Abstract
Urinary tract infection (UTI) is the most common bacterial disease in childhood worldwide and may have significant adverse consequences, particularly for young children. In this guideline, we provide the most up-to-date information for the diagnosis and management of community-acquired UTI in infants and children aged over 90 days up to 14 years. The current recommendations given by the American Academy of Pediatrics Practice guidelines, Canadian Pediatric Society guideline, and other international guidelines are considered as well as regional variations in susceptibility patterns and resources. This guideline covers the diagnosis, therapeutic options, and prophylaxis for the management of community-acquired UTI in children guided by our local antimicrobial resistance pattern of the most frequent urinary pathogens. Neonates, infants younger than three months, immunocompromised patients, children recurrent UTIs, or renal abnormalities should be managed individually because these patients may require more extensive investigation and more aggressive therapy and follow up, so it is considered out of the scope of these guidelines. Establishment of children-specific guidelines for the diagnosis and management of community-acquired UTI can reduce morbidity and mortality. We present a clinical statement from the Saudi Pediatric Infectious Diseases Society (SPIDS), which concerns the diagnosis and management of community-acquired UTI in children.
Keywords: Urinary tract infection, UTI, Community-acquired UTI, Acute pyelonephritis, Children, Escherichia coli, Saudi children, Saudi Arabia, Cystitis, Prophylaxis, Vesicoureteral reflux
1. Purpose and scope of the guidelines
-
•
The purpose of this guideline is to provide evidence-based guidance on the most effective diagnosis and management of community-acquired urinary tract infection (UTI) in infants and children.
-
•
The target population is mainly a pediatric age group from 3 months of age up to 14 years who presented with uncomplicated community-acquired UTI. Neonates, infants younger than 3 months, children with complicated UTI, hospital-acquired UTI, or renal disorders should be managed individually because these patients may require more different management routes; hence, it is considered out of the scope of these guidelines.
-
•
We aim to avoid a delayed diagnosis of UTI particularly in infants and young children with nonspecific signs and symptoms as well as to avoid frequent diagnosis of contaminated urine specimens as UTI. Delay in the diagnosis and management of UTIs may potentially result in renal damage and loss of renal function. The main need for this guideline is to facilitate decision-making in clinical practice and improve outcome in patients with UTI.
-
•
The target audience for these guidelines includes general physicians, family medicine specialist, pediatricians, infectious diseases specialists, pediatric urologist, radiologist, and any clinicians and healthcare providers caring for this condition.
2. Definitions
2.1. Urinary tract infection (UTI)
Defined as significant bacteriuria of a urinary pathogen in a symptomatic patient.
2.2. Significant bacteriuria
The diagnosis of significant bacteriuria depends upon the method of urine sample collection and quantitative urine culture result on the basis of the number of colony-forming units (CFUs) that grow in the culture medium [1].
Our threshold for significant bacteriuria is as follows:
-
•
For Suprapubic urine aspirate: any number of CFUs per mL of a uropathogen [2].
-
•
For Catheter sample: the presence of at least 50,000 CFUs per mL of a uropathogen [1].
-
•
For Clean-voided sample: growth of ≥100,000 CFU/mL of a uropathogen [1].
-
o
Growth of ≥10,000 CFU/mL of a single uropathogen from a urinary sample collected by either a catheter or clean-voided method should be considered sufficient for UTI diagnosis in symptomatic patients with fever and/or urinary symptoms, particularly when pyuria is present [[1], [2], [3], [4]].
2.3. Pyuria
Defined by one of the following [1]:
-
•
Any leukocyte esterase on dipstick analysis
-
•
≥5 WBC/high-power field in centrifuged urine sample
-
•
≥10 leukocytes/mm3 in centrifuged urine sample
2.4. Asymptomatic bacteriuria
Defined as the isolation of a significant quantitative count of bacteria in an appropriately collected urine specimen from an individual without symptoms of UTI [5].
2.5. Cystitis (lower UTI)
Cystitis is an inflammation of the urinary bladder mucosa with symptoms, including dysuria, frequency, urgency, malodorous urine, incontinence, hematuria, and suprapubic pain. However, in newborns and infants, these symptoms are rarely diagnosed accurately [6].
2.6. Pyelonephritis (upper UTI)
Pyelonephritis is a diffuse pyogenic infection of the renal pelvis and parenchyma with symptoms of fever, flank pain, or costovertebral angle tenderness with other clinical manifestations of systemic illness (including chills, rigors, fatigue, or malaise). In infants and young children, these classical manifestations may be absent and differentiation between cystitis and pyelonephritis is often difficult [6].
2.7. Recurrent UTI
SPIDS defined recurrent UTI as two or more episodes of symptomatic UTI within 12 months.
2.8. Urosepsis
Urosepsis is defined as a type of sepsis that is caused by an infection originating from the urinary tract and may become life-threatening. Sepsis is a systemic inflammatory response syndrome that includes abnormalities in two or more of the following parameters: body temperature, heart rate, respiratory rate, and leukocyte count [7].
2.9. Severe urosepsis
Severe urosepsis is a sepsis associated with cardiovascular dysfunction, acute respiratory distress syndrome, or dysfunction in two or more other organ systems (e.g., hematology, renal, hepatic, or neurologic) [7].
2.10. Evidence of urinary tract obstruction
Features that raise the suspicion for urinary tract obstruction include: elevated serum creatinine, hypertension, hematuria, poor urine output, or the presence of abdominal or bladder mass [8].
2.11. Complicated UTI
Defined by any of the following [2]:
-
o
UTI in a critically ill patient (e.g., Severe Urosepsis or shock)
-
o
UTI in a patient with the evidence of urinary tract obstruction
-
o
UTI in a patient who failed to respond after 48 h of starting an appropriate antibiotic therapy
3. Epidemiology and causative agents
-
•
UTI is the most common bacterial disease in childhood worldwide and may have significant adverse consequences, particularly in young children. It became one of the major problems in primary healthcare, general practice, and emergency departments of Saudi Arabia [9]. UTI is relatively common in infants and children; by the age of seven years, approximately 1.6% of boys and 7.8% of girls will experience at least one episode of UTI [10].
-
•
Escherichia coli (E. coli) is the most common bacterial uropathogen in pediatrics [11]. Other uropathogens include Klebsiella, Pseudomonas, Proteus, Enterobacter, Citrobacter, Enterococcus, Staphylococcus saprophyticus in the adolescent girl, and rarely Staphylococcus aureus [11].
-
•
In the Saudi pediatric population, E. coli is the most frequently isolated uropathogens reported in (70%–80%) of infants and children presented with UTI followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus species [[12], [13], [14]].
4. Pathogenesis and risk factors
-
•
The majority of UTIs begin with the colonization of the periurethral area by either the urinary pathogen or fecal flora; although, the presence of pathogens alone in the periurethral is not enough for the development of UTI [15,16]. The uropathogens will attach to the uroepithelium and trigger the cytokine's release after the recruitment of the toll-like receptors, this will lead to the generation of local inflammatory response [16,17]. The uropathogens will ascend from the periuretheral area to the bladder through the urethra and may also ascend to the kidneys through the ureters causing pyelonephritis. Bacteremia may develop, particularly in young infants and in those with underlying urological abnormalities [18].
-
•
There are also many host-associated factors that can increase the risk of UTI in children, including the following:
- a)
-
b)Circumcision status
-
oAn uncircumcised male infant with fever has up to eightfold higher prevalence of UTI than a circumcised boy with the highest prevalence in those less than 3 months [19].
-
o
-
c)Urinary obstruction
-
oChildren with functional or anatomical obstructive urological abnormalities such as posterior urethral valves, ureteropelvic junction obstruction (UPJO), and neurogenic bladder are at a high risk to develop UTI. Retention and stasis of urine with suboptimal clearance of bacteria from the urinary tract are excellent factors for most urinary pathogens [20].
-
o
-
d)Vesicoureteral reflux (VUR)
-
oVUR predisposes patients to recurrent UTI [21].
-
o
- e)
5. Clinical presentation
-
•
Clinical symptoms and signs for UTI are very broad and nonspecific, particularly in infant and young children. Clinical presentation becomes more obvious as the child grows older.
-
•
In infant and nonverbal children: UTI can present with fever, irritability, lethargy, poor feeding, or gastrointestinal symptoms (e.g., vomiting and diarrhea) [6]. Parents may report crying or discomfort of their infant with micturition in addition to poor urinary stream or foul-smelling urine.
-
•
In older verbal children: Symptoms and signs of UTI include fever, urinary symptoms (dysuria, frequency, urgency, secondary incontinence, change in urine color cloudy, or bloody), vomiting, abdominal, or back pain, suprapubic tenderness, and costovertebral angle tenderness [6].
The presence of fever, chills, or rigors with flank pain or costovertebral angle tenderness is highly suggestive of pyelonephritis in older children [6].
6. Diagnosis
6.1. SPIDS recommendation for UTI diagnosis
-
6.1.1
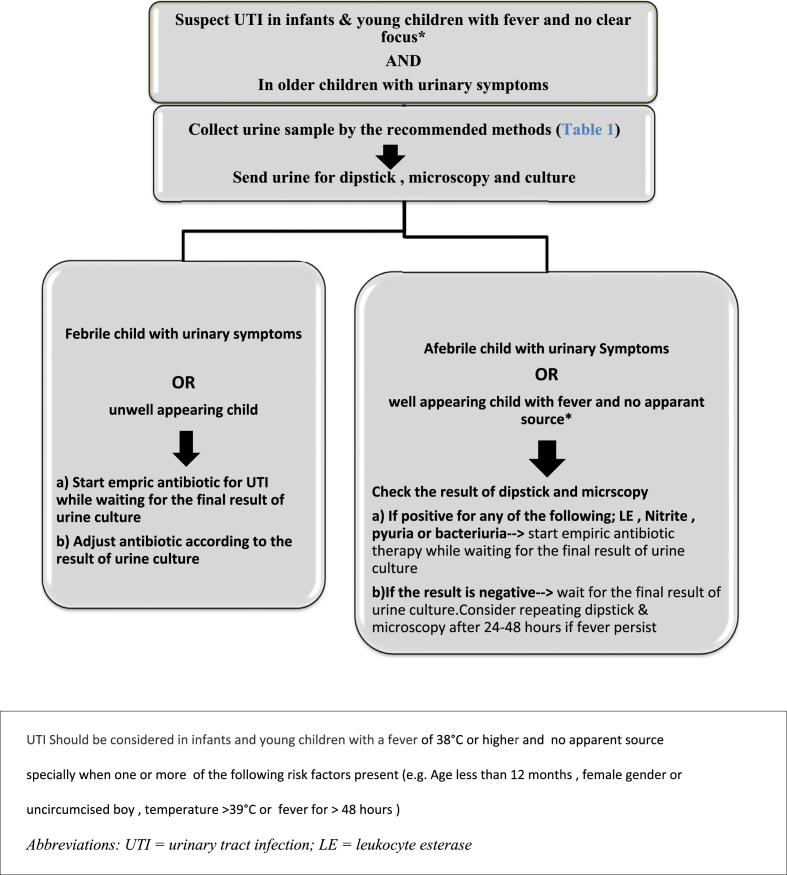
UTI should be considered in infants and young children with a fever of 38 °C or higher and no apparent source particularly when one or more of the following risk factors are present (e.g., age less than 12 months, female gender or uncircumcised male, temperature >39 °C, or fever for >48 h) (Fig. 1) [4].
-
6.1.2
UTI should be considered in older verbal children with urinary symptoms (e.g., dysuria, frequency, urgency, and abdominal pain)
-
6.1.3
In infants and children with suspected UTI, urine specimens should be collected for dipstick, microscopy, and culture before starting antimicrobial therapy, and the urine sample should be processed as soon as possible after collection
-
6.1.4
Diagnosis of UTI should only be made based on the combination of clinical symptoms and positive quantitative urine culture according to the method of urine sample collection (see the definition of significant bacteriuria)
Fig. 1.
Algorithm for urine testing and treatment of children with suspected UTI.
6.2. Clinical evaluation: [23]
-
o
Infant and children with suspected UTI should be evaluated promptly, including a detailed history for the acute illness (e.g., Fever, urinary symptoms, and antibiotic administration) in addition to the past medical and family history should be provided.
-
o
A complete physical examination is necessary to exclude any other source of fever. Physical examination should include the most important aspects in children with suspected UTI, such as the temperature degree, blood pressure, growth parameters, detailed abdominal, and flank examination looking for palpable masses or tenderness as well as the external genitalia for anatomical disorders and for circumcision status. The lower back should be evaluated for signs of occult meningomyelocele (e.g., area of pigmentation, sinus, and tuft of hair or lipoma).
6.3. Laboratory evaluation
6.3.1. Urine specimen collection, preservation, dipstick, microscopy, and culture
6.3.1.1. Background and rationale
-
o
Urine samples should be collected for dipstick, microscopy, and culture before starting antimicrobial therapy
-
o
There are four main methods of urine sample collection: urinary bag, clean-voided urine (CVU), transurethral bladder catheterization (BC), and suprapubic aspiration (SPA). SPA and transurethral BC are the least likely to yield a contaminated growth and recommended for nontoilet-trained children. CVU sample is an easy method with a good rate of accuracy in toilet-trained children. Urinary bag is a technique that is commonly used in daily practice by many physicians; however, it is not recommended as a result of the contamination rate, which is extremely high. It is helpful mainly in children with a negative culture result to exclude UTI [1,2]. SPIDS-recommended methods for urine sample collection are summarized in (Table 1). Urine specimen needs to be transferred to the laboratory and processed as soon as possible, preferably in less than 2 h, if the sample is kept at room temperature to prevent the growth of organisms [24].
-
o
Urine analysis (Urine dipstick and microscopy) remains a useful tool for screening of UTI. Leukocyte esterase (LE) is an enzyme present in leukocytes and is an indirect measure of pyuria [4,25]. The nitrite test measures the conversion of dietary nitrate to nitrite by some gram-negative bacteria, which requires approximately 4 h to complete the conversion in the bladder [26] Neither LE nor nitrites are fully sensitive or specific for UTI, but they are a useful screening test, particularly when used in combination. The AAP 2011 guideline [4] reported pooled sensitivity and specificity for the result of macroscopic and microscopic urine analyses (Table 2). Some conditions other than UTI can cause a false-positive result of LE, such as group A streptococcal infections and Kawasaki disease after vigorous exercise [4]. If UTI is thought unlikely, dipsticks have a good negative predictive value to exclude the diagnosis [27]. Urine microscopy can be done in either of two ways, (manual analysis of the centrifuged urine sample or automated analysis “enhanced analysis” of an uncentrifuged urine sample). The presence of pyuria (see definition) and bacteriuria can increase the likelihood of UTI [4]. Sensitivity, specificity, condition associated with false-positive and false-negative results of bacteriuria, or pyuria are highlighted in (Table 2).
-
•
Urine culture is the gold standard for UTI diagnosis [2,4,23,25]. Urine is sterile, so the presence of bacteria in sufficient quantity, in an appropriate specimen with concurrent evidence of symptoms with/without supported urine analysis, is highly suggestive of UTI. Many guidelines defer in the definition of significant microorganism growth based on colony-forming units (CFUs/mL) found on incubated medium plates. American guidelines suggest the threshold of 50,000 CFU/mL with concurrent pyuria for SPA and catheter specimens [4]. However, more recent evidence suggests that some are true.
-
•
UTI may not present with significant pyuria or positive analysis [28]. Additionally, newer data showed that an even lower threshold of 10,000 CFU/mL would slightly increase sensitivity without the reduction of diagnostic specificity of UTI [29]. The recommended cut-off number of CFUs/ml for significant bacteriuria according to the method of urine sample collection is outlined in (Table 1).
Table 1.
Recommended method for urine sample collection and cut-off number of colony-forming units/ml for significant bacteriuria according to the method of urine sample collection.
| Method of urine sample collection | Recommendation | Cut-off number for significant bacteriuria |
|---|---|---|
| Transurethral BC | First choice for Infants and nontoilet-trained children | >50.000 CFU/mL[1–3] OR >10.000 CFU/mL if fever and pyuria[1–3] |
| A clean-voided specimen | First choice for toilet-trained children Second choice for nontoilet-trained children who cannot undergo transurethral BC(e.g., increased bleeding tendency) |
>100.000 CFU/ml[1–3] OR >10.000 CFU/mL if fever and pyuria present [3] |
| Suprapubic aspiration (SPA) | Reliable method in neonates and an alternative method for males with phimosis or females with severe labial adhesions. | Any number of CFU per mL[2,3] |
AbbreviationsUTI = urinary tract infection; CFU = colony-forming units, and transurethral bladder catheterization = transurethral BC.
Table 2.
Sensitivity and specificity of components of urinalysis, alone and in combination [4].
|
Test |
Sensitivity (Range),% | Specificity (Range),% |
|---|---|---|
| Leukocyte esterase test | 83 (67–94) | 78 (64–92) |
| Nitrite test | 53 (15–82) | 98 (90–100) |
| Leukocyte esterase or nitrite test positive | 93 (90–100) | 72 (58–91) |
| Microscopy, WBCs | 73 (32–100) | 81 (45–98) |
| Microscopy, bacteriuria | 81 (16–99) | 83 (11–100) |
| Leukocyte esterase test, nitrite test, or microscopy positive | 99.8 (99–100) | 70 (60–92) |
Table reproduced with permission from Pediatrics, volume 128, pages 595–610, copyright © 2011 by the American Academy of Pediatrics [4].
6.3.1.2. SPIDS recommendation for the method of urine sample collection and preservation
-
•
The urine sample must be performed prior to the initiation of any antimicrobial agent in infant and children with suspected UTI.
-
•
For infants and nontoilet-trained children: Urinary catheterization is the recommended method of urine sample collection, an alternative method is an SPA preferably ultrasound-guided reserved for males with phimosis or females with severe labial adhesions [1,2].
-
•
For children who are toilet-trained: A CVU specimen is the recommended method of urine collection, provided proper instruction is given to the patient/caregiver with regard to hygiene and collection techniques [1,2].
-
•
Nontoilet-trained patients who cannot undergo either SPA or catheterized sample (e.g., increased bleeding tendency), a CVU specimen can be considered [1,2]. The recommended cut-off number of colony-forming units/ml for significant bacteriuria according to the urine collection method is summarized (Table 1).
-
•
We recommend against the use of a sterile urinary bag for urine sample collection in all age groups [1,2].
-
•
All urine specimens should be examined as soon as possible after collection, preferably in less than 2 h, if the sample is kept at room temperature [24].
6.3.1.3. SPIDS recommendation for urine dipstick and microscopy request and interpenetration
-
o
SPIDS recommends using urine dipsticks and microscopy as a screening tool for suspected cases of UTI [2,4,25].
-
o
Positive nitrites on dipstick analysis indicate that UTI is likely; although, a negative nitrite test does not exclude a UTI [4,26].
-
o
Positive leukocyte esterase on dipstick analysis is suggestive of UTI but is nonspecific (see differential diagnosis of pyuria without bacteriuria) [4,28].
-
o
UTI is unlikely when both results of LE and nitrites are negative; however, in a febrile high-risk child with no clear focus, a new urine dipstick and microscopy should be done after 24–48 h if fever persists.
6.3.1.4. SPIDS recommendation for positive urine culture result
-
•
Quantitative urine culture with significant bacteriuria based on the method of urine sample collection is required for the diagnosis of UTI (see the definition for significant bacteriuria) (Table 1).
6.3.2. SPIDS recommendation for other laboratory tests
-
o
Other laboratory tests (e.g., complete blood count, inflammatory markers, serum creatinine, blood culture, or lumber puncture) should not be routinely obtained in infants older than 3 months of age with suspected UTI who appear to be healthy, not dehydrated, immunocompetent, and with no other reasons to be obtained [30,31].
-
o
Additional laboratory tests should be considered selectively for patients who appear to be healthy, dehydrated, immunocompromised, have recurrent UTI, and admission to the hospital.
7. Differential diagnosis
Differential diagnosis of UTI should include other conditions that may present with clinical or laboratory findings similar to UTI such as any of the following conditions:
-
•
Fever without focus on children aged from 3 to 36 months: occult bacteremia and occult pneumonia
-
•
Urinary symptoms without bacteriuria: Children with BBD are frequently presented with urinary symptoms (e.g., urgency and dysuria). In such patients, urine culture is typically negative and pyuria is not present [22].
-
•
Asymptomatic bacteriuria: (see definitions) the child might present with nonspecific symptoms (e.g., fever and abdominal pain) caused by infection other than UTI such as gastroenteritis. Pyuria can also be detected in patients with asymptomatic bacteriuria and is not an indication of a real UTI. Spontaneous resolution is common and antibiotic treatment is not recommended [5].
-
•
Appendicitis, Kawasaki disease, and Group a streptococcal infection; these conditions can present with fever, abdominal pain, and pyuria but with a negative urine culture result
-
•
Urethritis, nonspecific vulvovaginitis, and vaginal foreign body: can present with urinary symptoms and bacteriuria with or without pyuria
8. Treatment
8.1. SPIDS recommendation for UTI treatment
For any suspected community-acquired UTI, obtain a urine analysis and culture and initiate empiric antibiotics based on local resistance patterns of urinary pathogens if available and change based on the susceptibility of the isolated pathogen (Fig. 1).
-
•
SPIDS recommends against treating asymptomatic bacteriuria in infants and children [5].
-
•
Check any previous urine culture and susceptibility findings, antibiotic prescription, and select antibiotics appropriately. A breakthrough infection while the patient on antibiotics prophylaxis needs treatment with different antibiotics.
8.2. SPIDS recommendations for the hospitalization of infants and children with UTI
Propose, under those conditions, hospitalization for the following infants and children:
-
•
Infants and children who require IV fluids
-
•Infants and children who require IV antibiotics due to
-
oIll-appearance, hemodynamically unstable, or clinical suspicion of urosepsis.
-
oSevere illness with vomiting and dehydration
-
oInability to tolerate oral antibiotics
-
oLack of response to oral antibiotics (worsening symptoms or persistent fever for more than 48 h).
-
oEvidence of urinary tract obstruction (see definition)
-
o
8.3. Empiric choice of antimicrobial therapy (oral vs. intravenous)
8.3.1. Background and rationale
-
•
E. coli and K. pneumonia are the most frequent uropathogens causing UTIs in Saudi children. Reviewed data from antibiograms of several tertiary care organizations in the regions of Saudi Arabia show significant changes in the susceptibility pattern of the most frequently isolated uropathogens to the usual empirical antibiotics in the last several years [9,14,32]. Among those isolates, data showed a very high prevalence of resistance against ampicillin and co-trimoxazole (nearly 70% and 60%, respectively). However, most of the isolated uropathogens were highly susceptible to a third-generation cephalosporin and aminoglycoside [9,14,32]. Therefore, ampicillin and co-trimoxazole should be avoided as empiric therapy; although, third generation cephalosporin or aminoglycoside remains an appropriate empiric antibiotic therapy for children admitted with community-acquired UTI.
-
•
Most patients with CA-UTI can be treated at an outpatient setting. Evidence showed that oral antibiotics are usually appropriate and in children aged ≥2 months, initiating treatment orally or parentally is equally effective [4]. IV antibiotics are indicated for all infants (≤60 days old), high-risk children unable to tolerate oral treatment, poor response to oral treatment, any child who is seriously unwell, or with complicated UTI [2,25]. Based on the Cochrane review of 23 randomized trials, the evaluation of antibiotics in 3295 children with proven UTI and acute pyelonephritis, oral antibiotics appear as effective as sequential short-course IV antibiotics followed by oral therapy [33]. For acute pyelonephritis or febrile UTIs, oral antibiotics can be an appropriate line of management in nontoxic children with no known structural urological abnormality, which assumes that they are likely to receive and tolerate every dose [33,34]. For infants or children with complicated pyelonephritis/urosepsis initial IV antibiotics are recommended followed by oral therapy as appropriate. In most of the patients who were started on appropriate UTI treatment; fever normalized within 24-48 h, urine became sterile after about 24 h, and pyuria usually disappeared within 3-4 days. [34].
8.3.2. SPIDS recommendations oral antibiotic therapy
-
•
Empirical antibiotic choice should be based on local antimicrobial sensitivity patterns if available and be adjusted based on sensitivity testing of the isolated pathogen.
-
•SPIDS recommends one of the following oral antibiotics as a first-line therapy for individuals with community-acquired UTI.
-
1Amoxicillin-clavulanic acid
-
2Cephalexin
-
3Cefuroxime or cefprozil
-
1
-
•
The recommended doses and duration of oral antibiotic therapy outlined in (Table 3, Table 4).
Table 3.
List of the preferred empiric antibiotic agents and alternative for infants and children with community-acquired UTI.
| Age | Treatment sitting | Empirical therapy |
Duration of treatment | |
|---|---|---|---|---|
| First line (one of the following) | Alternativea | |||
| ≥3 months – 14 Years | Outpatient | Amoxicillin-clavulanic acid | Cefixime | 3–7 days |
| Cephalexin | ||||
| Cefuroxime | ||||
| Cefprozil | ||||
| Inpatienta | Ceftriaxone |
Gentamycinb +/-Ceftriaxonec |
7–14 days | |
Check any previous urine culture and susceptibility results and choose antibiotics accordingly.
Alternative: If no improvement of fever and UTI symptoms at least 48 h after starting the first choice or when first choice not suitable.
Gentamicin should be considered in children with previous UTI caused by ESBL-producing bacteria and in those who have been recently exposed to cephalosporin antibiotic treatment during the last 3 months.
Broader or combined antimicrobial therapy of ceftriaxone and aminoglycoside may be indicated in critically ill patients and in those whose clinical condition worsens after starting the first-line antimicrobial therapy.
Table 4.
Oral antibiotics commonly used to treat urinary tract infections (UTIs) in children 3 months of age and older if the isolate is susceptible.
| Drug | Dosage per day |
|---|---|
| Amoxicillin | 50 mg/kg/day (divided in three doses) |
| Amoxicillin/clavulanate | 45 mg/kg/day of Amoxicillin component (divided in three doses) |
| Co-trimoxazole | 8 mg/kg/day of the trimethoprim component, (divided in two doses) |
| Cefixime | 8 mg/kg/day (given as a single dose) |
| Cefuroxime | 30 mg/kg/day (divided in two doses) |
| Cefprozil | 30 mg/kg/day (divided in two doses) |
| Cephalexin | 50 mg/kg/day (divided in four doses) |
| Nitrofurantoin | 5–7 mg/kg/day (divided in four doses) |
8.3.3. SPIDS recommendation for intravenous antibiotic therapy (IV)
-
•
Third-generation parenteral cephalosporin (e.g., ceftriaxone) is an appropriate first-line treatment option for hospitalized infants and children with community-acquired UTI [9,14,32].
-
•
Aminoglycosides (e.g., gentamicin) should be considered in children with previous UTI caused by ESBL-producing bacteria and in those who have been recently exposed to cephalosporin antibiotic treatment during the last 3 months [35,36].
-
•
Broader or combined antimicrobial therapy of ceftriaxone and aminoglycoside may be indicated in critically ill patients and in those whose clinical condition worsens after starting the first-line antimicrobial therapy.
-
•The recommended doses and duration of IV antibiotic therapy are outlined in Table 3, Table 5
-
•Adjust the antibiotics therapy according to the results of culture and sensitivity.
-
•Step down to oral antibiotics according to the culture and sensitivity results should be considered as soon as clinically possible.
-
•
Table 5.
IV antibiotics commonly used to treat urinary tract infections (UTIs) in children 3 months of age and older if the isolate is susceptible.
| Parenteral antibiotics | |
|---|---|
| Drug | Dosage per day |
| Ampicillin | 200 mg/kg/day IV (divided every 6 h) |
| Ceftriaxone | 50–75 mg/kg IV/IM every 24 h |
| Cefotaxime | 150 mg/kg/day IV (divided every 8 h) |
| Gentamicin | Initially 5–7.5 mg/kg once a day, subsequent doses adjusted according to serum gentamicin concentration |
| Amikacin | Initially 15 mg/kg once a day, subsequent doses adjusted according to serum amikacin concentration |
8.4. Duration of antimicrobial therapy
8.4.1. Background and rationale
The optimal duration of antibiotic therapy for the treatment of UTI remains controversial [1]. In a Cochrane systematic review of 10 randomized and quasi-randomized controlled studies involving 652 children with lower UTI found no significant difference between 2 and 4 days and 7 and 14 days of oral antibiotic therapy in the eradication of lower tract UTI [37]. For febrile children with UTI, there is no clear evidence available with regard to the recommended duration of antimicrobial therapy. A large multicenter randomized controlled trial has a still ongoing evaluation of the effectiveness of 5 days versus 10 days of treatment [38].
The clinician should choose from 7 to 14 days as the duration of antimicrobial therapy for the treatment of febrile UTI [1,25,39].
8.4.2. SPIDS recommendation for antimicrobial therapy duration
-
•
SPIDS recommends treatment courses of 3–7 days for most cases of lower UTI (cystitis) managed on an outpatient basis.
-
•
SPIDS recommends a treatment duration of 7–14 days for uncomplicated cases of upper UTI (Acute pyelonephritis).
-
•
Long treatment antibiotic courses may be required for complicated lower or upper UTI.
8.5. SPIDS recommendation for consultation and referral
-
•
Refer to pediatric specialist children of any age with high risk or serious illness and all children <3 months old with possible community-acquired UTI where bacterial sepsis could be considered according to the clinical presentation.
-
•
SPIDS recommends pediatric infectious diseases consultation for the infections caused by multiresistant-specific pathogens, particularly in the so-called SPICE organisms (Serratia, indole-positive Proteus, Citrobacter, and Enterobacter as well as Providencia, Morganella, and Hafnia), extended spectrum beta-lactamase (ESBL-producing bacteria) or carbapenem-resistant (CR-producing bacteria) because those organisms may require specific therapy and further follow up [40].
-
•SPIDS suggests referral to nephrologist or urologist in the following conditions:
-
oChildren with moderate to high-grade reflux (Grade III or more)
-
oChildren with congenital renal anomalies and urinary tract obstruction (e.g., UPJO, Obstructive megaureter, duplex system, multicystic dysplastic kidney, etc.)
-
oChildren with BBD
-
oChildren with impaired kidney function or hypertension
-
oChildren with recurrent febrile UTIs and abnormal result of renal and bladder imaging
-
o
9. Imaging studies
9.1. Ultrasonography
9.1.1. Background and rationale
-
•
Renal and bladder ultrasonography (RBUS) is a noninvasive, safe, and easy test that can detect renal and urinary bladder abnormalities. RBUS can also identify complicated renal infections (e.g., pyonephrosis, renal, or perirenal abscess) in children with acute UTI who fail to improve with appropriate antimicrobial therapy.41 The major advantages of RBUS are the lack of exposure to radiation and the detection of anatomic abnormalities that may require further evaluation, such as additional imaging or urological consultation. Although, RBUS is not a reliable study for the diagnosis of VUR or renal scarring, abnormalities in RBUS is useful in predicting the risk of renal scarring [42].
-
•
In Saudi Arabia, a study on 130 children, 12 years of age and younger, who were admitted with a first episode UTI, abnormal RBUS results were found in 38 patients (30.3%) [12]. Another study on 153 children younger than 5 years, abnormalities in RBUS were found on only 10% of patients with first UTI; however, RBUS was found on 50.7% of children with recurrent UTIs [43].
-
•
In a meta-analysis, including nine studies on children with UTI, 68% of children with high-grade VUR had either abnormal RBUS or a pathogen other than E. coli [42]. Data showed a high association between non E.coli UTI and renal abnormalities [44,45]. Children with UTI caused by an organism other than E-coli should undergo renal and bladder imaging to exclude associated renal anomalies particularly VUR.23,25,41
-
•
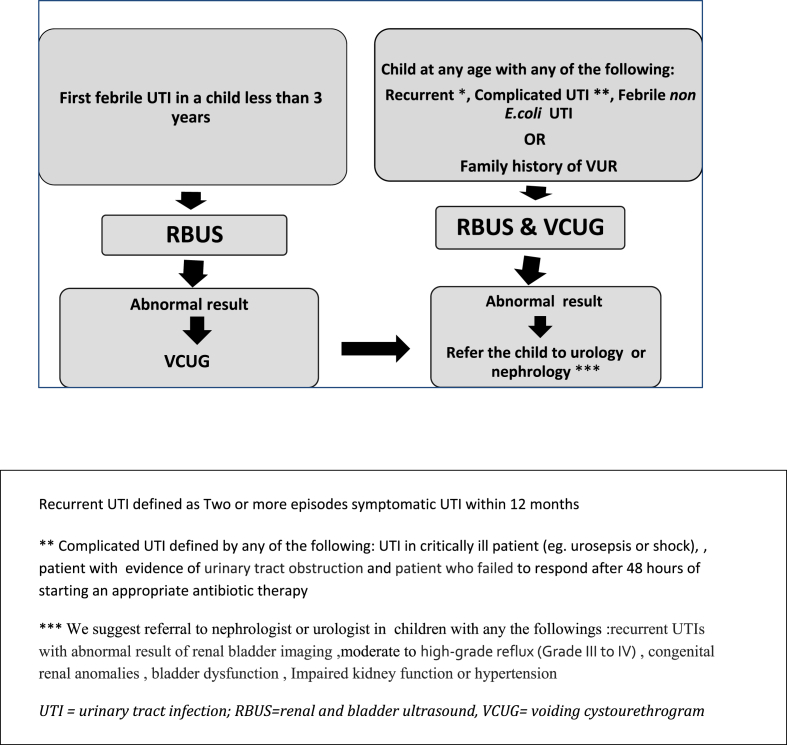
Fig. 2 shows an algorithm that summarizes the SPIDS recommendation for renal and bladder imaging in children with UTI
Fig. 2.
Algorithm for renal and bladder imaging in child with UTI.
9.1.2. SPIDS recommends RBUS to be performed in any of the following situations
-
•
First febrile UTI in children less than three years old
- •
9.1.3. Timing
The timing of RBUS depends on the clinical condition.
-
•
RBUS should be performed 2–6 weeks after the acute phase of infection to reduce the risk of false-positive results secondary to renal parenchymal inflammation during acute infection [4,23].
-
•
RBUS is recommended early during the acute phase of infection in infants and children who are severely ill or in those with complicated UTI or evidence of urinary tract obstruction to identify serious renal complications, such as renal or perirenal abscesses and pyonephrosis [4,23].
9.2. Voiding cystourethrogram (VCUG)
9.2.1. Background and rationale
-
•
VCUG is the radiological test of choice to diagnose and grade the severity of VUR (grade 1 through 5). VCUG can also identify the anatomy of male urethra, obstructive uropathies, and other abnormalities of the bladder.41 There are several disadvantages of performing VCUG, including high cost, excessive exposure to radiation, invasive catheterization, and the risk of infection.
-
•
Data from Saudi Arabia showed that about 33% of Saudi children presented with UTI have VUR [12,13,43]. A small number of cases with VUR ultimately requires surgical intervention for VUR [13].
-
•
The presence of an organism other than E. coli is significantly predicted of VUR [42,44,45].
-
•
SPIDS does not recommend VCUG routinely after the first febrile UTI due to the invasive nature and doubtful benefit of VCUG in many cases, Fig. 2.
9.2.2. SPIDS recommends VCUG to be performed in any of the following scenarios
-
o
Recurrent UTI
-
o
Complicated UTI (see definition)
-
o
Abnormalities in RBUS results (e.g., hydronephrosis, renal scarring, or any other findings that suggest either high-grade VUR or obstructive uropathy)
-
o
Febrile UTI caused by an organism other than E. coli.23,25,41
-
o
Child with UTI and family history of VUR in first-degree relatives [46].
9.2.3. Timing
9.3. Renal scintigraphy
9.3.1. Background and rationale
-
•
Renal scintigraphy using dimercaptosuccinic acid (DMSA) is the most sensitive test used to diagnose pyelonephritis during the acute phase of infection, and furthermore, detect the area of renal scar when performed months after the acute infection [48]. DMSA scan is an invasive test that involves an intravenous injection, several hours in hospital, and radiation exposure to the child [48].
-
•
SPIDS does not recommend using DMSA in the routine evaluation of children after first UTI.
9.3.2. SPIDS recommend DMSA to be performed in the following situations
-
•
Febrile UTI in children with severe VUR (grades IV and V) [42].
-
•
Clinician's concern about impaired renal function related to UTI BVU (e.g., elevated serum creatinine, hypertension, or proteinuria)
9.3.3. Timing
10. Prevention of recurrence and antibiotic prophylaxis
10.1. Background and rationale
-
•
Early diagnosis and prompt treatment of UTI is an effective method to reduce renal scarring associated with VUR and UTI. The role of antibiotic prophylaxis in the prevention of renal scarring in children with VUR remains disputed [49]. Moreover, the potential for the emergence of antimicrobial-resistant bacteria has been a major concern [50].
-
•
Randomized Intervention for Children with Vesicoureteral reflux (RIVUR) trials failed to reveal differences in the incidence of new renal scarring between the prophylaxis and placebo groups [51]. While the Swedish reflux trial has found that chemoprophylaxis reduces the rate of UTI recurrence and new renal scars in girls aged 1 to <2 years with VUR grade III or IV [52].
-
•
We do not recommend routine antibiotic prophylaxis in infants and children with normal urinary system or mild VUR (Grade I and II) after the first UTI because such cases do not benefit from the prophylaxis therapy [2,4,23,25].
-
•
We suggest an individualized decision for antibiotic prophylaxis after the assessment of the possible risks and benefits.
10.2. SPIDS recommends antibiotic prophylaxis in following conditions
-
•
Children with moderate to high-grade reflux (Grade III to IV)
-
•
Uncircumcised males with any grade of VUR
-
•
Children with BBD and any grade of VUR
10.3. Choice of antibiotic prophylaxis
10.3.1. An ideal agent depends on the local antimicrobial susceptibility pattern. We recommend to check the previous urine culture and susceptibility results and to choose antibiotics accordingly
10.3.2. SPIDS recommends a single daily prophylactic dose of any of the following options
-
1.
Nitrofurantoin: dose: 1–2 mg/kg/day.
-
2.Trimethoprim/sulfamethoxazole: dose based on trimethoprim at 2 mg/kg/day.
-
•Amoxicillin and cephalosporins should not routinely be used in prophylaxis because of the increased risk of development of resistant organisms [53]. However, these regimens can be used in infants less than two months of age or in patients who cannot tolerate or developed adverse effects related to the use of trimethoprim or nitrofurantoin.
-
•
11. Conclusion
-
•
UTIs are common in infants and children. It should be suspected in nonverbal children with unexplained fever and in older children with urinary symptoms suggestive of UTI. An appropriate urine sample should be collected for dipstick, microscopy, and culture before initiating an empirical antibiotic. Diagnosis of the UT should be made based on the combination of clinical symptoms and positive quantitative urine culture according to the recommended method of urine sample collection. Once UTI is diagnosed, an appropriate antibiotic therapy should be initiated as soon as possible to prevent complications and renal scarring. RBUS is recommended after first febrile UTI in all children less than 3 years and in children with complicated or recurrent at any age as well as in a child with febrile UTI caused by an organism other than E. coli. A VCUG is not routinely recommended after first UTI; however, it is indicated mainly in specific conditions (e.g., Abnormal RBUS result, recurrent UTI, and others). Prophylactic antibiotics do not seem to be beneficial after the first febrile UTI in children with mild VUR; although, it may be considered in high-grade reflux after the assessment of the possible risks and benefits.
Ethical statement
Hereby, I Dr.May ALbarrak consciously assure that for the manuscript (Diagnosis and Management of community-acquired urinary tract infection in infants and children: Clinical practice guideline endorsed by the Saudi Pediatric infectious Diseases Society) the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper properly credits the meaningful contributions of co-authors
-
4)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Roberts K.B., Downs S.M., Finnell S.M.E., Hellerstein S., Shortliffe L.D., Wald E.R. Reaffirmation of aap clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138 doi: 10.1542/peds.2016-3026. [DOI] [PubMed] [Google Scholar]
- 2.Robinson J.L., Finlay J.C., Lang M.E., Bortolussi R., Canadian Paediatric Society, Community Paediatrics Committee Urinary tract infection in infants and children: diagnosis and management. Paediatr Child Health. 2014 Jun 13;19(6):315–319. doi: 10.1093/pch/19.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald E.R., Roberts K.B. The diagnosis of UTI : colony count criteria revisited. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3239. [DOI] [PubMed] [Google Scholar]
- 4.Roberts K.B., Downs S.M., Finnell S.M.E., Hellerstein S., Shortliffe L.D., Wald E.R. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle L.E., Gupta K., Bradley S.F., Colgan R., DeMuri G.P., Drekonja D. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:E75–E83. doi: 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 6.Zorc J.J., Kiddoo D.A., Shaw K.N. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev. 2005;18:417–422. doi: 10.1128/CMR.18.2.417-422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein B., Giroir B., Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6 doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 8.Frokiaer J., Zeidel M. vol. 9. Elsevier; New York: 2011. Urinary tract obstruction. (Brenner and rector's the kidney). [Google Scholar]
- 9.Alanazi M.Q. An evaluation of community-acquired urinary tract infection and appropriateness of treatment in an emergency department in Saudi Arabia. Therapeut Clin Risk Manag. 2018;14:2363–2373. doi: 10.2147/TCRM.S178855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellstrom A., Hanson E., Hansson S., Hjalmas K., Jodal U. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991;66:232–234. doi: 10.1136/adc.66.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat R.G., Katy T.A., Place F.C. Pediatric urinary tract infections. Emerg Med Clin. 2011;29:637–653. doi: 10.1016/j.emc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Alshamsan L., Harbi A Al, Fakeeh K., Banyan E Al. First episode of urinary tract infection. Ann Saudi Med. 2009;29:12–2009. doi: 10.4103/0256-4947.51817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharifian M., Ahmadi M., Karimi A., Zand R.E., Moghadar R. vol. 24. 2013. pp. 731–736. (Of kidney diseases and transplantation original article urinary endothellin-1 level in children with pyelonephritis and). [DOI] [PubMed] [Google Scholar]
- 14.Hameed T., Al Nafeesah A., Chishti S., Al Shaalan M., Al Fakeeh K. Community-acquired urinary tract infections in children: resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int J Pediatr Adolesc Med. 2019;6:51–54. doi: 10.1016/j.ijpam.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mrild S., Wettergren B., Hellström M., Jodal U., Lincoln K., Ørskov I. Bacterial virulence and inflammatory response in infants with febrile urinary tract infection or screening bacteriuria. J Pediatr. 1988;112:348–354. doi: 10.1016/S0022-3476(88)80311-1. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S., Tsukamoto T., Terai A., Kurazono H., Takeda Y., Yoshida O. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;1127–9 doi: 10.1097/00005392-199703000-00119. [DOI] [PubMed] [Google Scholar]
- 17.Svanborg C., Frendéus B., Godaly G., Hang L., Hedlund M., Wachtler C. Toll-like receptor signaling and chemokine receptor expression influence the severity of urinary tract infection. J Infect Dis. 2001;183:61–65. doi: 10.1086/318858. [DOI] [PubMed] [Google Scholar]
- 18.Honkinen O., Jahnukainen T., Mertsola J., Eskola J., Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19:630–634. doi: 10.1097/00006454-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Shaikh N., Morone N.E., Bost J.E., Farrell M.H. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302–308. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 20.Chang S.L., Shortliffe L.D. Pediatric urinary tract infections. Pediatr Clin. 2006;53:379–400. doi: 10.1016/j.pcl.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Keren R., Shaikh N., Pohl H., Gravens-Mueller L., Ivanova A., Zaoutis L. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136:e13–21. doi: 10.1542/peds.2015-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman A.S., Bauer S.B. Diagnosis and management of dysfunctional voiding. Curr Opin Pediatr. 2006 Apr 1;18(2):139–147. doi: 10.1097/01.mop.0000193289.64151.49. [DOI] [PubMed] [Google Scholar]
- 23.Mori R., Lakhanpaul M., Verrier-Jones K. Diagnosis and management of urinary tract infection in children: summary of NICE guidance. Bmj. 2007 Aug 23;335(7616):395–397. doi: 10.1136/bmj.39286.700891.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards (Nccls) GP-16A2, volume 21, No. 19, Urinalysis and collection, transportation, and preservation of urine specimens; Approved guideline-second edition, p. 4-21.
- 25.Ammenti A., Alberici I., Brugnara M., Chimenz R., Guarino S., La Manna A. Updated Italian recommendations for the diagnosis, treatment and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr Int J Paediatr. 2020;109:236–247. doi: 10.1111/apa.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell H.R., McCredie D.A., Ritchie M.A. Urinary nitrite in symptomatic and asymptomatic urinary infection. Arch Dis Child. 1987;62:138–140. doi: 10.1136/adc.62.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritzenwanker M., Imirzalioglu C., Chakraborty T., Wagenlehner F.M. Modern diagnostic methods for urinary tract infections. Expert Rev Anti Infect Ther. 2016;14:1047–1063. doi: 10.1080/14787210.2016.1236685. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh N., Shope T.R., Hoberman A., Vigliotti A., Kurs-Lasky M., Martin J.M. Association between uropathogen and pyuria. Pediatrics. 2016;138 doi: 10.1542/peds.2016-0087. [DOI] [PubMed] [Google Scholar]
- 29.Primack W., Bukowski T., Sutherland R., Gravens-Mueller L., Carpenter M. What urinary colony count indicates a urinary tract infection in children? J Pediatr. 2017;191:259–261. doi: 10.1016/j.jpeds.2017.08.012. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaikh N., Borrell J.L., Evron J., Leeflang M.M. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev. 2015;2017 doi: 10.1002/14651858.CD009185.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachur R., Caputo G.L. Bacteremia and meningitis among infants with urinary tract infections. Pediatr Emerg Care. 1995;11:280–284. doi: 10.1097/00006565-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Albalawi S.K., Albalawi B.K., Shwameen M.O., Alharbi M.H. Bacterial susceptibility to antibiotics in urinary tract infections in children, KSAFH, Saudi Arabia, tabuk. The Egyptian Journal of Hospital Medicine. 2018 Oct 1;73(6):6952–6954. [Google Scholar]
- 33.Strohmeier Y., Hodson E.M., Willis N.S., Webster A.C., Craig J.C. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014;(7) doi: 10.1002/14651858.CD003772.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffrey H. Are oral antibiotics equivalent to intravenous antibiotics for the initial management of pyelonephritis in children? Paediatr Child Health (Oxford) 2010;15:150–152. doi: 10.1093/pch/15.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calbo E., Romaní V., Xercavins M., Gómez L., Vidal C.G., Quintana S. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum β-lactamases. J Antimicrob Chemother. 2006;57:780–783. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 36.Bryce A., Costelloe C., Wootton M., Butler C.C., Hay A.D. Comparison of risk factors for, and prevalence of, antibiotic resistance in contaminating and pathogenic urinary Escherichia coli in children in primary care: prospective cohort study. J Antimicrob Chemother. 2018;73:1359–1367. doi: 10.1093/jac/dkx525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michael M., Hodson E.M., Craig J.C., Martin S., Moyer V.A. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD003966. [DOI] [PubMed] [Google Scholar]
- 38.Hoberman A. The SCOUT study: short course therapy for urinary tract infections in children. https://clinicaltrials.gov/ct2/show/NCT01595529 Accessed October 14, 2016.
- 39.Strohmeier Y., Hodson E.M., Willis N.S., Webster A.C., Craig J.C. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014;(7):CD003772. doi: 10.1002/14651858.CD003772.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moy S., Sharma R. Treatment outcomes in infections caused by “SPICE” (Serratia, Pseudomonas, indole-positive Proteus, Citrobacter, and Enterobacter) organisms: carbapenem versus noncarbapenem regimens. Clin Therapeut. 2017;39:170–176. doi: 10.1016/j.clinthera.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Shaikh N, Hoberman A. Urinary tract infections in infants older than one month and young children: acute management, imaging, and prognosis. In: Post T.W., editor. UpToDate. Waltham, MA:.
- 43.Shaikh N., Craig J.C., Rovers M.M., Da Dalt L., Gardikis S., Hoberman A. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 2014;168:893–900. doi: 10.1001/jamapediatrics.2014.637. [DOI] [PubMed] [Google Scholar]
- 44.Garout W.A., Kurdi H.S., Shilli A.H., Kari J.A. Urinary tract infection in children younger than 5 years. Etiology and associated urological anomalies. Saudi Med J. 2015;36:497–501. doi: 10.15537/smj.2015.4.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman S., Reif S., Assia A., Levy I. Clinical and laboratory characteristics of non-E coli urinary tract infections. Arch Dis Child. 2006;91:845–846. doi: 10.1136/adc.2005.080721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberici I., La Manna A., Pennesi M., Starc M., Scozzola F., Nicolini G. First urinary tract infections in children: the role of the risk factors proposed by the Italian recommendations. Acta Paediatr Int J Paediatr. 2019;108:544–550. doi: 10.1111/apa.14506. [DOI] [PubMed] [Google Scholar]
- 47.Skoog S.J., Peters C.A., Arant B.S., Copp H.L., Elder J.S., Hudson R.G. Pediatric vesicoureteral reflux guidelines panel summary report: clinical practice guidelines for screening siblings of children with vesicoureteral reflux and neonates/infants with prenatal hydronephrosis. J Urol. 2010;184:1145–1151. doi: 10.1016/j.juro.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 48.Mazzi S., Rohner K., Hayes W., Weitz M. Timing of voiding cystourethrography after febrile urinary tract infection in children: a systematic review. Arch Dis Child. 2020;105:264–269. doi: 10.1136/archdischild-2019-316958. [DOI] [PubMed] [Google Scholar]
- 49.American College of Radiology ACR appropriateness criteria. Urinary tract infection--child. https://acsearch.acr.org/docs/69444/Narrative/ Accessed on July 30, 2019. [PubMed]
- 50.Robinson J.L., Finlay J.C., Lang M.E., Bortolussi R. Prophylactic antibiotics for children with recurrent urinary tract infections. Paediatr Child Health. 2015;20:45–47. doi: 10.1093/pch/20.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.RIVUR Trial Investigators. Hoberman A., Greenfield S.P., Mattoo T.K., Keren R., Mathews R., Pohl H.G., Kropp B.P., Skoog S.J., Nelson C.P., Moxey-Mims M., Chesney R.W., Carpenter M.A. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367–2376. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandström P., Jodal U., Sillén U., Hansson S. The Swedish reflux trial: review of a randomized, controlled trial in children with dilating vesicoureteral reflux. J Pediatr Urol. 2011 Dec 1;7(6):594–600. doi: 10.1016/j.jpurol.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Cheng C.H., Tsai M.H., Huang Y.C., Su L.H., Tsau Y.K., Lin C.J. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics. 2008 Dec 1;122(6):1212–1217. doi: 10.1542/peds.2007-2926. [DOI] [PubMed] [Google Scholar]