Abstract
Purpose
Cumulative evidence has demonstrated that breast cancer was the most commonly diagnosed cancer in women. Despite growing evidence for a link between serotonin and tumorigenesis, research on the expression of serotoninergic systems in the human breast cancer cell and tissue has only rarely been reported.
Methods
First, immunofluorescence staining, ELISA and Western blotting were used to detect serotonin and melatoninergic systems in various breast cancer cell types. Then, serotonin expression was evaluated in the cultures of TPBC cell line BT-474 and TNBC cell line MDA-MB-231 using immunofluorescence assay. To further explore the diagnostic role of serotonin in breast cancer, serotonin expression was conducted in the TPBC and TNBC tumor sections by immunostaining analysis.
Results
Our results suggested that both human breast cancer cells and human breast epithelial cell line could synthesize serotonin and melatonin. Unlike melatonin, serotonin levels varied significantly between human breast cancer and breast epithelial cell line (p<0.01). In addition, serotonin N-acetyltransferase (NAT) and acetylserotonin methyltransferase (ASMT), the key enzymes in the pathway of melatonin synthesis from serotonin, were also detectable. In agreement with these findings of human breast cancer cell and human breast epithelial cell line, serotonin expression was also much higher in triple-negative (PR−, ER−, HER-2−) breast cancer (TNBC) and triple-positive breast cancer (TPBC) compared to para-carcinoma tissues (PCTs).
Conclusion
Here, we provided evidence that the human breast cancer cell (MCF-7, Bcap-37) and human breast epithelial cell (MCF-10A) could synthesize intrinsic serotonin and melatonin, and serotonin expression was higher in the breast cancer tissue compared with PCT. The findings suggested that serotonin might be used as a predictive marker for breast cancer patients.
Keywords: serotonin, melatonin, breast cancer, serotonin N-acetyltransferase, acetylserotonin methyltransferase
Introduction
Breast cancer is the most commonly diagnosed type of cancer in women.1 Serotonin, a well-known neurotransmitter, also functions as a hormone outside of the central nervous system via its different receptor subtypes.2,3 It was shown to be involved in the regulation of cell proliferation, survival and metastasis.4 Receptors of serotonin have been detected in human breast cancer.5
Serotonin serves as a precursor of melatonin, and the biosynthesis pathway of melatonin from serotonin has been studied thoroughly.6 Serotonin is the product of a multistep metabolic pathway that starts with the hydroxylation of the aromatic amino acid L-tryptophan by tryptophan hydroxylase (TPH).7 The resulting metabolite hydroxytryptophan is decarboxylated by aromatic L-amino acid decarboxylase (AADC) to generate serotonin. TPH catalyzes the rate limiting step in serotonin synthesis. TPH has two isoforms, TPH1 and TPH2. Next, serotonin is catalyzed by the N-acetyltransferase (NAT) to N-acetyl-5- hydroxytryptamine, which is in turn catalytically converted by acetylserotonin methyltransferase (ASMT) to melatonin. However, serotonin can also be oxidized by monoamine oxidase A (MAOA) into 5-hydroxyindoleacetic acid (5-HIAA) in the bypass of melatonin synthesis.7
In addition to being produced in the brain, serotonin is also produced in gut,8 and retina.9 Our group reported that cultured rat cortical astrocytes and glioma C6 cells synthesize serotonin and melatonin.10 In addition, apolipoprotein E genotype influences melatonin biosynthesis by regulating NAT and MAOA expression in C6 cells.11 Furthermore, we investigated that serotoninergic and melatoninergic systems are expressed in the mouse embryonic fibroblast NIH3T3 cells.12 Most recently, our group investigated that serotonin and melatoninergic components were expressed in cultured human ovarian cumulus cells, and melatonin might reduce oxidative stress of human oocytes by ameliorating mitochondrial function.13 Furthermore, the results from recent studies strongly support the important role of serotonin in the regulation of normal mammary gland tissue and breast cancer.14
Breast cancer is classified into different subtypes based on the presence of progesterone receptor (PR), estrogen receptor (ER) and growth factor receptor epidermal 2 (HER-2). Compared with triple-positive (PR+, ER+, HER2+) breast cancer (TPBC), triple-negative (PR−, ER−, HER2−) breast cancer (TNBC) is generally considered as the most severe subgroup of breast cancer and is characterized by aggressive behavior, poor prognosis, poor differentiation, and an increased rate of node invasion.10
Although emerging evidence links serotonin and melatonin with tumorigenesis, no one systematically reported whether there is expression in human breast cancer cell line and human breast cancer tissue. In the current study, we investigated whether serotonin and melatoninergic systems were expressed in human breast cancer cell (MCF-7, Bcap-37, BT-474, MDA-MB-231), human breast epithelial cell (MCF-10A) and human breast cancer tissue, and whether serotonin could be used as a predictive marker for breast cancer patients.
Materials and Methods
Subjects
Samples of 37 breast cancer patients (20 TPBC, 17 TNBC) were obtained from the First Affiliated Hospital of Anhui Medical University. Clinical parameters and correlation analysis in TPBC and TNBC patients are summarized in Table 1. Ethics approval was obtained from the ethics committee of clinical medical research, First Affiliated Hospital of Anhui Medical University, and all patients signed informed consent prior to sample collection (ethics approval number: PJ2017-08-05). This was conducted in accordance with the Declaration of Helsinki.
Table 1.
Correlations Between Serotonin Expression and Clinical Parameters in TPBC and TNBC Patients
| Clinical Parameters | TNBC | TPBC | Serotonin Expression | r | p |
|---|---|---|---|---|---|
| TNBC TPBC | |||||
| n | 17 | 20 | |||
| Age (years) | −0.29 | 0.09 | |||
| <35 | 0 | 0 | 0 0 | ||
| ≥35 <50 | 7 | 6 | 32.59±3.62 33.38±1.21 | ||
| ≥50 | 10 | 14 | 33.85±2.07 34.70±1.21 | ||
| Tumor size (cm) | −0.24 | 0.16 | |||
| 1.0 to <2.0 | 4 | 9 | 33.74±2.47 33.69±1.86 | ||
| 2.0 to <5.0 | 13 | 11 | 32.39±3.89 34.73±1.11 | ||
| Histologic grade | 0.18 | 0.28 | |||
| I | 3 | 4 | 36.71±6.55 36.24±2.75 | ||
| II | 7 | 14 | 30.78±3.76 34.15±1.16 | ||
| III | 7 | 2 | 32.68±2.45 33.34±2.05 | ||
| Lymphatic invasion | 0.12 | 0.47 | |||
| (+) | 4 | 6 | 36.32±2.28 34.00±0.89 | ||
| (-) | 13 | 14 | 29.66±3.35 35.05±1.22 |
Notes: r: Correlation Coefficients. The correlation analysis was performed by Spearman correlation test, The data were presented as mean ± S.E.M. The statistical significance was defined as p <0.05.
Reagents
Dulbecco's modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco. HEPES (N-2-Hydroxyethylpiperazine-N’-2-ethanesulfonic acid), L-glutamine, penicillin, streptomycin, rabbit anti-serotonin, and anti-serotonin N-acetyltransferase N-terminal were obtained from Sigma-Aldrich. Anti-ASMT antibody was from Abcam Corporation. FITC-goat anti-rabbit IgG were supplied by Santa Cruz Biotechnology Corporation. HRP conjugated goat anti-rabbit IgG was purchased from Promega Corporation. The ECL chemiluminescence detection kit for Western blots was offered by Amersham Life Science. Melatonin ELISA kit was from Cloud-Clone Corporation.
Cell Culture
Human breast cancer cell line (MCF-7, BT-474, MDA-MB-231), human breast epithelial cell line (MCF-10A) and mouse breast cancer cells (4T1) were from ATCC, and Bcap-37 cell line was purchased from Shanghai Cell bank, Chinese Academy of Sciences (CAS). These cells were maintained in DMEM supplemented with 10% fetal calf serum, 55 µg/mL sodium pyruvate, 4 mg/mL glucose, 2mM L-glutamine, 25mM HEPES, 0.1 mg/L streptomycin, and 100 units/mL penicillin G were added to a 100-mm culture dish at a density of 3.0×105 cells/mL. Cells were cultured in a humidified 5% CO2 incubator at 37°C.
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described by our group. The Human breast cancer cell line (MCF-7, Bcap-37, BT-474, MDA-MB-231), human breast epithelial cell line (MCF-10A) and mouse breast cancer cells (4T1) were fixed with 4% paraformaldehyde in TBS (Tris- buffered saline: 0.05M Tris, 0.9% NaCl, pH 7.6) for 15 min at room temperature, rinsed in TBS for 3×10 min, and treated with 0.3% hydrogen peroxide in TBS for 30 min to quench endogenous peroxidase activity. Subsequently, these cells were incubated with anti-serotonin antibody. Confocal microscopy was performed and the data were processed as described previously.13
Immunohistochemistry Staining
The TPBC and TNBC sections were hydrated and treated with 1.5% hydrogen peroxide for 1 hr at 37°C, followed by treatment in 0.05 M citrate-buffered saline (pH 6.0) at 95°C for antigen retrieval. The TPBC and TNBC sections were then incubated in 5% goat serum for 1 h at 37°C prior to incubation with the primary antibodies anti-serotonin for 1 h at 37°C and overnight at 4°C. Subsequently, the TPBC and TNBC sections were incubated with the biotinylated goat anti-rabbit IgG for 1 h at 37°C, followed by incubation with avidin-biotin peroxidase complex and dehydration with ethanol. Data are presented as the percentages of positive areas of serotonin relative to the total area.
Western Blotting
The protocols used for the preparation of the cell lysate and Western blotting have been described previously. For Western blotting, cells were cultured up to to 106 cells in 100mm dishes and harvested, lysed in pre-chilled lysis buffer (50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% Nonidet p-40, 0.5% sodium deoxycholate, and a mixture of protease inhibitors) for 30 min and centrifuged at 12,000 rpm for 15 min. The supernatant was collected as the total protein of cells, which was mixed with the same amount of sample buffer. After boiling for 5 min, the protein sample was resolved on 15% SDS-PAGE gels and subsequently transferred onto nitrocellulose membranes and detected by anti-serotonin N-acetyltransferase N-terminal antibody, and anti-ASMT antibody.
Culture Medium Collection
The culture media used for melatonin determination in the human breast cancer cell (Bcap-37, MCF-7, BT-474, MDA-MB-231) and human breast epithelial cell (MCF-10A) were collected when the cells were cultured up to a cell number of 106. Fetal bovine serum (FBS)–supplemented medium was used to negative control. Light was avoided when the cell culture medium was collected and the time for collecting the cell culture medium was less than 3 minutes. Subsequently, the medium was centrifuged for 10 min at 12,000 rpm at 4°C. The supernatant was collected and kept at –80°C until assayed.
Melatonin Determination (ELISA)
The melatonin concentrations in the human breast cancer cells (Bcap-37, MCF-7, BT-474, MDA-MB-231) and human breast epithelial cell (MCF-10A) culture medium and negative controls were measured by ELISA kit. The assay was conducted according to the procedure of the commercial kit. The standard of melatonin levels in the melatonin research ELISA Kit range from 0 pg/mL to 1000 pg/mL. The standard curve of melatonin assay was highly reproducible, with an average correlation coefficients of 0.99. The average intra- and inter-assay coefficient of variation (CV) was <10% and <12%, respectively. The sensitivity of the assay is 5.17pg/mL.
Statistical Analysis
Statistical data was analyzed using t-test, which was conducted with SPSS 24.0 for Windows. Values were expressed as mean ± S.E.M. The statistical significance was defined as p <0.05.
Results
Serotonin Expression in the Cultured Human Breast Cancer Cell Line
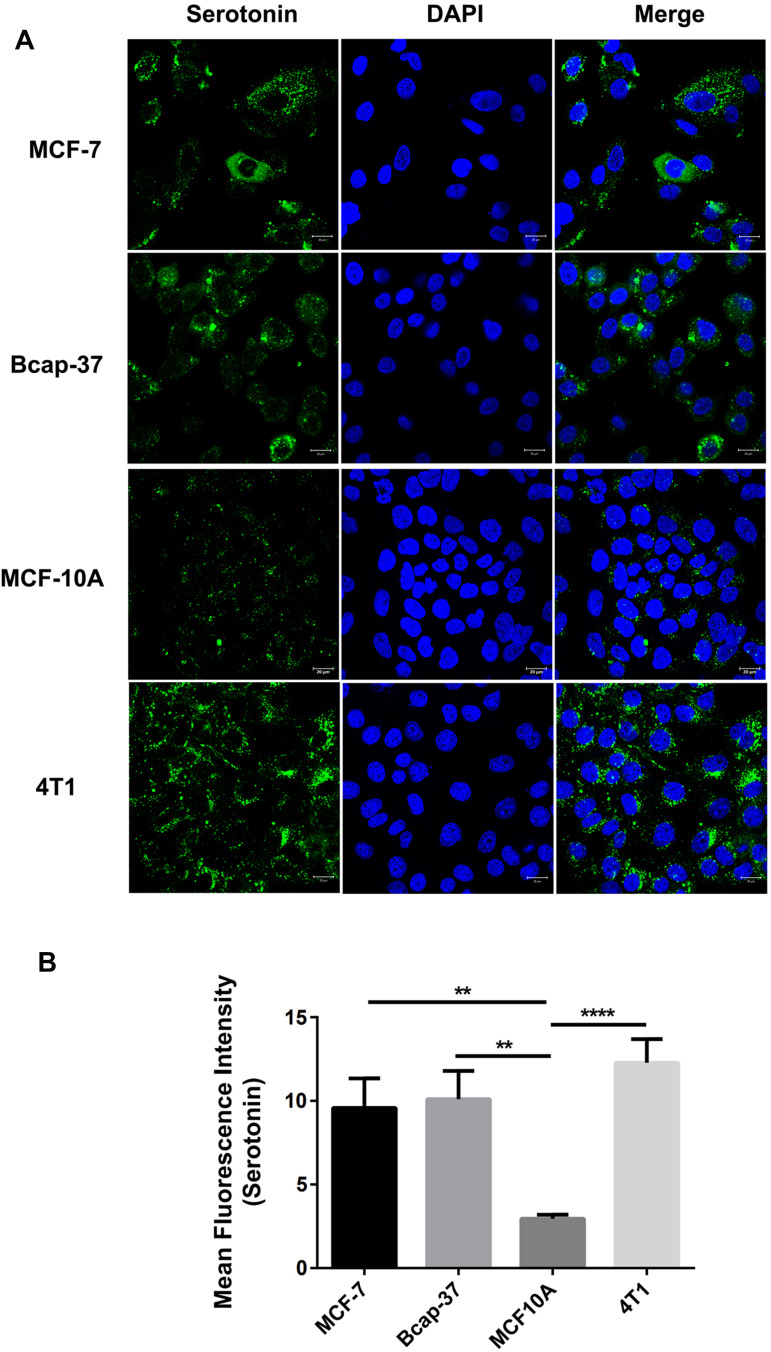
Serotonin is the precursor of melatonin synthesis. First, we investigated the presence of serotonin in the human breast cancer cells (MCF-7, Bcap-37), human breast epithelial cell (MCF-10A) and mouse breast cancer cell (4T1). Immunofluorescence staining showed that there were many serotonin-containing cells in these cells (Figure 1). In addition, the serotonin expression in the human breast cancer cell (MCF-7, Bcap-37) are much higher than that in human breast epithelial cell (MCF-10A)(p <0.01).
Figure 1.
Serotonin expression in the cultured human breast cancer cell line. Immunofluorescence staining was used in order to identify whether cultured human breast cancer cell lines (MCF-7, Bcap-37), human breast epithelial cell line (MCF-10A), and mouse breast cancer cells (4T1) expressed serotonin. Serotonin immunoreactivity (green) in the cultured human breast cancer cell lines (MCF-7, Bcap-37), human breast epithelial cell line (MCF-10A), and mouse breast cancer cells (4T1) was clearly present (A). It shows that serotonin expression was increased in the cultured human breast cancer cell lines (MCF-7, Bcap-37) compared with human breast epithelial cell line (MCF-10A) (B). Values were expressed as mean ± S.E.M.; **p<0.01, ****p<0.0001.
The Expression of Melatoninergic Systems in the Cultured Human Breast Cancer Cell
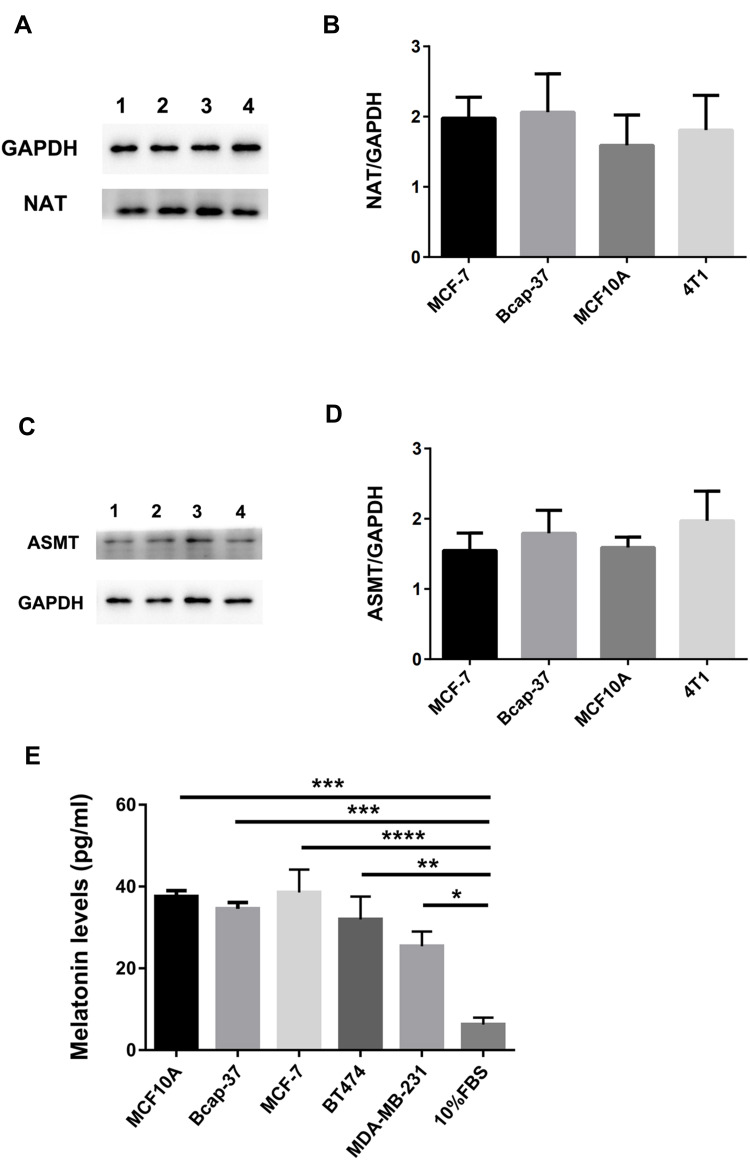
Melatonin synthesis requires the presence of the key enzymes including NAT and ASMT involved in its synthesis pathway. So, the expression of NAT and ASMT in the human breast cancer cells (MCF-7, Bcap-37), human breast epithelial cell (MCF-10A) and mouse breast cancer cell (4T1) was detected. Western blotting analysis indicated that both NAT (Figure 2A and B) and ASMT (Figure 2C and D) proteins were present in these cells.
Figure 2.
The expression of melatoninergic systems in the cultured human breast cancer cell line. Western blotting analysis was used in order to determine whether NAT and ASMT was expressed in the cultured human breast cancer cell lines (MCF-7, Bcap-37), human breast epithelial cell line (MCF-10A), and mouse breast cancer cells (4T1). NAT (A and B) and ASMT (C and D) protein expression measured by Western blotting and quantification of NAT and ASMT level relative to GAPDH. GAPDH was used as an internal control in order to measure the quality of protein. (E) Melatonin level was determined in the human breast cancer cell lines (Bcap-37, MCF-7, BT474, MDA-MB-231) and human breast epithelial cell line (MCF-10A) culture medium. All the cultured cells were incubated up to a number to 2×105 in vitro. There was a very low amount of melatonin in the 10%FBS medium. Values were expressed as mean ± S.E.M.; ****p<0.0001; ***p<0.001; **p<0.01; *p<0.05.
Melatonin levels in the culture supernatants of human breast cancer cell (Bcap-37, 34.57±1.54pg/mL; MCF-7, 38.55±5.62pg/mL; BT474, 31.97±5.62pg/mL; MDA-MB-231, 25.44±3.60pg/mL), and human breast epithelial cell (MCF-10A, 37.58±1.44 pg/mL) were measured by ELISA. A very low amount of melatonin concentration was detected in FBS–supplemented medium (6.27±1.70 pg/mL). The results suggested that there were no statistically significant differences in the melatonin levels between human breast epithelial cell (MCF-10A) and human breast cancer cell (Bcap-37, MCF-7, BT474, MDA-MB-231) (Figure 2E, p>0.05).
Serotonin Expression in the Cultured BT-474 and MDA-MB-231 Cells
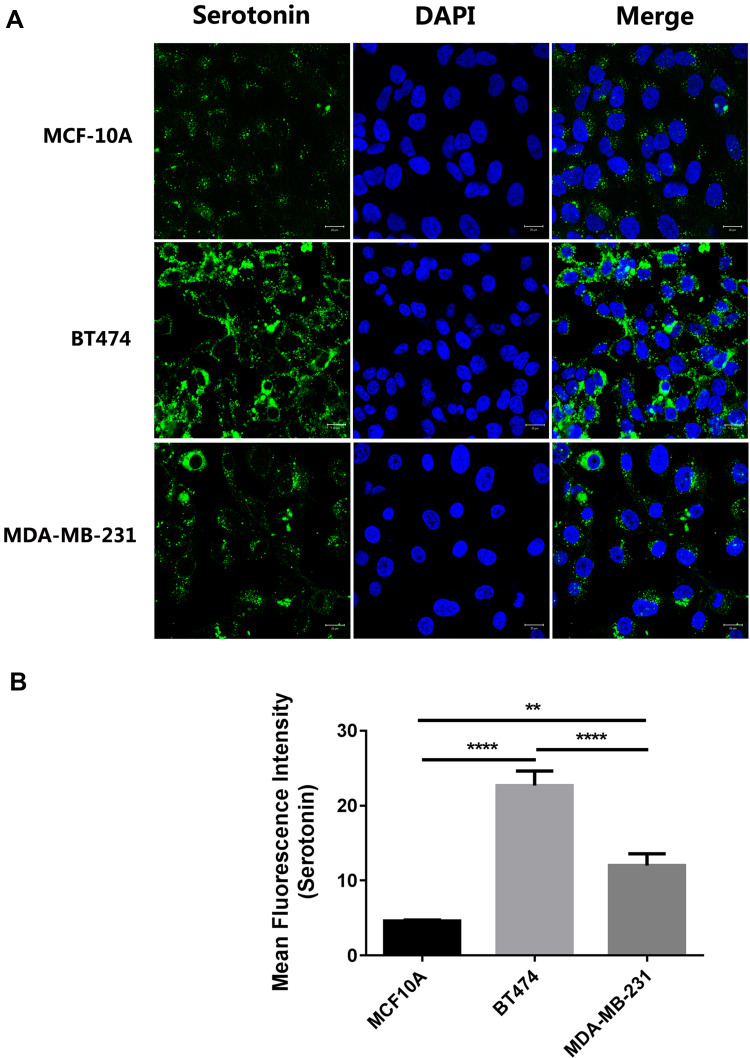
Next, we investigated the serotonin expression in the TPBC cell line BT-474 and TNBC cell line MDA-MB-231. Serotonin immunoreactivity (green) in the cultured BT-474 and MDA-MB-231 cells was clearly present (Figure 3A). It indicates that serotonin expression was increased in both BT474 and MDA-MB-231 cells compared to human breast epithelial cell line (MCF-10A). In addition, serotonin expression was much higher in BT474 compared with MDA-MB-231 cells (Figure 3B). Values were expressed as mean ± S.E.M., **p<0.01 (n=10); ****p<0.0001.
Figure 3.
Serotonin expression in the cultured BT-474 and MDA-MB-231 cells. Serotonin immunoreactivity (green) in the cultured BT-474 and MDA-MB-231 cells was clearly present (A). It indicates that serotonin expression was increased in both BT474 and MDA-MB-231 cells compared with human breast epithelial cell line (MCF-10A). In addition, serotonin expression was much higher in BT474 compared with MDA-MB-231 cells (B). Values were expressed as mean ± S.E.M.; **p<0.01 (n=10); ****p<0.0001.
Serotonin Expression in the TPBC and TNBC Tumor Sections
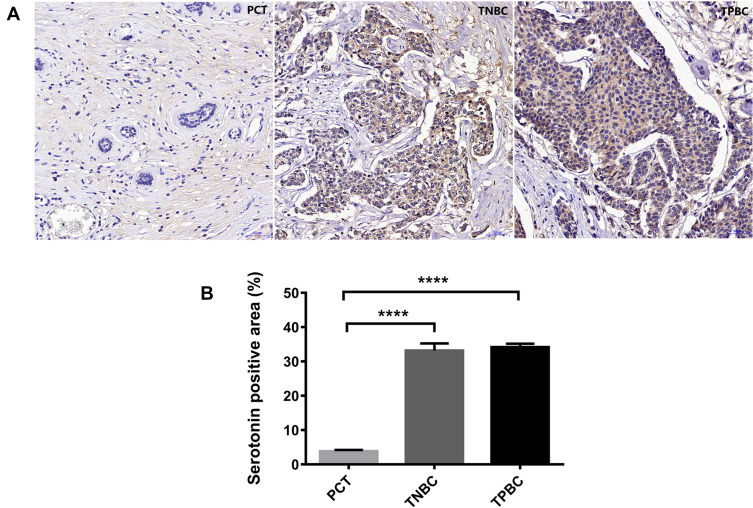
Lastly, serotonin expression in the tumor sections of TPBC and TNBC was investigated. Although there was no difference between TPBC and TNBC tumor sections in regards to serotonin expression, serotonin expression was increased in both TPBC and TNBC tumor sections compared to PCT (Figure 4). Data are the mean ± S.E.M. ****p<0.0001.
Figure 4.
Serotonin expression in the TPBC and TNBC tumor sections. Immunostaining analysis using antibodies against serotonin on tumor sections and PCT of TPBC and TNBC. The nuclei were stained with Hematoxylin (blue) (A). Quantification of serotonin positive areas relative to total areas (B). Values were expressed as mean ± S.E.M.; ****p<0.0001.
Discussion
In this study, the expression of serotonin and the key enzymes including NAT and ASMT in the melatonin synthesis pathway in human breast cancer cell (MCF-7, Bcap-37), human breast epithelial cell (MCF-10A) and mouse breast cancer cell (4T1) indicated their capability to synthesize melatonin and metabolize serotonin. In addition, the above results suggested that these cells synthesized serotonin and melatonin via traditional synthetic pathway. Despite no significant differences in the melatonin levels between human breast cancer cell (Bcap-37, MCF-7, BT474, MDA-MB-231) and human breast epithelial cell (MCF-10A), the serotonin expression in the human breast cancer cell (MCF-7, Bcap-37) was much higher than that in human breast epithelial cell (MCF-10A) (p <0.01).
Several reasons may contribute to the inconsistence. As previously mentioned, TPH is the rate limiting enzyme in serotonin synthesis, and serotonin is the precursor of melatonin. However, serotonin can also be oxidized by monoamine oxidase A (MAOA) into 5-hydroxyindoleacetic acid (5-HIAA) in the bypass of melatonin biosynthesis. In this study, the increased serotonin expression in the human breast cancer cells (MCF-7, Bcap-37) might be due to the TPH expression or TPH enzyme activity increase in its synthesis, or the oxidization by MAOA into 5-HIAA in the bypass of melatonin biosynthesis.
It has been demonstrated that serotonin receptors were expressed in the human dendritic cells,15 gastrointestinal tract,16 and breast cancer patients,17 serotonin receptor 5-HTR1B and 5- HTR2B were predominantly expressed in the cytoplasm of breast cancer cells, and correlation analysis revealed a significant correlation of 5-HTR2B with estrogen receptor-α (ER-α) and 5-HTR4 with ER-αand progesterone (PR), and the Type 7 serotonin receptor, 5-HT7, was essential in the mammary gland for regulation of mammary epithelial structure and function.18 We speculated that the difference in the amount of serotonin between TPBC and TNBC strains might be caused by different expression patterns of serotonin receptors (5-HTRs) in human breast cancer specimens. On the other hand, as a critical local regulator of epithelial homeostasis in the breast, serotonin exerted its actions through its receptors, and dysregulation of serotonin signaling was reported to contribute to breast cancer pathophysiology by enhancing cell proliferation and promoting resistance to apoptosis.19 In addition, serotonin activated glycolysis and mitochondrial biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively.20 In this study, the result that the higher serotonin expression in both breast cancer cell line (BT474, MDA-MB-231) and breast cancer tissue compared with controls indicated that serotonin might be used as a predictive marker for breast cancer patients.
This study has some limitations. Firstly, TPH expression or TPH enzyme activity in serotonin synthesis pathway was not detected in breast cancer tissue. In addition, serotonin receptors in these tumor tissues were not investigated. Furthermore, in contrast to triple-positive breast cancer (TPBC), triple-negative breast cancer (TNBC) were characterized by poor prognosis, poor differentiation, aggressive behavior, and increased rate of node invasion.21 The mechanisms of action of serotonin in regulating breast cancer markers were not investigated in this study, which were our interests for further research. Furthermore, the sample size should be increased in the future studies.
Taken together, these data provide definitive evidence of the capability of human breast cancer cells (MCF-7, Bcap-37), and human breast epithelial cell (MCF-10A) to metabolize L-tryptophan to serotonin and further to melatonin. Given the multiple systemic actions of serotonin, as a neurotransmitter, growth factor, and immunomodulator, and serotonin expression was higher in the breast cancer tissue compared with PCT, the current findings potentially had significant physiologic or pathologic implications. Especially, serotonin might be used as a predictive marker for breast cancer patients.
Funding Statement
The present work was supported by the National Natural Science Foundation of China (81771653), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT310002), National Natural Science Foundation of Anhui Province (1808085QH240), Key research and development program of Anhui Province (202004j07020043), the National key research and development program of China (2017YFC1001300), the Key Science Research Project in Universities of Anhui Province (KJ2017A194), the University Synergy Innovation Program of Anhui Province (GXXT-2019-044), and the Key Excellent Young Talents Support Program in Universities of Anhui Province (gxyqZD2017031). Key research and development program of Anhui Province.
Abbreviations
NAT, serotonin N-acetyltransferase; TPH, tryptophan hydroxylase; MAOA, monoamine oxidase A; AADC, aromatic L-amino acid decarboxylase; HIOMT, hydroxyndole-O-methyltransferase; 5-HIAA, 5-hydroxyindoleacetic acid; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle’s medium; HEPES, N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid; TBS, Tris-buffered saline; ASMT, acetylserotonin methyltransferase.
Compliance with Ethical Standards
Ethics approval was obtained from the ethics committee of clinical medical research, First Affiliated Hospital of Anhui Medical University (case number: PJ2017-08-05), and all patients signed informed consent prior to sample collection.
Disclosure
The authors declare that they have no competing interests.
References
- 1.da Silva JL, Cardoso Nunes NC, Izetti P, et al. Triple negative breast cancer: a thorough review of biomarkers. Crit Rev Oncol Hematol. 2020;145:102855. doi: 10.1016/j.critrevonc.2019.102855 [DOI] [PubMed] [Google Scholar]
- 2.Crispino M, Volpicelli F, Perrone-Capano C. Role of the serotonin receptor 7 in brain plasticity: from development to disease. Int J Mol Sci. 2020;21(2):505. doi: 10.3390/ijms21020505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H, Denna TH, Storkersen JN, et al. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–114. [DOI] [PubMed] [Google Scholar]
- 4.Ballou Y, Rivas A, Belmont A, et al. 5-HT serotonin receptors modulate mitogenic signaling and impact tumor cell viability. Mol Clin Oncol. 2018;9(3):243–254. doi: 10.3892/mco.2018.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hejazi SH, Ahangari G, Pornour M, et al. Evaluation of gene expression changes of serotonin receptors, 5-HT3AR and 5-HT2AR as main stress factors in breast cancer patients. Asian Pac J Cancer Prev. 2014;15(11):4455–4458. doi: 10.7314/APJCP.2014.15.11.4455 [DOI] [PubMed] [Google Scholar]
- 6.Acuna-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swami T, Weber HC. Updates on the biology of serotonin and tryptophan hydroxylase. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):12–21. doi: 10.1097/MED.0000000000000383 [DOI] [PubMed] [Google Scholar]
- 8.Banskota S, Ghia JE, Khan WI. Serotonin in the gut: blessing or a curse. Biochimie. 2019;161:56–64. doi: 10.1016/j.biochi.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 9.Masson J. Serotonin in retina. Biochimie. 2019;161:51–55. doi: 10.1016/j.biochi.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ, Zhuang J, Zhu HY, et al. Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res. 2007;43(3):232–238. doi: 10.1111/j.1600-079X.2007.00466.x [DOI] [PubMed] [Google Scholar]
- 11.Liu YJ, Meng F-T, Wang -L-L, et al. Apolipoprotein E influences melatonin biosynthesis by regulating NAT and MAOA expression in C6 cells. J Pineal Res. 2012;52(4):397–402. doi: 10.1111/j.1600-079X.2011.00954.x [DOI] [PubMed] [Google Scholar]
- 12.Liu YJ. Serotoninergic and melatoninergic systems are expressed in mouse embryonic fibroblasts NIH3T3 cells. Neuro Endocrinol Lett. 2013;34(3):236–240. [PubMed] [Google Scholar]
- 13.Liu YJ. Melatonin maintains mitochondrial membrane potential and decreases excessive intracellular Ca2+ levels in immature human oocytes. Life Sci. 2019;235:116810. doi: 10.1016/j.lfs.2019.116810 [DOI] [PubMed] [Google Scholar]
- 14.Sarrouilhe D, Mesnil M. Serotonin and human cancer: a critical view. Biochimie. 2019;161:46–50. doi: 10.1016/j.biochi.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 15.Szabo A, Gogolak P, Koncz G, et al. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. 2018;8(1):1765. doi: 10.1038/s41598-018-20173-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasi EA, Allen AA, Sugianto W, et al. Identification of three antimicrobials activating serotonin receptor 4 in colon cells. ACS Synthetic Biology. 2019;8(12):2710–2717. doi: 10.1021/acssynbio.9b00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopparapu PK. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33(2):363–370. [PubMed] [Google Scholar]
- 18.Pai VP, Hernandez LL, Stull MA, et al. The Type 7 Serotonin Receptor, 5-HT 7, is essential in the mammary gland for regulation of mammary epithelial structure and function. Biomed Res Int. 2015;2015:364746. doi: 10.1155/2015/364746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jose J, Tavares CDJ, Ebelt ND, et al. Serotonin analogues as inhibitors of breast cancer cell growth. ACS Med Chem Lett. 2017;8(10):1072–1076. doi: 10.1021/acsmedchemlett.7b00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sola-Penna M, Paixão LP, Branco JR, et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122(2):194–208. doi: 10.1038/s41416-019-0640-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma D, Kumar S, Narasimhan B. Estrogen alpha receptor antagonists for the treatment of breast cancer: a review. Chem Cent J. 2018;12(1):107. doi: 10.1186/s13065-018-0472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]