 2020. [PubMed]
- 7.Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease (2019) COVID-19 pandemic. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed November 18, 2020.
- 8. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol 2020;41:1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Ferro EG, Zhou G, Hashimoto D and Bhatt DL. Association between universal masking in a healthcare system and SARS-CoV-2 positivity among healthcare workers. JAMA 2020;324:703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhee C, Baker M, Vaidya V, et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3:e2020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Advani S, Smith B, Lewis S, Anderson D, Sexton D. Universal masking in hospitals in the COVID-19 era: is it time to consider shielding? Infect Control Hosp Epidemiol 2020;41:1066–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA 2020. doi: 10.1001/jama.2020.21399. [DOI] [PubMed]
- 13. Klompas M, Morris CA, Sinclair J, Pearson M, Shenoy ES. Universal masking in hospitals in the COVID-19 era. N Engl J Med 2020;382(21):e63. [DOI] [PubMed] [Google Scholar]
- 14. Wong SC, Lam GK, AuYeung CH, et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: implication of universal masking in hospitals. Infect Control Hosp Epidemiol 2021;42:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kingston L, O’Connell NH, Dunne CP. Hand hygiene–related clinical trials reported since 2010: a systematic review. J Hosp Infect 2016;92:309–320. [DOI] [PubMed] [Google Scholar]
- 16. Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev 2017;9:CD005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruiz-Fernández MD, Ramos-Pichardo JD, Ibáñez-Masero O, et al. Compassion fatigue, burnout, compassion satisfaction and perceived stress in healthcare professionals during the COVID-19 health crisis in Spain. J Clin Nurs 2020;29:4321–4330. [DOI] [PubMed] [Google Scholar]
- 18. Renardy M, Eisenberg M, Kirschner D. Predicting the second wave of COVID-19 in Washtenaw County, MI. J Theor Biol 2020;507:110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Standards for Quality Improvement Reporting Excellence, SQUIRE 2.0. Equator Network website. https://www.equator-network.org/reporting-guidelines/squire/. Accessed November 19, 2020.
- 20. Sinkowitz-Cochran RL, Garcia-Williams A, Hackbarth A, et al. Evaluation of organizational culture among different levels of healthcare staff participating in the Institute for Healthcare Improvement’s 100,000 Lives campaign. Infect Control Hosp Epidemiol 2012;33:135–143. [DOI] [PubMed] [Google Scholar]
- 21. Wise ME, Weber SG, Schneider A, et al. Hospital staff perceptions of a legislative mandate for methicillin-resistant Staphylococcus aureus screening. Infect Control Hosp Epidemiol 2011;32:573–578. [DOI] [PubMed] [Google Scholar]
- 22. Borkan J. Immersion/crystallization. In: Crabtree B, Miller W, eds. Doing Qualitative Research, 2nd ed. Thousand Oaks, CA: Sage, 1999:179–194. [Google Scholar]
- 23. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt 2014;34:502–508. [DOI] [PubMed] [Google Scholar]
- 24.Connecticut COVID-19 response, daily data report. Connecticut government website. https://portal.ct.gov/coronavirus/covid-19-data-tracker. Accessed November 23, 2020.
- 25. Smiddy MP, Murphy OM, Savage E, et al. Efficacy of observational hand hygiene audit with targeted feedback on doctors’ hand hygiene compliance: a retrospective time series analysis. J Infect Prev 2019;20:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madden JM, Soumerai SB, Lieu TA, Mandl KD, Zhang F, Ross-Degnan D. Effects of a law against early postpartum discharge on newborn follow-up, adverse events, and HMO expenditures. N Engl J Med 2002;347:2031–2038. [DOI] [PubMed] [Google Scholar]
- 27. Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2006;43:971–978. [DOI] [PubMed] [Google Scholar]
- 28. Glied SA, Hartz J, Giorgi G. Consider it done? The likely efficacy of mandates for health insurance. Health Affairs 2007;26:1612–1621. [DOI] [PubMed] [Google Scholar]
- 29. Advani S, Yarrington ME, Smith BA, Anderson DJ, Sexton DJ. Are we forgetting the “universal” in universal masking? Current challenges and future solutions. Infect Control Hosp Epidemiol 2020. doi: 10.1017/ice.2020.333. [DOI] [PMC free article] [PubMed]
- 30. Fakih MG, Sturm LK, Fakih RR. Overcoming COVID-19: addressing the perception of risk and transitioning protective behaviors to habits Infect Control Hosp Epidemiol 2021;42:489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gostin LO, Cohen IG, Koplan JP. Universal masking in the United States: the role of mandates, health education, and the CDC. JAMA 2020;324:837–838. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz JL. Evaluating and deploying COVID-19 vaccines—the importance of transparency, scientific integrity, and public trust. N Engl J Med 2020;383:1703–1705. [DOI] [PubMed] [Google Scholar]
- 33. Mello MM, Silverman RD, Omer SB. Ensuring uptake of vaccines against SARS-CoV-2. N Engl J Med 2020;383:1296–1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.205.
click here to view supplementary material


